Outpatient Testing for HIV in Italy, 2018–2023—Preliminary Data
Abstract
1. Introduction
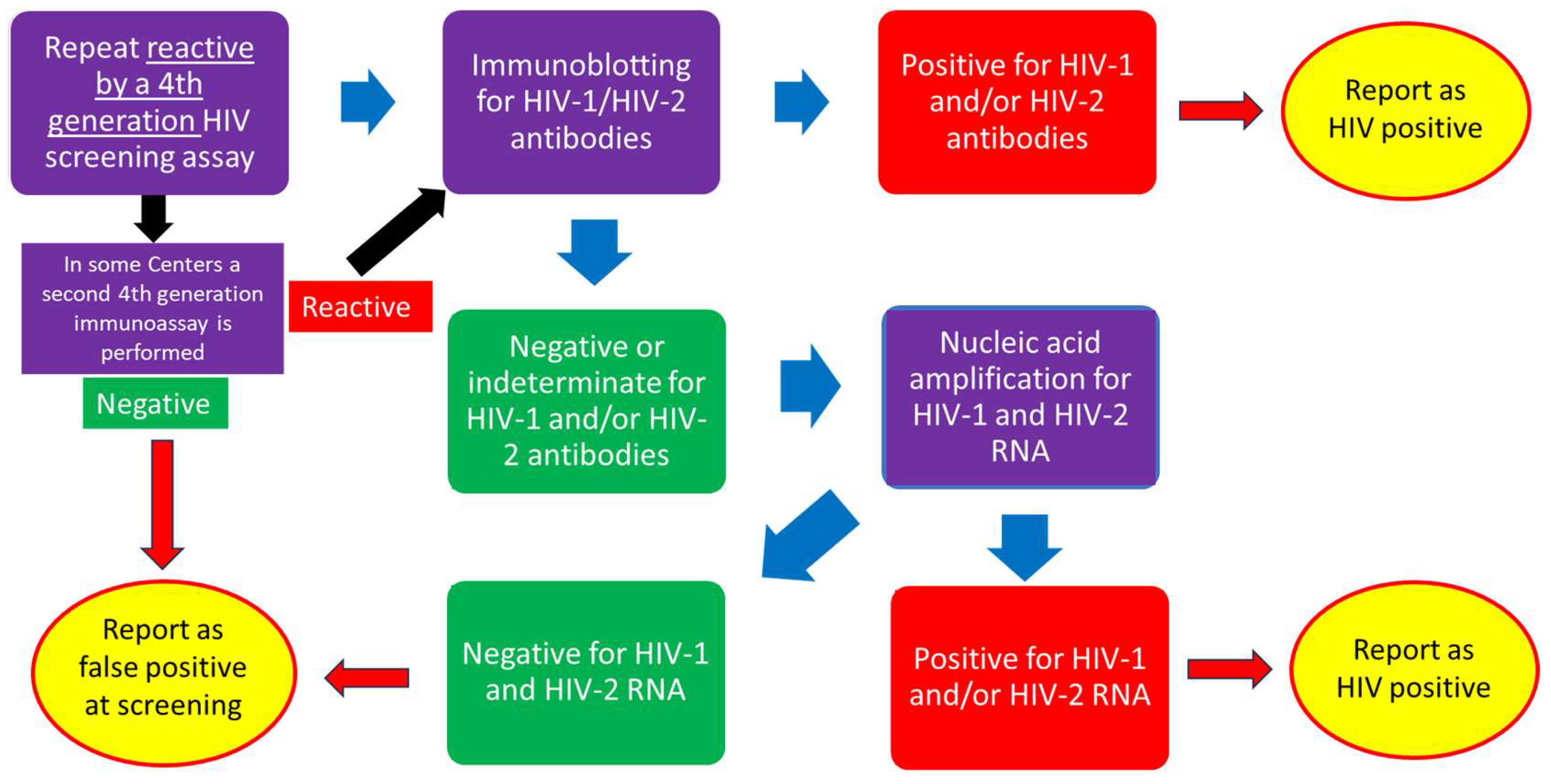
2. Materials and Methods
3. Results
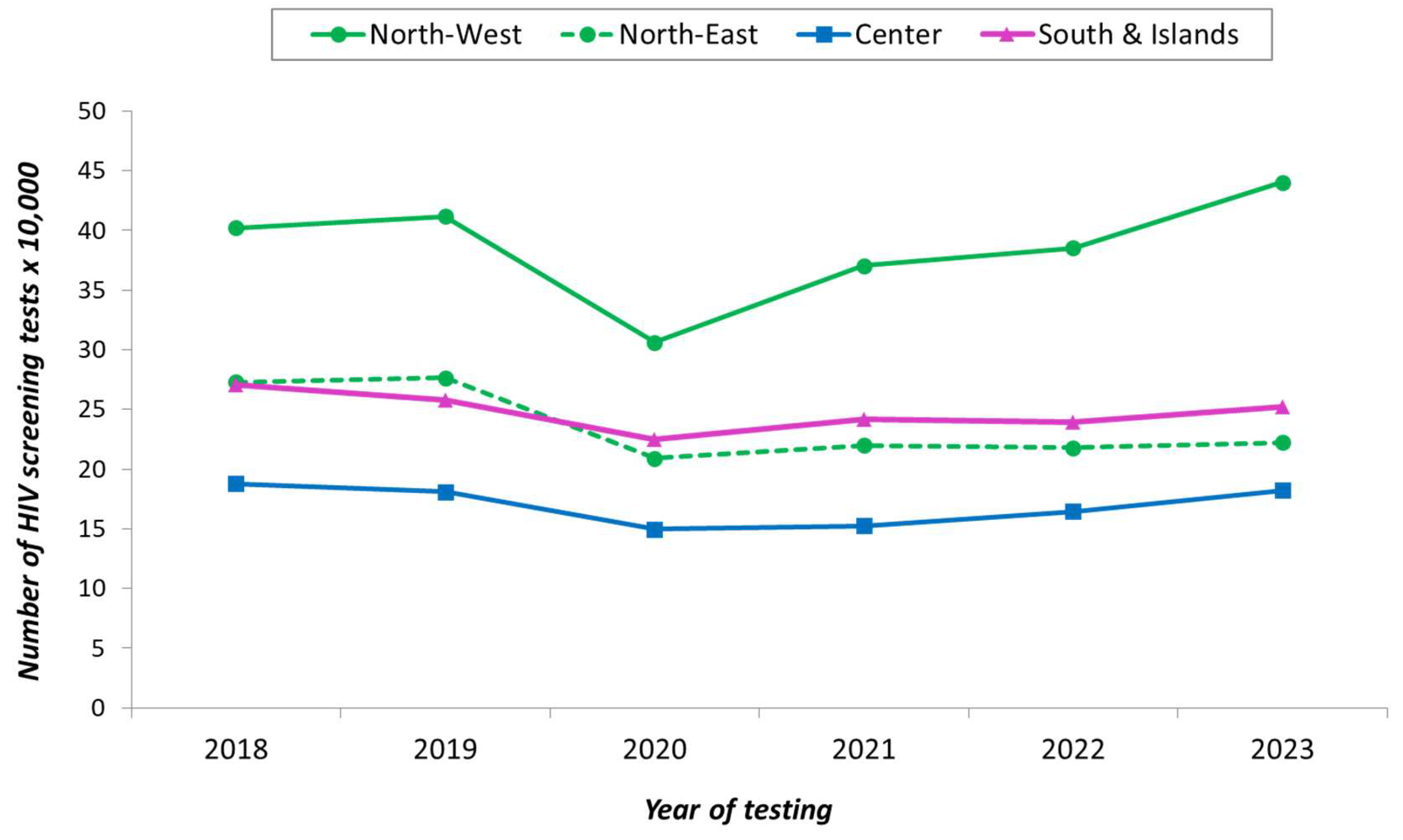
3.1. Overview on HIV Tests
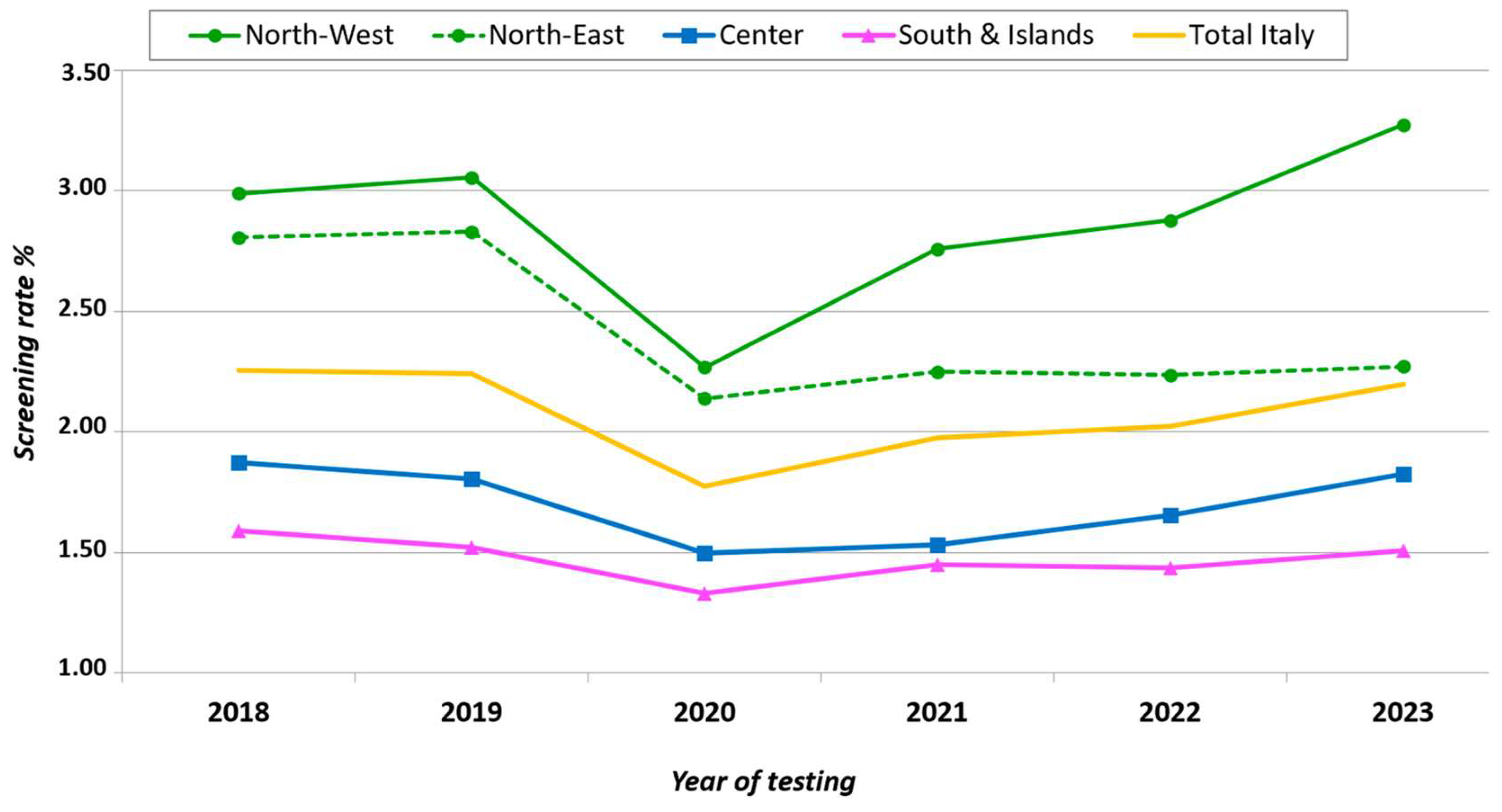
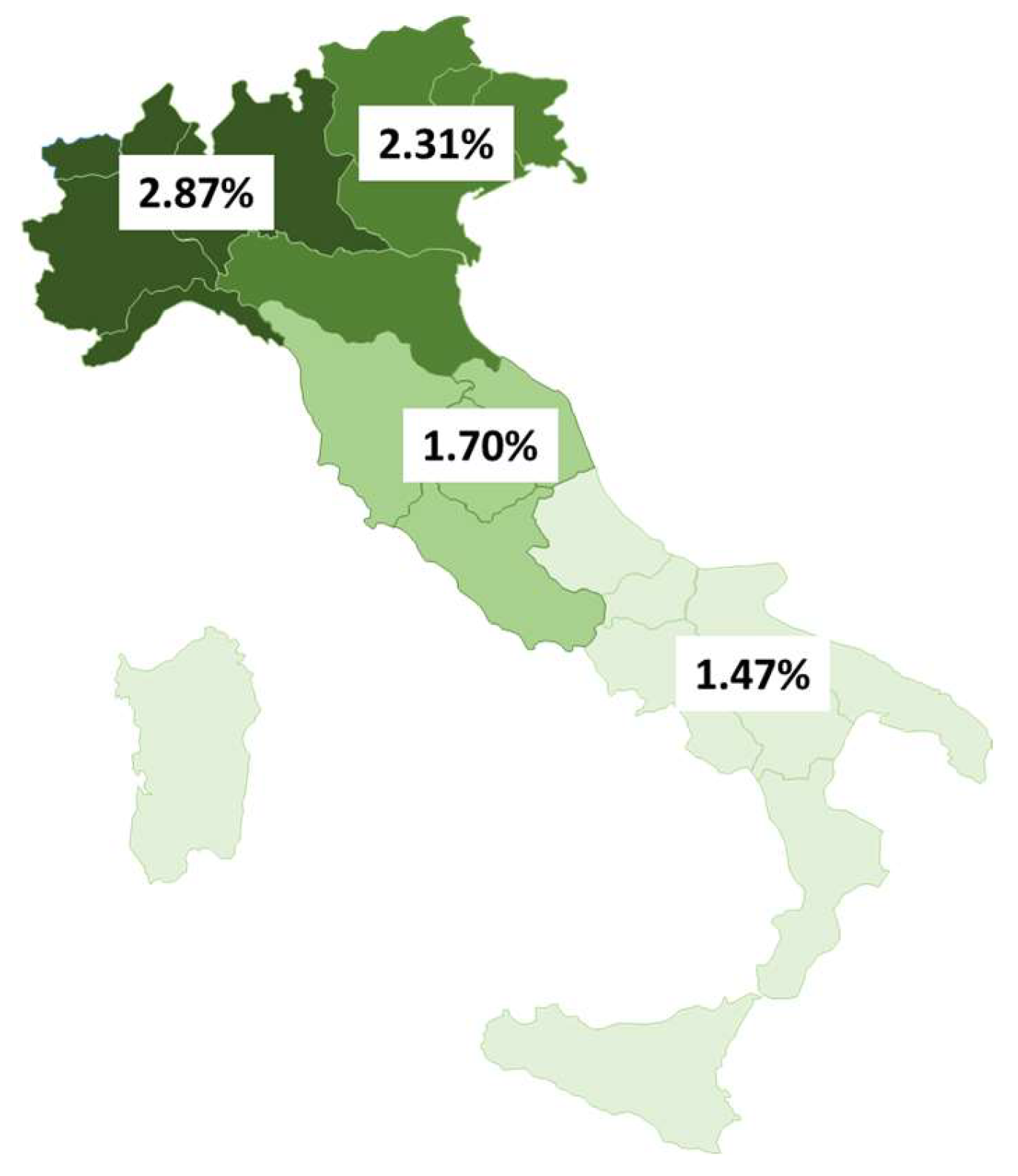
3.2. HIV Testing Rates Among Adult Residents, New HIV Diagnoses, and Patients Receiving ART
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immune Deficiency Syndrome |
| UN | United Nations |
| UNAIDS | United Nations Programme on HIV/AIDS |
| HIV | human immunodeficiency virus |
| GBD | Global Burden of Disease |
| MoH | Ministry of Health |
| AMCLI | Associazione Italiana Microbiologi Clinici |
| NAT | nucleic acid tests |
| ISTAT | Istituto Nazionale di Statistica |
| NHD | new HIV diagnosis |
| ART | antiretroviral therapy |
| WHO | World Health Organization |
| CDC | Centers for Disease Control |
| US | United States |
References
- UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. UNAIDS/JC2684 (English original, October 2014). Available online: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (accessed on 23 January 2025).
- The Path That Ends AIDS: UNAIDS Global AIDS Update 2023. Geneva: Joint United Nations Programme on HIV/AIDS. Licence: CC BY-NC-SA 3.0 IGO. 2023. Available online: https://thepath.unaids.org/wp-content/themes/unaids2023/assets/files/2023_report.pdf (accessed on 23 January 2025).
- GBD 2021 HIV Collaborators. Global, regional, and national burden of HIV/AIDS, 1990–2021, and forecasts to 2050, for 204 countries and territories: The Global Burden of Disease Study 2021. Lancet HIV 2024, 11, e807–e822. [Google Scholar] [CrossRef] [PubMed]
- Regine, V.; Pugliese, L.; Ferri, M.; Santaquilani, M.; Suligoi, B. Aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di AIDS in Italia al 31 dicembre 2023. Not. Ist. Sup. Sanità 2024, 37, 3–59. [Google Scholar]
- AMCLI ETS. Percorso Diagnostico “Infezioni da HIV”–Rif. 2023-03, rev. 2023. Available online: https://www.amcli.it/wp-content/uploads/2024/02/2023-03_INFEZIONI-DA-HIV.pdf (accessed on 23 January 2025).
- ISTAT Population Data by Region 2018–2023. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_RICPOPRES2011# (accessed on 23 January 2025).
- Italian Ministry Decree 31 March 2008. Istituzione del Sistema di Sorveglianza delle Nuove Diagnosi di Infezione da HIV. Gazzetta Ufficiale, 31 March 2008; no. 175.
- Regine, V.; Pugliese, L.; Ferri, M.; Santaquilani, M.; Suligoi, B. Aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di AIDS in Italia al 31 dicembre 2022. Not. Ist. Sup. Sanità. 2023, 36, 3–59. [Google Scholar]
- Branson, B.M. HIV Diagnostics: Current recommendations and Opportunities for Improvement. Infect. Dis. Clin. N. Am. 2019, 33, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Ciccullo, A.; Baldin, G.; Cauda, R.; Rusconi, S.; Giacomelli, A.; Oreni, L.; Borghi, V.; Mussini, C.; Guaraldi, G.; et al. Has COVID-19 changed the approach to HIV diagnosis? Medicine 2021, 100, e27418. [Google Scholar] [CrossRef] [PubMed]
- Rick, F.; Odoke, W.; van den Hombergh, J.; Benzaken, A.S.; Avelino-Silva, V.I. Impact of coronavirus disease (COVID-19) on HIV testing and care provision across four continents. HIV Med. 2022, 23, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Marziano, V.; Guzzetta, G.; Menegale, F.; Sacco, C.; Petrone, D.; Uriales, A.M.; Del Manso, M.; Bella, A.; Fabiani, M.; Vescio, M.F.; et al. Estimating SARS-CoV-2 infections and associated changes in COVID-19 severity and fatality. Influenza Other Respir. Viruses 2023, 17, e13181. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe, European Centre for Disease Prevention and Control. HIV/AIDS Surveillance in Europe 2024–2023 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2024; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/HIV_Surveillance_Report_2024.pdf (accessed on 30 January 2025).
- Adhikari, E.H.; Macias, D.; Gaffney, D.; White, S.; Rogers, V.L.; McIntire, D.D.; Roberts, S.W. Diagnostic accuracy of fourth-generation ARCHITECT HIV Ag/Ab Combo test and utility of signal-to-cutoff ratio to predict false-positive HIV tests in pregnancy. Am. J. Obs. Gynecol. 2018, 19, 408.e1–408.e9. [Google Scholar] [CrossRef] [PubMed]
- Baltaro, R.J.; Malenie, R.; Melbourne, H.; Garcia, F.; Gould, E.W.; Renshaw, A.A. Risk stratification of HIV infection for patients needing molecular confirmation with the Abbott 4th generation Architect System. J. Clin. Virol. 2019, 113, 31–34. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS Joint United Nations. Programme on HIV/AIDS Global HIV & AIDS Statistics. Available online: https://www.unaids.org/en/regionscountries/countries/italy (accessed on 23 January 2025).
- WHO. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 25 January 2025).
- Kondo, M.; Sudo, K.; Sano, T.; Kawahata, T.; Itoda, I.; Iwamuro, S.; Yoshimura, Y.; Tachikawa, N.; Kojima, Y.; Mori, H.; et al. Comparative evaluation of the Geenius HIV 1/2 Confirmatory Test and the HIV-1 and HIV-2 Western blots in the Japanese population. PLoS ONE 2018, 13, e0198924. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 2018. Available online: https://stacks.cdc.gov/view/cdc/50872 (accessed on 28 January 2025).
- Eshleman, S.H.; Piwowar-Manning, E.; Wilson, E.A.; Lennon, D.; Fogel, J.M.; Agyei, Y.; Sullivan, P.A.; Weng, L.; Moore, A.; Laeyendecker, O.; et al. Determination of HIV status and identification of incident HIV infections in a large, community-randomized trial: HPTN 071 (PopART). J. Int. AIDS Soc. 2020, 23, e25452. [Google Scholar] [CrossRef] [PubMed]
- Delaney, K.P.; Heffelfinger, J.D.; Wesolowski, L.G.; Owen, M.; Meyer, W.A.; Kennedy, S.; Uniyal, A.; Kerndt, P.R.; Branson, B.M. Performance of an alternative laboratory-based algorithm for HIV diagnosis in a high-risk population. J. Clin. Virol. 2011, 52 (Suppl. S1), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, C.; (Piedmont Regional Service for the Epidemiology of Infectious Diseases (SeREMI) and Research and Innovation Department (DAIRI), SS Antonio e Biagio e C Arrigo, University Hospital, Alessandria, Italy). Personal communication, 2025.
- Duffus, W.A.; Kintziger, K.W.; Heffelfinger, J.D.; Delaney, K.P.; Stephens, T.; Gibson, J.J. Repeat Western Blot Testing After Receiving an HIV Diagnosis and Its Association with Engagement in Care. Open AIDS J. 2012, 6 (Suppl. S1), 196–204. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, S.; Shallal, A.; Peterson, E.; Cook, B.; Markowitz, N. Increase in False-Positive Fourth-Generation Human Immunodeficiency Virus Tests in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 77, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart-Jongbloed, K.J.; Vasylyev, M.; Jordans, C.C.E.; Bernardino, J.L.; Nozza, S.; Psomas, C.K.; Voit, F.; Barber, T.J.; Skrzat-Klapaczynska, A.; Sandulescu, O.; et al. Systematic Review: Strategies for Improving HIV Testing and Detection Rates in European Hospitals. Microorganisms 2024, 12, 254. [Google Scholar] [CrossRef] [PubMed]
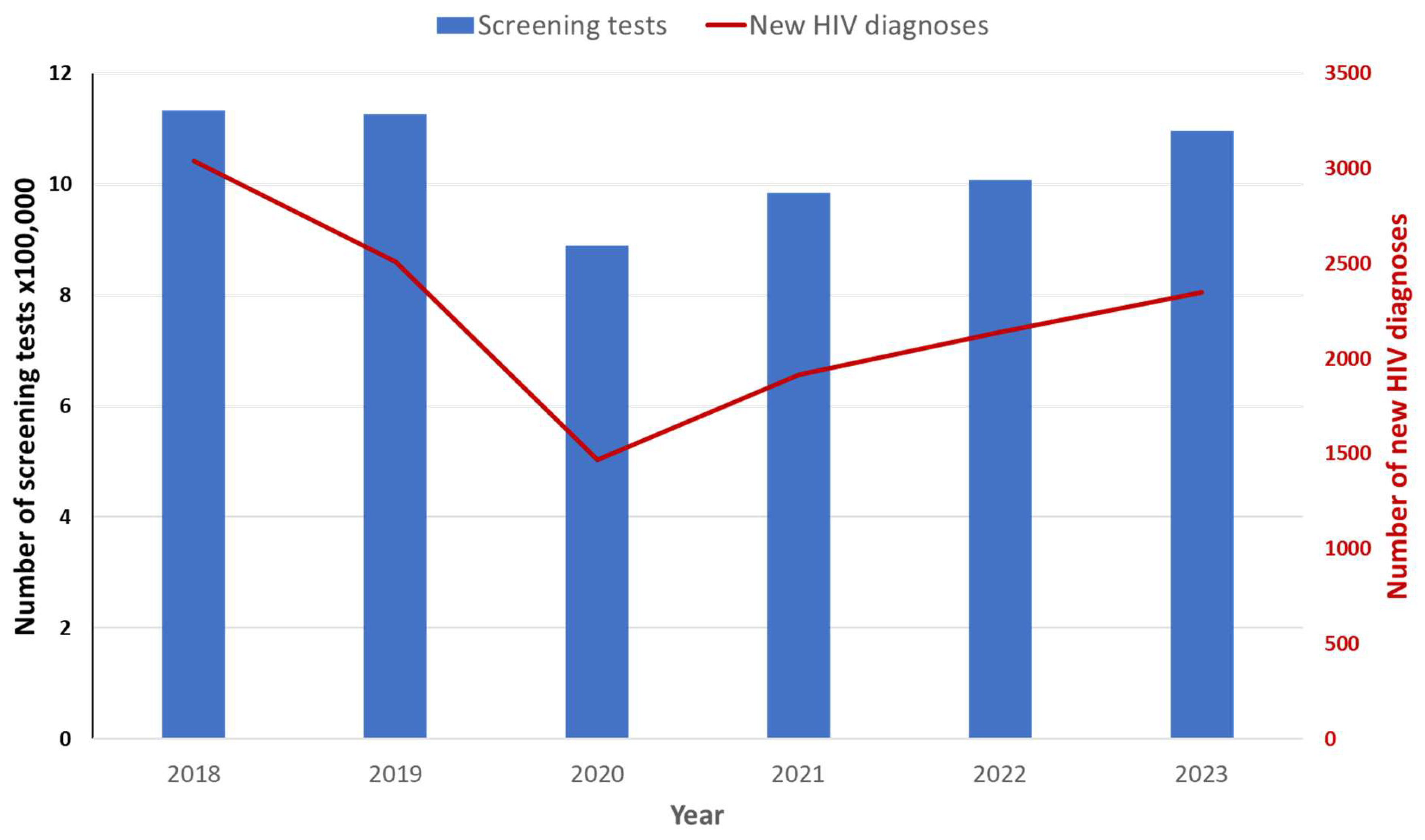
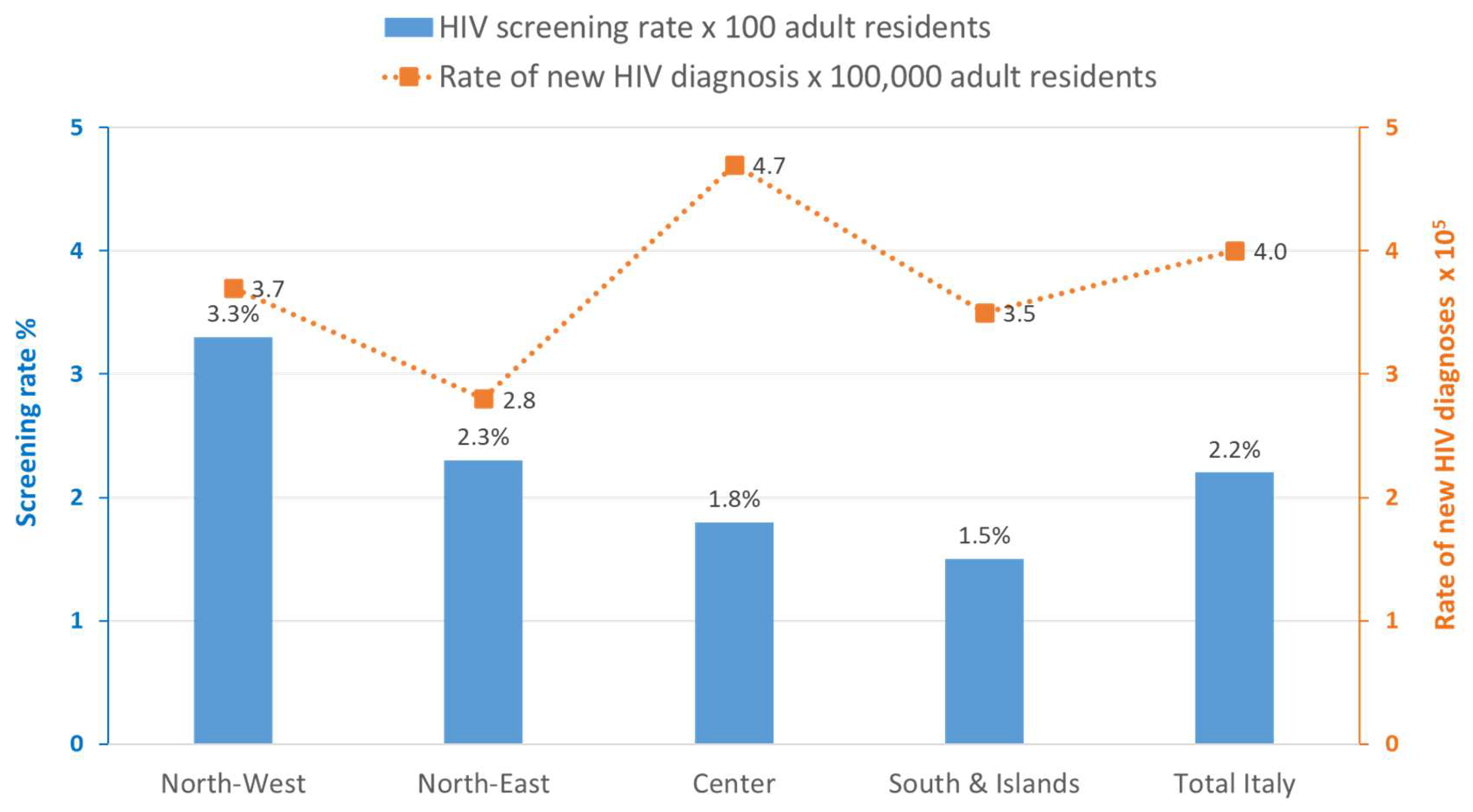
| Type of HIV Test | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Total |
|---|---|---|---|---|---|---|---|
| All HIV tests N. | 1,355,413 | 1,345,857 | 1,045,246 | 1,187,581 | 1,196,361 | 1,297,921 | 7,428,379 |
| % of screening tests out of all HIV tests | 83.6% | 83.7% | 85.1% | 82.9% | 84.2% | 84.5% | 84.0% |
| Screening rate/100 resident adults | 0.5% | 0.5% | 0.7% | 0.8% | 0.5% | 0.6% | 0.6% |
| Confirmatory HIV immunoblot N. | 5909 | 5931 | 6313 | 7755 | 5272 | 6417 | 37,597 |
| % of confirmatory immunoblot out of HIV screening tests | 0.5% | 0.5% | 0.7% | 0.8% | 0.5% | 0.6% | 0.6% |
| HIV-RNA NAT N. | 199,021 | 193,908 | 127,306 | 160,122 | 157,877 | 168,824 | 1,007,058 |
| % of HIV-RNA NAT out of all HIV tests | 14.7% | 14.4% | 12.2% | 13.5% | 13.2% | 13.0% | 13.6% |
| Other HIV tests N. | 17,106 | 19,072 | 21,655 | 34,994 | 25,681 | 25,858 | 144,366 |
| % of all HIV tests | 1.3% | 1.4% | 2.1% | 2.9% | 2.1% | 2.0% | 1.9% |
| 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Total | |
|---|---|---|---|---|---|---|---|
| Number of new HIV diagnoses (NHD) | 3038 | 2510 | 1470 | 1914 | 2140 | 2349 | 13,421 |
| Average n. of screening tests per NHD | 373 | 44 | 605 | 514 | 471 | 467 | 465 |
| Average n. of confirmatory immunoblot tests per NHD | 1.9 | 2.4 | 4.3 | 4.1 | 2.5 | 2.7 | 2.8 |
| Number of patients receiving ART | 121,000 | 123,000 | 125,000 | 127,000 | 130,000 | 131,000 | 757,000 |
| Average n. of HIV RNA tests per patient receiving ART | 1.6 | 1.6 | 1.0 | 1.3 | 1.2 | 1.3 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, C.; Regine, V.; Caraglia, A.; Centrone, F.; Chironna, M.; Cruschelli, G.; Farinella, M.; Orlando, V.A.; Pasqualini, C.; Puglia, M.; et al. Outpatient Testing for HIV in Italy, 2018–2023—Preliminary Data. Microorganisms 2025, 13, 655. https://doi.org/10.3390/microorganisms13030655
Galli C, Regine V, Caraglia A, Centrone F, Chironna M, Cruschelli G, Farinella M, Orlando VA, Pasqualini C, Puglia M, et al. Outpatient Testing for HIV in Italy, 2018–2023—Preliminary Data. Microorganisms. 2025; 13(3):655. https://doi.org/10.3390/microorganisms13030655
Chicago/Turabian StyleGalli, Claudio, Vincenza Regine, Anna Caraglia, Francesca Centrone, Maria Chironna, Gianluca Cruschelli, Massimo Farinella, Valentina Annachiara Orlando, Chiara Pasqualini, Monia Puglia, and et al. 2025. "Outpatient Testing for HIV in Italy, 2018–2023—Preliminary Data" Microorganisms 13, no. 3: 655. https://doi.org/10.3390/microorganisms13030655
APA StyleGalli, C., Regine, V., Caraglia, A., Centrone, F., Chironna, M., Cruschelli, G., Farinella, M., Orlando, V. A., Pasqualini, C., Puglia, M., Pugliese, L., Rancilio, L., Tavoschi, L., Voller, F., & Suligoi, B. (2025). Outpatient Testing for HIV in Italy, 2018–2023—Preliminary Data. Microorganisms, 13(3), 655. https://doi.org/10.3390/microorganisms13030655






