Biodegradation of S-Triazine Herbicides Under Saline Conditions by Paenarthrobacter ureafaciens PC, a New Halotolerant Bacterial Isolate: Insights into Both the Degradative Pathway and Mechanisms of Tolerance to High Salt Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.2.1. Isolation and Identification of the Halotolerant Prometryne-Degrading Bacterial Strain
2.2.2. Prometryne Degradation by Growing Cells of the Target Strain
2.2.3. Optimization of Conditions for Prometryne Degradation by Growing Cells of the Halotolerant Strain
2.2.4. Possible Prometryne-Degrading Pathways and Halotolerance Mechanisms of the Target Strain
2.3. Assays
2.3.1. Density of Bacterial Cell Suspension
2.3.2. Concentration of S-Triazine Herbicides
2.3.3. Analysis of Possible Degradation Intermediates of Prometryne
2.3.4. Genome Sequencing
2.4. Statistical Analysis
3. Results and Discussion
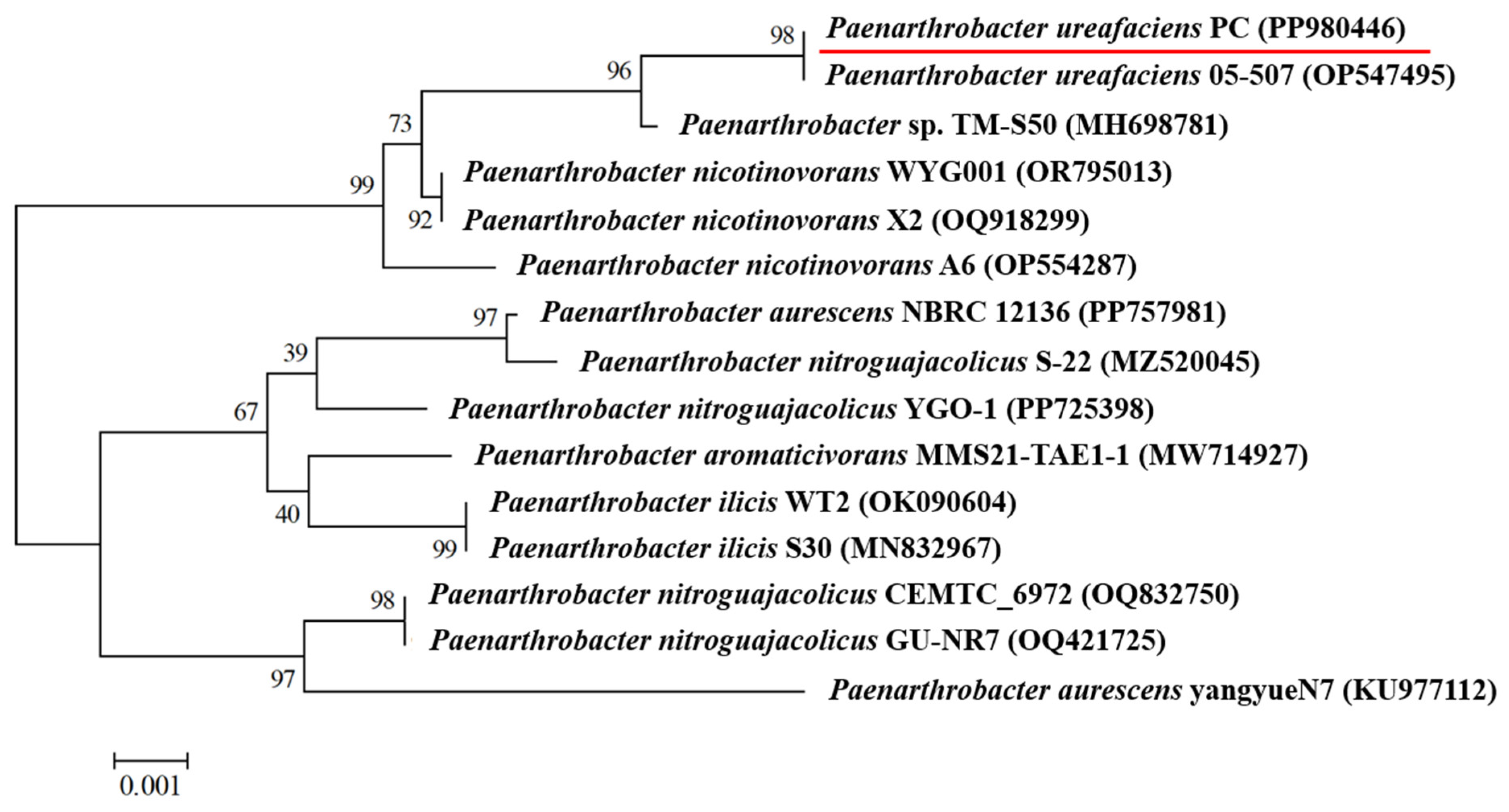
3.1. Isolation and Identification of a Halotolerant Bacterium Capable of Degrading Prometryne Efficiently
3.2. Prometryne Degradation by Growing Cells of the Strain PC
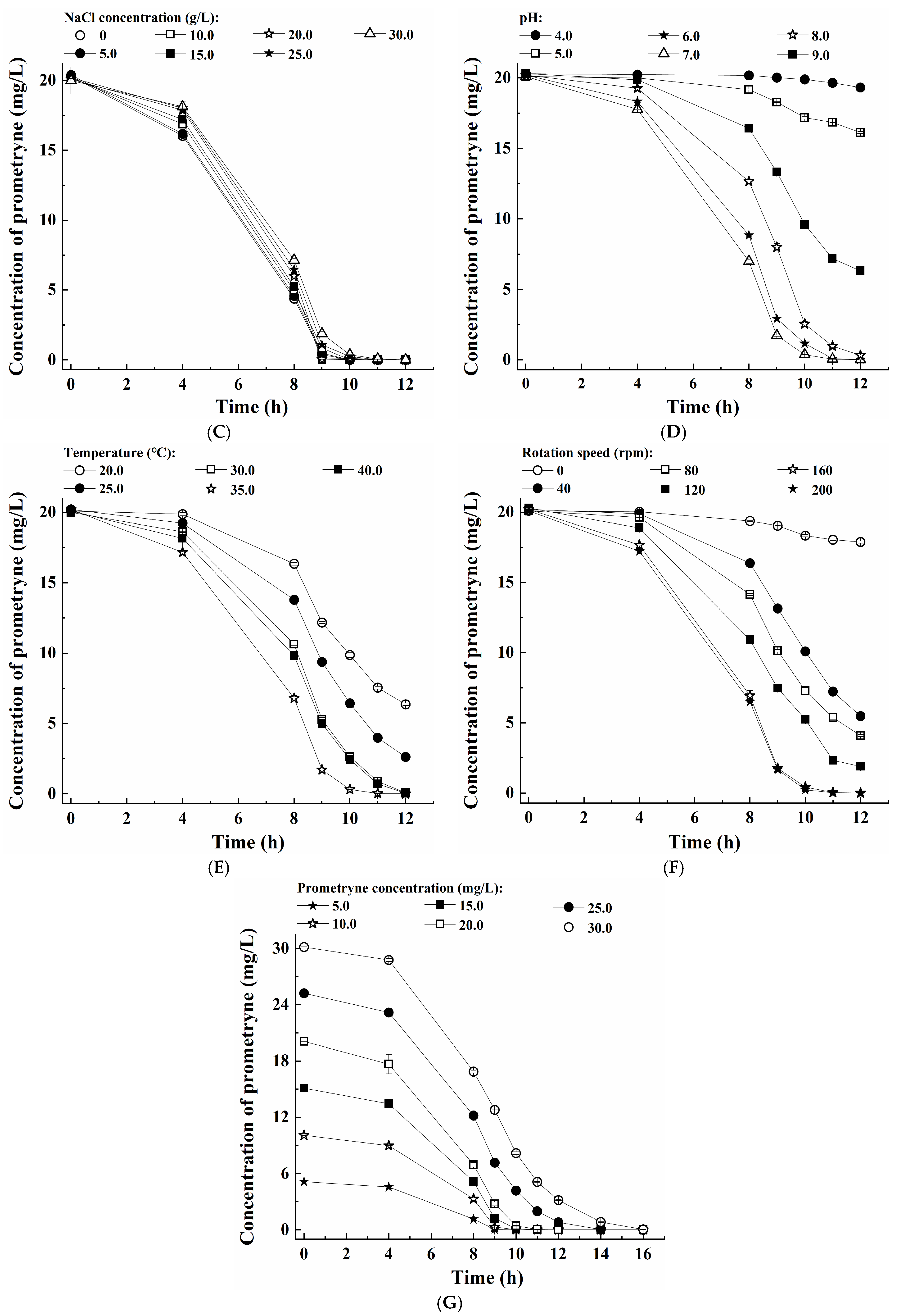
3.3. Optimization of Conditions for Prometryne Degradation by Growing Cells of the Strain PC
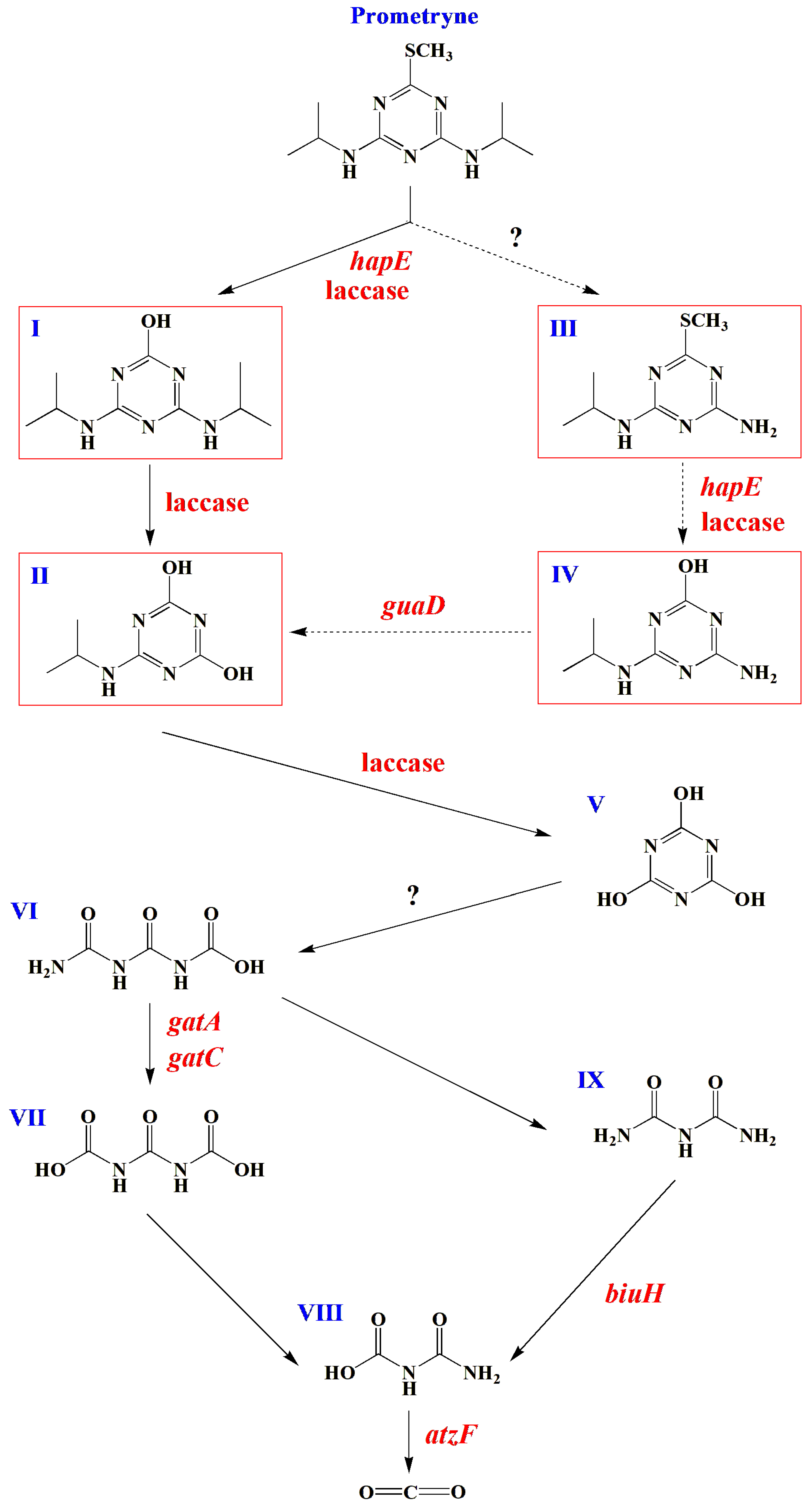
3.4. Speculation of Degradation Pathways of Prometryne by the Strain PC
3.4.1. Possible Degradation Intermediates of Prometryne by the Strain PC
3.4.2. Genome Sequencing of the Strain PC
3.4.3. Possible Degradation Pathways of Prometryne by the Strain PC
3.5. Possible Halotolerance Mechanisms of the Strain PC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viegas, C.A.; Silva, V.P.; Varela, V.M.; Correia, V.; Ribeiro, R.; Moreira-Santos, M. Evaluating formulation and storage of Arthrobacter aurescens strain TC1 as a bioremediation tool for terbuthylazine contaminated soils: Efficacy on abatement of aquatic ecotoxicity. Sci. Total Environ. 2019, 668, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.S.; Weihermüller, L.; Tappe, W.; Mukherjee, S.; Spielvogel, S. Field scale boscalid residues and dissipation half-life estimation in a sandy soil. Chemosphere 2016, 145, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Graymore, M.; Stagnitti, F.; Allinson, G. Impacts of atrazine in aquatic ecosystems. Environ. Int. 2001, 26, 483–495. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, C.; Song, F.; Li, H.; Liu, R.; Gao, J. Complete genome sequence and function gene identify of prometryne-degrading strain Pseudomonas sp. DY-1. Microorganisms 2021, 9, 1261. [Google Scholar] [CrossRef]
- Mnyandu, H.M.; Mahlambi, P.N. Optimization and application of QuEChERS and SPE methods followed by LC-PDA for the determination of triazines residues in fruits and vegetables from Pietermaritzburg local supermarkets. Food Chem. 2021, 360, 129818. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef]
- Pham, T.L.; Boujelbane, F.; Bui, H.N.; Nguyen, H.T.; Bui, X.T.; Nguyen, D.N.; Nguyen, H.T.T.; Phan, H.A.; Duong, H.T.G.; Bui, H.M. Pesticide production wastewater treatment by Electro-Fenton using Taguchi experimental design. Water Sci. Technol. 2021, 84, 3155–3171. [Google Scholar] [CrossRef]
- Huang, X.; He, J.; Yan, X.; Hong, Q.; Chen, K.; He, Q.; Zhang, L.; Liu, X.; Chuang, S.; Li, S.; et al. Microbial catabolism of chemical herbicides: Microbial resources, metabolic pathways and catabolic genes. Pestic. Biochem. Physiol. 2017, 143, 272–297. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Zhang, B.; Ni, Y.; Liu, J.; Yan, T.; Zhu, X.; Li, Q.X.; Hua, R.; Pan, D.; Wu, X. Bead-immobilized Pseudomonas stutzeri Y2 prolongs functions to degrade s-triazine herbicides in industrial wastewater and maize fields. Sci. Total Environ. 2020, 731, 139183. [Google Scholar] [CrossRef]
- Cao, D.; He, S.; Li, X.; Shi, L.; Wang, F.; Yu, S.; Xu, S.; Ju, C.; Fang, H.; Yu, Y. Characterization, genome functional analysis, and detoxification of atrazine by Arthrobacter sp. C2. Chemosphere 2021, 264, 128514. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.C.; Rosendahl, C.; Johnson, G.; Sadowsky, M.J.; Wackett, L.P. Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl. Environ. Microbiol. 2002, 68, 5973–5980. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hua, R.; Lv, P.; Tang, J.; Wang, Y.; Cao, H.; Wu, X.; Li, Q.X. Novel hydrolytic de-methylthiolation of the s-triazine herbicide prometryn by Leucobacter sp. JW-1. Sci. Total Environ. 2017, 579, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Satsuma, K. Mineralization of s-triazine herbicides by a newly isolated Nocardioides species strain DN36. Appl. Microbiol. Biotechnol. 2010, 86, 1585–1592. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, J.; Wang, W.; Wu, X. Purification, characterization, and catalytic mechanism of N-Isopropylammelide isopropylaminohydrolase (AtzC) involved in the degradation of s-triazine herbicides. Environ. Pollut. 2020, 268, 115803. [Google Scholar] [CrossRef]
- Sajjaphan, K.; Shapir, N.; Wackett, L.P.; Palmer, M.; Blackmon, B.; Tomkins, J.; Sadowsky, M.J. Arthrobacter aurescens TC1 atrazine catabolism genes trzN, atzB, and atzC are linked on a 160-kilobase region and are functional in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 4402–4407. [Google Scholar] [CrossRef]
- Esquirol, L.; Peat, T.S.; Wilding, M.; Liu, J.W.; French, N.G.; Hartley, C.J.; Onagi, H.; Nebl, T.; Easton, C.J.; Newman, J.; et al. An unexpected vestigial protein complex reveals the evolutionary origins of an s-triazine catabolic enzyme. J. Biol. Chem. 2018, 293, 7880–7891. [Google Scholar] [CrossRef]
- McMartin, D.W.; Headley, J.V.; Wood, B.P.; Gillies, J.A. Photolysis of atrazine and ametrynee herbicides in Barbados sugar cane plantation soils and water. J. Environ. Sci. Health B. 2003, 38, 293–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Wang, W.; Ru, S.; Zhao, L.; Ma, Y.; Song, X.; Liu, L.; Wang, J. Pollution level and ecological risk assessment of triazine herbicides in Laizhou Bay and derivation of seawater quality criteria. J. Hazard. Mater. 2024, 477, 135270. [Google Scholar] [CrossRef]
- Liu, J.; Pan, D.; Wu, X.; Chen, H.; Cao, H.; Li, Q.X.; Hua, R. Enhanced degradation of prometryn and other s-triazine herbicides in pure cultures and wastewater by polyvinyl alcohol-sodium alginate immobilized Leucobacter sp. JW-1. Sci. Total Environ. 2018, 615, 78–86. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Chen, Z. Microalgae for saline wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1224–1265. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, X.; Zhao, H.; Zhang, W.; Liu, G.; Zhang, X. Isolation of two salt-tolerant strains from activated sludge and its COD degradation characteristics from saline organic wastewater. Sci. Rep. 2020, 10, 18421. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Diversity of halophilic microorganisms: Environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 2002, 28, 56–63. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World J. Microbiol. Biotechnol. 2016, 32, 135. [Google Scholar] [CrossRef]
- Mokashe, N.; Chaudhari, B.; Patil, U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int. J. Biol. Macromol. 2018, 117, 493–522. [Google Scholar] [CrossRef]
- Padan, E.; Venturi, M.; Gerchman, Y.; Dover, N. Na+/H+ antiporters. Biochim. Biophys. Acta 2001, 505, 144–157. [Google Scholar] [CrossRef]
- Shapir, N.; Mandelbaum, R.T.; Gottlieb, H. Atrazine degradation in saline wastewater by Pseudomonas sp strain ADP. J. Ind. Microbiol. Biotechnol. 1998, 20, 153–159. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Du, L.; Yang, J.; Dong, J.; Ma, F. Optimization of culturing conditions for isolated Arthrobacter sp. ZXY-2, an effective atrazine-degrading and salt-adaptive bacterium. RSC Adv. 2017, 7, 33177–33184. [Google Scholar] [CrossRef]
- Layoun, P.; López-Pérez, M.; Haro-Moreno, J.M.; Haber, M.; Thrash, J.C.; Henson, M.W.; Kavagutti, V.S.; Ghai, R.; Salcher, M.M. Flexible genomic island conservation across freshwater and marine Methylophilaceae. ISME J. 2024, 18, wrad036. [Google Scholar] [CrossRef]
- Jiang, C.; Lu, Y.C.; Xu, J.Y.; Song, Y.; Song, Y.; Zhang, S.H.; Ma, L.Y.; Lu, F.F.; Wang, Y.K.; Yang, H. Activity, biomass and composition of microbial communities and their degradation pathways in exposed propazine soil. Ecotoxicol. Environ. Saf. 2017, 145, 398–407. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, M.; Zou, H.; Nurwono, G.; Zgonc, D.; Birch, Q.; Nadagouda, M.N.; Park, J.O.; Blotevogel, J.; Liu, C.; et al. Laccase immobilized on arginine-functionalized boron nitride nanosheets for enhanced atrazine degradation. Environ. Sci. Technol. 2024, 58, 15111–15119. [Google Scholar] [CrossRef] [PubMed]
- Seffernick, J.L.; Dodge, A.G.; Sadowsky, M.J.; Bumpus, J.A.; Wackett, L.P. Bacterial ammeline metabolism via guanine deaminase. J. Bacteriol. 2010, 19, 1106–1112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rathnayake, U.M.; Wood, W.N.; Hendrickson, T.L. Indirect tRNA aminoacylation during accurate translation and phenotypic mistranslation. Curr. Opin. Chem. Biol. 2017, 41, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.M.; Durchschein, K.; Richman, J.E.; Sadowsky, M.J.; Wackett, L.P. A new family of biuret hydrolases involved in s-triazine ring metabolism. ACS Catal. 2011, 2011, 1075–1082. [Google Scholar] [CrossRef]
- Esquirol, L.; Peat, T.S.; Sugrue, E.; Balotra, S.; Rottet, S.; Warden, A.C.; Wilding, M.; Hartley, C.J.; Jackson, C.J.; Newman, J.; et al. Bacterial catabolism of s-triazine herbicides: Biochemistry, evolution and application. Adv. Microb. Physiol. 2020, 76, 129–186. [Google Scholar]
- Aislabie, J.; Bej, A.K.; Ryburn, J.; Lloyd, N.; Wilkins, A. Characterization of Arthrobacter nicotinovorans HIM, an atrazine-degrading bacterium, from agricultural soil New Zealand. FEMS Microbiol. Ecol. 2005, 52, 279–286. [Google Scholar] [CrossRef]
- Esquirol, L.; Peat, T.S.; Wilding, M.; Lucent, D.; French, N.G.; Hartley, C.J.; Newman, J.; Scott, C. Structural and biochemical characterization of the biuret hydrolase (BiuH) from the cyanuric acid catabolism pathway of Rhizobium leguminasorum bv. viciae 3841. PLoS ONE 2018, 13, e0192736. [Google Scholar] [CrossRef]
- Castillo-Carvajal, L.C.; Sanz-Martín, J.L.; Barragán-Huerta, B.E. Biodegradation of organic pollutants in saline wastewater by halophilic microorganisms: A review. Environ. Sci. Pollut. Res. 2014, 21, 9578–9588. [Google Scholar] [CrossRef]
- Tanudjaja, E.; Hoshi, N.; Yamamoto, K.; Ihara, K.; Furuta, T.; Tsujii, M.; Ishimaru, Y.; Uozumi, N. Two Trk/Ktr/HKT-type potassium transporters, TrkG and TrkH, perform distinct functions in Escherichia coli K-12. J. Biol. Chem. 2023, 299, 102846. [Google Scholar] [CrossRef]
- Johnson, H.A.; Hampton, E.; Lesley, S.A. The Thermotoga maritima Trk potassium transporter—From frameshift to function. J. Bacteriol. 2009, 191, 2276–2284. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Y.; Liu, J.; Wang, Q.; Ma, Y. Characterization and function analysis of a Halo-alkaline-adaptable Trk K+ uptake system in Alkalimonas amylolytica strain N10. Sci. China C: Life Sci. 2009, 52, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yuda, R.; Unemoto, T.; Bakker, E.P. KtrAB, a new type of bacterial K+-uptake system from Vibrio alginolyticus. J. Bacteriol. 1998, 180, 3491–3494. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.; Bakker, E.P.; Uozumi, N.; Bremer, E. KtrAB and KtrCD: Two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 2003, 185, 1289–1298. [Google Scholar] [CrossRef]
- Masrati, G.; Dwivedi, M.; Rimon, A.; Gluck-Margolin, Y.; Kessel, A.; Ashkenazy, H.; Mayrose, I.; Padan, E.; Ben-Tal, N. Broad phylogenetic analysis of cation/proton antiporters reveals transport determinants. Nat. Commun. 2018, 9, 4205. [Google Scholar] [CrossRef]
- Casey, D.; Sleator, R.D. A genomic analysis of osmotolerance in Staphylococcus aureus. Gene 2021, 767, 145268. [Google Scholar] [CrossRef]
- Fujisawa, M.; Kusumoto, A.; Wada, Y.; Tsuchiya, T.; Ito, M. NhaK, a novel monovalent cation/H+ antiporter of Bacillus subtilis. Arch. Microbiol. 2005, 183, 411–420. [Google Scholar] [CrossRef]
- Fujisawa, M.; Ito, M.; Krulwich, T.A. Three two-component transporters with channel-like properties have monovalent cation/proton antiport activity. Proc. Natl. Acad. Sci. USA 2007, 104, 13289–13294. [Google Scholar] [CrossRef]
- Sereika, M.; Petriglieri, F.; Jensen, T.B.N.; Sannikov, A.; Hoppe, M.; Nielsen, P.H.; Marshall, I.P.G.; Schramm, A.; Albertsen, M. Closed genomes uncover a saltwater species of Candidatus Electronema and shed new light on the boundary between marine and freshwater cable bacteria. ISME J. 2023, 17, 561–569. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, S.; Ren, J.; Li, K.; Li, J.; Guo, Y. Uptake of selenite by Rahnella aquatilis HX2 involves the aquaporin AqpZ and Na+/H+ antiporter NhaA. Environ. Sci. Technol. 2023, 57, 2371–2379. [Google Scholar] [CrossRef]
- Hashimotoa, M.; Hamamotoa, T.; Kitadaa, M.; Hino, M.; Kudo, T.; Horikoshi, K. Characteristics of alkali-sensitive mutants of alkaliphilic Bacillus sp. strain C-125 that show cellular morphological abnormalities. Biosci. Biotechnol. Biochem. 1994, 58, 2090–2092. [Google Scholar] [CrossRef]
- Sperling, E.; Górecki, K.; Drakenberg, T.; Hägerhäll, C. Functional differentiation of antiporter-like polypeptides in complex I; a site-directed mutagenesis study of residues conserved in MrpA and NuoL but not in MrpD, NuoM, and NuoN. PLoS ONE 2016, 11, e0158972. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.H.; Ikewada, S.; Ishikawa, O.; Ito, M.; Krulwich, T.A. The Mrp system: A giant among monovalent cation/proton antiporters? Extremophiles 2005, 9, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Thermodynamic limits to microbial life at high salt concentrations. Environ. Microbiol. 2011, 13, 1908–1923. [Google Scholar] [CrossRef]
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol. Biochem. 2007, 45, 705–710. [Google Scholar] [CrossRef]
- Saum, S.H.; Sydow, J.F.; Palm, P.; Pfeiffer, F.; Oesterhelt, D.; Müller, V. Biochemical and molecular characterization of the biosynthesis of glutamine and glutamate, two major compatible solutes in the moderately halophilic bacterium Halobacillus halophilus. J. Bacteriol. 2006, 188, 6808–6815. [Google Scholar] [CrossRef]
- Cardoso, F.S.; Castro, R.F.; Borges, N.; Santos, H. Biochemical and genetic characterization of the pathways for trehalose metabolism in Propionibacterium freudenreichii, and their role in stress response. Microbiology 2007, 153, 270–280. [Google Scholar] [CrossRef]
- Rafaeli-Eshkol, D.; Avi-Dor, Y. Studies on halotolerance in a moderately halophilic bacterium. Effect of betaine on salt resistance of the respiratory system. Biochem. J. 1968, 109, 687–691. [Google Scholar] [CrossRef]
- Shao, Y.H.; Guo, L.Z.; Zhang, Y.Q.; Yu, H.; Zhao, B.S.; Pang, H.Q.; Lu, W.D. Glycine betaine monooxygenase, an unusual rieske-type oxygenase system, catalyzes the oxidative N-demethylation of glycine betaine in Chromohalobacter salexigens DSM 3043. Appl. Environ. Microbiol. 2018, 84, e00377–e00418. [Google Scholar] [CrossRef]
- Jensen, J.B.; Peters, N.K.; Bhuvaneswari, T.V. Redundancy in periplasmic binding protein-dependent transport systems for trehalose, sucrose, and maltose in Sinorhizobium meliloti. J. Bacteriol. 2002, 184, 2978–2986. [Google Scholar] [CrossRef]
- Boscari, A.; Mandon, K.; Poggi, M.C.; Le Rudulier, D. Functional expression of Sinorhizobium meliloti BetS, a high-affinity betaine transporter, in Bradyrhizobium japonicum USDA110. Appl. Environ. Microbiol. 2004, 70, 5916–5922. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yokoi, H.; Onishi, H. Ca-enzyme complex of halophilic nuclease H of halophilic Micrococcus varians subsp. halophilus for 5’-nucleotide production by RNA degradation. Agr. Biol. Chem. 1990, 54, 2573–2578. [Google Scholar] [CrossRef]
- Graziano, G.; Merlino, A. Molecular bases of protein halotolerance. Biochim. Biophys. Acta 2014, 1844, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, H.; Ruan, J.; Qian, W.; Fang, X.; Shi, Z.; Li, Y.; Li, S.; Shan, G.; Kristiansen, K.; et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Simpson, J.T.; Wong, K.; Jackman, S.D.; Schein, J.E.; Jones, S.J.; Birol, I. ABySS: A parallel assembler for short read sequence data. Genome Res. 2009, 19, 1117–1123. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Gardner, P.P.; Daub, J.; Tate, J.G.; Nawrocki, E.P.; Kolbe, D.L.; Lindgreen, S.; Wilkinson, A.C.; Finn, R.D.; Griffiths-Jones, S.; Eddy, S.R.; et al. Rfam: Updates to the RNA families database. Nucleic Acids Res. 2009, 37, D136–D140. [Google Scholar] [CrossRef]
- Bertelli, C.; Brinkman, F.S.L. Improved genomic island predictions with IslandPath-DIMOB. Bioinformatics 2018, 34, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Aziz, R.K.; Edwards, R.A. PhiSpy: A novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res. 2012, 40, e126. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Mai, G.; Wang, P.; Zhou, M.; Luo, Y.; Cai, Y.; Zhou, F. CRISPRdigger: Detecting CRISPRs with better direct repeat annotations. Sci. Rep. 2016, 6, 32942. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef]
- Li, W.; Jaroszewski, L.; Godzik, A. Tolerating some redundancy significantly speeds up clustering of large protein databases. Bioinformatics 2002, 18, 77–82. [Google Scholar] [CrossRef]
- Saier, M.H., Jr.; Reddy, V.S.; Tamang, D.G.; Västermark, A. The transporter classification database. Nucleic Acids Res. 2014, 42, D251–D258. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Eichinger, V.; Nussbaumer, T.; Platzer, A.; Jehl, M.A.; Arnold, R.; Rattei, T. EffectiveDB—Updates and novel features for a better annotation of bacterial secreted proteins and Type III, IV, VI secretion systems. Nucleic Acids Res. 2016, 44, D669–D674. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Pant, R.; Raghunath, A.; Irvine, A.G.; Pedro, H.; Hammond-Kosack, K.E. The Pathogen-Host Interactions database (PHI-base): Additions and future developments. Nucleic Acids Res. 2015, 43, D645–D655. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Pop, M. ARDB—Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009, 37, D443–D447. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]




| Gene ID | Database | Functional Annotation | Gene Name | Ko/COG ID | E-Value |
|---|---|---|---|---|---|
| GM002825 | KEGG | 4-Hydroxyacetophenone monooxygenase | hapE | K14520 | 0.00 |
| GM000956 | KEGG | Guanine deaminase | guaD | K01487 | 4.50 × 10−88 |
| GM002083 | KEGG | Guanine deaminase | guaD | K01487 | 7.90 × 10−88 |
| GM001055 | KEGG | Aspartyl-tRNA (Asn)/glutamyl-tRNA (Gln) amidotransferase subunit A | gatA | K02433 | 1.10 × 10−289 |
| GM001056 | KEGG | Aspartyl-tRNA (Asn)/glutamyl-tRNA (Gln) amidotransferase subunit C | gatC | K02435 | 4.00 × 10−47 |
| GM002811 | KEGG | Biuret amidohydrolase | biuH | K23359 | 2.70 × 10−134 |
| GM002397 | COG | Copper oxidase (laccase) domain | None | COG1496 | 6.90 × 10−78 |
| GM001342 | COG | Allophanate hydrolase subunit 1 | None | COG2049 | 8.80 × 10−142 |
| GM002988 | COG | Allophanate hydrolase subunit 1 | None | COG2049 | 1.20 × 10−256 |
| GM003301 | COG | Allophanate hydrolase subunit 1 | None | COG2049 | 2.30 × 10−108 |
| GM001343 | COG | Allophanate hydrolase subunit 2 | None | COG1984 | 3.20 × 10−137 |
| GM001611 | COG | Allophanate hydrolase subunit 2 | None | COG1984 | 1.30 × 10−88 |
| GM003302 | COG | Allophanate hydrolase subunit 2 | None | COG1984 | 7.00 × 10−172 |
| Description | Gene ID | Functional Annotation | Gene Name | Ko ID | E-Value |
|---|---|---|---|---|---|
| The “hypersaline-in” strategy | GM000608 | Monovalent cation/hydrogen antiporter | nhaK | K24163 | 4.20 × 10−260 |
| GM001133 | Na+: H+ antiporter, NhaA family | nhaA | K03313 | 5.40 × 10−229 | |
| GM001783 | Na+: H+ antiporter, NhaA family | nhaA | K03313 | 4.40 × 10−234 | |
| GM002340 | Multicomponent Na+: H+ antiporter subunit A | mrpA | K05565 | 0.00 | |
| GM002339 | Multicomponent Na+: H+ antiporter subunit C | mrpC | K05567 | 3.30 × 10−81 | |
| GM002338 | Multicomponent Na+: H+ antiporter subunit D | mrpD | K05568 | 2.40 × 10−279 | |
| GM002337 | Multicomponent Na+: H+ antiporter subunit E | mrpE | K05569 | 3.40 × 10−95 | |
| GM002336 | Multicomponent Na+: H+ antiporter subunit F | mrpF | K05570 | 3.30 × 10−35 | |
| GM002335 | Multicomponent Na+: H+ antiporter subunit G | mrpG | K05571 | 4.90 × 10−61 | |
| GM000828 | Trk/Ktr system potassium uptake protein | trkA, ktrA, ktrC | K03499 | 4.50 × 10−112 | |
| GM002457 | Trk/Ktr system potassium uptake protein | trkA, ktrA, ktrC | K03499 | 6.00 × 10−132 | |
| GM002458 | Trk/Ktr system potassium uptake protein | trkA, ktrA, ktrC | K03499 | 3.30 × 10−121 | |
| GM000827 | Trk/Ktr system potassium uptake protein | trkG, trkH, ktrB, ktrD | K03498 | 4.30 × 10−259 | |
| GM001785 | Trk/Ktr system potassium uptake protein | trkG, trkH, ktrB, ktrD | K03498 | 2.00 × 10−203 | |
| GM003781 | Trk/Ktr system potassium uptake protein | trkG, trkH, ktrB, ktrD | K03498 | 2.40 × 10−230 | |
| The “organic-solutes-in” strategy | GM000083 | Glycerol-3-phosphate dehydrogenase (NAD(P)+) | gpsA | K00057 | 1.00 × 10−185 |
| GM000457 | Glycerol-3-phosphate dehydrogenase | glpA, glpD | K00111 | 0.00 | |
| GM002023 | Glycerol-3-phosphate dehydrogenase | glpA, glpD | K00111 | 0.00 | |
| GM002508 | Glutamate synthase (NADPH) large chain | gltB | K00265 | 0.00 | |
| GM002509 | Glutamate synthase (NADPH) small chain | gltD | K00266 | 6.60 × 10−284 | |
| GM000823 | Glutamate dehydrogenase (NADP+) | gdhA | K00262 | 9.00 × 10−252 | |
| GM001516 | Glutamine synthetase | glnA | K01915 | 4.30 × 10−250 | |
| GM002413 | Glutamine synthetase | glnA | K01915 | 3.30 × 10−261 | |
| GM002418 | Glutamine synthetase | glnA | K01915 | 1.70 × 10−279 | |
| GM003074 | Glutamine synthetase | glnA | K01915 | 7.60 × 10−272 | |
| GM002414 | Glutamine synthetase adenylyltransferase | glnE | K00982 | 0.00 | |
| GM003815 | Trehalose 6-phosphate synthase | otsA | K00697 | 8.90 × 10−292 | |
| GM003814 | Trehalose 6-phosphate phosphatase | otsB | K01087 | 1.00 × 10−140 | |
| GM001556 | Trehalose/maltose transport system substrate-binding protein | thuE | K10236 | 3.30 × 10−234 | |
| GM002154 | Trehalose/maltose transport system substrate-binding protein | thuE | K10236 | 5.50 × 10−237 | |
| GM001555 | Trehalose/maltose transport system permease protein | thuF | K10237 | 2.50 × 10−184 | |
| GM002153 | Trehalose/maltose transport system permease protein | thuF | K10237 | 4.80 × 10−149 | |
| GM001554 | Trehalose/maltose transport system permease protein | thuG | K10238 | 3.70 × 10−160 | |
| GM002152 | Trehalose/maltose transport system permease protein | thuG | K10238 | 1.80 × 10−154 | |
| GM001511 | Glycine betaine monooxygenase A | bmoA | K00479 | 5.40 × 10−261 | |
| GM001510 | Glycine betaine monooxygenase B | bmoB | K21832 | 3.80 × 10−268 | |
| GM001698 | MFS transporter, MHS family, proline/betaine transporter | proP | K03762 | 1.20 × 10−305 | |
| GM002272 | MFS transporter, MHS family, proline/betaine transporter | proP | K03762 | 1.40 × 10−240 | |
| GM003303 | MFS transporter, MHS family, proline/betaine transporter | proP | K03762 | 1.40 × 10−240 | |
| GM003596 | MFS transporter, MHS family, proline/betaine transporter | proP | K03762 | 8.20 × 10−244 | |
| Halophilic enzymes | GM001455 | Ribonuclease HI | rnhA | K03469 | 5.40 × 10−171 |
| GM000017 | Ribonuclease HII | rnhB | K03470 | 2.10 × 10−140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Jiang, Y.; Xu, B.; Fu, X.; Tan, L.; Jin, M. Biodegradation of S-Triazine Herbicides Under Saline Conditions by Paenarthrobacter ureafaciens PC, a New Halotolerant Bacterial Isolate: Insights into Both the Degradative Pathway and Mechanisms of Tolerance to High Salt Concentrations. Microorganisms 2025, 13, 649. https://doi.org/10.3390/microorganisms13030649
Fu C, Jiang Y, Xu B, Fu X, Tan L, Jin M. Biodegradation of S-Triazine Herbicides Under Saline Conditions by Paenarthrobacter ureafaciens PC, a New Halotolerant Bacterial Isolate: Insights into Both the Degradative Pathway and Mechanisms of Tolerance to High Salt Concentrations. Microorganisms. 2025; 13(3):649. https://doi.org/10.3390/microorganisms13030649
Chicago/Turabian StyleFu, Chunqing, Yifan Jiang, Bingwen Xu, Xinmei Fu, Liang Tan, and Mei Jin. 2025. "Biodegradation of S-Triazine Herbicides Under Saline Conditions by Paenarthrobacter ureafaciens PC, a New Halotolerant Bacterial Isolate: Insights into Both the Degradative Pathway and Mechanisms of Tolerance to High Salt Concentrations" Microorganisms 13, no. 3: 649. https://doi.org/10.3390/microorganisms13030649
APA StyleFu, C., Jiang, Y., Xu, B., Fu, X., Tan, L., & Jin, M. (2025). Biodegradation of S-Triazine Herbicides Under Saline Conditions by Paenarthrobacter ureafaciens PC, a New Halotolerant Bacterial Isolate: Insights into Both the Degradative Pathway and Mechanisms of Tolerance to High Salt Concentrations. Microorganisms, 13(3), 649. https://doi.org/10.3390/microorganisms13030649






