Abstract
Phyllanthus emblica Linn. (commonly known as Amla or Indian Gooseberry) is commonly used in Ayurvedic medicine to treat respiratory infections, skin disorders, and gastrointestinal issues. The fruit contains an abundance of polyphenols, which contribute to its strong antioxidant properties. The antibacterial activity of fruit extracts derived from P. emblica against Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae was determined along with the antibiotic-resistant variants extended-spectrum β-lactamase (ESBL) E. coli, methicillin-resistant S. aureus (MRSA), and ESBL K. pneumoniae. Disc diffusion and broth dilution assays were conducted to assess the activity of aqueous, methanolic, and ethyl acetate extracts, with large zones of inhibition of up to 15 mm on agar observed for S. aureus and MRSA. Minimum inhibitory concentration (MIC) values ranging from 158 to 1725 µg/mL were calculated. The aqueous and methanolic extracts of P. emblica were less active against E. coli, ESBL E. coli, K. pneumoniae, and ESBL K. pneumoniae, with the only noteworthy MIC (633 µg/mL) observed for the aqueous extract against K. pneumoniae. Interestingly, a lack of inhibition was observed on agar for any of the extracts against these bacteria. Liquid chromatography–mass spectrometry (LC-MS) analysis identified several notable flavonoids, phenolic acids, terpenoids, and tannins. Notably, Artemia nauplii bioassays indicated that all extracts were nontoxic. The antibacterial activity and absence of toxicity in P. emblica extracts suggest their potential as candidates for antibiotic development, highlighting the need for further mechanistic and phytochemical investigations.
1. Introduction
Antimicrobial resistance (AMR) poses a critical and growing challenge to global public health, resulting in heightened rates of illness, death, and economic hardship. Indeed, AMR resulted in approximately 1.27 million fatalities in 2019 [1]. The six primary pathogens associated with fatalities due to resistance include Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, which together contributed to 929,000 deaths linked to AMR [2]. Multidrug-resistant strains encompass third-generation cephalosporin-resistant E. coli, fluoroquinolone-resistant E. coli, carbapenem-resistant K. pneumoniae, and third-generation cephalosporin-resistant K. pneumoniae, with each of these responsible for between 50,000 and 100,000 deaths attributable annually to their antibiotic resistance [2]. Additionally, the effectiveness of contemporary medical practices is compromised, increasing the risks associated with infections and procedures such as surgeries and cancer treatments. AMR also presents considerable economic implications, with projections indicating an extra $1 trillion required by healthcare to treat antibiotic-resistant infections by 2050, alongside a substantial effect on global GDP [1]. Combatting AMR necessitates the implementation of infection prevention strategies, ensuring access to diagnostics and treatments, and the pursuit of innovative solutions through extensive investigation. Notably, only less than 40 new antibacterial compounds are presently in the clinical trial stages [3]. It is important to note that the candidates aimed at World Health Organisation (WHO) priority pathogens are primarily derivatives of existing classes. Indeed, fewer than 25% of the drugs currently in the clinical development pipeline belong to a novel class or operate through a novel mechanism, and none of these show potential activity against Gram-negative WHO critical threat pathogens [3].
There is a growing interest in plant-derived antimicrobials, which offer distinctive bioactive compounds, extensive activity against various strains, and synergistic effects [4]. In contrast to synthetic antibiotics, numerous compounds present in medicinal plants, including flavonoids, terpenoids, and tannins, operate via mechanisms that diverge from conventional antibiotics, thereby complicating the ability of pathogens to rapidly develop resistance [4,5]. This alternative approach has the potential to reduce the spread of AMR by providing unique, multi-targeted pathways for bacterial inhibition, thereby lessening the rapid adaptation frequently observed with conventional drugs. Additionally, some medicinal plants can potentiate the action of clinical antibiotics, improving their effectiveness against resistant bacteria. Several plants demonstrate additive and/or synergistic effects when used alongside antibiotics, boosting antimicrobial potency and often enabling lower dosages, which can help minimise the selective pressure that drives resistance [4,6]. For instance, Mangifera indica Linn. ethanol extracts induce a fourfold reduction in the MIC of tetracycline and erythromycin when used in combination against S. aureus [7].
Recent studies reported significant antibacterial activity from a 70% ethanol extract of Phyllanthus emblica Linn. (Emblica officinalis Gaertn.) fruits and its phytochemicals (gallic acid, ellagic acid, rutin, and quercetin) against S. aureus, E. coli, and K. pneumoniae, with MIC values between 3.13 and 6.25 µg/mL [8,9]. That research also noted synergistic effects when combining the extract or compounds (gallic acid, ellagic acid, rutin, and quercetin) with ampicillin and chloramphenicol against the tested bacteria. However, the extracts and pure compounds were suspended in 10% dimethyl sulfoxide (DMSO) in that study. It is likely that this high DMSO concentration may inhibit bacterial growth, potentially producing falsely low MIC values. Indeed, standardised guidelines recommend that DMSO only be used in concentrations under 1% to prevent such interference [10]. Moreover, the extract volume used in the assays was not specified in that study, which may also compromise MIC accuracy. Another study reported synergistic interaction for 90% ethanol P. emblica extracts in combination with ciprofloxacin and azithromycin against S. aureus and E. coli, utilising disc diffusion assays [11]. The study demonstrated that the combination of extracts and antibiotics resulted in increased zone of inhibition (ZOI) values compared to their individual ZOI values. Notably, that study lacked important information regarding extract concentration, synergy testing protocols, and appropriate experimental controls, which constrains the reliability and reproducibility of the findings. Additionally, the final sample preparation for the disc diffusion assay used high concentrations of ethanol, which could impact bacterial growth and skew results, providing false positive results.
To address inconsistencies in the previous research on the antibacterial activities of P. emblica and the lack of comprehensive phytochemical analyses, we evaluated the antibacterial activity of aqueous, methanolic, and ethyl acetate fruit extracts against selected bacterial pathogens, including resistant strains of S. aureus, E. coli, and K. pneumoniae. Additionally, interactions between active extracts and reference antibiotics were investigated using fractional inhibitory concentration (FIC) assays to determine potential synergistic effects. Furthermore, LC-MS analysis was performed to characterise the phytochemical profiles of the extracts, identifying key flavonoids, phenolic acids, terpenoids, and tannins. Lastly, extract toxicity was assessed through Artemia franciscana nauplii lethality assays, providing preliminary understandings into their safety for therapeutic applications.
2. Materials and Methods
2.1. Plant Sources
The fruit powder of Phyllanthus emblica (batch no: AMP1020), manufactured by Aarshaveda, was purchased online from Sattvic (Melbourne, Australia). The supplier’s website was searched using the conventional Ayurvedic nomenclature of P. emblica (Amla, Indian gooseberry) to locate the product. The authenticity and purity of the plant material were verified by the provider, with the product sourced from India and the tree-ripened whole berries dried at room temperature and finely ground into the powdered form. Proper labelling was ensured, and voucher specimens (NBG-EO0220GU) for P. emblica were deposited at the School of Pharmacy and Medical Sciences, Griffith University (Southport, Australia).
2.2. Extract Preparation
The extracts were prepared using a previously developed methodology established by our group [12]. Briefly, one gram of P. emblica fruit powder was incubated in 50 mL of sterile deionised water (EO-Aq), methanol (EO-MeOH), or ethyl acetate (EO-EtOAc) for 24 h at room temperature with gentle oscillation. Organic solvents were of analytical grade and purchased from Thermo Fisher Scientific Inc. (Melbourne, Australia). The extracts were then vacuum filtered, and the aqueous extracts were dried using a laboratory freeze dryer (Martin Christ, Germany) for 72 h while organic extracts were evaporated at 42 °C. Dried extracts were weighed, reconstituted in 10 mL of 1% DMSO (Merck Life Science Pty. Ltd., Bayswater, Australia), and passed through 0.2 µm filters (Sarstedt Australia Pty. Ltd., Mawson Lakes, Australia), and stored at −20 °C.
2.3. Antibiotics and Bacterial Strains
Powdered and disc antibiotics, including penicillin G, ciprofloxacin, polymyxin B, oxacillin, amoxicillin, erythromycin, tetracycline, chloramphenicol, gentamicin, vancomycin, Augmentin®, and cefoxitin, were obtained from Merck Life Science Pty. Ltd. (Bayswater, Australia). Powdered antibiotics were prepared as 1 mg/mL stock solutions for broth microdilution and stored at −20 °C. Amoxicillin discs were prepared by applying 10 µL of a 10 µg/mL stock solution to sterile filter paper discs. Reference strains of Klebsiella pneumoniae (ATCC 13883), ESBL K. pneumoniae (ATCC 700603), Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and MRSA (ATCC 43300) were sourced from American Type Culture Collection (ATCC) via In Vitro Technologies (Noble Park North, Australia), while a clinical isolate of ESBL E. coli was acquired from Gold Coast University Hospital (Southport, Australia). Mueller–Hinton (MH) agar and broth were used for bacterial cultures, with MRSA maintained at 35 °C [13] and other strains incubated at 37 °C for 18–24 h.
2.4. Antibacterial Susceptibility Screening
Antibacterial activities of P. emblica fruit extracts in MH agar were examined through a modified Kirby–Bauer disc diffusion method [12]. The abbreviations used in this study include EO-AQ (P. emblica aqueous extract), EO-MeOH (P. emblica methanol extract), and EO-EtOAc (P. emblica ethyl acetate extract). Reference antibiotics (Merck Life Sciences Pty. Ltd., Bayswater, Australia) are penicillin G (PEN G), erythromycin (ERY), tetracycline (TET), chloramphenicol (CHL), ciprofloxacin (CIP), polymyxin B (POL B), oxacillin (OXA), amoxicillin (AMX), gentamicin (GEN), vancomycin (VAN), Augmentin® (AUG), and cefoxitin (CEF). Negative controls included 1% DMSO, while sterile water served as the blank.
2.5. Minimum Inhibitory Concentration (MIC)
The MIC values were assessed using a 96-well microdilution assay, where extracts and antibiotics were serially diluted and inoculated with bacterial suspensions [12]. Plates were incubated for 24 h at 37 °C (with the exception of MRSA, which was incubated at 35 °C), followed by p-iodonitrotetrazolium violet (Merck Life Sciences Pty. Ltd.) staining, which was employed to visualise bacterial inhibition. MIC was recorded as the lowest concentration preventing colour change. Activity was classified into six categories, ranging from inactive to highly active [14]. All experiments were performed in duplicate.
2.6. Assessment of Fractional Inhibitory Concentration (FIC)
Reference antibiotics and plant extracts with antibacterial activity were selected for combination studies at a 50:50 ratio to evaluate their interactions against susceptible bacterial pathogens. The interactions were evaluated by calculating the FIC for each component and determining the total FIC (ΣFIC) as described in previous methods [12]. Based on ΣFIC values, interactions were categorised as synergistic (≤0.5), additive (>0.5–1.0), indifferent (>1.0–4.0), or antagonistic (>4.0) [15].
2.7. Toxicity Evaluation
The toxicity of plant extracts was assessed following a previously described method using Artemia franciscana nauplii lethality assays (ALA) [12,16] with brine shrimp purchased from Aquabuy (Silverwater, Australia). Mortality was evaluated by counting live nauplii, and LC50 values were determined using probit analysis, representing the concentrations causing 50% lethality.
2.8. Non-Targeted Headspace Quantitative Analysis Using LC-MS
A comprehensive headspace metabolic profiling of all extracts was performed using a Vanquish Ultra High-Performance Liquid Chromatography (UHPLC) system coupled with an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher, Melbourne, Australia). The system parameters, gradient flow, mobile phases, and data analysis of eluted compounds were conducted according to the methodology established by our group in previous research [12]. This methodology confirmed uniform gradient programming, optimal system configurations, and thorough examination of compound elution profiles, enabling precise and reproducible outcomes for the present study. Compound lists for each extract were exported to Excel (Version 2412), where potential compounds were identified by comparison. Duplicate entries were combined using pivot table analysis, and relative abundance was calculated as a percentage of the total peak area.
3. Results
3.1. Antibacterial Assays
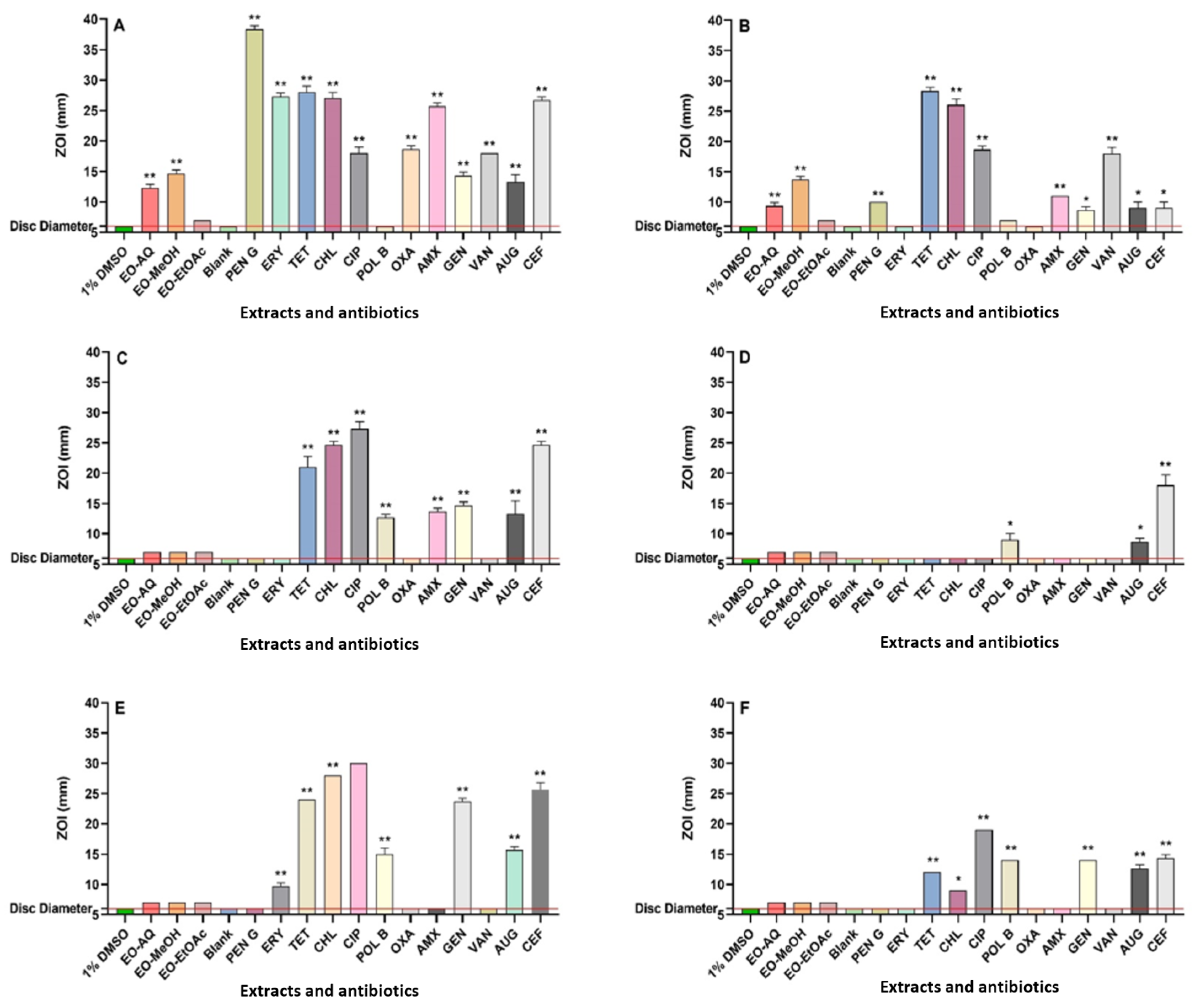
Following extraction, the final concentrations of the P. emblica fruit extracts were 40.5, 47.3, and 6.9 mg/mL, respectively. The antibacterial efficacy of all extracts and control antibiotics was calculated using disc diffusion assays, quantified as ZOI values. Additionally, broth microdilution assays were used as a more quantitative assessment of antibacterial potency, reported as MIC values. The aqueous and methanol extracts demonstrated varying levels of antibacterial activity against S. aureus, E. coli, and K. pneumoniae, as well as their resistant strains, in both the disc diffusion and broth microdilution assays (Figure 1 and Table 1). The disc diffusion assays demonstrated that both the aqueous and methanolic extracts were active against S. aureus and MRSA, with inhibition zones measuring between 9.5 and 15 mm. In contrast, the ethyl acetate extract exhibited no activity against S. aureus and MRSA on agar. In broth microdilution assays, both aqueous and methanol extracts demonstrated good antibacterial activity against S. aureus and MRSA, with MIC values varying from 158 to 315 µg/mL. In contrast, the ethyl acetate extract demonstrated noteworthy to moderate activity against S. aureus and MRSA, with MIC values of 863 and 1725 µg/mL, respectively. The aqueous and methanol P. emblica extracts exhibited substantial antibacterial activity against MRSA, comparable to the efficacy of the conventional antibiotics penicillin G, amoxicillin, oxacillin, erythromycin, Augmentin®, and cefoxitin. This highlights the promise of plant-derived compounds to be effective alternatives or complementary agents in the fight against antibiotic-resistant infections.
Figure 1.
Antimicrobial activities of P. emblica fruit extracts were assessed using disc diffusion assays against the following: (A) S. aureus, (B) MRSA, (C) E. coli, (D) ESBL E. coli, (E) K. pneumoniae, and (F) ESBL K. pneumoniae. The horizontal red line at 6 mm on the y-axis marks the disc diameter used in the assay. A single asterisk (*) indicates p-values < 0.01, while a double asterisk (**) represents p-values < 0.001.

Table 1.
Minimum inhibitory concentration (MIC) values (µg/mL) for aqueous (EO-AQ), methanol (EO-MeOH), and ethyl acetate (EO-EtOAc) fruit extracts of P. emblica, and reference antibiotics as positive controls, against six bacterial species.
None of the fruit extracts demonstrated antibacterial activity against E. coli, ESBL E. coli, K. pneumoniae, and ESBL K. pneumoniae in the disc diffusion assay. In contrast, the aqueous and methanol extracts exhibited low to noteworthy antibacterial activity against these pathogens in broth microdilution assays. The aqueous extracts showed low antibacterial activity against E. coli, ESBL E. coli, and ESBL K. pneumoniae, with MIC values of 5063 and 10,125 µg/mL, respectively. Notably, the aqueous extract displayed noteworthy antibacterial activity against K. pneumoniae, with an MIC value of 633 µg/mL. Furthermore, the methanolic extract exhibited low antibacterial activity against E. coli, K. pneumoniae, and ESBL K. pneumoniae, with a MIC of 2956 µg/mL. Interestingly, the methanol extract demonstrated moderate antibacterial activity against ESBL E. coli, with an MIC of 1478 µg/mL.
3.2. Combinatorial Studies: Fractional Inhibitory Concentration Determinations
Also examined were the interactions between combinations of P. emblica extracts and conventional antibiotics against the bacterial strains tested (Table 2). No synergistic interactions were detected for any combination in this study. Nine combinations exhibited additive effects, while thirty-eight combinations showed indifference. Furthermore, nine combinations exhibited antagonistic effects.

Table 2.
∑FIC values for combinations of P. emblica fruit extracts and conventional antibiotics.
3.3. LC-MS Metabolomic Analysis and Compound Characterisation
Extract metabolomic profiles were acquired using LC-MS profiling. Compound identification was carried out by cross-referencing various databases whenever possible. The study sought to profile a diverse array of compounds, with a specific focus on flavonoids, tannins, and terpenoids. The majority of compounds in the extracts were of high polarity, as they were found to elute during the 30% to 90% acetonitrile gradient phase in the chromatogram (Supplementary Materials Figure S1) [17]. In contrast, organic acids and amines show a tendency to favour polar environments, leading to their earlier elution in the chromatogram. Conversely, lipophilic compounds that are of low polarity were eluted later in the gradient at elevated acetonitrile concentrations due to their enhanced affinity for the non-polar stationary phase. To create a thorough list of identified phytochemicals, only those compounds that corresponded with the data reported from at least one of the screened databases were incorporated (Supplementary Materials Table S1). This study concentrated on phenolic acids, tannins, flavonoids, and terpenoids (Table 3) because of their significant biological properties [18]. These compounds are appreciated for their medicinal properties, including their antioxidant, anti-inflammatory, and antibacterial effects. Furthermore, their functions in plant defence underscore their promise as natural resources for drug development and therapeutic uses, preventing disease and increasing general health.

Table 3.
LC-MS analysis using negative ionisation mode showing the putative identification and % relative abundance of phytochemicals in the fruit extracts of P. emblica. Phytochemicals absent in any extract are indicated as (-).
3.4. Toxicity Analysis
The toxicity of plant extracts was evaluated using Artemia franciscana lethality assays. The extracts were classified as toxic if they yielded LC50 values of 1000 µg/mL or lower following 24 h of exposure to the extracts [19]. The results for all extracts were comparable to the negative control (containing artificial seawater) (p > 0.05), as they did not cause ≥50% mortality at 1000 µg/mL. As such, they were deemed non-toxic.
4. Discussion
This study evaluated the antibacterial activity of P. emblica fruit extracts against both antibiotic-susceptible and resistant bacterial pathogens. Aqueous and methanolic extracts demonstrated antibacterial activity ranging from low to good, with methanolic extracts being the most potent on agar and in broth dilution assays. Ethyl acetate extracts showed moderate to notable activity, particularly against S. aureus and MRSA. The observed differences in antibacterial potency are likely attributable to variations in extract yields and phytochemical concentrations associated with solvent polarity. Methanol and water, as polar solvents, extract greater quantities of mid-to-high polarity phytochemicals [20], whereas ethyl acetate predominantly extracts mid-to-low polarity compounds. These variations in phytochemical composition influence antibacterial activity, as low-polarity or larger phytochemicals diffuse more slowly through solid agar, thereby affecting disc diffusion outcomes [21]. Additionally, the solubility of these phytochemicals in broth may influence the accuracy and consistency of MIC measurements [22]. Furthermore, prior research has highlighted that factors such as agar thickness and uniformity can significantly impact the size of inhibition zones in agar diffusion assays [23].
The MRSA strain demonstrated significant resistance to several widely used antibiotics, including β-lactams such as penicillin G, oxacillin, and amoxicillin, as well as macrolides including erythromycin. This resistance is further exacerbated by the rise of extended-spectrum β-lactamase (ESBL) enzymes, which render many β-lactam antibiotics less effective [24]. Similarly, macrolides, which are commonly used to inhibit bacterial protein synthesis, face challenges as MRSA exhibits resistance to these drugs. Such resistance substantially limits treatment options, emphasising the critical need for novel therapeutic approaches and drug candidates capable of bypassing or overcoming these resistance mechanisms. In this context, identifying innovative compounds with unique antibacterial properties becomes increasingly important. Interestingly, all extracts from P. emblica exhibited good to moderate antibacterial activity against S. aureus and MRSA, with MIC ranging from 158 µg/mL to 1725 µg/mL. These results suggest that the MRSA resistance mechanisms may have minimal impact on the efficacy of the active compounds within these extracts. This could imply that the compounds either target distinct bacterial pathways or actively inhibit the mechanisms that confer antibiotic resistance. These findings underscore the potential of such plant-based extracts as promising candidates for addressing the growing challenge of antibiotic resistance.
The mecA gene is a pivotal factor in conferring resistance to MRSA, as it encodes a unique penicillin-binding protein, PBP2a, which exhibits a reduced affinity for β-lactam antibiotics [25]. This protein allows MRSA to continue synthesising its cell walls even in the presence of β-lactam antibiotics, rendering these medications largely ineffective. As a result, the mode of action of phytochemicals present in the extracts may differ significantly from that of β-lactam antibiotics, even in strains resistant to these drugs. Alternatively, the phytochemicals in these extracts could interfere with the bacterial defence mechanisms, effectively sensitising the bacteria to the antibiotics and enhancing their potency [25]. This finding is particularly noteworthy, as the MRSA strain examined in this study displayed substantially higher levels of resistance (when compared to the susceptible strain) to a broad spectrum of antibiotics, including the β-lactam, macrolide, and fluoroquinolone classes. Such observations highlight the potential of these phytochemical-rich extracts as a promising avenue for overcoming antibiotic resistance and addressing the growing challenges posed by resistant bacterial strains.
The aqueous P. emblica extracts exhibited low antibacterial activity against E. coli and ESBL E. coli, with a MIC of 5063 µg/mL. In contrast, methanol extracts demonstrated better antibacterial efficacies against both pathogens, with MIC values of 2956 µg/mL and 1478 µg/mL, respectively. Similarly, methanol extracts exhibited the same MIC of 2956 µg/mL against K. pneumoniae and ESBL K. pneumoniae. Interestingly, the aqueous extract showed noteworthy antibacterial activity against K. pneumoniae, with an MIC of 633 µg/mL. These findings indicate that aqueous and methanol extracts may contain bioactive compounds with broad-spectrum activity against E. coli and K. pneumoniae, including resistant strains. This efficacy may result from mechanisms of action distinct from those of β-lactam antibiotics. For instance, the extracts might interfere with bacterial cell wall formation, membrane functionality, or other vital processes unrelated to β-lactam mechanisms [26]. Furthermore, the antibacterial activity of these extracts may not directly inhibit ESBL enzymes but could operate through alternative pathways. Further investigation is required to determine whether the extracts directly disrupt resistance in the ESBL-producing pathogens or act via other, unidentified antibiotic mechanisms. Such studies should assess the inhibitory effects of the extracts or isolated components on β-lactamase activity and evaluate their influence on the expression of ESBL resistance genes.
Our study also investigated the potential of combining P. emblica extracts with conventional antibiotics. This approach shows significant promise for the development of novel antibiotic therapies, particularly as bacterial resistance to conventional antibiotics continues to rise. Plant-derived compounds may offer unique mechanisms to inhibit or block these resistance pathways, enhancing the efficacy of existing antibiotics [27]. Our objective was to enhance antibiotic efficacy and counteract bacterial resistance mechanisms by combining them with plant extracts. A well-known example of this approach is Augmentin®, a formulation that contains amoxicillin and clavulanate, which improves treatment outcomes by addressing resistance [28]. The clavulanate inhibits β-lactamase enzymes that are present within resistant species, allowing amoxicillin to be effective. It achieves this by permanently binding to the active site of the enzyme, preventing antibiotic destruction.
Our study observed additive interactions between the antibiotics and plant extracts, including penicillin G, tetracycline, ciprofloxacin, and vancomycin, against S. aureus and MRSA. Additive interactions suggest that the overall impact of the plant extracts and antibiotics is equivalent to the total of their separate effects. Although this does not indicate a synergistic enhancement, it illustrates that the plant extracts offer complementary antibacterial support in conjunction with the antibiotics, and therefore the use in combination may be beneficial.
The additive effect observed may originate from the plant extracts interacting with bacterial pathways or mechanisms that are different from those influenced by the antibiotics. For instance, penicillin G and vancomycin target bacterial cell wall synthesis, while plant extracts may interfere with bacterial membranes or intracellular processes [29], thereby increasing the stress on the bacterial cells. Similarly, tetracycline inhibits protein synthesis, and ciprofloxacin interferes with DNA replication, but the plant extracts might simultaneously target metabolic pathways or reduce efflux pump activity [29], thereby contributing to the overall antibacterial effect. Furthermore, the methanol P. emblica extract demonstrated an additive interaction with tetracycline in combating K. pneumoniae, likely due to their interference with tetracycline-specific efflux pumps [30], which is a key mechanism of resistance. Inhibiting these pumps may enable the extracts to prolong the retention of tetracycline within bacterial cells, thereby increasing its efficacy. Whilst ribosomal modifications may also play a role in tetracycline resistance, this mechanism is not as common, indicating that the extracts may primarily focus on inhibiting efflux pump activity to enhance the antibacterial effects of tetracycline.
These findings underscore the potential of integrating plant-derived compounds [29] with conventional antibiotics to combat bacterial resistance. Such combinations could be particularly valuable in addressing multidrug-resistant pathogens, including MRSA. Further research to elucidate the mechanisms underlying these interactions is essential to optimise their application in clinical settings and develop innovative therapeutic approaches.
Notably, the polymyxin B and P. emblica extract combination exhibited substantial antagonistic effects against E. coli, ESBL E. coli, K. pneumoniae, and ESBL K. pneumoniae. This may be influenced by pH variations in the broth, as polymyxin B’s membrane-disrupting activity is highly pH-sensitive, since its efficacy is suppressed under alkaline and acidic conditions [31]. The presence of plant extracts could alter the pH, potentially diminishing the antibacterial potency of both polymyxin B and the phytochemicals. Furthermore, bioactive phytochemicals may bind to polymyxin B, impairing its absorption and effectiveness [32]. Understanding these dynamics is essential for optimising combination therapies, and future studies will be critical to explore these interactions in greater detail.
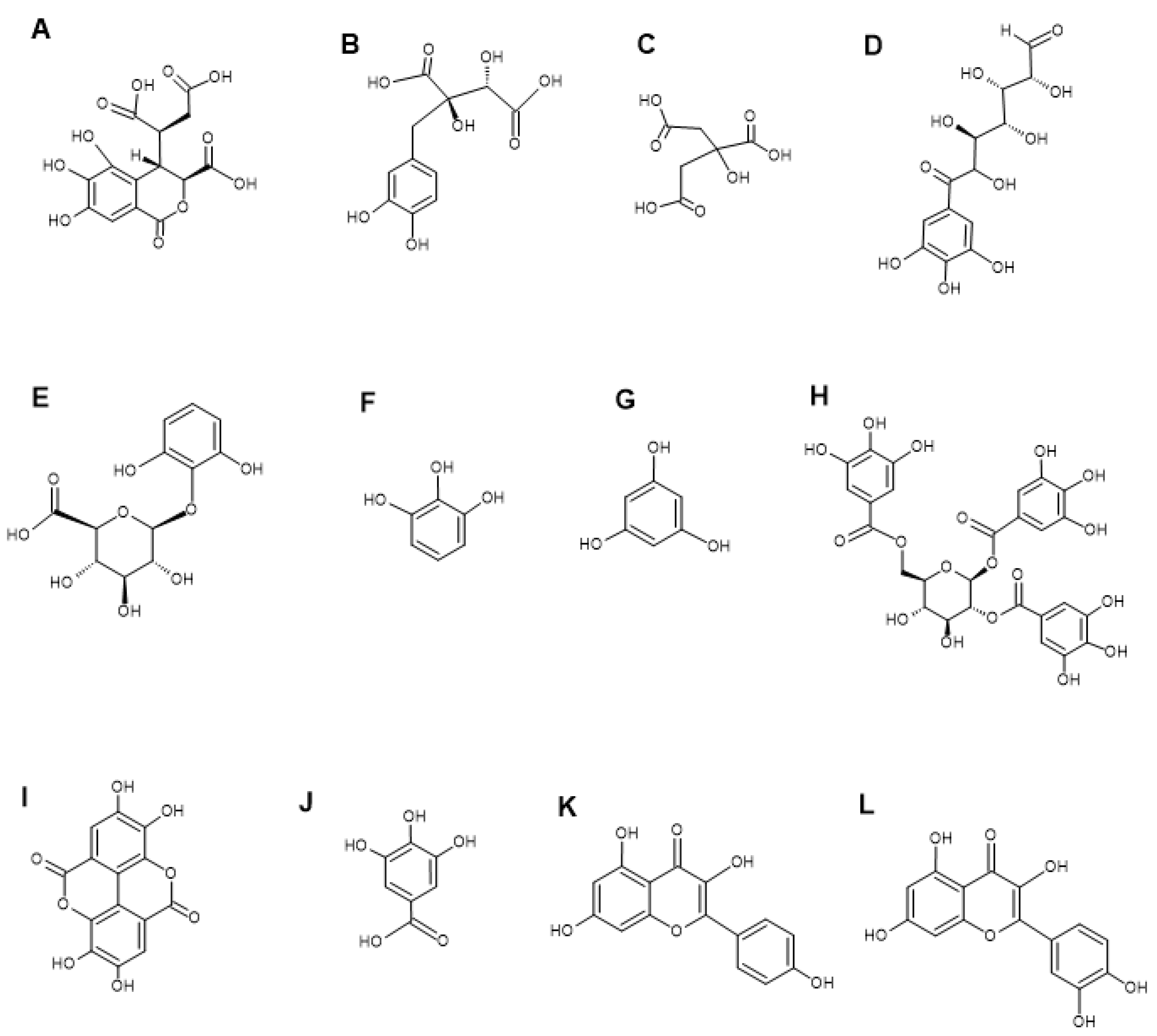
LC-MS metabolomics analysis of the P. emblica extracts showed the presence of multiple phytochemicals, including flavonoids, tannins, terpenoids, and phenolic acids (Table 3). Notable phytochemicals identified in the P. emblica extracts include chebulic acid (Figure 2A), fukiic acid (Figure 2B), citric acid (Figure 2C), 6-galloylglucose (Figure 2D), pyrogallol-2-O-glucuronide (Figure 2E), pyrogallol (Figure 2F), phloroglucinol (Figure 2G), 1,2,6-tri-O-galloyl-β-D-glucopyranose (Figure 2H), ellagic acid (Figure 2I), gallic acid (Figure 2J), kaempferol (Figure 2K), and quercetin (Figure 2L). Previous studies also identified other various phytochemicals in the fruit extracts of P. emblica, including ellagic acid, gallic acid, ascorbic acid, chebulic acid, chebulinic acid, kaempferol, quercetin, corilagin, emblicanin, and pedunculagin [13,33,34]. Our previous study conducted GC-MS analysis on the water, methanol, and ethyl acetate fruit extracts of P. emblica and reported the presence of 2-butoxy-ethanol, octanal, 2-ethyl-1-hexanol, nonanal, methoxycitronellal, endo-borneol, terpinen-4-ol, 2-methyl-decane, carvone, 2-phenylbutanal, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, 2-ethyl-3-hydroxyhexyl 2-methylpropanoate and 1-isobutyl 4-isopropyl 3-isopropyl-2,2-dimethylsuccinate [19].
Figure 2.
Structures of notable compounds identified in the fruit extracts of P. emblica. Chebulic acid (A), fukiic acid (B), citric acid (C), 6-galloylglucose (D), pyrogallol-2-O-glucuronide (E), pyrogallol (F), phloroglucinol (G), 1,2,6-tri-O-galloyl-β-D-glucopyranose (H), ellagic acid (I), gallic acid (J), kaempferol (K), and quercetin (L). The Chemsketch software (version 2023.2.4) was used to make the chemical structures.
Our study identified chebulic acid in all extracts of P. emblica. Studies in the literature are lacking in terms of the antibacterial potential of chebulic acid. However, previous studies reported that chebulic acid showed significant protection against endothelial cell dysfunction and has antioxidant properties [35,36]. Our study also identified fukiic acid, a polyphenol in the ethyl acetate extracts of P. emblica. Currently, there is limited scientific literature specifically addressing the antibacterial properties of fukiic acid.
The fruit extracts of P. emblica were found to be relatively abundant in citric acid and isocitric acid. Citric acid has demonstrated moderate antimicrobial activity against E. coli and S. aureus. Studies have reported MICs of 60 mg/mL for E. coli and S. aureus [37]. Another study noted MICs of >1000 µg/mL for S. aureus and 500 µg/mL for E. coli [38]. The antimicrobial efficacy of citric acid is influenced by pH, as it exhibits greater activity at low pH levels, particularly between its first and second pKa values (3.1 and 4.7) [39]. At these pH levels, citric acid remains largely undissociated, enhancing its ability to penetrate microbial membranes. A previous study demonstrated that citric acid induces antibiotic tolerance in bacteria through significant alterations in metabolism and oxidative stress [40]. Specifically, citric acid activates the glyoxylate cycle, suppresses the tricarboxylic acid (TCA) cycle, reduces ATP production, and mitigates oxidative stress, collectively impairing the efficacy of antibiotics [40]. The findings advocate for a balanced approach to citric acid usage, especially in medical and dietary contexts, to prevent compromising antibiotic effectiveness.
We have also identified galloyl-glucose derivatives in the P. emblica fruit extracts, including 6-galloylglucose, methyl 6-O-galloyl-β-D-glucopyranoside, 1,6-bis-O-(3,4,5-trihydroxybenzoyl) hexopyranose, methyl 4,6-di-O-galloyl-β-D-glucopyranoside, 1,2,6-trigalloyl-β-D-glucopyranose, 1,3,4-trigalloyl-β-D-glucopyranose, and 2-cinnamoyl-1,6-digalloyl-β-D-glucopyranose. Previous studies also identified several galloyl-glucose derivatives in the P. emblica fruit extracts [13,33,34,41,42]. Additionally, efflux pump inhibitory activity has been reported for 1,2,6-tri-O-galloyl-β-d-glucopyranose against MDR uropathogenic E. coli [43]. That compound showed efflux pump inhibition, which was confirmed by ethidium bromide accumulation and efflux assays, suggesting this mechanism may contribute to its antibacterial effects. Furthermore, this compound exhibited the MIC of 10–16 µg/mL (15.72–25.15 µM) against E. coli strains and demonstrated synergistic antibiofilm activity in combination with gentamicin and trimethoprim [44].
Our study also identified some other notable phytochemicals in the P. emblica extracts, including gallic acid, pyrogallol, ellagic acid, kaempferol, and quercetin. Previous studies have reported that gallic acid and pyrogallol have notable synergistic effects when combined with conventional antibiotics against Staphylococcus aureus. Gallic acid reduced the MIC of norfloxacin from 156.3 μg/mL to 49.21 μg/mL and gentamicin from 49.21 μg/mL to 2.44 μg/mL, indicating substantially enhanced antibacterial efficacy [45]. Similarly, pyrogallol showed significant synergy, reducing the MIC of norfloxacin by 49.98% (from 156.3 μg/mL to 78.13 μg/mL) and achieving an even greater effect with gentamicin, reducing the MIC from 49.21 μg/mL to 2.44 μg/mL. Among the tested combinations, gentamicin and pyrogallol yielded the most substantial reduction, highlighting its potential as an effective therapeutic strategy against S. aureus. In addition, pyrogallol also exhibited notable antimicrobial activity against methicillin-susceptible S. aureus, methicillin-resistant S. aureus, E. coli, colistin-resistant E. coli, and colistin-resistant K. pneumoniae, with an MIC of 250 μg/mL and a minimal bactericidal concentration (MBC) of 250–500 μg/mL [46]. Ellagic acid reduced the MIC of tetracycline, chloramphenicol, and tobramycin by up to fourfold against MDR E. coli [47].
Kaempferol exhibits significant antibacterial activity, particularly when combined with colistin, showing synergistic effects against colistin-resistant Gram-negative bacteria such as K. pneumoniae and E. coli. For instance, kaempferol reduced MICs of colistin by up to fourfold in resistant strains [48]. Additionally, kaempferol disrupts bacterial cell walls and biofilm formation, highlighting its potential as an adjunctive agent in combating antibiotic resistance. Quercetin exhibits notable antibacterial effects against S. aureus and E. coli, with MICs of 20 and 500 μg/mL, respectively [49]. Additionally, quercetin exhibited synergistic interactions with gentamicin and ceftriaxone against MDR E. coli [50].
Toxicity assessments using Artemia nauplii confirmed that all fruit extracts of P. emblica are non-toxic, suggesting their potential safety as antimicrobial agents. However, further evaluation using diverse mammalian cell lines is necessary to confirm their suitability for medical applications. Overall, this study highlights fruit extracts of P. emblica as promising sources of antimicrobial compounds for future research and combating bacterial infections.
5. Conclusions
The growing prevalence of antibiotic-resistant bacteria highlights the urgent need for novel antibacterial agents, with natural products emerging as promising candidates. Our study demonstrated that fruit extracts of P. emblica effectively inhibit the growth of both resistant and susceptible bacterial strains. Furthermore, the extracts additively enhanced the efficacy of conventional antibiotics, including penicillin G, ciprofloxacin, vancomycin, and tetracycline, potentially restoring their activity against resistant bacteria. This potentiation may be attributed to the extracts’ ability to deactivate bacterial β-lactamase enzymes and efflux pumps, thereby increasing intracellular antibiotic concentrations. Several phytochemicals identified in the extracts likely contribute to these effects, making them valuable targets for the development of new antibacterial agents. Future research should focus on confirming the potentiating mechanisms of phytochemicals and exploring additional pathways. In particular, studies evaluating the extracts for the ability to inhibit β-lactamase enzymes and efflux mechanisms are required. Additionally, qPCR studies would be useful to determine if the expression of the enzymes and efflux proteins is affected by the extract components.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13030611/s1, Figure S1. LC-MS total compound chromatograms of (A) EO-Aq (P. emblica aqueous), (B) EO-MeOH (P. emblica methanol), and (C) EO-EtOAc (P. emblica ethyl acetate); Table S1. LC-MS putative identification and % relative abundance of phytochemicals identified in the aqueous (AQ), methanolic (MeOH), and ethyl acetate (EtOAc) fruit extracts of P. emblica using the negative ionisation mode.
Author Contributions
Conceptualization, investigation, resources, M.J.C. and I.E.C.; methodology, G.T. and M.J.C.; validation, formal analysis, data curation, G.T.; writing—original draft preparation, G.T.; writing—review and editing, supervision, M.J.C. and I.E.C.; project administration and funding acquisition, I.E.C. and M.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work was provided by the Environmental Futures Research Institute, Griffith University, Australia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank Muhammad Jawad Zai for assistance with compound identification.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 31 January 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. A review of ayurvedic principles and the use of ayurvedic plants to control diarrhoea and gastrointestinal infections. Pharmacogn. Commun. 2023, 13, 152–162. [Google Scholar] [CrossRef]
- Mundy, L.; Pendry, B.; Rahman, M. Antimicrobial resistance and synergy in herbal medicine. J. Herb. Med. 2016, 6, 53–58. [Google Scholar] [CrossRef]
- De Oliveira, S.M.S.; Falcão-Silva, V.S.; Siqueira-Junior, J.P.; Costa, M.J.C.; Diniz, M.F.F.M. Modulation of drug resistance in Staphylococcus aureus by extract of mango (Mangifera indica L., anacardiaceae) peel. Rev. Bras. Farmacogn. 2011, 21, 190–193. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, V.; Sourirajan, A.; Dev, K. Fruit extract and phenolic compounds of Phyllanthus emblica fruits as bioactivity enhancer of chloramphenicol against bacterial species. Plant Foods Hum. Nutr. 2024, 79, 656–661. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, V.; Patel, C.N.; Sourirajan, A.; Dev, K. Synergistic antibacterial activity of Phyllanthus emblica fruits and its phytocompounds with ampicillin: A computational and experimental study. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 397, 857–871. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Hollanders, M.; Benkendorff, K. Out of control: The need for standardised solvent approaches and data reporting in antibiofilm assays incorporating dimethyl-sulfoxide (DMSO). Biofilm 2022, 4, 100081. [Google Scholar] [CrossRef]
- Jahan, N.; Akter, S. Assessment of the antimicrobial activity of the ethanolic extract of Phyllanthus emblica in combination with different classes of antibiotics against single and multi-drug resistant strains. J. Pharmacogn. Phytochem. 2015, 4, 142–155. [Google Scholar]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phyllanthus niruri Linn.: Antibacterial activity, phytochemistry, and enhanced antibiotic combinatorial strategies. Antibiotics 2024, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Vitko, N.P.; Richardson, A.R. Laboratory maintenance of methicillin-resistant Staphylococcus aureus (MRSA). Curr. Protoc. Microbiol. 2013, 28, 9C-2. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Ruebhart, D.R.; Wickramasinghe, W.; Cock, I.E. Protective efficacy of the antioxidants vitamin E and Trolox against Microcystis aeruginosa and Microcystin-LR in Artemia franciscana Nauplii. J. Toxicol. Environ. Health A 2009, 72, 1567–1575. [Google Scholar] [CrossRef]
- Cajka, T.; Hricko, J.; Rudl Kulhava, L.; Paucova, M.; Novakova, M.; Kuda, O. Optimization of mobile phase modifiers for fast LC-MS-based untargeted metabolomics and lipidomics. Int. J. Mol. Sci. 2023, 24, 31987. [Google Scholar] [CrossRef]
- Angelini, P. Plant-derived antimicrobials and their crucial role in combating antimicrobial resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; White, A.; Cheesman, M.J. Use of specific combinations of the triphala plant component extracts to potentiate the inhibition of gastrointestinal bacterial growth. J. Ethnopharmacol. 2020, 260, 112937. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.N.; Steck, T.R. The relationship between agar thickness and antimicrobial susceptibility testing. Indian J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 2000, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Mwangi, M.; Chung, M.; Milheirco, C.; De Lencastre, H.; Tomasz, A. The mechanism of heterogeneous beta-lactam resistance in MRSA: Key role of the stringent stress response. PLoS ONE 2013, 8, e82814. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Cheesman, M.; Ilanko, A.; Blonk, B.; Cock, I. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129. [Google Scholar] [CrossRef]
- Cai, X.; Javor, S.; Gan, B.H.; Köhler, T.; Reymond, J.L. The antibacterial activity of peptide dendrimers and polymyxin B increases sharply above pH 7.4. Chem. Commun. 2021, 57, 5654–5657. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Subedi, L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. 2014, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, H.; Yasin, K.A.; Choudhary, M.A.; Khaliq, N.; Ur-Rahman, A.; Choudhary, M.I.; Malik, S. Studies on the chemical constituents of Phyllanthus emblica. Nat. Prod. Res. 2007, 21, 775–781. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B.; Grewal, A.S.; Guarve, K. Pharmacological properties of chebulinic acid and related ellagitannins from nature: An emerging contemporary bioactive entity. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100163. [Google Scholar] [CrossRef]
- Yang, Z.N.; Su, B.J.; Wang, Y.Q.; Liao, H.B.; Chen, Z.F.; Liang, D. Isolation, absolute configuration, and biological activities of chebulic acid and brevifolincarboxylic acid derivatives from Euphorbia hirta. J. Nat. Prod. 2020, 83, 985–995. [Google Scholar] [CrossRef]
- Ayşe, E.; Eliuz, E. Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against Gram-negative bacteria. Lett. Appl. Microbiol. 2021, 72, 332–340. [Google Scholar] [CrossRef]
- Li, X.-S.; Xue, J.-Z.; Qi, Y.; Muhammad, I.; Wang, H.; Li, X.-Y.; Luo, Y.-J.; Zhu, D.-M.; Gao, Y.-H.; Kong, L.-C.; et al. Citric acid confers broad antibiotic tolerance through alteration of bacterial metabolism and oxidative stress. Int. J. Mol. Sci. 2023, 24, 9089. [Google Scholar] [CrossRef]
- Ahmad, B.; Hafeez, N.; Rauf, A.; Bashir, S.; Linfang, H.; Rehman, M.U.; Mubarak, M.S.; Uddin, M.S.; Bawazeer, S.; Shariati, M.A.; et al. Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S. Afr. J. Bot. 2021, 138, 278–310. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Efflux-pump inhibitory activity of a gallotannin from Terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014, 28, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Synergistic antibiofilm efficacy of a gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose from Terminalia chebula fruit in combination with gentamicin and trimethoprim against multidrug-resistant uropathogenic Escherichia coli biofilms. PLoS ONE 2017, 12, e0178712. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Anek, P.; Kumpangcum, S.; Roytrakul, S.; Khanongnuch, C.; Saenjum, C.; Phannachet, K. Antibacterial activities of phenolic compounds in Miang extract: Growth inhibition and change in protein expression of extensively drug-resistant Klebsiella pneumoniae. Antibiotics 2024, 13, 536. [Google Scholar] [CrossRef]
- Jenic, D.; Waller, H.; Collins, H.; Erridge, C. Reversal of tetracycline resistance by cepharanthine, cinchonidine, ellagic acid and propyl gallate in a multidrug-resistant Escherichia coli. Nat. Prod. Bioprospect. 2021, 11, 345–355. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Guo, W.; Yao, Z.; Du, X.; Chen, L.; Sun, Y.; Shi, S.; Cao, J.; Zhou, T. The antibacterial activity of kaempferol combined with colistin against colistin-resistant Gram-negative bacteria. Microbiol. Spectr. 2022, 10, e02265-22. [Google Scholar] [CrossRef]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Alnour, T.M.S.; Ahmed-Abakur, E.H.; Elssaig, E.H.; Abuduhier, F.M.; Ullah, M.F. Antimicrobial synergistic effects of dietary flavonoids rutin and quercetin in combination with antibiotics gentamicin and ceftriaxone against E. coli (MDR) and P. mirabilis (XDR) strains isolated from human infections: Implications for food–medicine interactions. Ital. J. Food Sci. 2022, 34, 34–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).