Molecular Epidemiology of Cryptosporidiosis on Lamb and Goat Kid Farms in Gran Canaria, Canary Islands (Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Farms, Animals, and Sampling
2.3. Microscopy Analysis
2.4. Molecular Analysis
2.4.1. DNA Extraction
2.4.2. PCR Primers and Conditions
2.4.3. DNA Sequence Analysis
2.4.4. Phylogenetic Analysis
2.5. Questionnaire
2.6. Statistical Analysis
3. Results
3.1. Parasitological Analysis and Faecal Score
3.2. Molecular Analysis
3.2.1. PCR Amplification of SSU rRNA and GP60
3.2.2. Correlation Analysis Between SSU rRNA Gene Results and Faecal Scores
3.2.3. Sequencing
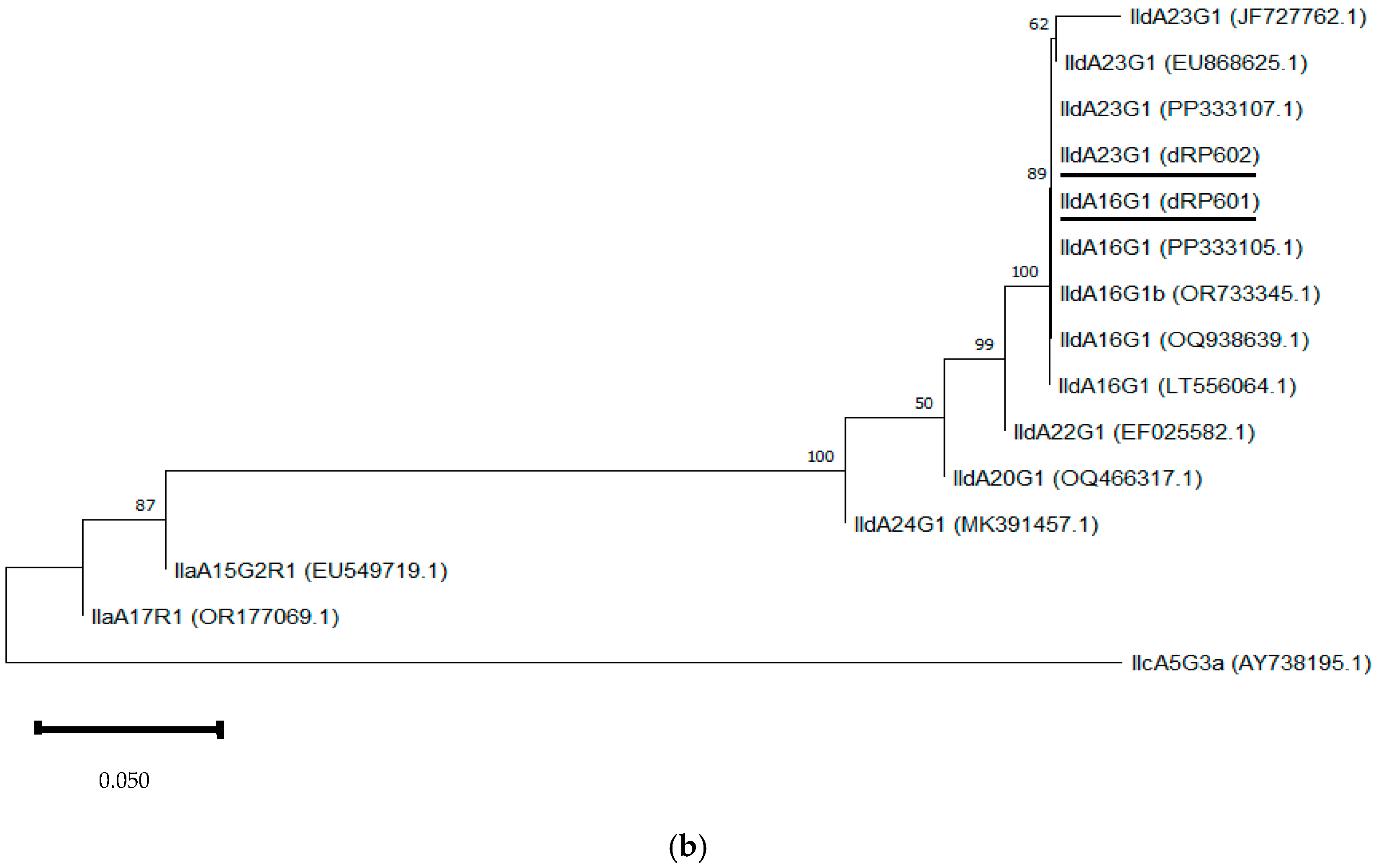
3.2.4. Phylogenetic Analysis
3.3. Questionnaire Analysis
3.3.1. Farm Characteristics and Management Data
3.3.2. Parasitological Knowledge
3.3.3. Economic Impact
3.3.4. Correlation Between Questionnaire and Parasitological Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fayer, R. Cryptosporidium: A water-borne zoonotic parasite. Vet. Parasitol. 2004, 126, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef]
- Adkins, P.R.F. Cryptosporidiosis. Vet. Clin. N. Am. Food Anim. Pract. 2022, 38, 121–131. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, X.; Guo, S.; Yang, F.; Yang, X.; Guo, Y.; Feng, Y.; Xiao, L.; Li, N. Subtyping Cryptosporidium xiaoi, a Common Pathogen in Sheep and Goats. Pathogens 2021, 10, 800. [Google Scholar] [CrossRef]
- Naguib, D.; Roellig, D.M.; Arafat, N.; Xiao, L. Genetic Characterization of Cryptosporidium cuniculus from Rabbits in Egypt. Pathogens 2021, 10, 775. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A.; Feng, Y.; Xiao, L. An Update on Zoonotic Cryptosporidium Species and Genotypes in Humans. Animals 2021, 11, 3307. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ryan, U.; Feng, Y.; Xiao, L. Emergence of zoonotic Cryptosporidium parvum in China. Trends Parasitol. 2022, 38, 335–343. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, Z.; Cheng, D.; Tao, J. Prevalence of Cryptosporidium spp. in Sheep and Goats in Jiangsu, China. Vet. Sci. 2024, 11, 144. [Google Scholar] [CrossRef]
- Golomazou, E.; Mamedova, S.; Eslahi, A.V.; Karanis, P. Cryptosporidium and agriculture: A review. Sci. Total Environ. 2024, 916, 170057. [Google Scholar] [CrossRef]
- Ibrahim, A.; El-Alfy, E.; Darwish, A.; Naguib, D.; Gad, M. Genetic Diversity of Cryptosporidium Causing Infections from Diarrheic Cases in Egypt and Co-infections with Other Intestinal Protozoan Parasites. Egyp. J. Vet. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Budu-Amoako, E.; Greenwood, S.J.; Dixon, B.R.; Barkema, H.W.; McClure, J.T. Foodborne illness associated with Cryptosporidium and Giardia from livestock. J. Food Prot. 2011, 74, 1944–1955. [Google Scholar] [CrossRef]
- Sander, V.A.; Sánchez López, E.F.; Mendoza Morales, L.; Ramos Duarte, V.A.; Corigliano, M.G.; Clemente, M. Use of Veterinary Vaccines for Livestock as a Strategy to Control Foodborne Parasitic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020, 132, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Roblin, M.; Canniere, E.; Barbier, A.; Daandels, Y.; Dellevoet-Groenewegen, M.; Pinto, P.; Tsaousis, A.; Leruste, H.; Brainard, J.; Hunter, P.R.; et al. Study of the economic impact of cryptosporidiosis in calves after implementing good practices to manage the disease on dairy farms in Belgium, France, and the Netherlands. Curr. Res. Parasitol. Vector Borne Dis. 2023, 4, 100149. [Google Scholar] [CrossRef]
- Zambriski, J.A.; Nydam, D.V.; Wilcox, Z.J.; Bowman, D.D.; Mohammed, H.O.; Liotta, J.L. Cryptosporidium parvum: Determination of ID₅₀ and the dose-response relationship in experimentally challenged dairy calves. Vet. Parasitol. 2013, 197, 104–112. [Google Scholar] [CrossRef]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef]
- Nasser, A.M. Removal of Cryptosporidium by wastewater treatment processes: A review. J. Water Health 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Shaw, H.J.; Innes, E.A.; Morrison, L.J.; Katzer, F.; Wells, B. Long-term production effects of clinical cryptosporidiosis in neonatal calves. Int. J. Parasitol. 2020, 50, 371–376. [Google Scholar] [CrossRef]
- Robertson, L.J.; Campbell, A.T.; Smith, H.V. Survival of Cryptosporidium parvum Oocysts under Various Environmental Pressures. Appl. Environ. Microbiol. 1992, 58, 3494–3500. [Google Scholar] [CrossRef]
- Santín, M.; Trout, J.M.; Xiao, L.; Zhou, L.; Greiner, E.; Fayer, R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004, 122, 103–117. [Google Scholar] [CrossRef]
- Santín, M.; Trout, J.M.; Fayer, R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet. Parasitol. 2008, 155, 15–23. [Google Scholar] [CrossRef]
- Majeed, Q.A.H.; El-Azazy, O.M.E.; Abdou, N.M.I.; Al-Aal, Z.A.; El-Kabbany, A.I.; Tahrani, L.M.A.; AlAzemi, M.S.; Wang, Y.; Feng, Y.; Xiao, L. Epidemiological observations on cryptosporidiosis and molecular characterization of Cryptosporidium spp. in sheep and goats in Kuwait. Parasitol. Res. 2018, 117, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.; Innes, E.A.; Jonsson, N.N.; Katzer, F. Shedding of Cryptosporidium in calves and dams: Evidence of re-infection and shedding of different gp60 subtypes. Parasitology 2019, 146, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Santín, M. Cryptosporidium and Giardia in Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 223–238. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, L.; Wang, Y.; Wang, L.; Amer, S.; Roellig, D.M.; Guo, Y.; Feng, Y. Periparturient transmission of Cryptosporidium xiaoi from ewes to lambs. Vet. Parasitol. 2013, 197, 627–633. [Google Scholar] [CrossRef]
- Bordes, L.; Houert, P.; Costa, D.; Favennec, L.; Vial-Novella, C.; Fidelle, F.; Grisez, C.; Prévot, F.; Jacquiet, P.; Razakandrainibe, R. Les infections asymptomatiques par Cryptosporidium chez les brebis et les agneaux sont une source de contamination environnementale par les génotypes zoonotiques de Cryptosporidium parvum. [Asymptomatic Cryptosporidium infections in ewes and lambs are a source of environmental contamination with zoonotic genotypes of Cryptosporidium parvum]. Parasite 2020, 27, 57. [Google Scholar] [CrossRef]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef]
- Weinreich, F.; Hahn, A.; Eberhardt, K.A.; Feldt, T.; Sarfo, F.S.; Di Cristanziano, V.; Frickmann, H.; Loderstädt, U. Comparison of Three Real-Time PCR Assays Targeting the SSU rRNA Gene, the COWP Gene and the DnaJ-Like Protein Gene for the Diagnosis of Cryptosporidium spp. in Stool Samples. Pathogens 2021, 10, 1131. [Google Scholar] [CrossRef]
- Baroudi, D.; Hakem, A.; Adamu, H.; Amer, S.; Khelef, D.; Adjou, K.; Dahmani, H.; Chen, X.; Roellig, D.; Feng, Y.; et al. Zoonotic Cryptosporidium species and subtypes in lambs and goat kids in Algeria. Parasit. Vectors 2018, 11, 582. [Google Scholar] [CrossRef]
- Gomes-Gonçalves, S.; Palmeira, J.D.; Ferreira, H.; Santos-Silva, S.; Mesquita, J.R. Occurrence and Phylogenetic Analysis of Zoonotic Enteropathogenic Protist Parasites in Asymptomatic Domestic Ruminants from Portugal. Pathogens 2023, 12, 1341. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Li, N.; Guo, Y.; Wang, W.; Feng, K.; He, W.; Li, F.; Huang, J.; Xu, Y.; et al. Comparative genomics analysis reveals sequence characteristics potentially related to host preference in Cryptosporidium xiaoi. Int. J. Parasitol. 2024, 54, 379–390. [Google Scholar] [CrossRef]
- Guo, Y.; Li, N.; Ryan, U.; Feng, Y.; Xiao, L. Small ruminants and zoonotic cryptosporidiosis. Parasitol. Res. 2021, 120, 4189–4198. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Navarro, E.; Prieto, A.; Pérez-Creo, A.; Viña, M.; Díaz-Cao, J.M.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; et al. Cryptosporidium species in post-weaned and adult sheep and goats from N.W. Spain: Public and animal health significance. Vet. Parasitol. 2018, 254, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Wang, X.; Huang, Y.; Mu, G.; Zhang, Y.; Jia, H.; Zhang, X.; Yang, H.; Wang, X.; Han, X.; et al. Sheep as a Potential Source of Zoonotic Cryptosporidiosis in China. Appl. Environ. Microbiol. 2018, 84, e00868-18. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Doblies, D.; Giles, M.; Elwin, K.; Smith, R.P.; Clifton-Hadley, F.A.; Chalmers, R.M. Distribution of Cryptosporidium species in sheep in the UK. Vet. Parasitol. 2008, 154, 214–219. [Google Scholar] [CrossRef]
- Quílez, J.; Torres, E.; Chalmers, R.M.; Hadfield, S.J.; Del Cacho, E.; Sánchez-Acedo, C. Cryptosporidium Genotypes and Subtypes in Lambs and Goat Kids in Spain. Appl. Environ. Microbiol. 2008, 74, 6026–6031. [Google Scholar] [CrossRef]
- Quílez, J.; Torres, E.; Chalmers, R.M.; Robinson, G.; Del Cacho, E.; Sánchez-Acedo, C. Cryptosporidium species and subtypes analysis from dairy calves in Spain. Parasitology 2008, 135, 1613–1620. [Google Scholar] [CrossRef]
- Papanikolopoulou, V.; Baroudi, D.; Guo, Y.; Wang, Y.; Papadopoulos, E.; Lafi, S.Q.; Abd El-Tawab, M.M.; Diakou, A.; Giadinis, N.D.; Feng, Y.; et al. Genotypes and subtypes of Cryptosporidium spp. in diarrheic lambs and goat kids in northern Greece. Parasitol. Int. 2018, 67, 472–475. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Li, F.; Li, N.; Feng, Y.; Xiao, L. Divergent Copies of a Cryptosporidium parvum-Specific Subtelomeric Gene. Microorganisms 2019, 7, 366. [Google Scholar] [CrossRef]
- Jang, D.H.; Cho, H.C.; Park, Y.J.; Park, J.; Choi, K.S. First report of Cryptosporidium andersoni and risk factors associated with the occurrence of Cryptosporidium spp. in pre-weaned native Korean calves with diarrhea. Front. Vet. Sci. 2023, 10, 1145096. [Google Scholar] [CrossRef]
- Efectivos de Ganado Según Especies. Municipios e Islas de Canarias Por Años. Desde 2014 Hasta 2023. Gobierno de Canarias. [Number of Livestock by Species. Municipalities and Islands of the Canary Islands by Year. From 2014 to 2023. Government of the Canary Islands]. Available online: https://www3.gobiernodecanarias.org/istac/statistical-visualizer/visualizer/data.html?resourceType=dataset&agencyId=ISTAC&resourceId=E01008B_000001&version=~latest#visualization/table (accessed on 17 April 2024).
- Abo-Shehada, M.N.; Abo-Farieha, H.A. Prevalence of Eimeria species among goats in northern Jordan. Small Rumin. Res. 2003, 49, 109–113. [Google Scholar] [CrossRef]
- Ruiz, A.; González, J.F.; Rodríguez, E.; Martín, S.; Hernández, Y.I.; Almeida, R.; Molina, J.M. Influence of climatic and management factors on Eimeria infections in goats from semi-arid zones. J. Vet. Med. B. Infect. Dis. Vet. Public Health 2006, 53, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Alvarez, M.; Lanza, I.; Cármenes, P. Role of enteric pathogens in the aetiology of neonatal diarrhoea in lambs and goat kids in Spain. Epidemiol. Infect. 1996, 117, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Quílez, J.; Prieto, A.; Navarro, E.; Pérez-Creo, A.; Fernández, G.; Panadero, R.; López, C.; Díez-Baños, P.; Morrondo, P. Cryptosporidium species and subtypes analysis in diarrhoeic pre-weaned lambs and goat kids from north-western Spain. Parasitol. Res. 2015, 114, 4099–4105. [Google Scholar] [CrossRef]
- Del Río, M.C.; Martín, S.; Quílez, J.; Vergara-Castiblanco, C.; Molina, J.M.; Ferrer, O.; Conde, M.M.; Molina, J.A.; Ruiz, A. Molecular analysis of cryptosporidiosis on cattle farms in Gran Canaria, Canary Islands (Spain). Int. J. Vet. Sci. Med. 2025, 13, 1–14. [Google Scholar] [CrossRef]
- García, L.S.; Bruckner, D.A.; Brewer, T.C.; Shimizu, R.Y. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J. Clin. Microbiol. 1983, 18, 185–190. [Google Scholar] [CrossRef]
- Ramo, A.; Quílez, J.; Del Cacho, E.; Sánchez-Acedo, C. Optimization of a fragment size analysis tool for identification of Cryptosporidium species and Gp60 alleles infecting domestic ruminants. Vet. Parasitol. 2014, 205, 466–471. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Hira, P.R.; Zhou, L.; Al-Ali, F.M.; Al-Shelahi, F.A.; Shweiki, H.M.; Iqbal, J.; Khalid, N.; Xiao, L. Unique Endemicity of Cryptosporidiosis in Children in Kuwait. J. Clin. Microbiol. 2005, 43, 2805–2809. [Google Scholar] [CrossRef]
- Maurya, P.S.; Rakesh, R.L.; Pradeep, B.; Kumar, S.; Kundu, K.; Garg, R.; Ram, H.; Kumar, A.; Banerjee, P.S. Prevalence and risk factors associated with Cryptosporidium spp. infection in young domestic livestock in India. Trop. Anim. Health Prod. 2013, 45, 941–946. [Google Scholar] [CrossRef]
- Jacobson, C.; Al-Habsi, K.; Ryan, U.; Williams, A.; Anderson, F.; Yang, R.; Abraham, S.; Miller, D. Cryptosporidium infection is associated with reduced growth and diarrhoea in goats beyond weaning. Vet. Parasitol. 2018, 260, 30–37. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, M.; Jing, B.; Jian, F.; Gong, P.; Lu, C.; Yan, Y.; Pei, Z.; Ning, C. Cryptosporidium spp. in large-scale sheep farms in China: Prevalence and genetic diversity. Sci. Rep. 2024, 14, 11218. [Google Scholar] [CrossRef]
- Aslan Çelik, B.; Çelik, Ö.Y.; Ayan, A.; Orunç Kılınç, Ö.; Akyıldız, G.; İrak, K.; Selçuk, M.A.; Ercan, K.; Baldaz, V.; Oktay Ayan, Ö. Occurence and genotype distribution of Cryptosporidium spp., and Giardia duodenalis in sheep in Siirt, Turkey. Pol. J. Vet. Sci. 2023, 26, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Romero-Salas, D.; Alvarado-Esquivel, C.; Cruz-Romero, A.; Aguilar-Domínguez, M.; Ibarra-Priego, N.; Merino-Charrez, J.O.; Pérez de León, A.A.; Hernández-Tinoco, J. Prevalence of Cryptosporidium in small ruminants from Veracruz, Mexico. BMC Vet. Res. 2016, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, G.; Cui, B.; Huang, J.; Cui, Z.; Zhang, S.; Dong, H.; Yue, D.; Zhang, L.; Ning, C.; et al. Prevalence, molecular characterization and zoonotic potential of Cryptosporidium spp. in goats in Henan and Chongqing, China. Exp. Parasitol. 2014, 142, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Causapé, A.C.; Quílez, J.; Sánchez-Acedo, C.; del Cacho, E.; López-Bernad, F. Prevalence and analysis of potential risk factors for Cryptosporidium parvum infection in lambs in Zaragoza (northeastern Spain). Vet. Parasitol. 2002, 104, 287–298. [Google Scholar] [CrossRef]
- Mišić, Z.; Katić-Radivojević, S.; Kulišić, Z. Cryptosporidium infection in lambs and goat kids in Serbia. Acta Veterinaria 2006, 56, 49–54. [Google Scholar] [CrossRef]
- Ahamed, I.; Yadav, A.; Katoch, R.; Godara, R.; Saleem, T.; Nisar, N.A. Prevalence and analysis of associated risk factors for Cryptosporidium infection in lambs in Jammu district. J. Parasit. Dis. 2015, 39, 414–417. [Google Scholar] [CrossRef]
- Adjou, K.T.; Chevillot, A.; Lucas, P.; Blanchard, Y.; Louifi, H.; Arab, R.; Mammeri, M.; Thomas, M.; Polack, B.; Karadjian, G.; et al. First identification of Cryptosporidium parvum virus 1 (CSpV1) in various subtypes of Cryptosporidium parvum from diarrheic calves, lambs and goat kids from France. Vet. Res. 2023, 54, 66. [Google Scholar] [CrossRef]
- Tako, S.; Fleiderovitz, L.; Markovich, M.P.; Mazuz, M.L.; Behar, A.; Yasur-Landau, D. Cryptosporidium parvum gp60 subtypes in diarrheic lambs and goat kids from Israel. Parasitol. Res. 2023, 122, 2237–2241. [Google Scholar] [CrossRef]
- Paz e Silva, F.M.; Lopes, R.S.; Bresciani, K.D.; Amarante, A.F.; Araujo, J.P., Jr. High occurrence of Cryptosporidium ubiquitum and Giardia duodenalis genotype E in sheep from Brazil. Acta Parasitol. 2014, 59, 193–196. [Google Scholar] [CrossRef]
- Paraud, C.; Pors, I.; Rieux, A.; Brunet, S. High excretion of Cryptosporidium ubiquitum by peri-parturient goats in one flock in western France. Vet. Parasitol. 2014, 202, 301–304. [Google Scholar] [CrossRef]
- Li, P.; Cai, J.; Cai, M.; Wu, W.; Li, C.; Lei, M.; Xu, H.; Feng, L.; Ma, J.; Feng, Y.; et al. Distribution of Cryptosporidium species in Tibetan sheep and yaks in Qinghai, China. Vet. Parasitol. 2016, 215, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, X.; Bo, X.; Zhang, B.; Zhang, Y.; Yu, F.; Zhao, A.; Zhang, Z.; Qi, M. Molecular evaluation of Cryptosporidium spp. in sheep in southern Xinjiang, China. Parasitol. Res. 2023, 122, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Vodovoza, T.; Atwill, E.R. Diverse Genotypes of Cryptosporidium in Sheep in California, USA. Pathogens 2022, 11, 1023. [Google Scholar] [CrossRef]
- Sweeny, J.P.; Ryan, U.M.; Robertson, I.D.; Yang, R.; Bell, K.; Jacobson, C. Longitudinal investigation of protozoan parasites in meat lamb farms in southern Western Australia. Prev. Vet. Med. 2011, 101, 192–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, R.; Jacobson, C.; Gardner, G.; Carmichael, I.; Campbell, A.J.; Ng-Hublin, J.; Ryan, U. Longitudinal prevalence; oocyst shedding and molecular characterisation of Cryptosporidium species in sheep across four states in Australia. Vet. Parasitol. 2014, 200, 50–58. [Google Scholar] [CrossRef]
- Connelly, L.; Craig, B.H.; Jones, B.; Alexander, C.L. Genetic diversity of Cryptosporidium spp. within a remote population of Soay Sheep on St. Kilda Islands, Scotland. Appl. Environ. Microbiol. 2013, 79, 2240–2246. [Google Scholar] [CrossRef]
- Tzanidakis, N.; Sotiraki, S.; Claerebout, E.; Ehsan, A.; Voutzourakis, N.; Kostopoulou, D.; Stijn, C.; Vercruysse, J.; Geurden, T. Occurrence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in sheep and goats reared under dairy husbandry systems in Greece. Parasite 2014, 21, 45. [Google Scholar] [CrossRef]
- Lang, J.; Han, H.; Dong, H.; Qin, Z.; Fu, Y.; Qin, H.; Zhang, J.; Zhao, J.; Li, X.; Zhao, G.; et al. Molecular characterization and prevalence of Cryptosporidium spp. in sheep and goats in western Inner Mongolia, China. Parasitol. Res. 2023, 122, 537–545. [Google Scholar] [CrossRef]
- Rieux, A.; Paraud, C.; Pors, I.; Chartier, C. Molecular characterization of Cryptosporidium spp. in pre-weaned kids in a dairy goat farm in western France. Vet. Parasitol. 2013, 192, 268–272. [Google Scholar] [CrossRef]
- Kaupke, A.; Michalski, M.M.; Rzeżutka, A. Diversity of Cryptosporidium species occurring in sheep and goat breeds reared in Poland. Parasitol. Res. 2017, 116, 871–879. [Google Scholar] [CrossRef]
- Dessì, G.; Tamponi, C.; Varcasia, A.; Sanna, G.; Pipia, A.P.; Carta, S.; Salis, F.; Díaz, P.; Scala, A. Cryptosporidium infections in sheep farms from Italy. Parasitol. Res. 2020, 119, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Geurden, T.; Thomas, P.; Casaert, S.; Vercruysse, J.; Claerebout, E. Prevalence and molecular characterisation of Cryptosporidium and Giardia in lambs and goat kids in Belgium. Vet. Parasitol. 2008, 155, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Imre, K.; Luca, C.; Costache, M.; Sala, C.; Morar, A.; Morariu, S.; Ilie, M.S.; Imre, M.; Dărăbuș, G. Zoonotic Cryptosporidium parvum in Romanian newborn lambs (Ovis aries). Vet. Parasitol. 2013, 191, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Alkathiri, B.; Lee, S.; Min, K.D.; Kim, S.; Lee, S.M.; Lee, W.K.; Kwak, D.; Lee, S.H. Outbreak of severe diarrhea due to zoonotic Cryptosporidium parvum and C. xiaoi in goat kids in Chungcheongbuk-do, Korea. Parasitol. Res. 2023, 122, 2045–2054. [Google Scholar] [CrossRef]
- Aydemir, S.; Barlık, F.; Ekici, A.; Barlık, D.H.; Alkan, S.; Gürbüz, E.; Yılmaz, H. Molecular Characterization of Giardia intestinalis and Cryptosporidium spp. Detected in Humans in Ağrı, Türkiye. Iran. J. Parasitol. 2024, 19, 9–17. [Google Scholar] [CrossRef]
- Quílez, J.; Vergara-Castiblanco, C.; Monteagudo, L.; Del Cacho, E.; Sánchez-Acedo, C. Host association of Cryptosporidium parvum populations infecting domestic ruminants in Spain. Appl. Environ. Microbiol. 2013, 79, 5363–5371. [Google Scholar] [CrossRef]
- Sahraoui, L.; Thomas, M.; Chevillot, A.; Mammeri, M.; Polack, B.; Vallée, I.; Follet, J.; Ain-Baaziz, H.; Adjou, K.T. Molecular characterization of zoonotic Cryptosporidium spp. and Giardia duodenalis pathogens in Algerian sheep. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100280. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olson, M.E.; Zhu, G.; Enomoto, S.; Abrahamsen, M.S.; Hijjawi, N.S. Cryptosporidium and cryptosporidiosis. Adv. Parasitol. 2005, 59, 77–158. [Google Scholar] [CrossRef]
- Starkey, S.R.; Kimber, K.R.; Wade, S.E.; Schaaf, S.L.; White, M.E.; Mohammed, H.O. Risk factors associated with Cryptosporidium infection on dairy farms in a New York state watershed. J. Dairy Sci. 2006, 89, 4229–4236. [Google Scholar] [CrossRef]
- Baillou, A.; Tomal, F.; Chaumeil, T.; Barc, C.; Levern, Y.; Sausset, A.; Pezier, T.; Schulthess, J.; Peltier-Pain, P.; Laurent, F.; et al. Characterization of intestinal mononuclear phagocyte subsets in young ruminants at homeostasis and during Cryptosporidium parvum infection. Front. Immunol. 2024, 15, 1379798. [Google Scholar] [CrossRef]
- Mwaba, F.; Robertson, L.J.; Tembo, R.; Zulu, M.; Ngalamika, O.; Phiri, A.M.; Siwila, J. Occurrence and factors associated with Cryptosporidium infection in livestock in three districts of Zambia. Vet. Parasitol. Reg. Stud. Rep. 2024, 52, 101057. [Google Scholar] [CrossRef] [PubMed]
- Naciri, M.; Mancassola, R.; Fort, G.; Danneels, B.; Verhaeghe, J. Efficacy of amine-based disinfectant KENO™COX on the infectivity of Cryptosporidium parvum oocysts. Vet. Parasitol. 2011, 179, 43–49. [Google Scholar] [CrossRef] [PubMed]
- de Andrés Aguayo, A.; Millet, J.P.; Álvarez-Bruned, L.; Palma, D.; Gómez, A.; Gallés, P.; Sabaté, S.; Álvarez, G.; Rodríguez, V.; Cornejo, T.; et al. Clostridium and Cryptosporidium outbreak linked to a splash pad. BMC Public Health 2024, 24, 1578. [Google Scholar] [CrossRef] [PubMed]



| Locus | Primer | Primer Sequence | Fragment Size Range (bp) | Reference |
|---|---|---|---|---|
| SSU rRNA | F1 | 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ | 386–399 | [48] |
| R1 | 5′-AAGGAGTAAGGAACAACCTCCA-3′ | |||
| F2 | 5′-AATTGGAGGGCAAGTCTGGT-3′ | |||
| R2 | 5′-AACATCCTTGGCAAATGCTT-3′ | |||
| GP60 | F | 5′-CCAGCCGTTCCACTCAGA-3′ | 333–366 | [48] |
| R | 5′-GGTACCTTCTCCGAACCACA-3′ |
| Locus | Nomenclature | GenBank Accession Number | Lambs | Sheep | Goat Kids | Goats | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nº of Isolates (n = 84) | Nº of Farms (n = 15) | Nº of Isolates (n = 86) | Nº of Farms (n = 15) | Nº of Isolates (n = 120) | Nº of Farms (n = 15) | Nº of Isolates (n = 120) | Nº of Farms (n = 15) | |||
| SSU rRNA gene | C. xiaoi | PQ345453 | 2 | 1 | 1 | 1 | 15 | 6 | 1 | 1 |
| C. parvum | PQ345455 | 2 | 2 | 1 | 1 | 13 | 5 | 0 | 0 | |
| C. ubiquitum | PQ345467 | 3 | 2 | 4 | 1 | 0 | 0 | 2 | 1 | |
| GP60 gene | C. parvum IIdA16G1 | PQ363713 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| C. parvum IIdA23G1 | PQ363714 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Río, M.C.; Martín, S.; Quílez, J.; Molina, J.M.; Ferrer, O.; Molina, J.A.; Melián, A.; Ruiz, A. Molecular Epidemiology of Cryptosporidiosis on Lamb and Goat Kid Farms in Gran Canaria, Canary Islands (Spain). Microorganisms 2025, 13, 644. https://doi.org/10.3390/microorganisms13030644
Del Río MC, Martín S, Quílez J, Molina JM, Ferrer O, Molina JA, Melián A, Ruiz A. Molecular Epidemiology of Cryptosporidiosis on Lamb and Goat Kid Farms in Gran Canaria, Canary Islands (Spain). Microorganisms. 2025; 13(3):644. https://doi.org/10.3390/microorganisms13030644
Chicago/Turabian StyleDel Río, María Cristina, Sergio Martín, Joaquín Quílez, José Manuel Molina, Otilia Ferrer, José Adrián Molina, Adrián Melián, and Antonio Ruiz. 2025. "Molecular Epidemiology of Cryptosporidiosis on Lamb and Goat Kid Farms in Gran Canaria, Canary Islands (Spain)" Microorganisms 13, no. 3: 644. https://doi.org/10.3390/microorganisms13030644
APA StyleDel Río, M. C., Martín, S., Quílez, J., Molina, J. M., Ferrer, O., Molina, J. A., Melián, A., & Ruiz, A. (2025). Molecular Epidemiology of Cryptosporidiosis on Lamb and Goat Kid Farms in Gran Canaria, Canary Islands (Spain). Microorganisms, 13(3), 644. https://doi.org/10.3390/microorganisms13030644







