A Low-Temperature-Active Pectate Lyase from a Marine Bacterium for Orange Juice Clarification
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids, Strains, Chemicals, and Media
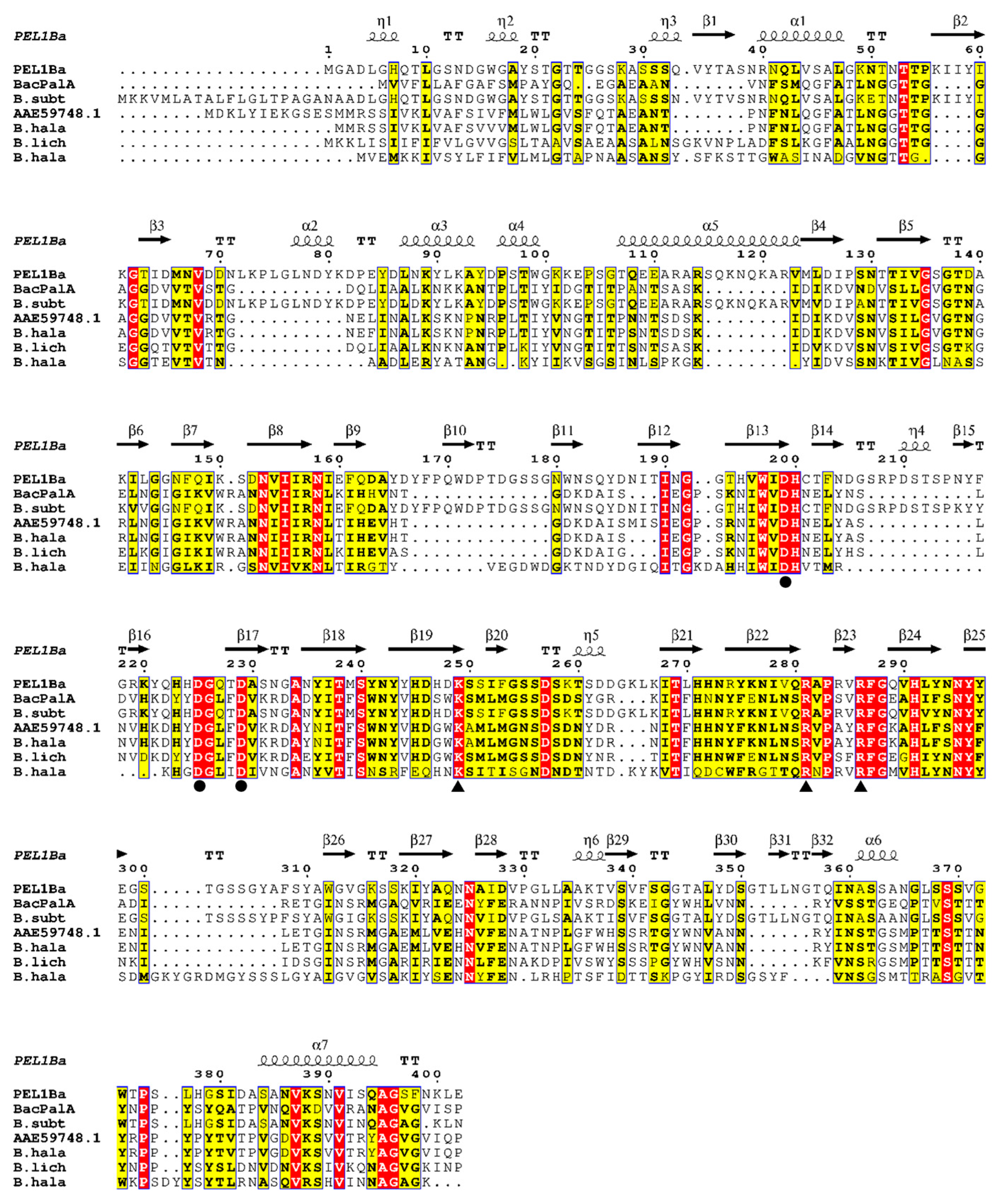
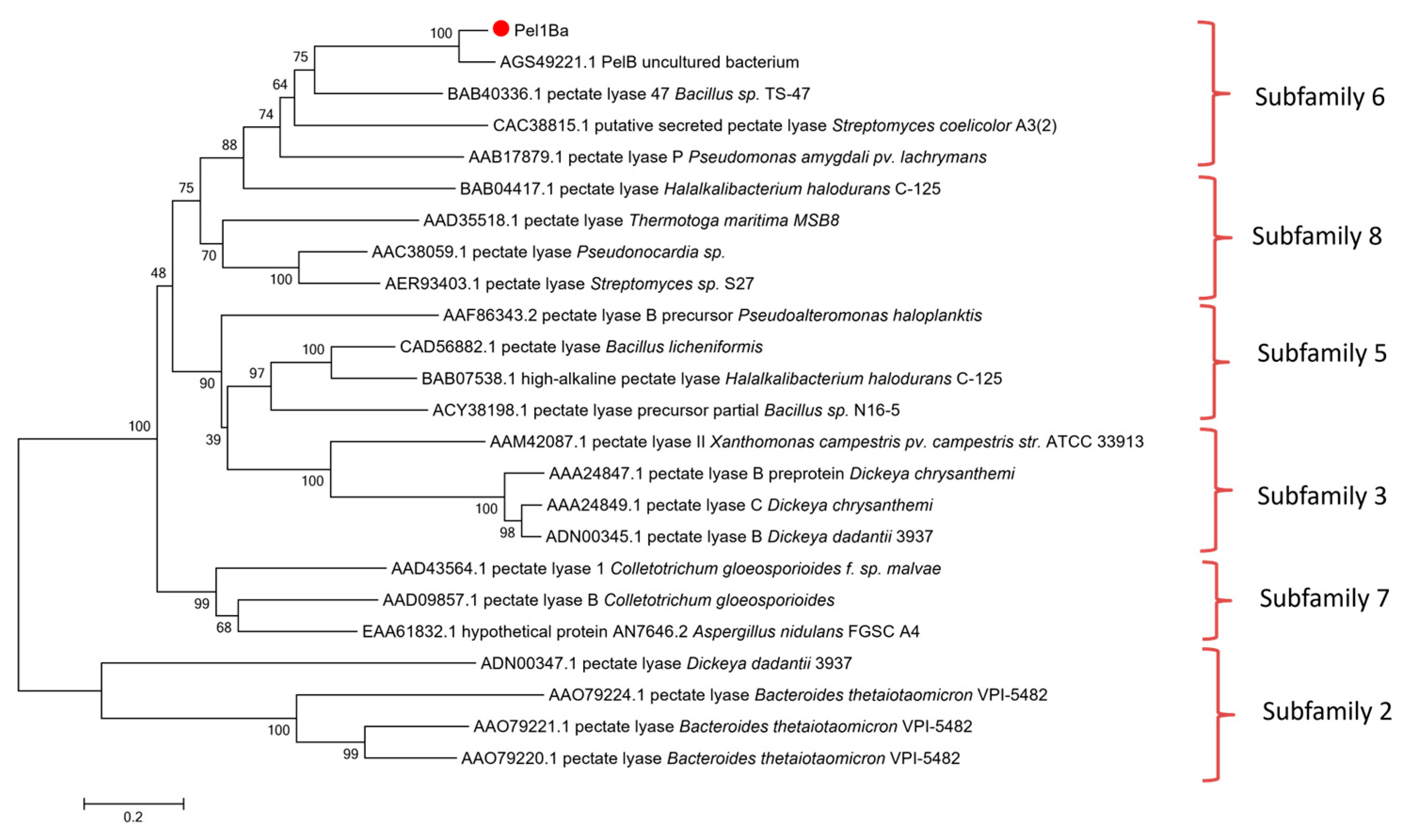
2.2. Sequence Analysis and Homology Modeling
2.3. Expression and Purification of Pectin Lyase Pel1Ba
2.4. Enzyme Activity Assay
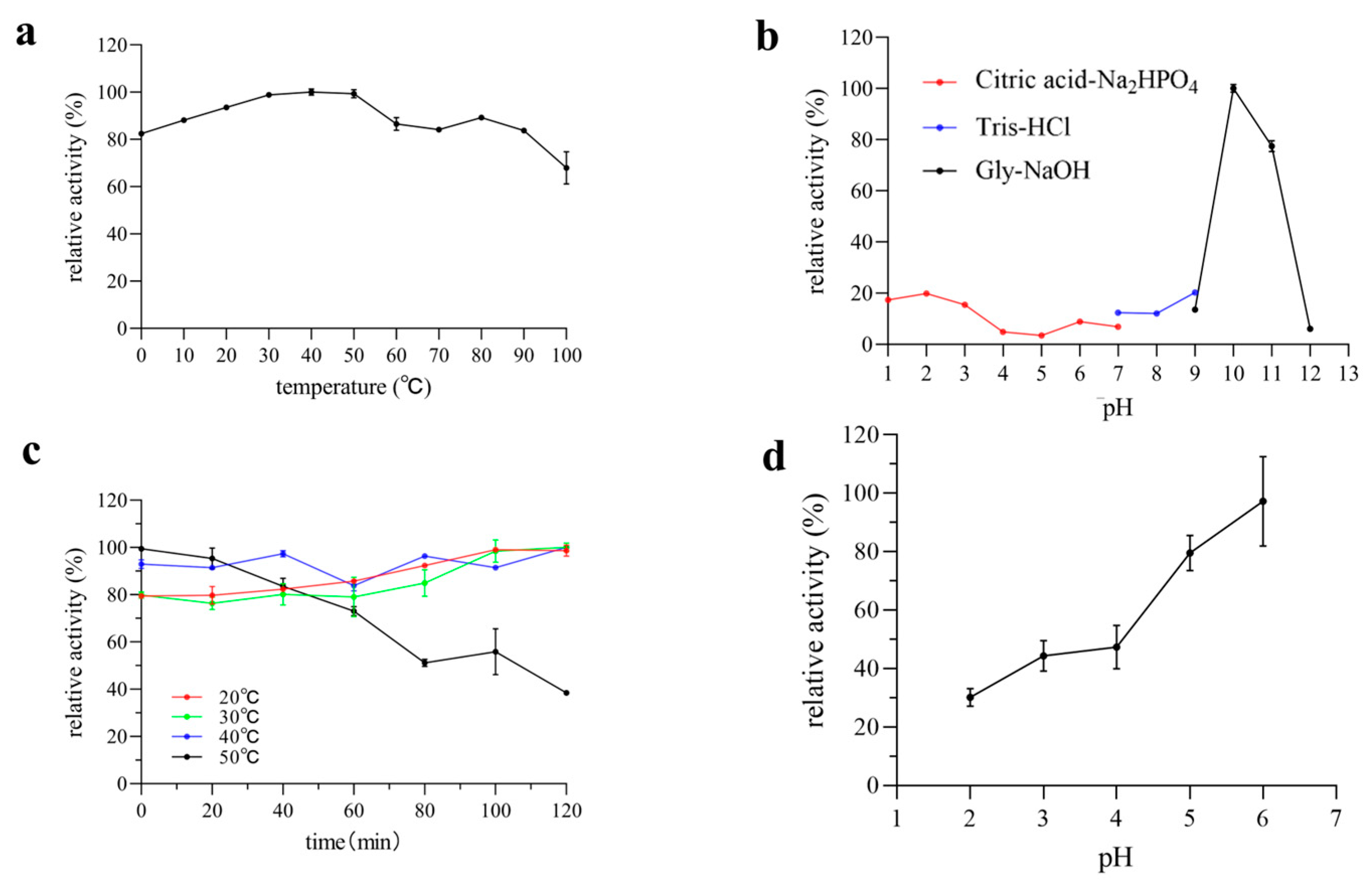
2.5. Biochemical Characterization of Pel1Ba
2.6. Effect of Metal Ions and Organic Solvents on Pel1Ba Activity
2.7. Determination of Kinetic Parameters
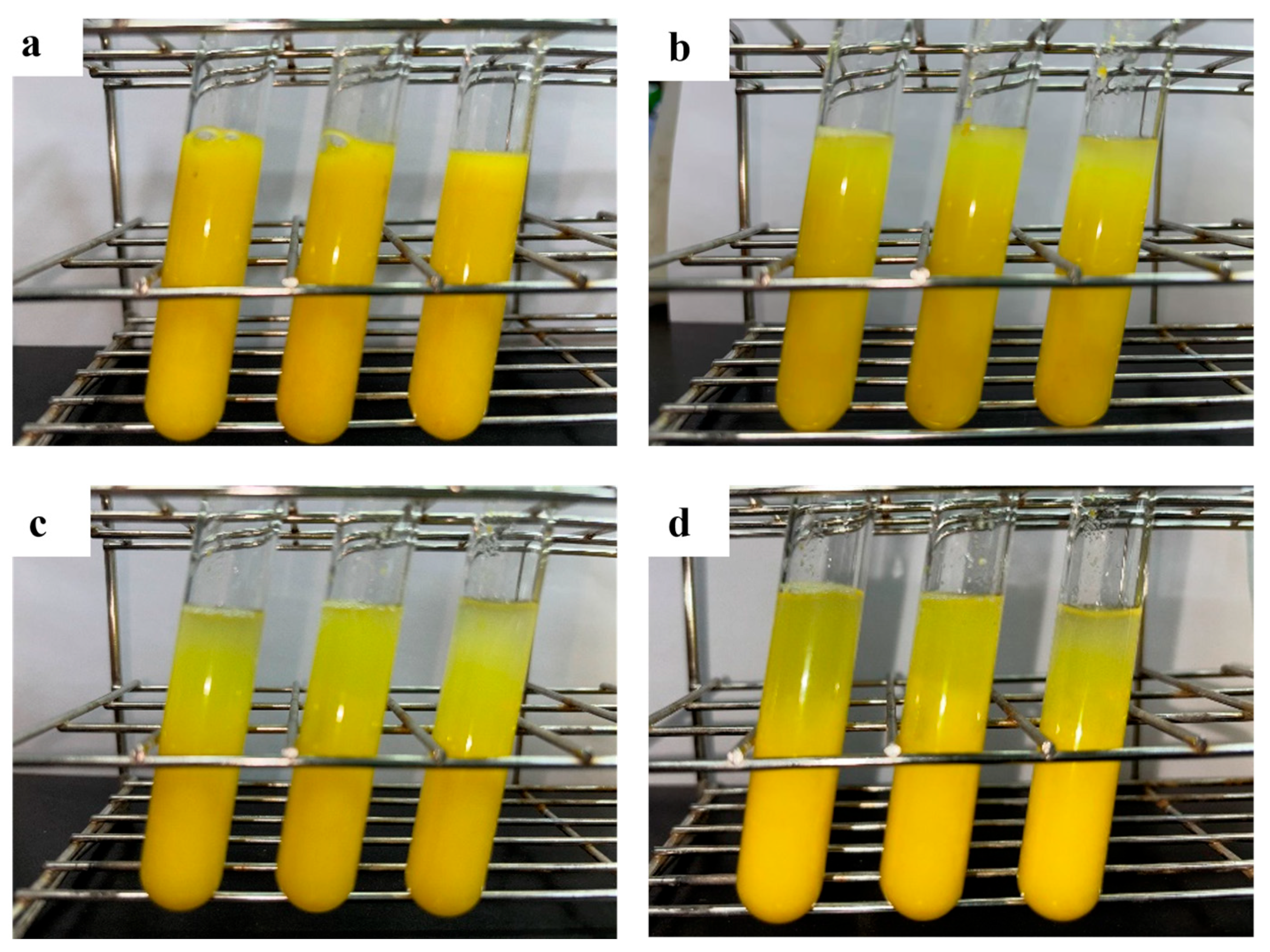
2.8. Application of Pel1Ba in Orange Juice Clarification
2.9. Site-Directed Mutagenesis
3. Results
3.1. Cloning and Sequence Analysis of the Pel1Ba Gene
3.2. Heterologous Expression and Purification of Pel1Ba
3.3. Effect of Temperature and pH on Enzyme Activity and Stability
3.4. Effects of Metal Ions and Organic Solvents on Enzyme Activity
3.5. Kinetic Parameters
3.6. Application of Pel1Ba in Orange Juice Processing
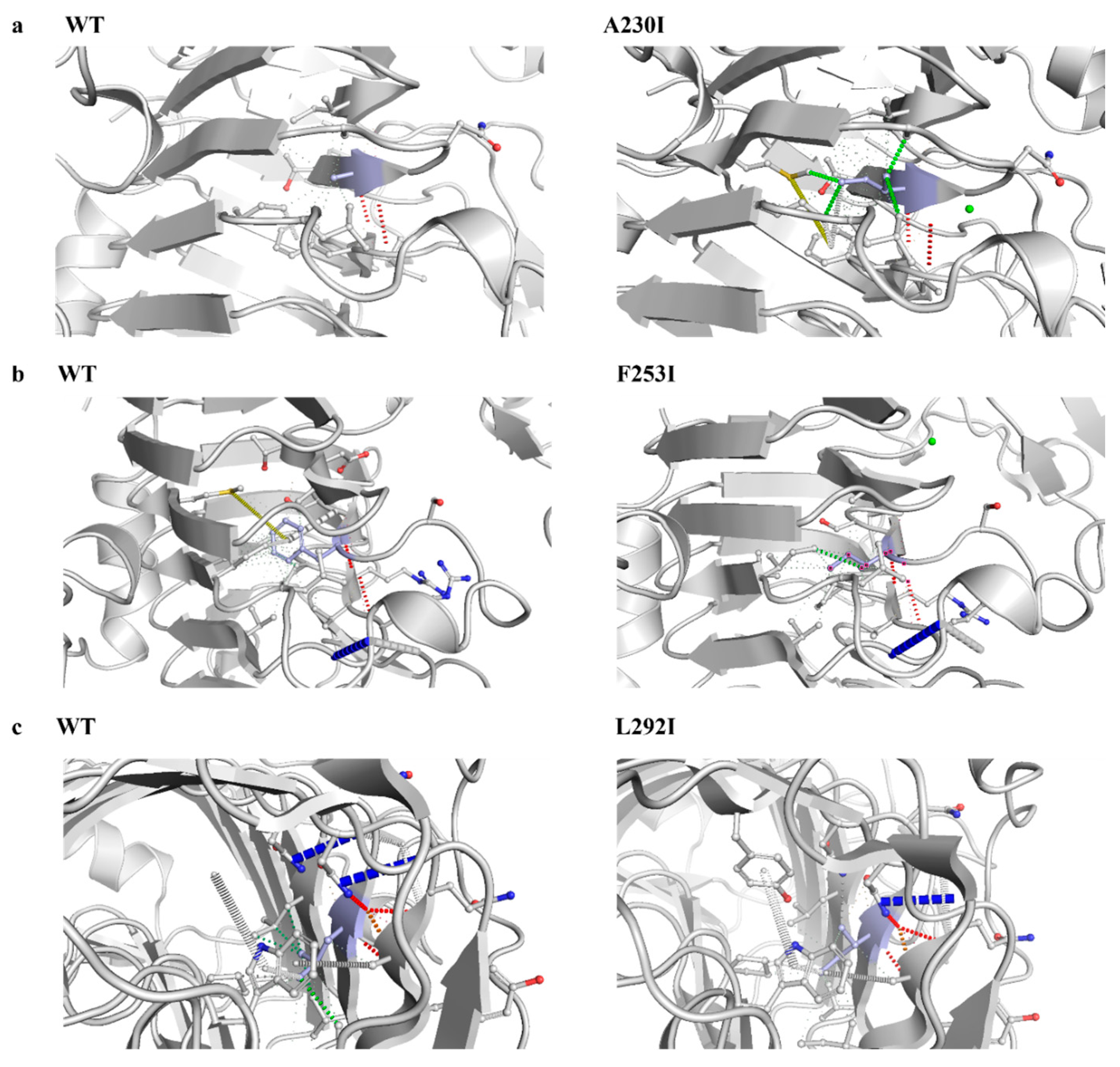
3.7. Structural Modeling and Identification of Catalytic Sites in Pel1Ba
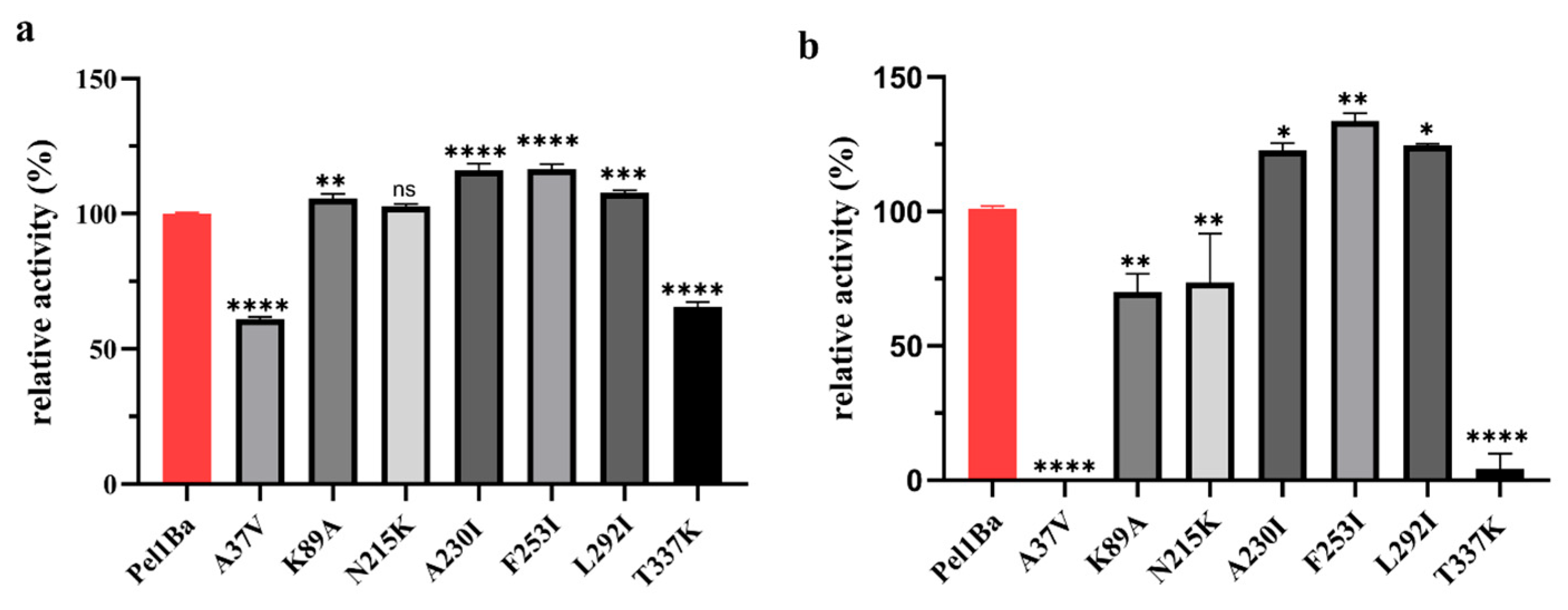
3.8. Enzymatic Properties of Pel1Ba Mutants
3.9. Kinetic Parameters of the Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, P.; Yang, S. Origins and features of pectate lyases and their applications in industry. Appl. Microbiol. Biotechnol. 2020, 104, 7247–7260. [Google Scholar] [CrossRef]
- Combo, A.M.M.; Aguedo, M. Enzymatic production of pectic oligosaccharides from polygalacturonic acid with commercial pectinase preparations. Food Bioprod. Process. 2001, 90, 588–596. [Google Scholar] [CrossRef]
- Truong, L.V.; Tuyen, H. Cloning of two pectate lyase genes from the marine Antarctic bacterium Pseudoalteromonas haloplanktis strain ANT/505 and characterization of the enzymes. Extremophiles 2020, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wu, J. The purification and characterization of a novel alkali-stable pectate lyase produced by Bacillus subtilis PB1. World J. Microbiol. Biotechnol. 2017, 33, 190. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Fauster, V. Characterization of cold-active pectate lyases from psychrophilic Mrakia frigida. Lett. Appl. Microbiol. 2005, 40, 453–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, F. A novel thermophilic chitinase directly mined from the marine metagenome using the deep learning tool Preoptem. Bioresour. Bioprocess. 2022, 9, 54. [Google Scholar] [CrossRef]
- Kunst, F.; Ogasawara, N.A. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Tatusova, T.; Ciufo, S. RefSeq microbial genomes database: New representation and annotation strategy. Nucleic Acids Res. 2015, 43, 3872. [Google Scholar] [CrossRef]
- Thomas, L.M.; Doan, C.N. Structure of pectate lyase A: Comparison to other isoforms. Biol. Crystallogr. 2002, 58, 1008–1015. [Google Scholar] [CrossRef]
- Pickersgill, R.; Jenkins, J. The structure of Bacillus subtilis pectate lyase in complex with calcium. Nat. Struct. Biol. 1994, 1, 717–723. [Google Scholar] [CrossRef]
- Lietzke, S.E.; Scavetta, R.D. The Refined Three-Dimensional Structure of Pectate Lyase E from Erwinia chrysanthemi at 2.2 A Resolution. Plant Physiol. 1996, 111, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, C.H. Crystal structure and substrate-binding mode of a novel pectate lyase from alkaliphilic Bacillus sp. N16-5. Biochem. Biophys. Res. Commun. 2012, 420, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Salmon, K. A vector set for systematic metabolic engineering in Saccharomyces cerevisiae. Yeast 2011, 28, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yuan, S. Expression, purification and characterization of pectate lyase A from Aspergillus nidulans in Escherichia coli. World J. Microbiol. Biotechnol. 2007, 23, 1057–1064. [Google Scholar] [CrossRef]
- Afifi, A.F.; Fawzi, E.M.; Foaad, M.A. Purification and characterization of pectin lyase produced by Curvularia inaequalis NRRL 13844 on orange peels waste, solid state culture. Ann. Microbiol. 2002, 52, 287–297. [Google Scholar]
- Yadav, S.; Yadav, P.K.; Yadav, D.; Yadav, K.D. Purification and characterization of pectin lyase secreted by Penicillium citrinum. Biochemistry 2009, 74, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xue, Y.; Ma, Y. Cloning, evaluation, and high-level expression of a thermo-alkaline pectate lyase from alkaliphilic Bacillus clausii with potential in ramie degumming. Appl. Microbiol. Biotechnol. 2017, 101, 3663–3676. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Su, Y.; Wang, W.; Feng, Y. Advances in separation and analysis of aromatic amino acids in food. Se Pu 2022, 40, 686–693. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, K.; Chen, H.; Wang, Z.; Zhao, W.; Zhu, L. Cold-adapted enzymes: Mechanisms, engineering and biotechnological application. Bioprocess. Biosyst. Eng. 2023, 46, 1399–1410. [Google Scholar] [CrossRef]
- Gerday, C.; Aittaleb, M.; Bentahir, M.; Chessa, J.P.; Claverie, P.; Collins, T.; D’Amico, S.; Dumont, J.; Garsoux, G.; Georlette, D.; et al. Cold-adapted enzymes: From fundamentals to biotechnology. Trends Biotechnol. 2000, 18, 103–107. [Google Scholar] [CrossRef]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.A.; Somero, G.N. Hot spots in cold adaptation: Localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc. Natl. Acad. Sci. USA 1998, 95, 11476–11481. [Google Scholar] [CrossRef]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Structures of the psychrophilic Alteromonas haloplanctis α-amylase give insights into cold adaptation at a molecular level. Structure 1998, 6, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Aghajari, N.; Van Petegem, F.; Villeret, V.; Chessa, J.P.; Gerday, C.; Haser, R.; Van Beeumen, J. Crystal structures of a psychrophilic metalloprotease reveal new insights into catalysis by cold-adapted proteases. Proteins 2003, 50, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Rathore, M.; Sharma, M. Microbial pectinase: Sources, characterization and applications. Rev. Environ. Sci. Bio/Technol. 2013, 12, 45–60. [Google Scholar] [CrossRef]
- Hoondal, G.; Tiwari, R.; Tewari, R.; Dahiya, N.; Beg, Q. Microbial alkalinepectinases and their industrial applications: A review, Appl. Microbiol. Biotechnol. 2002, 59, 409e418. [Google Scholar]
- Abdullah, A.G.L.; Sulaiman, N.M.; Aroua, M.K.; Megat Mohd Noor, M.J. Response surface optimization of conditions for clarification ofcarambola fruit juice using a commercial enzyme. J. Food Eng. 2007, 81, 65–71. [Google Scholar] [CrossRef]
- Sreenath, H.K.; Frey, M.D.; Scherz, H.; Radola, B.J. Degradation of a washed carrot preparation by cellulases and pectinases. Biotechnol. Bioeng. 1984, 26, 788–796. [Google Scholar] [CrossRef]
- Joshi, V.K.; Chauhan, S.K.; Lal, B.B. Extraction of juice from peaches, plumes and apricot by pectinolytic treatment. J. Food Proc. Technol. 1991, 28, 64–65. [Google Scholar]
- Adapa, V.; Ramya, L.N.; Pulicherla, K.K.; Sambasiva Rao, K.R.S. Cold active pectinases: Advancing the food industry to the next generation. Appl. Biochem. Biotechnol. 2014, 172, 2324–2337. [Google Scholar] [CrossRef]
- Pulicherla, K.; Ghosh, M.; Kumar, P.; Rao, K. Psychrozymes—The next generation industrial enzymes. J. Mar. Sci. Res. Dev. 2011, 1, 2. [Google Scholar] [CrossRef]
- Kumari, M.; Padhi, S.; Sharma, S.; Phukon, L.C.; Singh, S.P.; Rai, A.K. Biotechnological potential of psychrophilic microorganisms as the source of cold-active enzymes in food processing applications. 3 Biotech. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Ramirez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef] [PubMed]
- Ramya, L.N.; Pulicherla, K.K. Molecular insights into cold-active polygalacturonase enzyme for its potential application in food processing. J. Food Sci. Technol. 2015, 52, 5484–5496. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Baker, R.A.; Wicker, L. Current and potential applications of enzyme infusion in the food industry. Trends Food Sci. Technol. 1996, 7, 279–284. [Google Scholar] [CrossRef]
- Francisco, J.B.; Guitian, R.; Moreno, J.; Francisco, J.; Galdo, F. Effect of Antiinflammatory Drugs on COX-1 and COX-2 Activity in human Articular Chondrocytes. J. Rheumatol. 1999, 26, 1366–1373. [Google Scholar]
- Kassara, S.; Li, S.; Smith, P.; Blando, F.; Bindon, K. Pectolytic enzyme reduces the concentration of colloidal particles in wine due to changes in polysaccharide structure and aggregation properties. Int. J. Biol. Macromol. 2019, 140, 546–555. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Qin, X.; Gao, L.; Han, B.; Zhang, D.; Li, J.; Huang, H.; Zhang, W. Efficient Expression of an Acidic Endo-polygalacturonase from Aspergillus niger and Its Application in Juice Production. J. Agric. Food Chem. 2017, 65, 2730–2736. [Google Scholar] [CrossRef] [PubMed]



| Metal Ion | Relative Activity (%) | |
|---|---|---|
| 1 mM | 10 mM | |
| control | 100 | 100 |
| Mg2+ | 30.20 ± 2.91 | 31.87 ± 0.98 |
| Mn2+ | 132.27 ± 1.12 | 125.34 ± 1.52 |
| Co2+ | 42.12 ± 1.17 | 40.58 ± 1.44 |
| Cu2+ | 48.40 ± 1.99 | 54.46 ± 1.05 |
| Fe3+ | 85.62 ± 2.64 | 2.80 ± 0.13 |
| Fe2+ | 82.97 ± 2.73 | 57.48 ± 2.92 |
| Ni2+ | 86.85 ± 0.33 | 87.38 ± 3.74 |
| Zn2+ | 92.09 ± 0.74 | 93.63 ± 1.53 |
| K+ | 123.49 ± 4.12 | 126.79 ± 1.67 |
| Ca2+ | 130.36 ± 3.07 | 146.64 ± 3.67 |
| EDTA | 86.67 ± 0.32 | 40.62 ± 1.30 |
| SDS | 73.58 ± 2.36 | 33.45 ± 1.24 |
| Enzyme | Vmax (μmol·min−1·mL−1) | Kcat (s−1) | Km (mol/mL) | Kcat/Km (×102 mL·mg−1·s−1) |
|---|---|---|---|---|
| Pel1Ba | 0.02997 | 12.70 | 8.98 | 1.41 |
| A230I | 0.02861 | 13.95 | 8.56 | 1.63 |
| F253I | 0.01853 | 11.32 | 5.69 | 1.99 |
| L292I | 0.02641 | 15.08 | 8.11 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Wang, J.; Yan, Y.; Zhan, Y.; Zhou, Z.; Lin, M. A Low-Temperature-Active Pectate Lyase from a Marine Bacterium for Orange Juice Clarification. Microorganisms 2025, 13, 634. https://doi.org/10.3390/microorganisms13030634
Bai Y, Wang J, Yan Y, Zhan Y, Zhou Z, Lin M. A Low-Temperature-Active Pectate Lyase from a Marine Bacterium for Orange Juice Clarification. Microorganisms. 2025; 13(3):634. https://doi.org/10.3390/microorganisms13030634
Chicago/Turabian StyleBai, Yujing, Jin Wang, Yongliang Yan, Yuhua Zhan, Zhengfu Zhou, and Min Lin. 2025. "A Low-Temperature-Active Pectate Lyase from a Marine Bacterium for Orange Juice Clarification" Microorganisms 13, no. 3: 634. https://doi.org/10.3390/microorganisms13030634
APA StyleBai, Y., Wang, J., Yan, Y., Zhan, Y., Zhou, Z., & Lin, M. (2025). A Low-Temperature-Active Pectate Lyase from a Marine Bacterium for Orange Juice Clarification. Microorganisms, 13(3), 634. https://doi.org/10.3390/microorganisms13030634






