Novel Co-Cultivation Bioprocess with Immobilized Paenibacillus polymyxa and Scenedesmus obliquus for Lipid and Butanediol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. The Preculture and Main Culture
2.1.1. Chitosan-Coated Carrageenan Beads
2.1.2. Chitosan-Coated Calcium Alginate Beads
2.2. The Cell Count
2.3. Substrate Analysis
2.4. Growth of the Microorganisms
2.5. Biomass Concentration
2.6. Lipid Content
2.7. Determination of the Pigment Composition
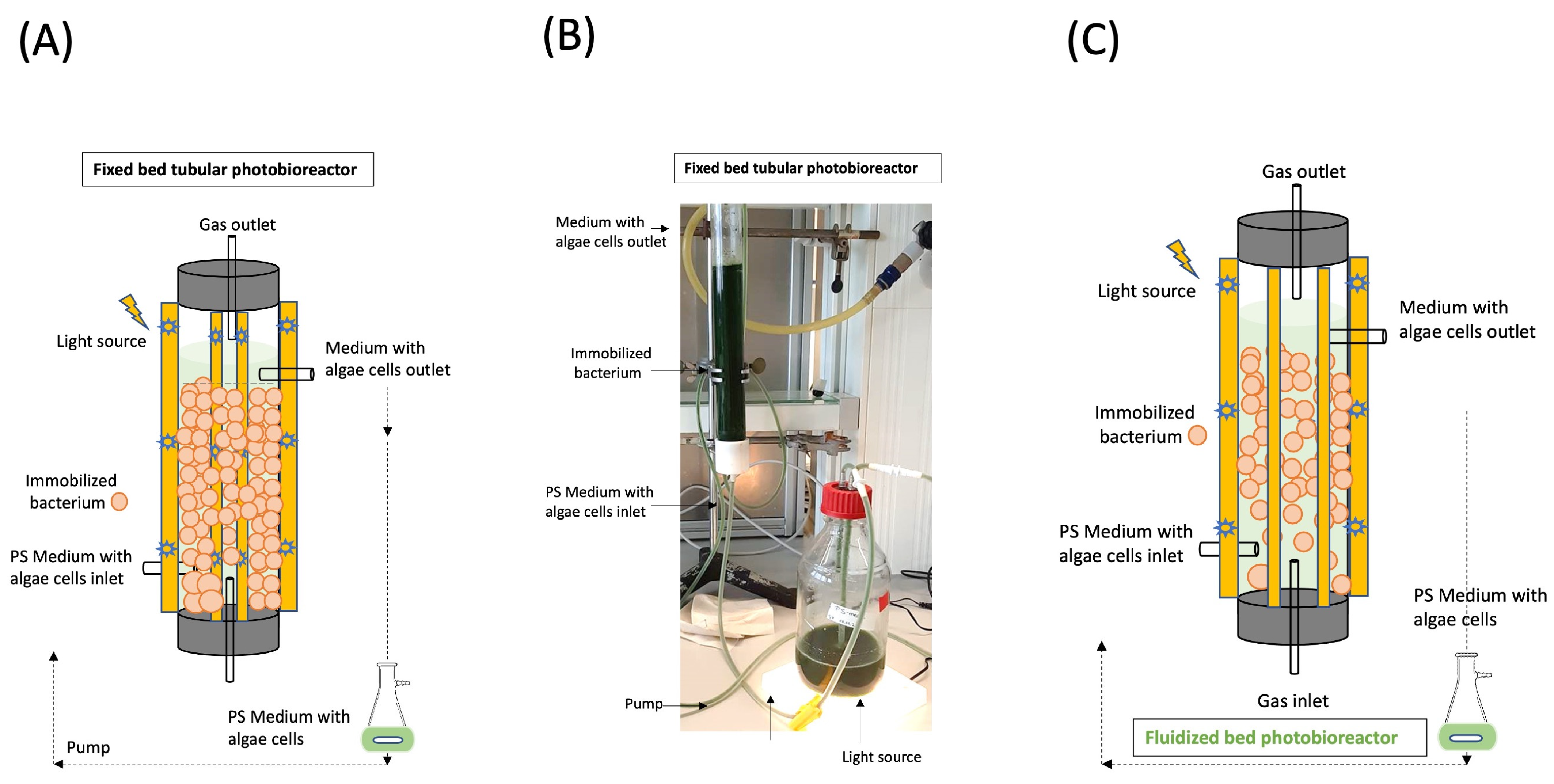
2.8. Photobioreactor Construction
2.9. Experimental Designs and Statistical Analyses
3. Results
3.1. Co-Cultivation with Immobilized Bacteria Showed Increased Growth and Total Chlorophyll Content in the Microalgal Cells
3.2. Co-Cultivation with the Immobilized Bacteria Improved the Biomass and Lipid Production by S. obliquus
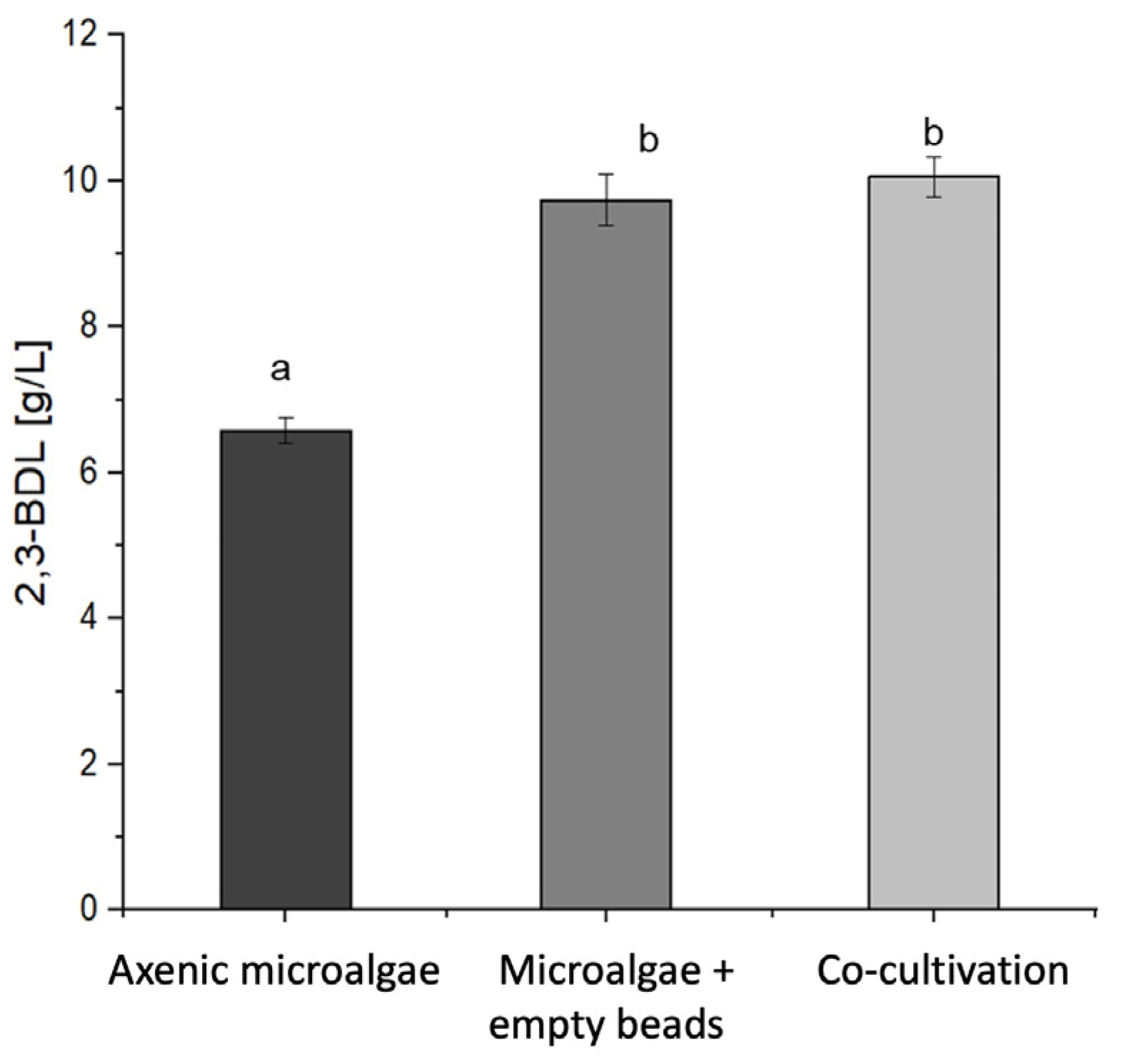
3.3. Co-Cultivation with the Microalgae Improved the 2,3-Butanediol Production by Immobilized P. polymyxa
3.4. The Implementation of a Novel Co-Cultivation Bioprocess for Lipid and 2,3-Butanediol Production Through Co-Cultivation of an Immobilized PGPR and Microalga for Combined Beneficial Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Wang, R.; Xue, S.; Zhang, D.; Zhang, Q.; Wen, S.; Kong, D.; Yan, C.; Cong, W. Construction and characteristics of artificial consortia of Scenedesmus obliquus-bacteria for S. obliquus growth and lipid production. Algal Res. 2015, 12, 436–445. [Google Scholar]
- Osman, M.E.H.; Abo-Shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the potential of microalgae cultivated on wastewater combined with salinity stress to improve biodiesel production. Environ. Sci. Pollut. Res. Int. 2023, 30, 114610–114624. [Google Scholar] [CrossRef] [PubMed]
- Barati, B.; Zafar, F.F.; Rupani, P.F.; Wang, S. Bacterial pretreatment of microalgae and the potential of novel nature hydrolytic sources. Environ. Technol. Innov. 2021, 21, 101362. [Google Scholar] [CrossRef]
- Bilad, M.R.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F. Coupled cultivation and pre-harvesting of microalgae in a membrane photobioreactor (MPBR). Bioresour. Technol. 2014, 155, 410–417. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar]
- Borowitzka, M.A.; Moheimani, N.R. Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013; pp. 17–36. [Google Scholar]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar]
- Afify, A.E.M.M.R.; El Baroty, G.S.; El Baz, F.K.; El Baky, H.H.A.; Murad, S.A. Scenedesmus obliquus: Antioxidant and antiviral activity of proteins hydrolysed by three enzymes. J. Genet. Eng. Biotechnol. 2018, 16, 399–408. [Google Scholar]
- Hegewald, E. Taxonomy and Phylogeny of Scenedesmus. In Phylogenetic Studies on Scenedesmaceae (Chlorophyta); Algological Studies/Archiv für Hydrobiologie, Supplement Volumes No. 100; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 2000; pp. 29–49. [Google Scholar]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on lipid production by Scendesmus obliquus triggered by low dose exposure to nanoparticles. Sci. Rep. 2017, 7, 15526. [Google Scholar]
- Matsunaga, T.; Matsumoto, M.; Maeda, Y.; Sugiyama, H.; Sato, R.; Tanaka, T. Characterization of marine microalga, Scenedesmus sp. strain JPCC GA0024 toward biofuel production. Biotechnol. Lett. 2009, 31, 1367–1372. [Google Scholar] [PubMed]
- Shao, Y.; Fang, H.; Zhou, H.; Wang, Q.; Zhu, Y.; He, Y. Detection and Imaging of Lipids of Scenedesmus obliquus Based on Confocal Raman Microspectroscopy. Ph.D. Thesis, Ernst-Moritz-Arndt-Universität Greifswald, Greifswald, Germany, 2017. [Google Scholar]
- Bucheli, J.; Cella, H.; Nader, C.; Oliveira, C.Y.B.; Bastolla, C.L.V.; Lopes, R.G.; Pereira, G.V.; Karam, J.; Derner, R.B. Bacterial assemblages structure in intensive cultivations of the microalga Tetradesmus obliquus. J. Basic Microbiol. 2023, 63, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A comparative study between fungal pellet-and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Bahar, K.; Islam, K.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.Z.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar]
- Ashraf, N.; Ahmad, F.; Lu, Y. Synergy between microalgae and microbiome in polluted waters. Trends Microbiol. 2023, 31, 9–21. [Google Scholar]
- Monteiro dos Santos, L.; Barbosa da Silva, J.C.; de Farias Silva, C.E.; Villar da Gama, B.M.; Almeida Medeiros, J.; Markou, G.; Rosas Garcia Almeida, R.M.; Abud, A.K.d.S. Co-Cultivation between the Microalga Tetradesmus obliquus and Filamentous Fungus Cunninghamella echinulata Improves Tertiary Treatment of Cheese Whey Effluent in Semicontinuous Mode. Processes 2024, 12, 1573. [Google Scholar] [CrossRef]
- Chekanov, K.; Zaytseva, A.; Mamedov, I.; Solovchenko, A.; Lobakova, E. The Dynamics of the Bacterial Community of the Photobioreactor-Cultivated Green Microalga Haematococcus lacustris during Stress-Induced Astaxanthin Accumulation. Biology 2021, 10, 115. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, N.; Singh, G.; Mishra, S.; Lodhiyal, N. Microalgae cultivation and value-based products from wastewater: Insights and applications. Blue Biotechnol. 2024, 1, 20. [Google Scholar] [CrossRef]
- Wang, X.; Gao, S.; Zhang, Y.; Zhao, Y.; Cao, W. Performance of different microalgae-based technologies in biogas slurry nutrient removal and biogas upgrading in response to various initial CO2 concentration and mixed light-emitting diode light wavelength treatments. J. Clean. Prod. 2017, 166, 408–416. [Google Scholar] [CrossRef]
- Cho, S.; Luong, T.T.; Lee, D.; Oh, Y.-K.; Lee, T. Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour. Technol. 2011, 102, 8639–8645. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-Del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, G.; Rizwan, M.; Lee, K. Removal of nutrients and COD from wastewater using symbiotic co-culture of bacterium Pseudomonas putida and immobilized microalga Chlorella vulgaris. J. Ind. Eng. Chem. 2017, 51, 297–303. [Google Scholar]
- Abate, R.; Oon, Y.S.; Oon, Y.L.; Bi, Y. Microalgae-bacteria nexus for environmental remediation and renewable energy resources: Advances, mechanisms and biotechnological applications. Heliyon 2024, 10, e31170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef]
- Joshi, J.; Langwald, S.V.; Kruse, O.; Patel, A. Immobilization of Paenibacillus polymyxa with biopolymers to enhance the production of 2,3-butanediol. Microb. Cell Fact. 2025, 24, 15. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed Oligosaccharides Stimulate Plant Growth by Enhancing Carbon and Nitrogen Assimilation, Basal Metabolism, and Cell Division. J. Plant Growth Regul. 2012, 32, 443–448. [Google Scholar]
- Arman, M.; Ul Qader, S.A. Structural analysis of kappa-carrageenan isolated from Hypnea musciformis (red algae) and evaluation as an elicitor of plant defense mechanism. Carbohydr. Polym. 2012, 88, 1264–1271. [Google Scholar]
- Castro, J.; Vera, J.; González, A.; Moenne, A. Oligo-Carrageenans Stimulate Growth by Enhancing Photosynthesis, Basal Metabolism, and Cell Cycle in Tobacco Plants (var. Burley). J. Plant Growth Regul. 2011, 31, 173–185. [Google Scholar]
- Chanda, M.-J.; Merghoub, N.; El Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.-T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2008, 149, 43–51. [Google Scholar]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Bi, F.; Iqbal, S.; Arman, M.; Ali, A.; Hassan, M. Carrageenan as an elicitor of induced secondary metabolites and its effects on various growth characters of chickpea and maize plants. J. Saudi Chem. Soc. 2011, 15, 269–273. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salama, E.-S.; Kim, H.-C.; Abou-Shanab, R.A.I.; Oh, Y.-K.; Jeon, B.-H. Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst. Eng. 2013, 36, 827–833. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef]
- Dias, C.; Reis, A.; Santos, J.A.L.; Gouveia, L.; Lopes da Silva, T. Primary brewery wastewater as feedstock for the yeast Rhodosporidium toruloides and the microalga Tetradesmus obliquus mixed cultures with lipid production. Process Biochem. 2022, 113, 71–86. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Wang, S.; Yang, G.; Zhao, L.; Pan, K. Co-culturing bacteria and microalgae in organic carbon containing medium. J. Biol. Res. Thessalon. 2016, 23, 8. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef]
- Danger, M.; Oumarou, C.; Benest, D.; Lacroix, G. Bacteria can control stoichiometry and nutrient limitation of phytoplankton. Funct. Ecol. 2007, 21, 202–210. [Google Scholar] [CrossRef]
- Patel, A.; Slaats, B.; Hallmann, J.; Tilcher, R.; Beitzen-Heineke, W.; Vorlop, K.D. Encapsulation and application of bacterial antagonists and a nematophagous fungus for biological pest control. Gesunde Pflanzen 2004, 57, 30–33. [Google Scholar] [CrossRef]
- Magdouli, S.K.; Brar, S.K.; Blais, J.F. Co-culture for lipid production: Advances and challenges. Biomass Bioenergy 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richmann, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Antoun, H.; Bashan, Y. Involvement of indole-3-acetic acid produced by the growth-promoting bacterium Azospirillum spp. in promoting growth of chlorella vulgaris 1. J. Phycol. 2008, 44, 938–947. [Google Scholar]
- Kitcha, S.; Cheirsilp, B. Enhanced Lipid Production by Co-cultivation and Co-encapsulation of Oleaginous Yeast Trichosporonoides spathulata with Microalgae in Alginate Gel Beads. Appl. Biochem. Biotechnol. 2014, 173, 522–534. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Ori, M.O.; Ekpan, F.M.; Samuel, H.S.; Egwuatu, O.P. Emerging Co-Cultivation Strategies for Microalgal Biomass and Biodiesel Production. Prog. Chem. Biochem. Res. 2024, 7, 198–224. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Wager, Y.Z.; Roostaei, J. Co-cultivation of microalgae and bacteria for optimal bioenergy feedstock production in wastewater by using response surface methodology. Sci. Rep. 2024, 14, 20703. [Google Scholar] [CrossRef]
- Torres, M.J.; Bellido-Pedraza, C.M.; Llamas, A. Applications of the Microalgae Chlamydomonas and Its Bacterial Consortia in Detoxification and Bioproduction. Life 2024, 14, 940. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef]
- Guo, Z.; Tong, Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 2014, 26, 1483–1492. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.W.; Zehr, J.P. Cellular interactions: Lessons from the nitrogen-fixing cyanobacteria. J. Phycol. 2013, 49, 1024–1035. [Google Scholar] [CrossRef]
- Naseema Rasheed, R.; Pourbakhtiar, A.; Mehdizadeh Allaf, M.; Baharlooeian, M.; Rafiei, N.; Alishah Aratboni, H.; Morones-Ramirez, J.R.; Winck, F.V. Microalgal co-cultivation—Recent methods, trends in omic-studies, applications, and future challenges. Front. Bioeng. Biotechnol. 2023, 11, 1193424. [Google Scholar] [CrossRef]
- Yong, S.C.; Roversi, P.; Lillington, J.; Rodriguez, F.; Krehenbrink, M.; Zeldin, O.B.; Garman, E.F.; Lea, S.M.; Berks, B.C. A complex iron-calcium cofactor catalyzing phosphotransfer chemistry. Science 2014, 345, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Homburg, S.V.; Kruse, O.; Patel, A. COMBINE: Co-cultivation of microalgae and bacteria. Biotechnol. Adv. 2018, 36, 1279–1296. [Google Scholar] [CrossRef][Green Version]
- Rojas-Pirela, M.; Carillo, P.; Lárez-Velásquez, C.; Romanazzi, G. Effects of chitosan on plant growth under stress conditions: Similarities with plant growth-promoting bacteria. Front. Plant Sci. 2024, 15, 1423949. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Tavares, G.M.; Prata, A.S.; Hubinger, M.D. Complexation of chitosan with gum Arabic, sodium alginate and κ-carrageenan: Effects of pH, polymer ratio and salt concentration. Carbohydr. Polym. 2019, 223, 11512. [Google Scholar] [CrossRef]
- Legrand, J.; Artub, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Covarrubias, S.A.; de-Bashan, L.E.; Moreno, M.; Bashan, Y. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012, 93, 2669–2680. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, P.W.; Chen, L.J. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour. Technol. 2015, 184, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Juliarnita, I.G.A.; Hadisoebroto, R.; Rinanti, A. Bioethanol production from mixed culture microalgae biomass with temperature hydrolysis variation. MATEC Web Conf. 2018, 156, 13010. [Google Scholar]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [PubMed]
- Ray, A.; Nayak, M.; Ghosh, A. A review on co-culturing of microalgae: A greener strategy towards sustainable biofuels production. Sci. Total Environ. 2021, 802, 149765. [Google Scholar] [CrossRef]
- Patrinou, V.; Patsialou, S.; Daskalaki, A.; Economou, C.N.; Aggelis, G.; Vayenas, D.V.; Tekerlekopoulou, A.G. Laboratory- and Pilot-Scale Cultivation of Tetraselmis striata to Produce Valuable Metabolic Compounds. Life 2023, 13, 480. [Google Scholar] [CrossRef]
- Cheah, Y.T.; Chan, D.J.C. Physiology of microalgal biofilm: A review on prediction of adhesion on substrates. Bioengineered 2021, 12, 7577–7599. [Google Scholar] [CrossRef]
- Rojas-Villalta, D.; Gómez-Espinoza, O.; Murillo-Vega, F.; Villalta-Romero, F.; Guerrero, M.; Guillén-Watson, R.; Núñez-Montero, K. Insights into Co-Cultivation of Photosynthetic Microorganisms for Novel Molecule Discovery and Enhanced Production of Specialized Metabolites. Fermentation 2023, 9, 941. [Google Scholar] [CrossRef]



| Cultivation | Chlorophyll Content (mg/Cells) |
|---|---|
| a. axenic algae in PS medium | 15 × 10−6 |
| b. axenic algae with empty chitosan-coated carrageenan beads | 18 × 10−6 |
| c. co-cultivation with immobilized bacteria | 26 × 10−6 |
| Cultivation | Biomass [g/L] | Biomass Yield [gX/gS] | Biomass-Specific Substrate Uptake Rates qS [gS/gXd] |
|---|---|---|---|
| a. axenic algae in PS medium | 0.31 | 0.04 | 0.06 |
| b. axenic algae with empty chitosan-coated carrageenan beads | 0.54 | 0.03 | |
| c. co-cultivation with immobilized bacteria | 0.51 | 0.02 |
| Cultivation | Total Lipids [g/L] | Lipid Concentration [%] |
|---|---|---|
| a. axenic algae in PS medium | 0.09 | 0.67 |
| b. axenic algae empty chitosan-coated carrageenan beads | 0.04 | 1.03 |
| c. co-cultivation with immobilized bacteria | 0.11 | 1.05 |
| Cultivation | Product Formation Rates qP [gP/gxd] | Product Yields YP/S [gP/gS] |
|---|---|---|
| a. Co-cultivation with free cells | 0.05 | 0.02 |
| b. Co-cultivation with chitosan–calcium alginate-immobilized bacteria | 0.07 | 0.04 |
| c. Co-cultivation with chitosan–carrageenan-immobilized bacteria | 0.55 | 0.06 |
| Cultivation | Growth Rate µmax [d] | Dry Biomass Concentration [g/L] |
|---|---|---|
| Co-cultivation with BP-2 beads in PS medium (flask) | 0.02 | 0.16 |
| Co-cultivation with BP-2 beads in PS medium (PBR) | 0.01 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, J.S.; Fladung, L.; Kruse, O.; Patel, A. Novel Co-Cultivation Bioprocess with Immobilized Paenibacillus polymyxa and Scenedesmus obliquus for Lipid and Butanediol Production. Microorganisms 2025, 13, 606. https://doi.org/10.3390/microorganisms13030606
Joshi JS, Fladung L, Kruse O, Patel A. Novel Co-Cultivation Bioprocess with Immobilized Paenibacillus polymyxa and Scenedesmus obliquus for Lipid and Butanediol Production. Microorganisms. 2025; 13(3):606. https://doi.org/10.3390/microorganisms13030606
Chicago/Turabian StyleJoshi, Jnanada Shrikant, Laura Fladung, Olaf Kruse, and Anant Patel. 2025. "Novel Co-Cultivation Bioprocess with Immobilized Paenibacillus polymyxa and Scenedesmus obliquus for Lipid and Butanediol Production" Microorganisms 13, no. 3: 606. https://doi.org/10.3390/microorganisms13030606
APA StyleJoshi, J. S., Fladung, L., Kruse, O., & Patel, A. (2025). Novel Co-Cultivation Bioprocess with Immobilized Paenibacillus polymyxa and Scenedesmus obliquus for Lipid and Butanediol Production. Microorganisms, 13(3), 606. https://doi.org/10.3390/microorganisms13030606






