A Novel In Vitro Primary Human Alveolar Model (AlveolAir™) for H1N1 and SARS-CoV-2 Infection and Antiviral Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Isolation and Culture
2.2. Histology
2.3. Immunofluorescence (IF)
2.4. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.5. Gene Expression
2.6. Trans-Epithelial Electrical Resistance (TEER)
2.7. Lucifer Yellow Assay
2.8. Virus
2.9. Viral Infection and Treatment in AlveolAir™
2.10. Virus Quantification
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Statistics
3. Results
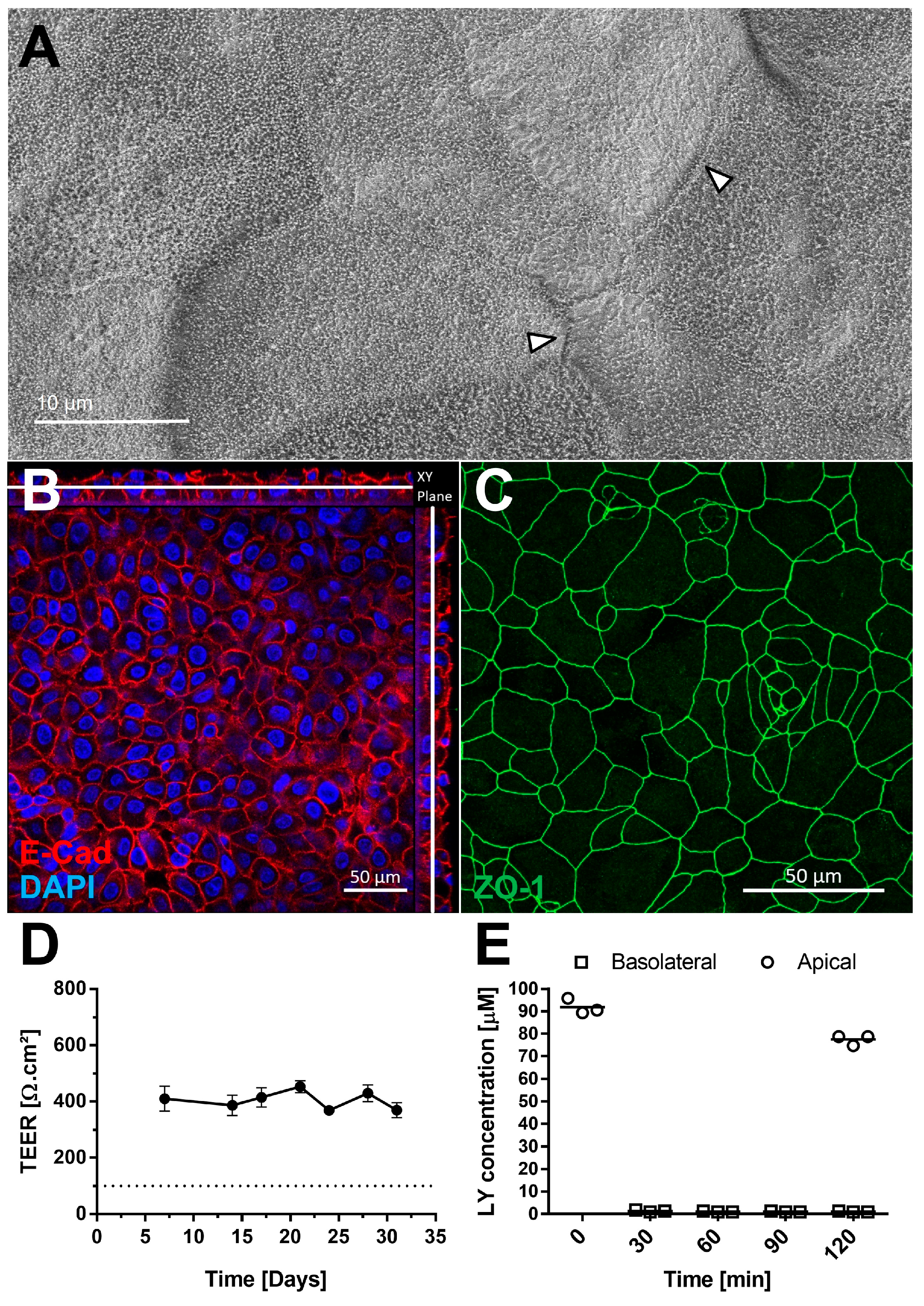
3.1. Morphological Characterization of AlveolAir™: The Presence of ATIs and ATIIs
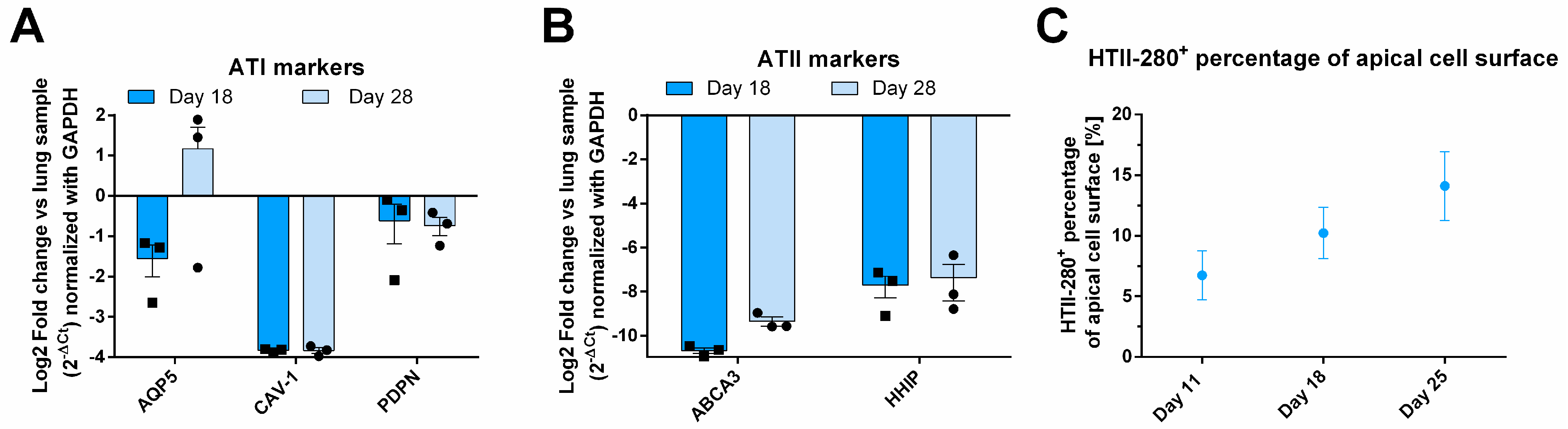
3.2. Molecular Characterization of AlveolAir™: An Increasing Number of ATIIs over Time
3.3. AlveolAir™ Exhibits a Long-Term Barrier Function
3.4. H1N1 Replication Alters Integrity of AlveolAir™ and Induces a Strong Inflammatory Response in Epithelial Cells
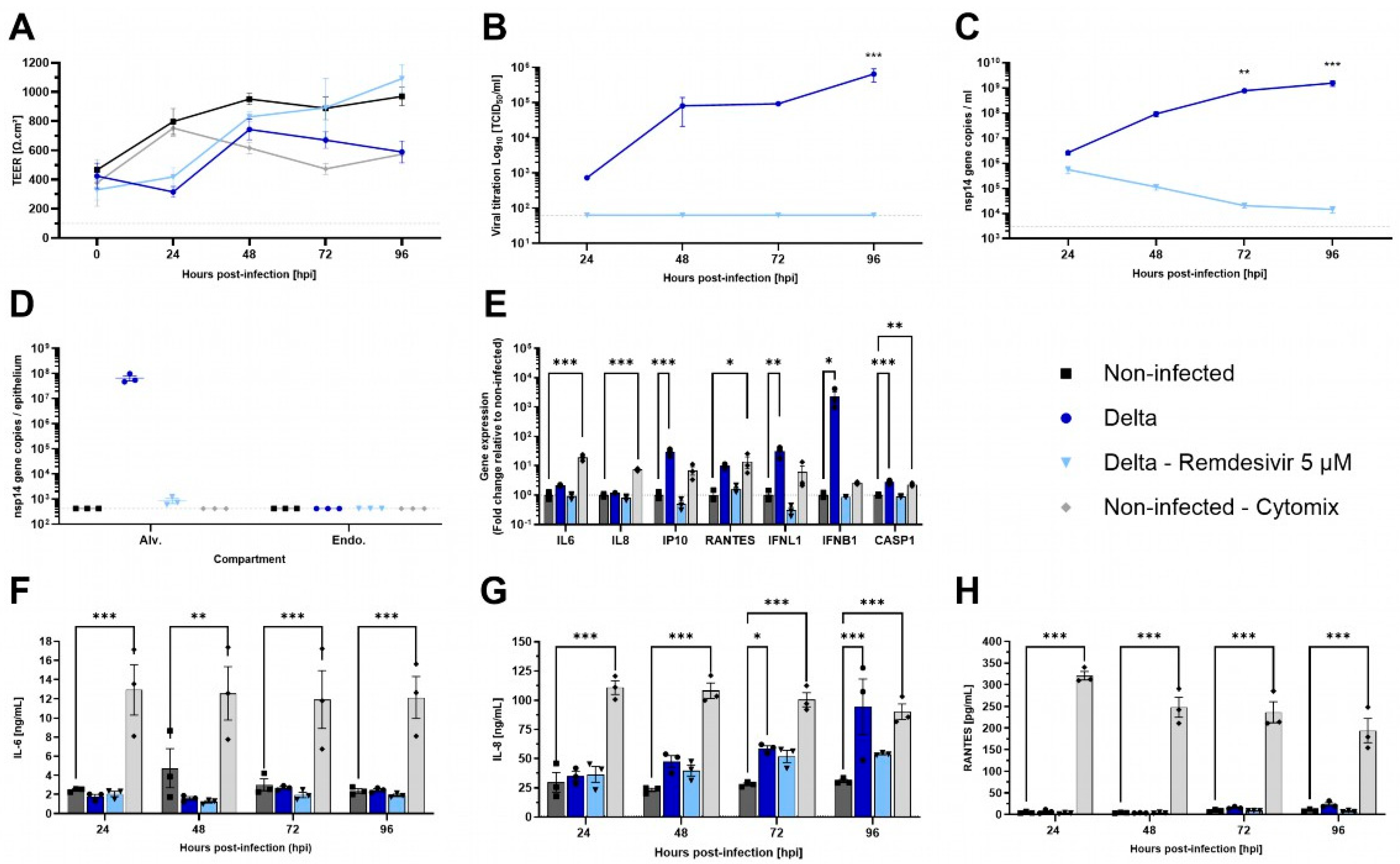
3.5. SARS-CoV-2 Delta Variant Infects AlveolAir™ with No Impact on Epithelium Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Stucki, A.O.; Sauer, U.G.; Allen, D.G.; Kleinstreuer, N.C.; Perron, M.M.; Yozzo, K.L.; Lowit, A.B.; Clippinger, A.J. Differences in the Anatomy and Physiology of the Human and Rat Respiratory Tracts and Impact on Toxicological Assessments. Regul. Toxicol. Pharmacol. 2024, 150, 105648. [Google Scholar] [CrossRef]
- Krewski, D.; Acosta, D.; Andersen, M.; Anderson, H.; Bailar, J.C.; Boekelheide, K.; Brent, R.; Charnley, G.; Cheung, V.G.; Green, S.; et al. Toxicity Testing in the 21st Century: A Vision and a Strategy. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 51–138. [Google Scholar] [CrossRef]
- Knudsen, L.; Ochs, M. The Micromechanics of Lung Alveoli: Structure and Function of Surfactant and Tissue Components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Beers, M.F.; Moodley, Y. When Is an Alveolar Type 2 Cell an Alveolar Type 2 Cell? A Conundrum for Lung Stem Cell Biology and Regenerative Medicine. Am. J. Respir. Cell Mol. Biol. 2017, 57, 18–27. [Google Scholar] [CrossRef]
- Chung, K.-P.; Cheng, C.-N.; Chen, Y.-J.; Hsu, C.-L.; Huang, Y.-L.; Hsieh, M.-S.; Kuo, H.-C.; Lin, Y.-T.; Juan, Y.-H.; Nakahira, K.; et al. Alveolar Epithelial Cells Mitigate Neutrophilic Inflammation in Lung Injury through Regulating Mitochondrial Fatty Acid Oxidation. Nat. Commun. 2024, 15, 7241. [Google Scholar] [CrossRef]
- Qian, Z.; Travanty, E.A.; Oko, L.; Edeen, K.; Berglund, A.; Wang, J.; Ito, Y.; Holmes, K.V.; Mason, R.J. Innate Immune Response of Human Alveolar Type II Cells Infected with Severe Acute Respiratory Syndrome–Coronavirus. Am. J. Respir. Cell Mol. Biol. 2013, 48, 742. [Google Scholar] [CrossRef]
- Daum, N.; Kuehn, A.; Hein, S.; Schaefer, U.F.; Huwer, H.; Lehr, C.-M. Isolation, Cultivation, and Application of Human Alveolar Epithelial Cells. In Human Cell Culture Protocols; Mitry, R.R., Hughes, R.D., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 806, pp. 31–42. [Google Scholar]
- Ehrhardt, C.; Kim, K.-J.; Lehr, C.-M. Isolation and Culture of Human Alveolar Epithelial Cells. In Human Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 2004; Volume 107, pp. 207–216. [Google Scholar]
- Chiu, M.C.; Li, C.; Liu, X.; Yu, Y.; Huang, J.; Wan, Z.; Xiao, D.; Chu, H.; Cai, J.-P.; Zhou, B.; et al. A Bipotential Organoid Model of Respiratory Epithelium Recapitulates High Infectivity of SARS-CoV-2 Omicron Variant. Cell Discov. 2022, 8, 57. [Google Scholar] [CrossRef]
- Kong, J.; Wen, S.; Cao, W.; Yue, P.; Xu, X.; Zhang, Y.; Luo, L.; Chen, T.; Li, L.; Wang, F.; et al. Lung Organoids, Useful Tools for Investigating Epithelial Repair after Lung Injury. Stem Cell Res. Ther. 2021, 12, 95. [Google Scholar] [CrossRef]
- Huang, D.; Liu, T.; Liao, J.; Maharjan, S.; Xie, X.; Pérez, M.; Anaya, I.; Wang, S.; Tirado Mayer, A.; Kang, Z.; et al. Reversed-Engineered Human Alveolar Lung-on-a-Chip Model. Proc. Natl. Acad. Sci. USA 2021, 118, e2016146118. [Google Scholar] [CrossRef]
- Harb, A.; Fakhreddine, M.; Zaraket, H.; Saleh, F.A. Three-Dimensional Cell Culture Models to Study Respiratory Virus Infections Including COVID-19. Biomimetics 2021, 7, 3. [Google Scholar] [CrossRef]
- Wijesekara, P.; Yadav, P.; Perkins, L.A.; Stolz, D.B.; Franks, J.M.; Watkins, S.C.; Reinoso Jacome, E.; Brody, S.L.; Horani, A.; Xu, J.; et al. Engineering Rotating Apical-out Airway Organoid for Assessing Respiratory Cilia Motility. iScience 2022, 25, 104730. [Google Scholar] [CrossRef]
- Vazquez-Armendariz, A.I.; Tata, P.R. Recent Advances in Lung Organoid Development and Applications in Disease Modeling. J. Clin. Investig. 2025, 133, e170500. [Google Scholar] [CrossRef]
- Si, L.; Bai, H.; Rodas, M.; Cao, W.; Oh, C.Y.; Jiang, A.; Moller, R.; Hoagland, D.; Oishi, K.; Horiuchi, S.; et al. A Human-Airway-on-a-Chip for the Rapid Identification of Candidate Antiviral Therapeutics and Prophylactics. Nat. Biomed. Eng. 2021, 5, 815–829. [Google Scholar] [CrossRef]
- Sengupta, A.; Roldan, N.; Kiener, M.; Froment, L.; Raggi, G.; Imler, T.; De Maddalena, L.; Rapet, A.; May, T.; Carius, P.; et al. A New Immortalized Human Alveolar Epithelial Cell Model to Study Lung Injury and Toxicity on a Breathing Lung-On-Chip System. Front. Toxicol. 2022, 4, 840606. [Google Scholar] [CrossRef]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T.; et al. Second-Generation Lung-on-a-Chip with an Array of Stretchable Alveoli Made with a Biological Membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]
- Baptista, D.; Moreira Teixeira, L.; Barata, D.; Tahmasebi Birgani, Z.; King, J.; Van Riet, S.; Pasman, T.; Poot, A.A.; Stamatialis, D.; Rottier, R.J.; et al. 3D Lung-on-Chip Model Based on Biomimetically Microcurved Culture Membranes. ACS Biomater. Sci. Eng. 2022, 8, 2684–2699. [Google Scholar] [CrossRef]
- Bai, H.; Si, L.; Jiang, A.; Belgur, C.; Zhai, Y.; Plebani, R.; Oh, C.Y.; Rodas, M.; Patil, A.; Nurani, A.; et al. Mechanical Control of Innate Immune Responses against Viral Infection Revealed in a Human Lung Alveolus Chip. Nat. Commun. 2022, 13, 1928. [Google Scholar] [CrossRef]
- Carius, P.; Jungmann, A.; Bechtel, M.; Grißmer, A.; Boese, A.; Gasparoni, G.; Salhab, A.; Seipelt, R.; Urbschat, K.; Richter, C.; et al. A Monoclonal Human Alveolar Epithelial Cell Line (“Arlo”) with Pronounced Barrier Function for Studying Drug Permeability and Viral Infections. Adv. Sci. 2023, 10, 2207301. [Google Scholar] [CrossRef]
- Salomon, J.J.; Muchitsch, V.E.; Gausterer, J.C.; Schwagerus, E.; Huwer, H.; Daum, N.; Lehr, C.-M.; Ehrhardt, C. The Cell Line NCl-H441 Is a Useful in Vitro Model for Transport Studies of Human Distal Lung Epithelial Barrier. Mol. Pharm. 2014, 11, 995–1006. [Google Scholar] [CrossRef]
- Koziol-White, C.; Gebski, E.; Cao, G.; Panettieri, R.A. Precision Cut Lung Slices: An Integrated Ex Vivo Model for Studying Lung Physiology, Pharmacology, Disease Pathogenesis and Drug Discovery. Respir. Res. 2024, 25, 231. [Google Scholar] [CrossRef]
- Liu, G.; Betts, C.; Cunoosamy, D.M.; Åberg, P.M.; Hornberg, J.J.; Sivars, K.B.; Cohen, T.S. Use of Precision Cut Lung Slices as a Translational Model for the Study of Lung Biology. Respir. Res. 2019, 20, 162. [Google Scholar] [CrossRef]
- BéruBé, K.; Pitt, A.; Hayden, P.; Prytherch, Z.; Job, C. Filter-Well Technology for Advanced Three-Dimensional Cell Culture: Perspectives for Respiratory Research. Altern. Lab. Anim. 2010, 38, 49–65. [Google Scholar] [CrossRef]
- Boda, B.; Benaoudia, S.; Huang, S.; Bonfante, R.; Wiszniewski, L.; Tseligka, E.D.; Tapparel, C.; Constant, S. Antiviral Drug Screening by Assessing Epithelial Functions and Innate Immune Responses in Human 3D Airway Epithelium Model. Antivir. Res. 2018, 156, 72–79. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Brito, F.; Benaoudia, S.; Royston, L.; Cagno, V.; Fernandes-Rocha, M.; Piuz, I.; Zdobnov, E.; Huang, S.; Constant, S.; et al. Propagation of Respiratory Viruses in Human Airway Epithelia Reveals Persistent Virus-Specific Signatures. J. Allergy Clin. Immunol. 2018, 141, 2074–2084. [Google Scholar] [CrossRef]
- Medaglia, C.; Kolpakov, I.; Zwygart, A.C.-A.; Zhu, Y.; Constant, S.; Huang, S.; Cagno, V.; Dermitzakis, E.T.; Stellacci, F.; Xenarios, I.; et al. An Anti-Influenza Combined Therapy Assessed by Single Cell RNA-Sequencing. Commun. Biol. 2022, 5, 1075. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Julien, T.; Trouillet-Assant, S.; Traversier, A.; Errazuriz-Cerda, E.; Fouret, J.; Dubois, J.; Gaymard, A.; Lescure, F.-X.; et al. Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia. Cell Rep. Med. 2020, 1, 100059. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Yu, W.C.L.; Chan, R.W.Y.; Wang, J.; Travanty, E.A.; Nicholls, J.M.; Peiris, J.S.M.; Mason, R.J.; Chan, M.C.W. Viral Replication and Innate Host Responses in Primary Human Alveolar Epithelial Cells and Alveolar Macrophages Infected with Influenza H5N1 and H1N1 Viruses. J. Virol. 2011, 85, 6844–6855. [Google Scholar] [CrossRef]
- Hönzke, K.; Obermayer, B.; Mache, C.; Fatykhova, D.; Kessler, M.; Dökel, S.; Wyler, E.; Baumgardt, M.; Löwa, A.; Hoffmann, K.; et al. Human Lungs Show Limited Permissiveness for SARS-CoV-2 Due to Scarce ACE2 Levels but Virus-Induced Expansion of Inflammatory Macrophages. Eur. Respir. J. 2022, 60, 2102725. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Lin, Y.-R.; Chu, Y.-L.; Chung, J.H.Y.; Lu, H.-E.; Chen, G.-Y. Tissue-Level Alveolar Epithelium Model for Recapitulating SARS-CoV-2 Infection and Cellular Plasticity. Commun. Biol. 2022, 5, 70. [Google Scholar] [CrossRef]
- Tanabe, I.; Ishimori, K.; Ishikawa, S. Development of an in Vitro Human Alveolar Epithelial Air-Liquid Interface Model Using a Small Molecule Inhibitor Cocktail. BMC Mol. Cell Biol. 2024, 25, 9. [Google Scholar] [CrossRef]
- Lamers, M.M.; van der Vaart, J.; Knoops, K.; Riesebosch, S.; Breugem, T.I.; Mykytyn, A.Z.; Beumer, J.; Schipper, D.; Bezstarosti, K.; Koopman, C.D.; et al. An Organoid-derived Bronchioalveolar Model for SARS-CoV-2 Infection of Human Alveolar Type II-like Cells. EMBO J. 2021, 40, e105912. [Google Scholar] [CrossRef]
- Bluhmki, T.; Traub, S.; Müller, A.-K.; Bitzer, S.; Schruf, E.; Bammert, M.-T.; Leist, M.; Gantner, F.; Garnett, J.P.; Heilker, R. Functional Human iPSC-Derived Alveolar-like Cells Cultured in a Miniaturized 96-Transwell Air–Liquid Interface Model. Sci. Rep. 2021, 11, 17028. [Google Scholar] [CrossRef]
- Kang, D.; Park, J.A.; Kim, W.; Kim, S.; Lee, H.; Kim, W.; Yoo, J.; Jung, S. All-Inkjet-Printed 3D Alveolar Barrier Model with Physiologically Relevant Microarchitecture. Adv. Sci. 2021, 8, 2004990. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Meldrum, K.; Bateman, J.W.P.; Tetley, T.D.; Doak, S.H.; Clift, M.J.D. Development and Characterisation of a Novel Complex Triple Cell Culture Model of the Human Alveolar Epithelial Barrier. Vitr. Models 2024, 3, 125–137. [Google Scholar] [CrossRef]
- Weinheimer, V.K.; Becher, A.; Tönnies, M.; Holland, G.; Knepper, J.; Bauer, T.T.; Schneider, P.; Neudecker, J.; Rückert, J.C.; Szymanski, K.; et al. Influenza A Viruses Target Type II Pneumocytes in the Human Lung. J. Infect. Dis. 2012, 206, 1685. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, Q.; Yao, Q.; Wang, X.; Zhang, H.; Chen, R.; Ren, L.; Min, J.; Deng, F.; et al. SARS-CoV-2 Cell Tropism and Multiorgan Infection. Cell Discov. 2021, 7, 17. [Google Scholar] [CrossRef]
- Short, K.R.; Kasper, J.; van der Aa, S.; Andeweg, A.C.; Zaaraoui-Boutahar, F.; Goeijenbier, M.; Richard, M.; Herold, S.; Becker, C.; Scott, D.P.; et al. Influenza Virus Damages the Alveolar Barrier by Disrupting Epithelial Cell Tight Junctions. Eur. Respir. J. 2016, 47, 954–966. [Google Scholar] [CrossRef]
- Wang, P.; Luo, R.; Zhang, M.; Wang, Y.; Song, T.; Tao, T.; Li, Z.; Jin, L.; Zheng, H.; Chen, W.; et al. A Cross-Talk between Epithelium and Endothelium Mediates Human Alveolar–Capillary Injury during SARS-CoV-2 Infection. Cell Death Dis. 2020, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Nicolas De Lamballerie, C.; Pizzorno, A.; Dubois, J.; Julien, T.; Padey, B.; Bouveret, M.; Traversier, A.; Legras-Lachuer, C.; Lina, B.; Boivin, G.; et al. Characterization of Cellular Transcriptomic Signatures Induced by Different Respiratory Viruses in Human Reconstituted Airway Epithelia. Sci. Rep. 2019, 9, 11493. [Google Scholar] [CrossRef] [PubMed]
- Carossino, M.; Izadmehr, S.; Trujillo, J.D.; Gaudreault, N.N.; Dittmar, W.; Morozov, I.; Balasuriya, U.B.R.; Cordon-Cardo, C.; García-Sastre, A.; Richt, J.A. ACE2 and TMPRSS2 Distribution in the Respiratory Tract of Different Animal Species and Its Correlation with SARS-CoV-2 Tissue Tropism. Microbiol. Spectr. 2024, 12, e03270-23. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, C.F.; Laurent, E.; Caul-Futy, M.; Dubois, J.; Mialon, C.; Chojnacki, C.; Sage, E.; Boda, B.; Huang, S.; Rosa-Calatrava, M.; et al. A Novel In Vitro Primary Human Alveolar Model (AlveolAir™) for H1N1 and SARS-CoV-2 Infection and Antiviral Screening. Microorganisms 2025, 13, 572. https://doi.org/10.3390/microorganisms13030572
Lopes CF, Laurent E, Caul-Futy M, Dubois J, Mialon C, Chojnacki C, Sage E, Boda B, Huang S, Rosa-Calatrava M, et al. A Novel In Vitro Primary Human Alveolar Model (AlveolAir™) for H1N1 and SARS-CoV-2 Infection and Antiviral Screening. Microorganisms. 2025; 13(3):572. https://doi.org/10.3390/microorganisms13030572
Chicago/Turabian StyleLopes, Cindia Ferreira, Emilie Laurent, Mireille Caul-Futy, Julia Dubois, Chloé Mialon, Caroline Chojnacki, Edouard Sage, Bernadett Boda, Song Huang, Manuel Rosa-Calatrava, and et al. 2025. "A Novel In Vitro Primary Human Alveolar Model (AlveolAir™) for H1N1 and SARS-CoV-2 Infection and Antiviral Screening" Microorganisms 13, no. 3: 572. https://doi.org/10.3390/microorganisms13030572
APA StyleLopes, C. F., Laurent, E., Caul-Futy, M., Dubois, J., Mialon, C., Chojnacki, C., Sage, E., Boda, B., Huang, S., Rosa-Calatrava, M., & Constant, S. (2025). A Novel In Vitro Primary Human Alveolar Model (AlveolAir™) for H1N1 and SARS-CoV-2 Infection and Antiviral Screening. Microorganisms, 13(3), 572. https://doi.org/10.3390/microorganisms13030572





