Vertical Distribution and Drivers of Antibiotic Resistance Genes in Agricultural Soil Irrigated with Livestock Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Chemical Properties of the Samples
2.3. Analysis of the Antibiotics in Soil
2.4. ARB Colony Counting
2.5. Metagenomic Sequencing and Bioinformatics Analysis
2.5.1. Library Preparation and Sequencing
2.5.2. Analysis of Sequencing Information
2.6. Statistical Analyses
3. Results
3.1. Distribution of Antibiotics in the Investigated Farmland
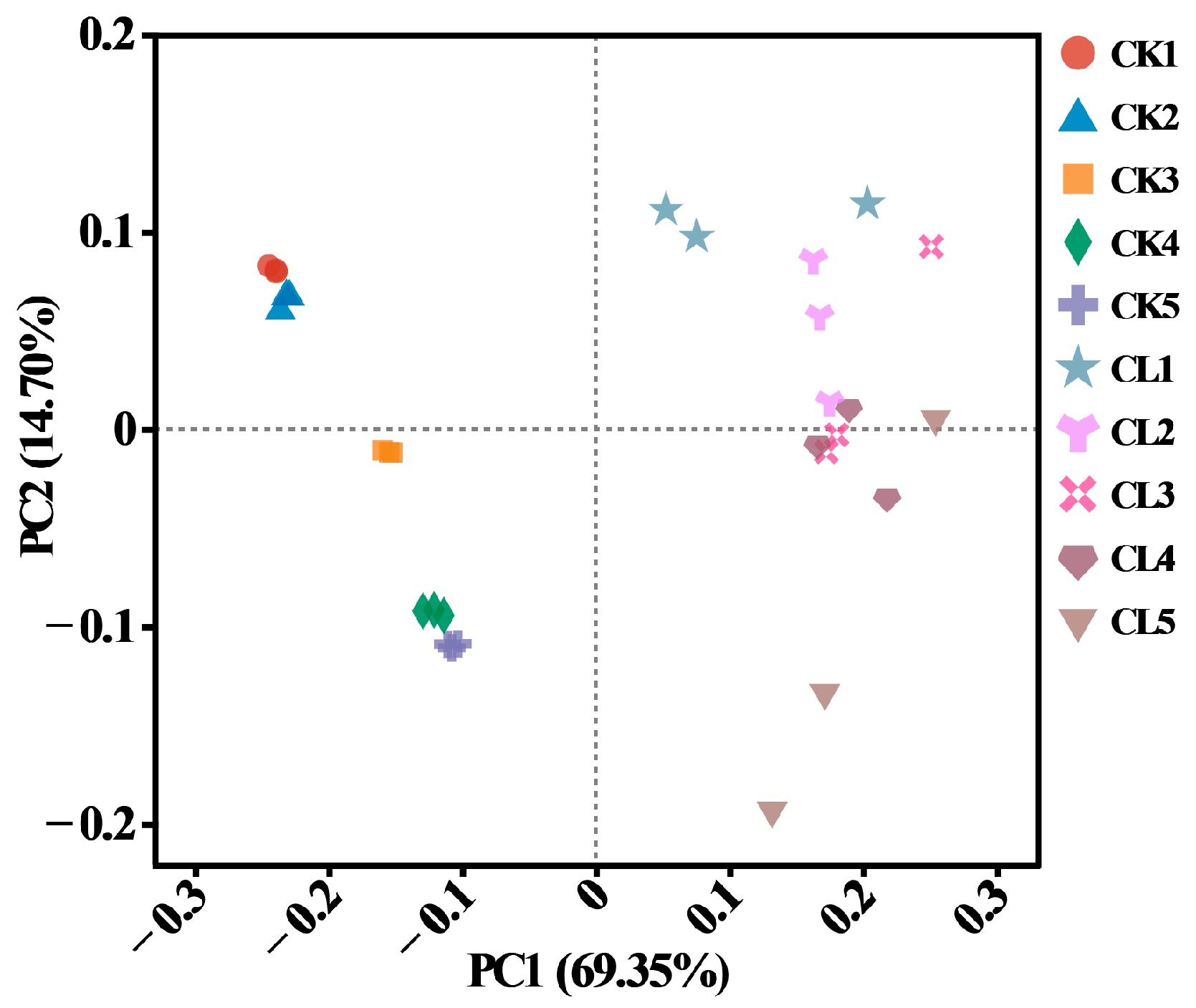
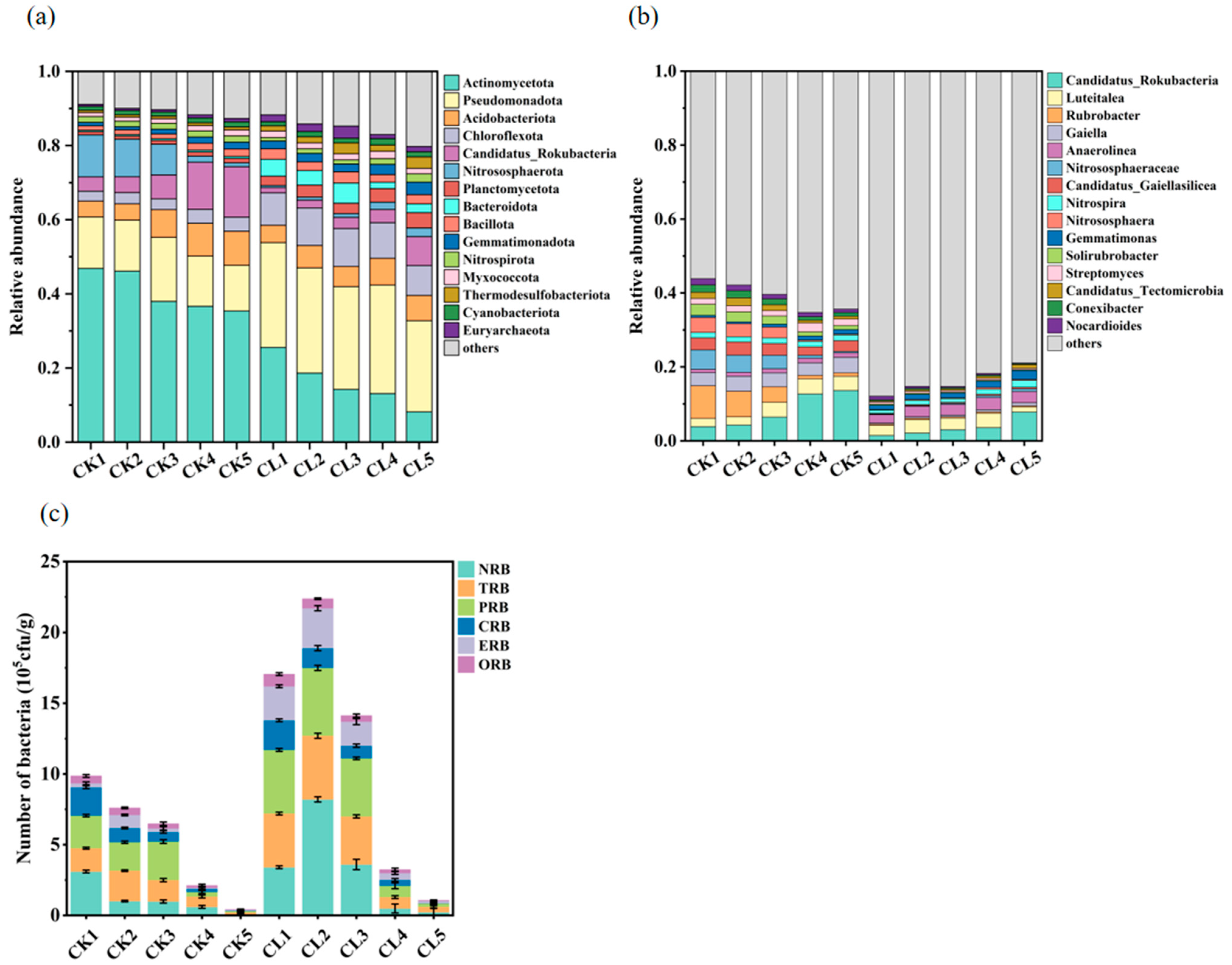
3.2. The Bacterial Community Profiles of the Investigated Farmland
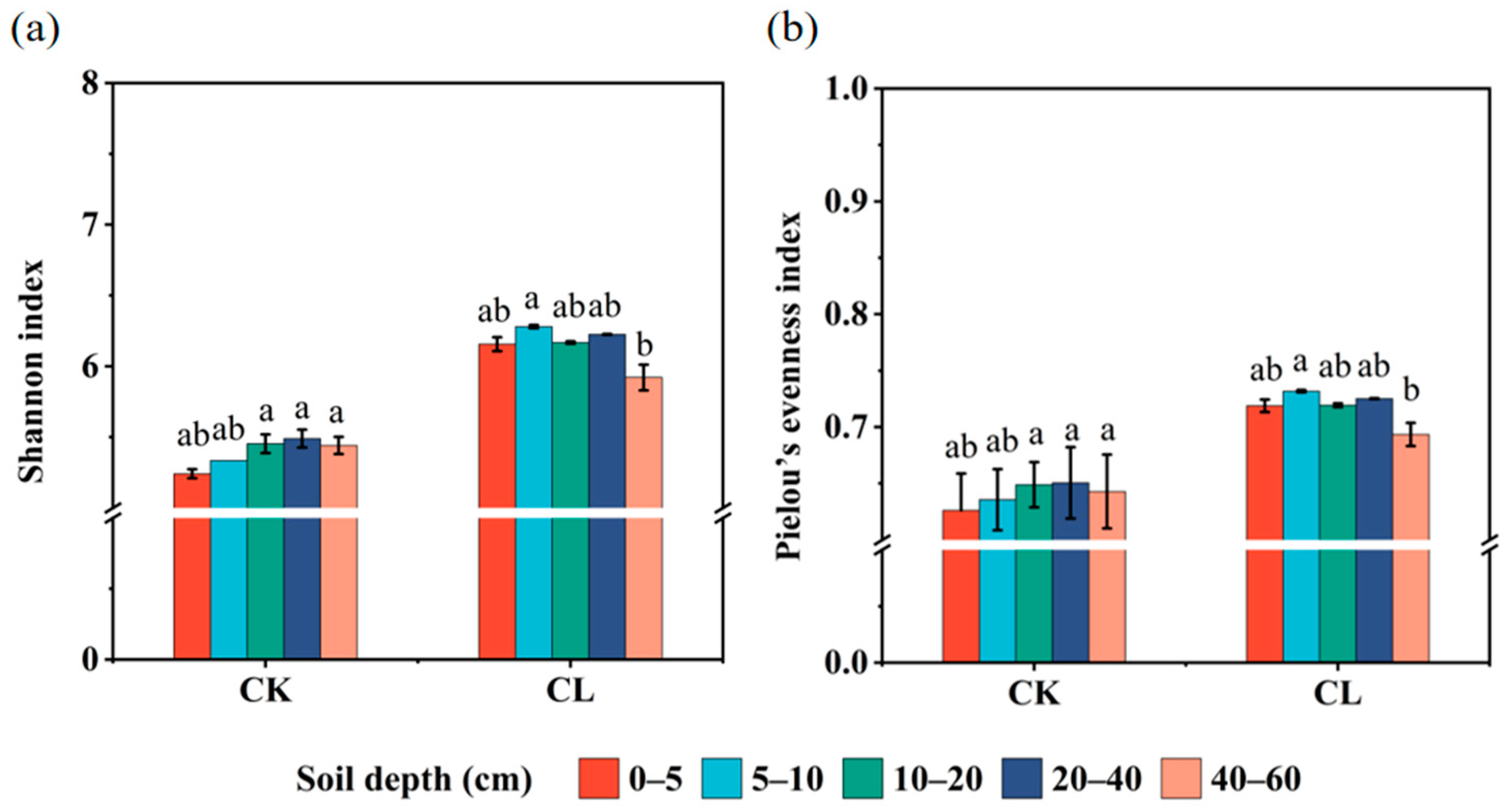
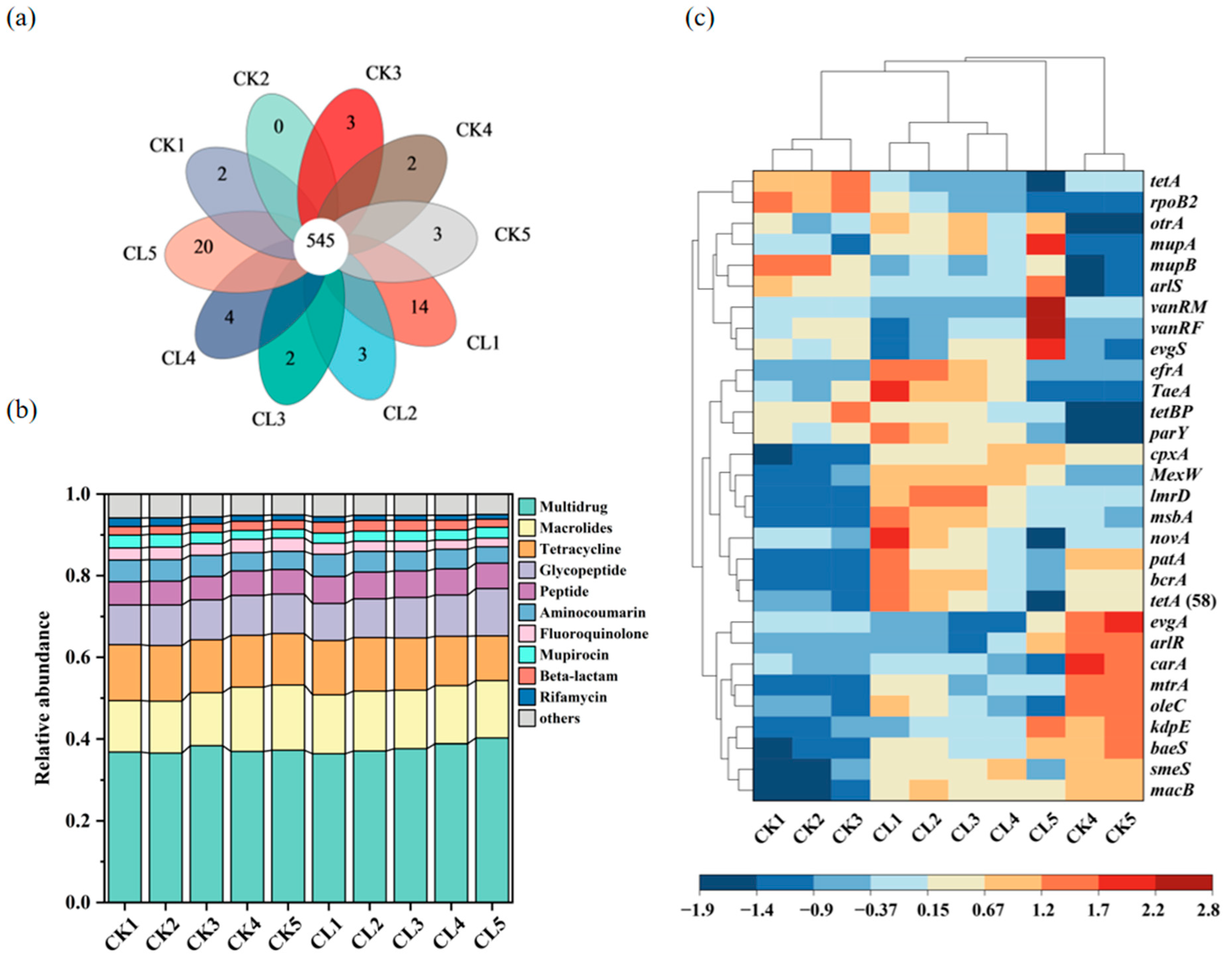
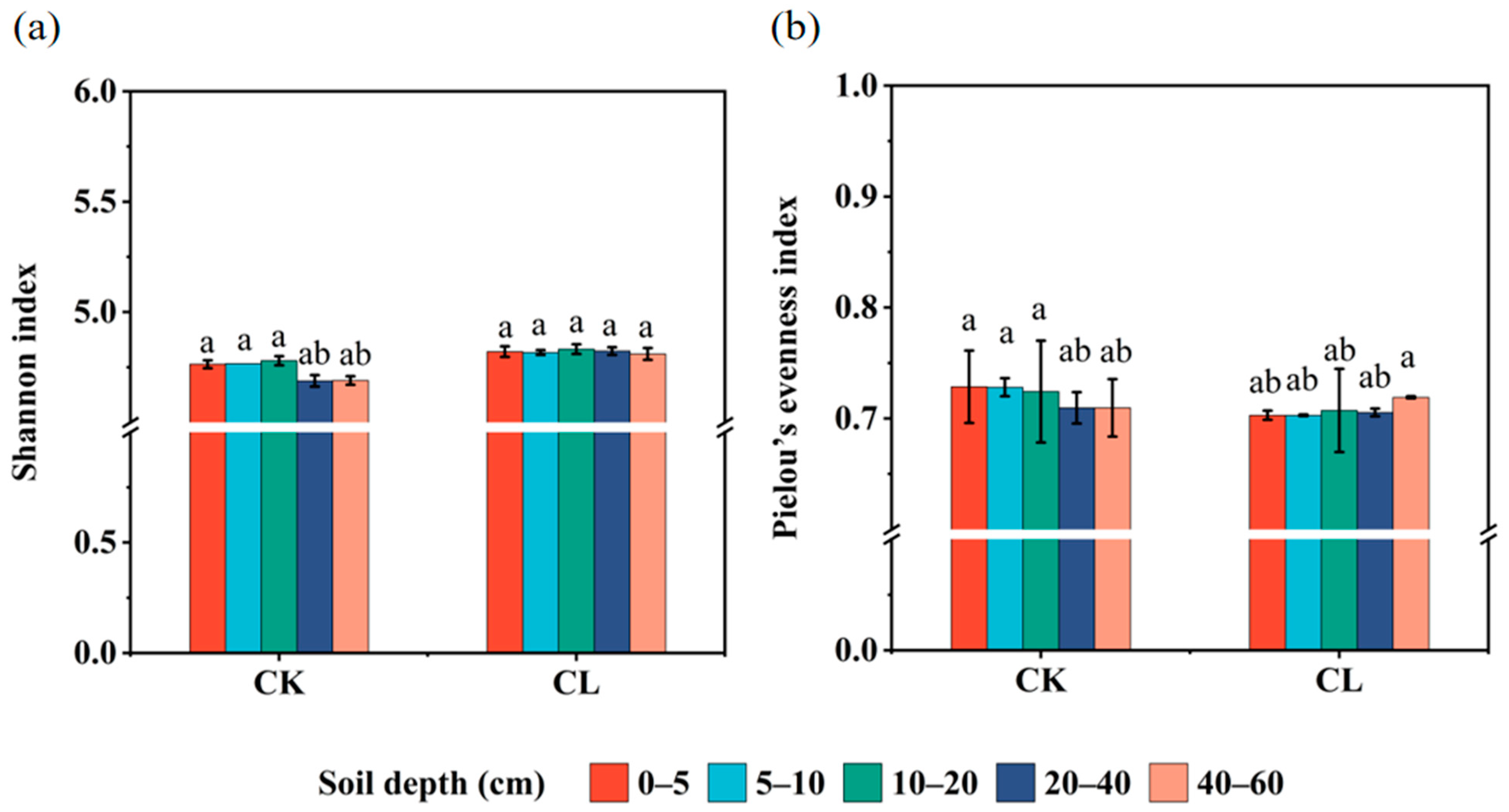
3.3. The Diversity and Abundance of the ARGs in the Investigated Farmland
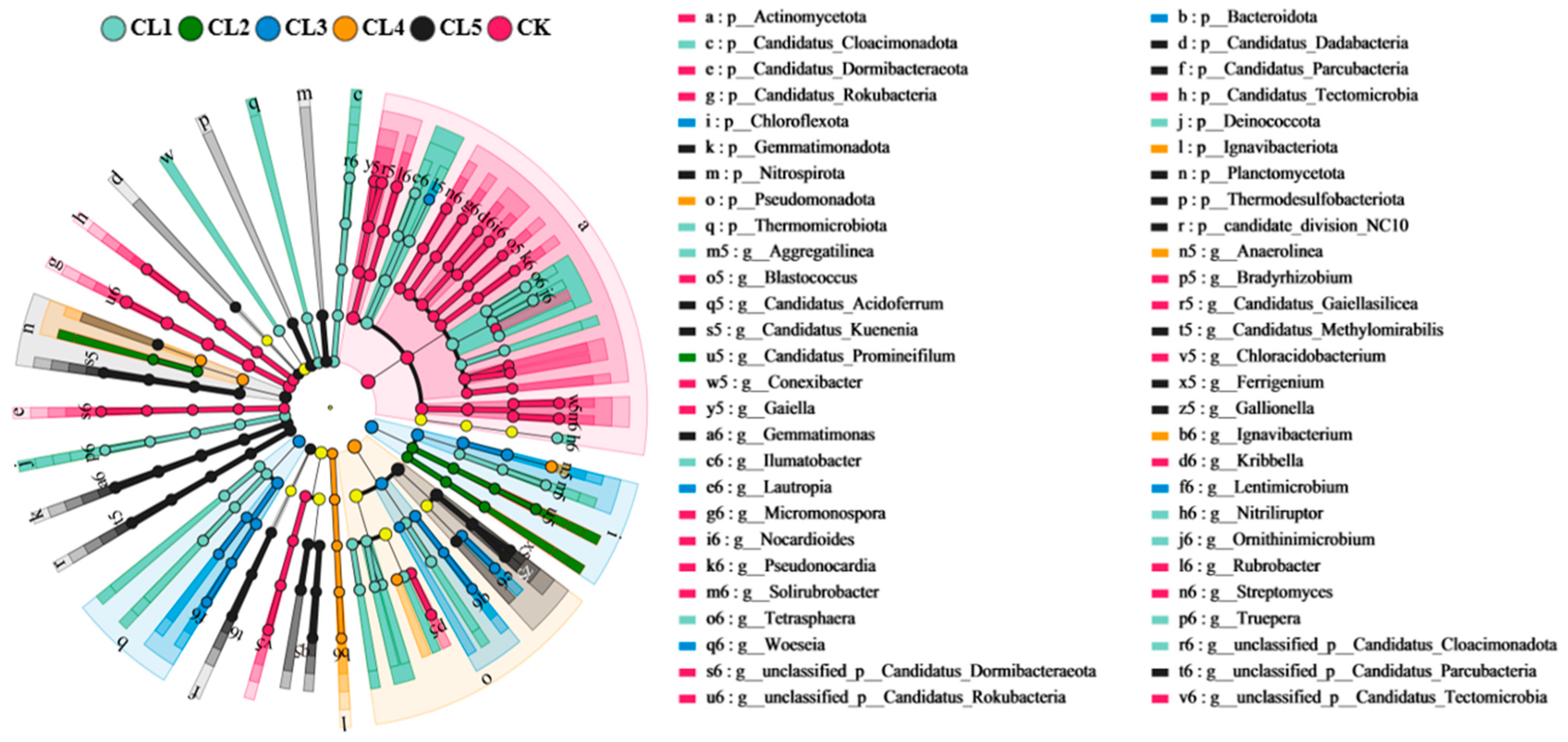
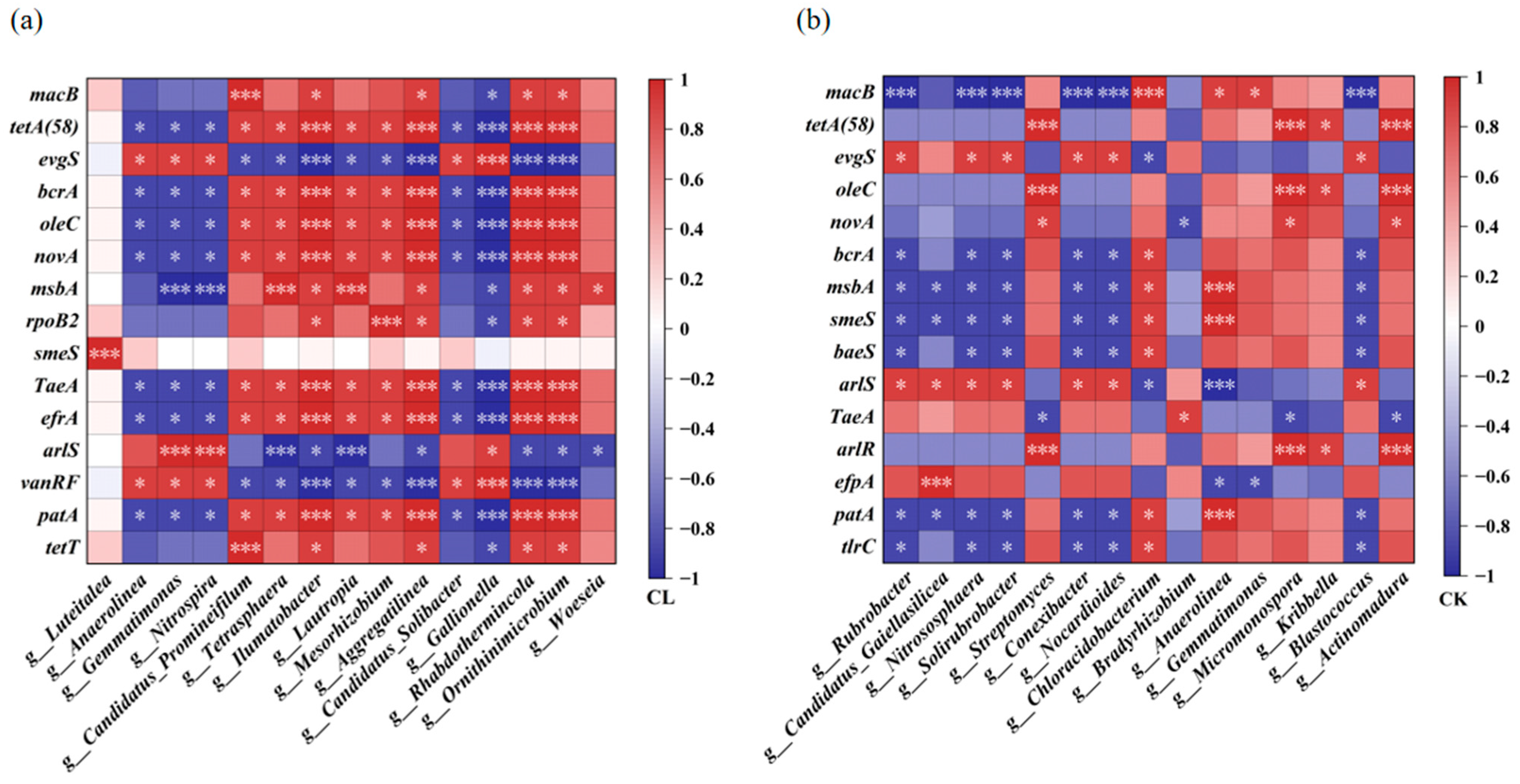
3.4. Relationships Between the Main ARGs and the Bacteria
3.5. Factors Influencing the Distribution of ARGs
4. Discussion
4.1. The Influence of Long-Term Livestock Wastewater Application on the Residual Antibiotic Concentration of the Farmland Soil
4.2. The Influence of Long-Term Livestock Wastewater Application on the Distribution of ARB and ARGs in the Farmland Soil
4.3. The Main Factor Influencing the Migration of ARGs in Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Class | Antibiotic | Abbreviation | RT (min) | Precursor (m/z) | Adduct | Production (m/z) | CE (V) |
|---|---|---|---|---|---|---|---|
| SAs | Sulfadiazine | SD | 1.44 | 215.1 | [M + H] + | 156.0/92.2 | 10/22 |
| Sulphadimethoxine | SDM | 2.875 | 311.00 | [M + H] + | 156.05/92.05 | −22/−35 | |
| Sulfamethizole | SMT | 2.058 | 271.00 | [M + H] + | 156.00/108.00 | −14/−23 | |
| Sulfamethoxydiazine | SMD | 2.699 | 281.10 | [M + H] + | 156.10/108.10 | −17/−26 | |
| Sulfamethoxazole | SMZ | 2.599 | 254.00 | [M + H] + | 156.00/92.00 | −16/−28 | |
| Sulfaphenazolum | SPP | 3.784 | 315.10 | [M + H] + | 158.10/156.10 | −28/−21 | |
| QNs | Ofloxacin | OFX | 2.114 | 362.20 | [M + H] + | 318.10/261.10 | −18/−28 |
| Ciprofloxacin | CIP | 2.393 | 332.20 | [M + H] + | 288.10/314.10 | −17/16 | |
| Enrofloxacin | ENR | 2.52 | 360.10 | [M + H] + | 316.10/342.10 | −20/−20 | |
| TCs | Oxytetracycline | OTC | 2.258 | 461.20 | [M + H] + | 425.95/443.10 | −18/−14 |
| MLs | Clarithromycin | CLR | 6.695 | 425.30 | [M + H] + | 126.10/377.30 | −50/−20 |
| Erythromycin | ERY | 6.455 | 734.30 | [M + H] + | 158.15/576.35 | −35/20 | |
| Roxithromycin | ROX | 6.72 | 837.50 | [M + H] + | 158.15/769.40 | −40/−25 |
| Time (min) | Elute A (%) | Elute B (%) | Flow Rate (mL/min) | Injection Volume (μL) | Spray Voltage (V) | ESI |
|---|---|---|---|---|---|---|
| 0 | 85 | 15 | 0.4 | 10 | 4500 | + |
| 2 | 70 | 30 | ||||
| 5 | 60 | 40 | ||||
| 7 | 5 | 95 | ||||
| 8.5 | 5 | 95 | ||||
| 8.51 | 85 | 15 | ||||
| 12.5 | stop |
| Sampling Site | Soil Layer | pH | EC (µS/cm) | MC (%) |
|---|---|---|---|---|
| CL | CL1 (0–5 cm) | 9.84 ± 0.17 a | 1461.3 ± 6.43 a | 25.20 ± 1.91 b |
| CL2 (5–10 cm) | 9.66 ± 0.16 a | 949.3 ± 13.28 b | 23.59 ± 1.21 b | |
| CL3 (10–20 cm) | 9.50 ± 0.21 a | 872.8 ± 9.68b | 33.16 ± 1.20 a | |
| CL4 (20–40 cm) | 9.83 ± 0.12 a | 679.3 ± 6.41 b | 17.33 ± 0.95 b | |
| CL5 (40–60 cm) | 9.95 ± 0.05 a | 678.4 ± 10.12 b | 20.40 ± 1.33 b | |
| CK | CK1 (0–5 cm) | 8.17 ± 0.012 a | 69.6 ± 2.10 a | 1.40 ± 0.29 d |
| CK2 (5–10 cm) | 8.15 ± 0.12 a | 51.8 ± 2.65 a | 4.19 ± 0.21 cd | |
| CK3 (10–20 cm) | 8.21 ± 0.05 a | 56.9 ± 2.20 a | 7.11 ± 0.05 bc | |
| CK4 (20–40 cm) | 8.41 ± 0.19 a | 64.3 ± 3.39 a | 10.61 ± 0.10ab | |
| CK5 (40–60 cm) | 8.25 ± 0.21 a | 54.7 ± 3.85 a | 12.96 ± 0.19 a |
References
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.H.; Huang, D.L.; Du, L.; Song, B.; Yin, L.S.; Chen, Y.S.; Gao, L.; Li, R.J.; Huang, H.; Zeng, G.M. Antibiotic resistance in soil-plant systems: A review of the source, dissemination, influence factors, and potential exposure risks. Sci. Total Environ. 2023, 869, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, Z.Y.; Wu, X.Y.; Zhao, L.; Wang, N.; Mao, D.Q.; Ren, H.Q.; Luo, Y. Antibiotic contamination amplifies the impact of foreign antibiotic-resistant bacteria on soil bacterial community. Sci. Total Environ. 2021, 758, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, D.M.; Xie, J.; Zhang, Y.T.; Wan, Y.; Zhang, Y.Q.; Shi, X.J. Impacts of farmland application of antibiotic-contaminated manures on the occurrence of antibiotic residues and antibiotic resistance genes in soil: A meta-analysis study. Chemosphere 2022, 300, 13. [Google Scholar] [CrossRef]
- Ying, G.G.; He, L.Y.; Ying, A.J.; Zhang, Q.Q.; Liu, Y.S.; Zhao, J.L. China Must Reduce Its Antibiotic Use. Environ. Sci. Technol. 2017, 51, 1072–1073. [Google Scholar] [CrossRef]
- Chuppava, B.; Keller, B.; Abd El-Wahab, A.; Meissner, J.; Kietzmann, M.; Visscher, C. Resistance of Escherichia coli in Turkeys after Therapeutic or Environmental Exposition with Enrofloxacin Depending on Flooring. Int. J. Environ. Res. Public Health 2018, 15, 1993. [Google Scholar] [CrossRef]
- Tsukayama, P.; Pehrsson, E.; Patel, S.; Berg, D.E.; Gilman, R.H.; Dantas, G. Transmission dynamics of antibiotic resistance genes in human and environmental microbiomes in Lima, Peru. Int. J. Infect. Dis. 2018, 73, 6. [Google Scholar] [CrossRef]
- Fang, L.F.; Chen, C.Y.; Li, S.Y.; Ye, P.P.; Shi, Y.J.; Sharma, G.; Sarkar, B.; Shaheen, S.M.; Lee, S.S.; Xiao, R.; et al. A comprehensive and global evaluation of residual antibiotics in agricultural soils: Accumulation, potential ecological risks, and attenuation strategies. Ecotox. Environ. Saf. 2023, 262, 16. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F.G.; Li, S.; Wang, J.; Hu, J.R.; Chen, S.; Chen, Q.; Li, Y.M.; Ha, X.; Sun, W.L. Insights into the driving factors of vertical distribution of antibiotic resistance genes in long-term fertilized soils. J. Hazard. Mater. 2023, 456, 12. [Google Scholar] [CrossRef]
- Tang, X.J.; Lou, C.L.; Wang, S.X.; Lu, Y.H.; Liu, M.; Hashmi, M.Z.; Liang, X.Q.; Li, Z.P.; Liao, Y.L.; Qin, W.J.; et al. Effects of long-term manure applications on the occurrence of antibiotics and antibiotic resistance genes (ARGs) in paddy soils: Evidence from four field experiments in south of China. Soil Biol. Biochem. 2015, 90, 179–187. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, H. Adsorption behavior of antibiotic in soil environment: A critical review. Front. Environ. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Wei, R.C.; Ge, F.; Zhang, L.L.; Hou, X.; Cao, Y.N.; Gong, L.; Chen, M.; Wang, R.; Bao, E.D. Occurrence of 13 veterinary drugs in animal manure-amended soils in Eastern China. Chemosphere 2016, 144, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.Y.; Zhang, L.P.; Li, F.R.; Huang, S.J.; Yang, J.; Ma, C.; Zhang, D.X.; Yu, C.P.; Hu, A.Y. Urban ponds as hotspots of antibiotic resistome in the urban environment. J. Hazard. Mater. 2021, 403, 12. [Google Scholar] [CrossRef] [PubMed]
- Kivits, T.; Broers, H.P.; Beeltje, H.; van Vliet, M.; Griffioen, J. Presence and fate of veterinary antibiotics in age-dated groundwater in areas with intensive livestock farming. Environ. Pollut. 2018, 241, 988–998. [Google Scholar] [CrossRef]
- Chi, S.L.; Xu, W.H.; Han, Y.R. ARGs distribution and high-risk ARGs identification based on continuous application of manure in purple soil. Sci. Total Environ. 2022, 853, 12. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, H.Y.; Song, D.; Chen, H.; Lin, X.G.; Wang, Y.M.; Ji, L.D. Distribution of antibiotic, heavy metals and antibiotic resistance genes in livestock and poultry feces from different scale of farms in Ningxia, China. J. Hazard. Mater. 2022, 440, 15. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Song, Y.Q.; Lin, H.; Yin, H.B. Dissecting horizontal and vertical gene transfer of antibiotic resistance plasmid in bacterial community using microfluidics. Environ. Int. 2019, 131, 9. [Google Scholar] [CrossRef]
- Chen, P.; Yu, K.F.; He, Y.L. The dynamics and transmission of antibiotic resistance associated with plant microbiomes. Environ. Int. 2023, 176, 15. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.E.; Lee, Y.B. Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: A critical view. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Shen, Y.P.; Zhang, B.Y.; Yao, Y.; Wang, H.B.; Chen, Z.L.; Hao, A.J.; Guo, P. Insights into the interactions of plant-associated bacteria and their role in the transfer of antibiotic resistance genes from soil to plant. J. Hazard. Mater. 2024, 480, 12. [Google Scholar] [CrossRef]
- Zalewska, M.; Blazejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure—Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Hu, H.W.; Chen, Q.L.; Singh, B.K.; Yan, H.; Chen, D.L.; He, J.Z. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ. Int. 2019, 130, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.B.; Yuan, T.; Zhou, L.; Cheng, S.; Qu, X.; Lu, P.; Feng, Q.Y. Impact factors of the accumulation, migration and spread of antibiotic resistance in the environment. Environ. Geochem. Health 2021, 43, 1741–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Gao, Y.C.; Zheng, L.W.; Ji, L.; Kong, X.; Du, J.H.; Wang, H.; Duan, L.C.; Niu, T.; Liu, J.H.; et al. Identification and Distribution of Antibiotic Resistance Genes and Antibiotic Resistance Bacteria in the Feces Treatment Process: A Case Study in a Dairy Farm, China. Water 2024, 16, 1575. [Google Scholar] [CrossRef]
- Topp, G.C.; Ferre, P.A. Methods for measurement of soil water content: Thermogravimetric using convective oven-drying. Methods Soil Anal. Part 4 Phys. Methods 2002, 4, 422–424. [Google Scholar]
- Wakim, L.M.; Descat, A.; Occelli, F.; Deram, A.; Goossens, J.F. Detection of 13 emerging soil pollutant compounds using a dual extraction method (QuEChERS and solid phase extraction) and a liquid chromatography/mass spectrometry LC-MS/MS method. MethodsX 2024, 12, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.W.; Zhang, R.J.; Du, X.; Yuan, B.; Zhang, Z.S.; Lin, Z.Y.; Wang, J.; Sun, Y. Development of a 3-step sequential extraction method to investigate the fraction and affecting factors of 21 antibiotics in soils. J. Hazard. Mater. 2024, 469, 10. [Google Scholar] [CrossRef]
- Sun, X.; Su, L.; Zhen, J.; Wang, Z.; Panhwar, K.A.; Ni, S.-Q. The contribution of swine wastewater on environmental pathogens and antibiotic resistance genes: Antibiotic residues and beyond. Chemosphere 2024, 364, 143263. [Google Scholar] [CrossRef]
- Ren, J.H.; Shi, H.C.; Liu, J.C.; Zheng, C.Y.; Lu, G.H.; Hao, S.F.; Jin, Y.; He, C. Occurrence, source apportionment and ecological risk assessment of thirty antibiotics in farmland system. J. Environ. Manag. 2023, 335, 11. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, S.D.; Zhao, L.X.; Li, X.J.; Weng, L.P.; Sun, Y.; Li, Y.T. Temporal and spatial variability of antibiotics in agricultural soils from Huang-Huai-Hai Plain, northern China. Chemosphere 2021, 272, 10. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.H.; Wang, F.F.; Antoine, G.; van der Hoek, J.P. Sulfonamides removal under different redox conditions and microbial response to sulfonamides stress during riverbank filtration: A laboratory column study. Chemosphere 2019, 220, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.G.; Zhou, Q.X.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Kroeze, C.; van de Schans, M.G.M.; Baartman, J.; Yang, J.; Li, S.; Xu, W.; Wang, M.; Ma, L.; et al. More inputs of antibiotics into groundwater but less into rivers as a result of manure management in China. Environ. Sci. Ecotechnol. 2025, 23, 100513. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Y.; Ling, C.; Shen, Y.; Zhan, X.; Xing, B. Fate of emerging antibiotics in soil-plant systems: A case on fluoroquinolones. Sci. Total Environ. 2024, 951, 175487. [Google Scholar] [CrossRef]
- Abril, C.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Uptake and translocation of multiresidue industrial and household contaminants in radish grown under controlled conditions. Chemosphere 2021, 268, 9. [Google Scholar] [CrossRef]
- Han, X.M.; Hu, H.W.; Chen, Q.L.; Yang, L.Y.; Li, H.L.; Zhu, Y.G.; Li, X.Z.; Ma, Y.B. Antibiotic resistance genes and associated bacterial communities in agricultural soils amended with different sources of animal manures. Soil Biol. Biochem. 2018, 126, 91–102. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, L.; Gu, J.; Feng, Y.C.; He, K.; Jiang, H.H. Effects of soil solarization combined with manure-amended on soil ARGs and microbial communities during summer fallow. Environ. Pollut. 2023, 333, 11. [Google Scholar] [CrossRef]
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, Virulence, and Infective Dose. PLoS Pathogens 2007, 3, 1372–1373. [Google Scholar] [CrossRef]
- D’Costa, V.M.; Mcgrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the Antibiotic Resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef]
- Li, M.M.; Ray, P.; Knowlton, K.F.; Pruden, A.; Xia, K.; Teets, C.; Du, P. Fate of pirlimycin and antibiotic resistance genes in dairy manure slurries in response to temperature and pH adjustment. Sci. Total Environ. 2020, 710, 9. [Google Scholar] [CrossRef]
- Sun, J.B.; Zhang, D.; Peng, S.; Yang, X.Q.; Hua, Q.Q.; Wang, W.; Wang, Y.M.; Lin, X.G. Critical insights into the Hormesis of antibiotic resistome in saline soil: Implications from salinity regulation. J. Hazard. Mater. 2024, 472, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fu, Q.; Wen, Q.; Wu, Y.; Bao, H.; Guo, J. Microbial community competition rather than high-temperature predominates ARGs elimination in swine manure composting. J. Hazard. Mater. 2022, 423, 127149. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, M.; Stedtfeld, R.D.; Sheng, H.; Fan, J.; Liu, M.; Chai, B.; Soares de Carvalho, T.; Li, H.; Li, Z.; et al. Long-Term Effect of Different Fertilization and Cropping Systems on the Soil Antibiotic Resistome. Environ. Sci. Technol. 2018, 52, 13037–13046. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.J.; Reyes, A.; Bin, W.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The Shared Antibiotic Resistome of Soil Bacteria and Human Pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.Y.; Shao, Z.L.; Li, X.C.; Zheng, X.Q.; Xu, J. Fate of antibiotic resistance genes in farmland soil applied with three different fertilizers during the growth cycle of pakchoi and after harvesting. J. Environ. Manag. 2021, 289, 7. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, M.; Gao, Y.; Zheng, L.; Ji, L.; Du, J.; Kong, X.; Wang, H.; Shi, F.; Wang, H.; Liu, J.; et al. Vertical Distribution and Drivers of Antibiotic Resistance Genes in Agricultural Soil Irrigated with Livestock Wastewater. Microorganisms 2025, 13, 610. https://doi.org/10.3390/microorganisms13030610
Shang M, Gao Y, Zheng L, Ji L, Du J, Kong X, Wang H, Shi F, Wang H, Liu J, et al. Vertical Distribution and Drivers of Antibiotic Resistance Genes in Agricultural Soil Irrigated with Livestock Wastewater. Microorganisms. 2025; 13(3):610. https://doi.org/10.3390/microorganisms13030610
Chicago/Turabian StyleShang, Ming, Yongchao Gao, Liwen Zheng, Lei Ji, Jianhua Du, Xue Kong, Hui Wang, Feng Shi, Hailun Wang, Jianhui Liu, and et al. 2025. "Vertical Distribution and Drivers of Antibiotic Resistance Genes in Agricultural Soil Irrigated with Livestock Wastewater" Microorganisms 13, no. 3: 610. https://doi.org/10.3390/microorganisms13030610
APA StyleShang, M., Gao, Y., Zheng, L., Ji, L., Du, J., Kong, X., Wang, H., Shi, F., Wang, H., Liu, J., Yang, X., & Wang, Z. (2025). Vertical Distribution and Drivers of Antibiotic Resistance Genes in Agricultural Soil Irrigated with Livestock Wastewater. Microorganisms, 13(3), 610. https://doi.org/10.3390/microorganisms13030610






