The Prevalence of Bacterial Vaginosis in Pregnant Women in Slovenia, Determined via Microscopy and Semi-Quantitative Relative Culture, and Its Association with Adverse Pregnancy Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Microbiological Methods
2.3. Detection of BV via Microscopy
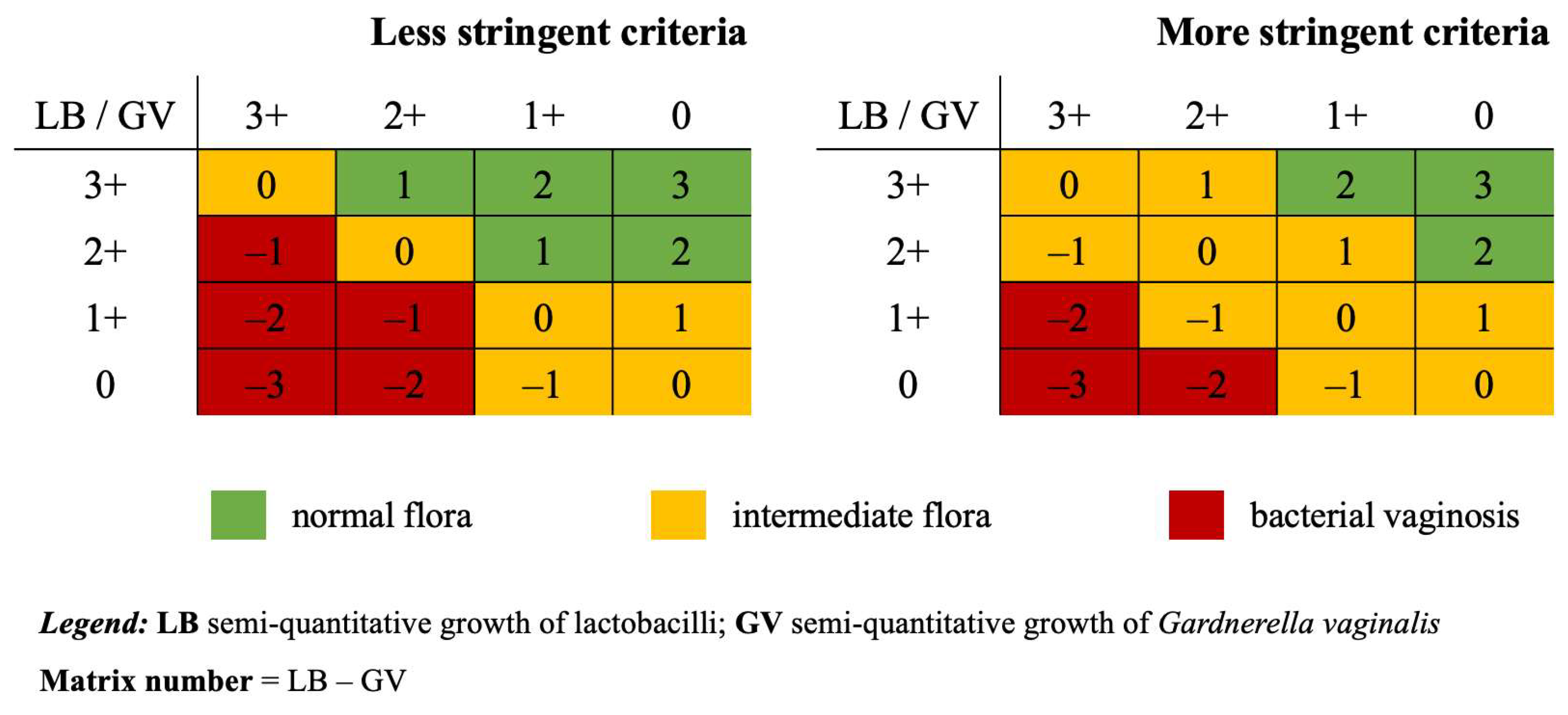
2.4. Detection of BV via SRC
2.5. Data Management and Statistical Analysis
3. Results
3.1. Study Population
3.2. Microbiological Characteristics
3.3. Prevalence of BV
3.4. Adverse Pregnancy Outcomes and BV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BV | Bacterial vaginosis |
| GBS | Group B streptococcus |
| GV | Gardnerella vaginalis |
| LB | Lactobacilli |
| MALDI-TOF | Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry |
| NPIS | National Perinatal Information System |
| PPROM | Preterm, premature rupture of membranes |
| SRC | Semi-quantitative relative culture |
References
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Colebunders, R.; Crucitti, T. The Global Epidemiology of Bacterial Vaginosis: A Systematic Review. Am. J. Obstet. Gynecol. 2013, 209, 505–523. [Google Scholar] [CrossRef]
- Sethi, N.; Narayanan, V.; Saaid, R.; Ahmad Adlan, A.S.; Ngoi, S.T.; Teh, C.S.J.; Hamidi, M., on behalf of WHOW research group. Prevalence, Risk Factors, and Adverse Outcomes of Bacterial Vaginosis among Pregnant Women: A Systematic Review. BMC Pregnancy Childbirth 2025, 25, 40. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Gerede, A.; Nikolettos, K.; Vavoulidis, E.; Margioula-Siarkou, C.; Petousis, S.; Giourga, M.; Fotinopoulos, P.; Salagianni, M.; Stavros, S.; Dinas, K.; et al. Vaginal Microbiome and Pregnancy Complications: A Review. J. Clin. Med. 2024, 13, 3875. [Google Scholar] [CrossRef]
- Yudin, M.H.; Money, D.M. No. 211-Screening and Management of Bacterial Vaginosis in Pregnancy. J. Obstet. Gynaecol. Can. 2017, 39, e184–e191. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Gordon, A.; Heatley, E.; Milan, S.J. Antibiotics for Treating Bacterial Vaginosis in Pregnancy. Cochrane Database Syst. Rev. 2013, 2013, CD000262. [Google Scholar] [CrossRef]
- Hadhoum, S.; Subtil, D.; Labreuche, J.; Couvreur, E.; Brabant, G.; Dessein, R.; Le Guern, R. Reassessing the Association between Bacterial Vaginosis and Preterm Birth: A Systematic Review and Meta-Analysis. J. Gynecol. Obstet. Hum. Reprod. 2025, 54, 102871. [Google Scholar] [CrossRef]
- Hillier, S.L.; Austin, M.; Macio, I.; Meyn, L.A.; Badway, D.; Beigi, R. Diagnosis and Treatment of Vaginal Discharge Syndromes in Community Practice Settings. Clin. Infect. Dis. 2021, 72, 1538–1543. [Google Scholar] [CrossRef]
- Amsel, R.; Totten, P.; Spiegel, C.; Chen, C.; Esenbach, D.; Holmes, K. Nonspecific Vaginitis. Diagnostic Criteria and Microbial and Epidemiologic Associations. Am. J. Med. 1983, 74, A28. [Google Scholar] [CrossRef]
- Bornstein, J.; Lakovsky, Y.; Lavi, I.; Bar-Am, A.; Abramovici, H. The Classic Approach to Diagnosis of Vulvovaginitis: A Critical Analysis. Infect. Dis. Obstet. Gynecol. 2001, 9, 105–111. [Google Scholar] [CrossRef]
- Muzny, C.A.; Cerca, N.; Elnaggar, J.H.; Taylor, C.M.; Sobel, J.D.; Van Der Pol, B. State of the Art for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 2023, 61, e00837-22. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Drouet, H.; Ly, C.; Bretelle, F.; Fenollar, F. Evaluation of Various Diagnostic Strategies for Bacterial Vaginosis, Including a New Approach Based on MALDI-TOF Mass Spectrometry. Microorganisms 2024, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, S.S.; Johnson, C.; Shupe, A.; Fine, J.; Dantas, G.; Burnham, C.-A.D.; Yarbrough, M.L. Assessment of the Urinary Microbiota of MSM Using Urine Culturomics Reveals a Diverse Microbial Environment. Clin. Chem. 2021, 68, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J.; dos Santos, C.O.; Damoiseaux, R.A.M.J.; Ruijs, G.J.H.M. Bacterial Agents in Vulvovaginitis and Vaginal Discharge: A 10-Year Retrospective Study in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2123–2128. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of Diagnosing Bacterial Vaginosis Is Improved by a Standardized Method of Gram Stain Interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L.; Krohn, M.A.; Edelman, R.; Regan, J.A. Association between Bacterial Vaginosis and Preterm Delivery of a Low-Birth-Weight Infant. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z. Inflammatory Mediators in Bacterial Vaginosis: The Role of Cytokines. APMIS 2024, 132, 245–255. [Google Scholar] [CrossRef]
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial Vaginosis as a Risk Factor for Preterm Delivery: A Meta-Analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef]
- Hauth, J.C.; Dubard, M.B. Reduced Incidence of Preterm Delivery with Metronidazole and Erythromycin in Women with Bacterial Vaginosis. N. Engl. J. Med. 1995, 333, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Subtil, D.; Brabant, G.; Tilloy, E.; Devos, P.; Canis, F.; Fruchart, A.; Bissinger, M.-C.; Dugimont, J.-C.; Nolf, C.; Hacot, C.; et al. Early Clindamycin for Bacterial Vaginosis in Pregnancy (PREMEVA): A Multicentre, Double-Blind, Randomised Controlled Trial. Lancet 2018, 392, 2171–2179. [Google Scholar] [CrossRef]
- Haahr, T.; Ersbøll, A.S.; Karlsen, M.A.; Svare, J.; Sneider, K.; Hee, L.; Weile, L.K.; Ziobrowska-Bech, A.; Østergaard, C.; Jensen, J.S.; et al. Treatment of Bacterial Vaginosis in Pregnancy in Order to Reduce the Risk of Spontaneous Preterm Delivery—A Clinical Recommendation. Acta Obstet. Gynecol. Scand. 2016, 95, 850–860. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for Bacterial Vaginosis in Pregnant Persons to Prevent Preterm Delivery: US Preventive Services Task Force Recommendation Statement. Obstet. Gynecol. Surv. 2020, 75, 537–538. [Google Scholar] [CrossRef]
- Ugwumadu, A.; Manyonda, I.; Reid, F.; Hay, P. Effect of Early Oral Clindamycin on Late Miscarriage and Preterm Delivery in Asymptomatic Women with Abnormal Vaginal Flora and Bacterial Vaginosis: A Randomised Controlled Trial. Lancet 2003, 361, 983–988. [Google Scholar] [CrossRef]
- Lamont, R. Intravaginal Clindamycin to Reduce Preterm Birth in Women with Abnormal Genital Tract Flora. Obstet. Gynecol. 2003, 101, 516–522. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus Iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Ratnam, S.; Fitzgerald, B.L. Semiquantitative Culture of Gardnerella Vaginalis in Laboratory Determination of Nonspecific Vaginitis. J. Clin. Microbiol. 1983, 18, 344–347. [Google Scholar] [CrossRef]
- Hay, P.E.; Morgan, D.J.; Ison, C.A.; Bhide, S.A.; Romney, M.; McKenzie, P.; Pearson, J.; Lamont, R.F.; Taylor-Robinson, D. A Longitudinal Study of Bacterial Vaginosis during Pregnancy. BJOG Int. J. O G 1994, 101, 1048–1053. [Google Scholar] [CrossRef]
- Desseauve, D.; Chantrel, J.; Fruchart, A.; Khoshnood, B.; Brabant, G.; Ancel, P.Y.; Subtil, D. Prevalence and Risk Factors of Bacterial Vaginosis during the First Trimester of Pregnancy in a Large French Population-Based Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 30–34. [Google Scholar] [CrossRef]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2016, 6, 1528. [Google Scholar] [CrossRef] [PubMed]
- Aduloju, O.P.; Akintayo, A.A.; Aduloju, T. Prevalence of Bacterial Vaginosis in Pregnancy in a Tertiary Health Institution, South Western Nigeria. Pan Afr. Med. J. 2019, 33, 9. [Google Scholar] [CrossRef] [PubMed]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations with Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef]
- Gratacoas, E.; Figueras, F.; Barranco, M.; Ros, R.; Andreu, A.; Alonso, P.; Cararach, V. Prevalence of Bacterial Vaginosis and Correlation of Clinical to Gram Stain Diagnostic Criteria in Low Risk Pregnant Women. Eur. J. Epidemiol. 1999, 15, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, B.; Pernevi, P.; Chidekel, L.; Platz-Christensen, J.J. Bacterial Vaginosis in Early Pregnancy May Predispose for Preterm Birth and Postpartum Endometritis. Acta Obstet. Gynecol. Scand. 2002, 81, 1006–1010. [Google Scholar] [CrossRef]
- Kalinka, J.; Hanke, W.; Wasiela, M.; Laudański, T. Socioeconomic and Environmental Risk Factors of Bacterial Vaginosis in Early Pregnancy. J. Perinat. Med. 2002, 30, 467–475. [Google Scholar] [CrossRef]
| Parameter | n | % (Isolates) | % (Swabs/Pregnancies) |
|---|---|---|---|
| Demographic characteristics | |||
| Pregnant women | 3284 | / | 95.5 |
| Swabs/pregnancies | 3437 | / | 100 |
| Pregnant women age (mean, range) | 31 (14–51) | / | / |
| Gestational age (median, range) | 28 (4–41) | / | / |
| Microbiological isolates * (n = 4922) | |||
| Lactobacilli | 2497 | 50.9 | § |
| Lactobacillus crispatus | 931 | 18.8 | 27.1 |
| Lactobacillus jensenii | 491 | 9.9 | 14.3 |
| Lactobacillus gasseri | 448 | 9.1 | 13.0 |
| Lactobacillus iners | 361 | 7.3 | 10.5 |
| Other lactobacilli | 266 | 5.8 | 7.7 |
| Gardnerella vaginalis | 577 | 11.7 | 16.8 |
| Mixed aerobic bacteria | 1152 | 23.3 | § |
| Streptococcus agalactiae | 489 | 9.9 | 14.2 |
| Enterococcus faecalis | 178 | 3.6 | 5.2 |
| Streptococcus anginosus | 171 | 3.5 | 5.0 |
| Escherichia coli | 140 | 2.8 | 4.1 |
| Staphylococcus aureus | 61 | 1.2 | 1.8 |
| Others | 113 | 2.3 | § |
| Yeast | 696 | 14.1 | 20.3 |
| Diagnostic Method/Vaginal Microbiota Category | n | % (Swabs/Pregnancies) |
|---|---|---|
| Microscopy (Nugent score) | ||
| Normal (0–3) | 2637 | 76.7 |
| Intermediate (4–6) | 576 | 16.8 |
| Bacterial vaginosis (7–10) | 224 | 6.5 |
| Total | 3437 | 100 |
| SRC # (less stringent criteria) | ||
| Normal | 2250 | 65.5 |
| Intermediate | 807 | 23.5 |
| Bacterial vaginosis | 380 | 11.1 |
| Total | 3437 | 100 |
| SRC # (more stringent criteria) | ||
| Normal | 2222 | 64.6 |
| Intermediate | 874 | 25.4 |
| Bacterial vaginosis | 341 | 9.9 |
| Total | 3437 | 100 |
| Vaginal Microbiota Categories | SRC # (Less Stringent Criteria) | SRC # (More Stringent Criteria) | ||||||
|---|---|---|---|---|---|---|---|---|
| Microscopy (Nugent Score) | Normal | Intermediate | BV * | Sum | Normal | Intermediate | BV * | Sum |
| Normal (0–3) | 2174 | 414 | 49 | 2637 | 2152 | 448 | 37 | 2637 |
| Intermediate (4–6) | 71 | 372 | 133 | 576 | 68 | 394 | 114 | 576 |
| BV * (7–10) | 5 | 21 | 198 | 224 | 2 | 32 | 190 | 224 |
| Sum | 2250 | 807 | 380 | 3437 | 2222 | 874 | 341 | 3437 |
| Pregnancy Outcome | Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|
| Diagnostic Method/Criteria | Crude | p-Value | Adjusted * | p-Value |
| Preterm Birth | ||||
| Microscopy (Nugent score) | ||||
| Normal | 0.38 (0.35 to 0.42) | <0.001 | 0.28 (0.25 to 0.31) | <0.001 |
| Intermediate | 2.41 (1.94 to 2.99) | <0.001 | 2.53 (2.01 to 3.178) | <0.001 |
| Bacterial vaginosis | 0.87 (0.60 to 1.26) | 0.458 | 0.87 (0.58 to 1.29) | 0.487 |
| SRC # (less stringent criteria) | ||||
| Normal | 0.36 (0.32 to 0.40) | <0.001 | 0.26 (0.23 to 0.29) | <0.001 |
| Intermediate | 1.89 (1.56 to 2.30) | <0.001 | 1.99 (1.61 to 2.45) | <0.001 |
| Bacterial vaginosis | 1.65 (1.26 to 2.17) | <0.001 | 1.72 (1.29 to 2.29) | <0.001 |
| SRC # (more stringent criteria) | ||||
| Normal | 0.36 (0.32–0.40) | <0.001 | 0.26 (0.23 to 0.29) | <0.001 |
| Intermediate | 1.77 (1.46–2.15) | <0.001 | 1.88 (1.53 to 2.31) | <0.001 |
| Bacterial vaginosis | 1.70 (1.28–2.26) | <0.001 | 1.76 (1.30 to 2.37) | <0.001 |
| PPROM † | ||||
| Microscopy (Nugent score) | ||||
| Normal | 0.11 (0.09 to 0.13) | <0.001 | 0.09 (0.08 to 0.11) | <0.001 |
| Intermediate | 3.21 (2.46 to 4.19) | <0.001 | 3.28 (2.55 to 4.23) | <0.001 |
| Bacterial vaginosis | 0.75 (0.41 to 1.38) | 0.356 | 0.84 (0.48 to 1.46) | 0.533 |
| SRC # (less stringent criteria) | ||||
| Normal | 0.10 (0.09 to 0.12) | <0.001 | 0.09 (0.07 to 0.10) | <0.001 |
| Intermediate | 2.24 (1.72 to 2.92) | <0.001 | 2.24 (1.74 to 2.87) | <0.001 |
| Bacterial vaginosis | 1.89 (1.31 to 2.72) | <0.001 | 1.99 (1.41 to 2.80) | <0.001 |
| SRC # (more stringent criteria) | ||||
| Normal | 0.10 (0.09 to 0.12) | <0.001 | 0.09 (0.07 to 0.10) | <0.001 |
| Intermediate | 2.10 (1.61 to 2.72) | <0.001 | 2.14 (1.67 to 2.74) | <0.001 |
| Bacterial vaginosis | 1.91 (1.30 to 2.80) | <0.001 | 1.97 (1.38 to 2.82) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starc, M.; Lučovnik, M.; Eržen Vrlič, P.; Jeverica, S. The Prevalence of Bacterial Vaginosis in Pregnant Women in Slovenia, Determined via Microscopy and Semi-Quantitative Relative Culture, and Its Association with Adverse Pregnancy Outcomes. Microorganisms 2025, 13, 588. https://doi.org/10.3390/microorganisms13030588
Starc M, Lučovnik M, Eržen Vrlič P, Jeverica S. The Prevalence of Bacterial Vaginosis in Pregnant Women in Slovenia, Determined via Microscopy and Semi-Quantitative Relative Culture, and Its Association with Adverse Pregnancy Outcomes. Microorganisms. 2025; 13(3):588. https://doi.org/10.3390/microorganisms13030588
Chicago/Turabian StyleStarc, Maja, Miha Lučovnik, Petra Eržen Vrlič, and Samo Jeverica. 2025. "The Prevalence of Bacterial Vaginosis in Pregnant Women in Slovenia, Determined via Microscopy and Semi-Quantitative Relative Culture, and Its Association with Adverse Pregnancy Outcomes" Microorganisms 13, no. 3: 588. https://doi.org/10.3390/microorganisms13030588
APA StyleStarc, M., Lučovnik, M., Eržen Vrlič, P., & Jeverica, S. (2025). The Prevalence of Bacterial Vaginosis in Pregnant Women in Slovenia, Determined via Microscopy and Semi-Quantitative Relative Culture, and Its Association with Adverse Pregnancy Outcomes. Microorganisms, 13(3), 588. https://doi.org/10.3390/microorganisms13030588






