Rhizosphere Growth-Promoting Fungi of Healthy Nicotiana tabacum L.: A Systematic Approach to Boosting Plant Growth and Drought Resistance
Abstract
1. Introduction
2. Materials and Methods
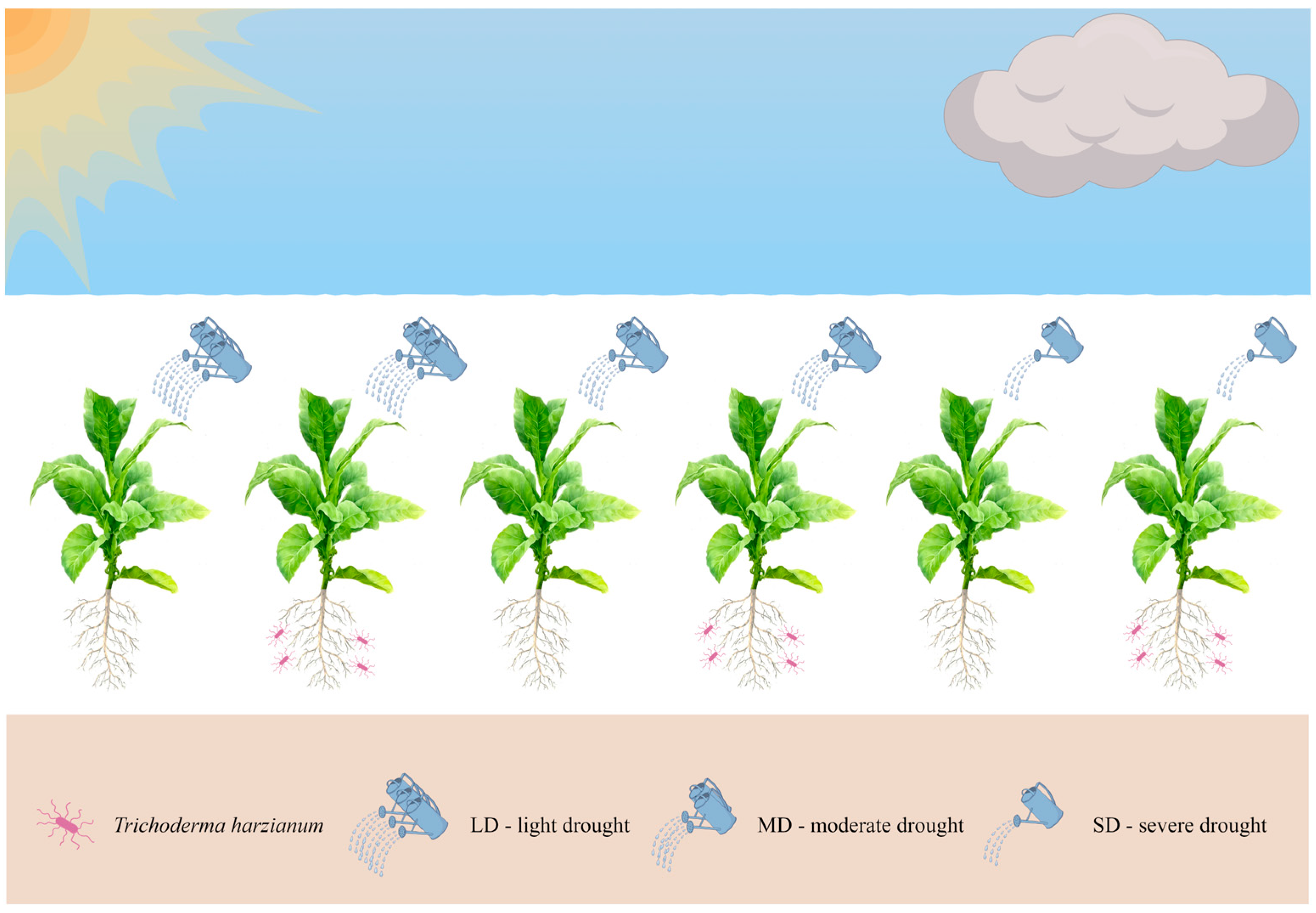
2.1. Trichoderma Harzianum Isolation and Drought Treatment
2.2. Agronomic Traits
2.3. Root Development
2.4. Relative Water Content of Leaves
2.5. Root Activity
2.6. Photosynthetic Parameters
2.7. Photosynthetic Pigments
2.8. Osmoprotectants
2.9. Leaf Protective Enzyme Activity
2.10. Membrane Lipid Peroxidation Index
2.11. Nitrate Reductase Activity
2.12. Data Processing
3. Results
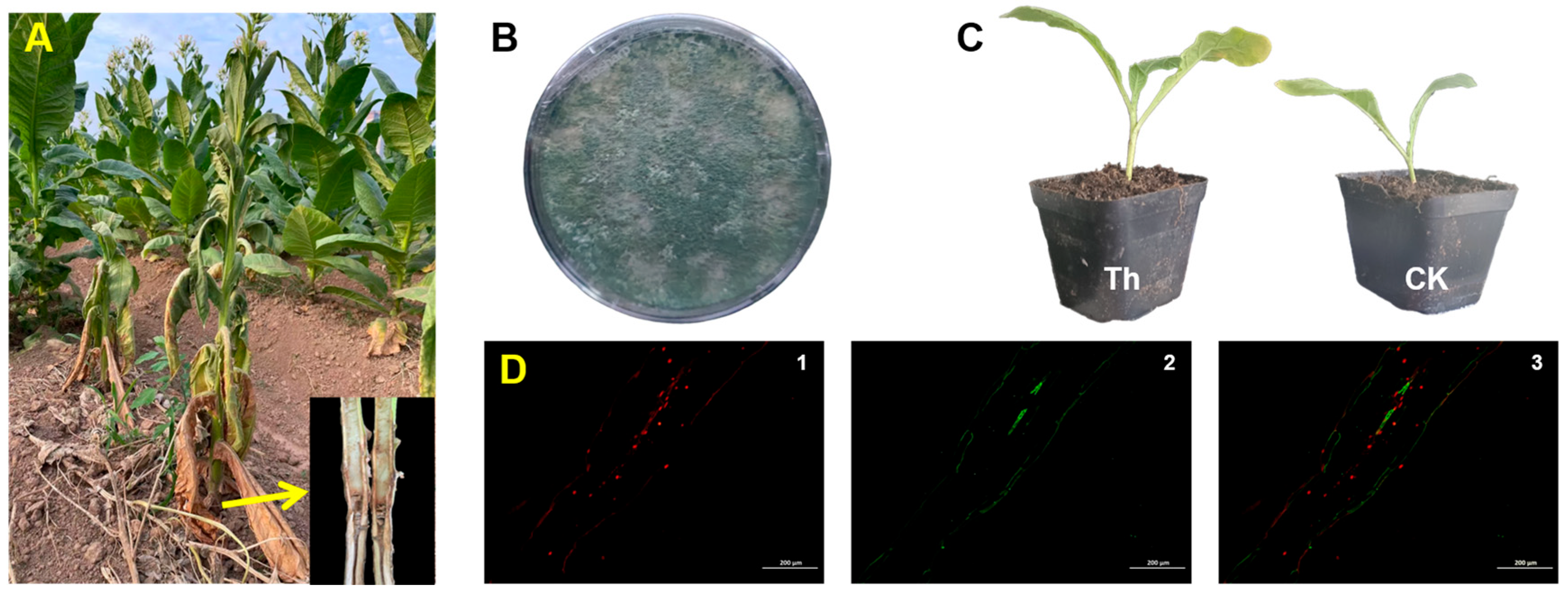
3.1. Plant Growth-Promoting Fungi Isolation and Its Effects Against Drought
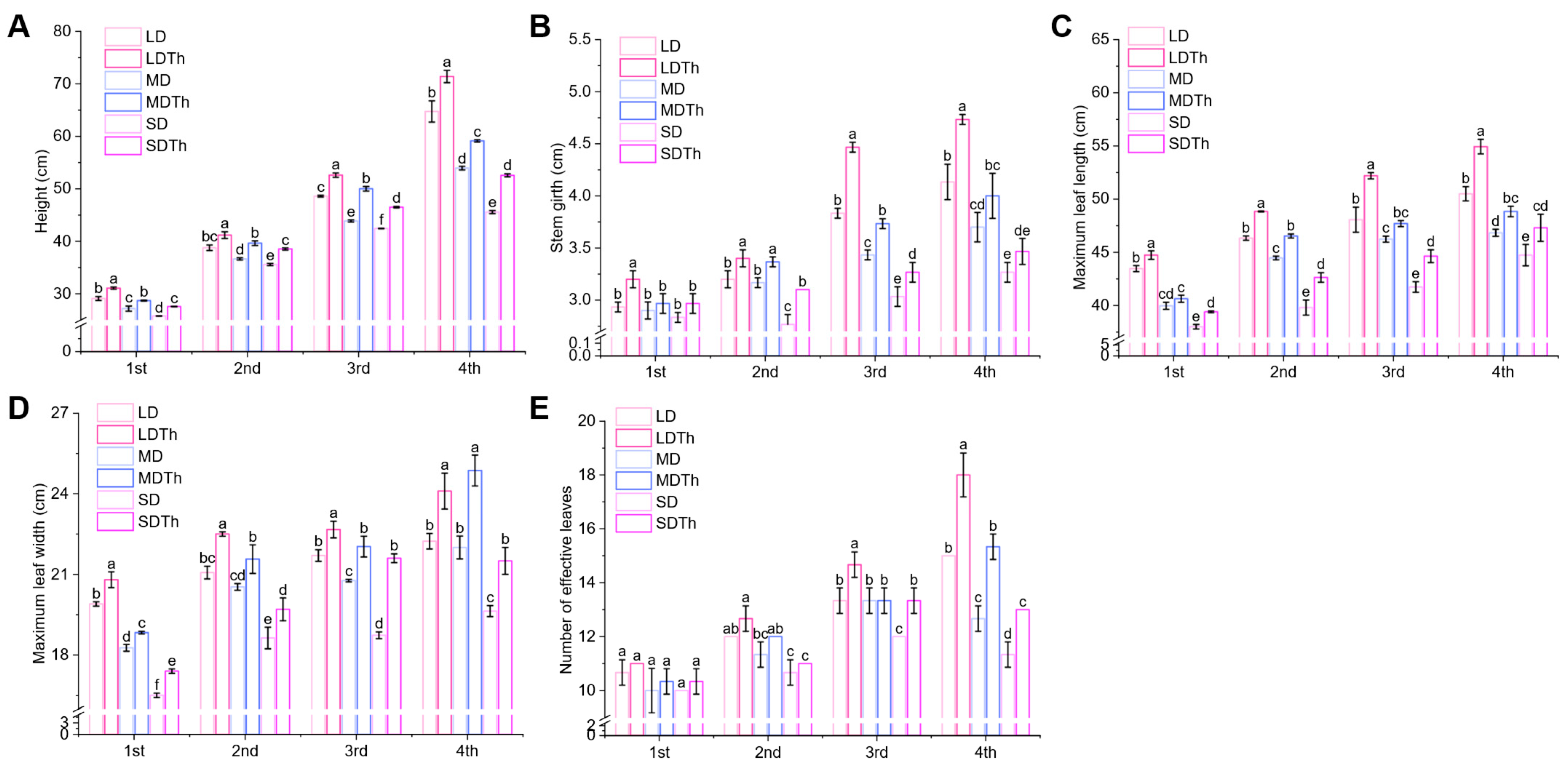
3.2. Agronomic Traits of N. tabacum L. After T. harzianum Inoculation During DS Treatment
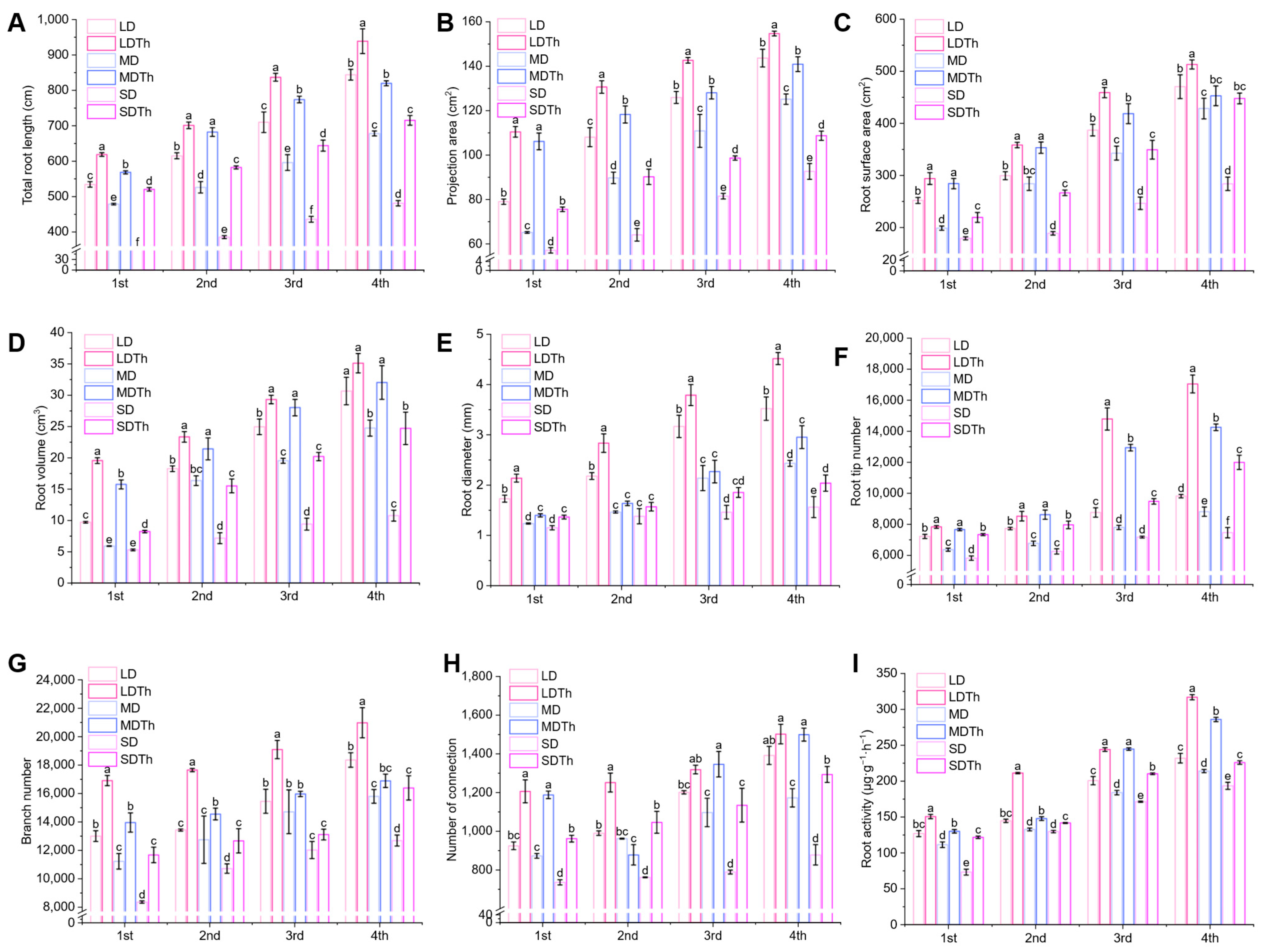
3.3. Root System of N. tabacum L. After T. harzianum Inoculation During DS Treatment
3.4. Photosynthetic Parameters of N. tabacum L. After T. harzianum Inoculation During DS Treatment
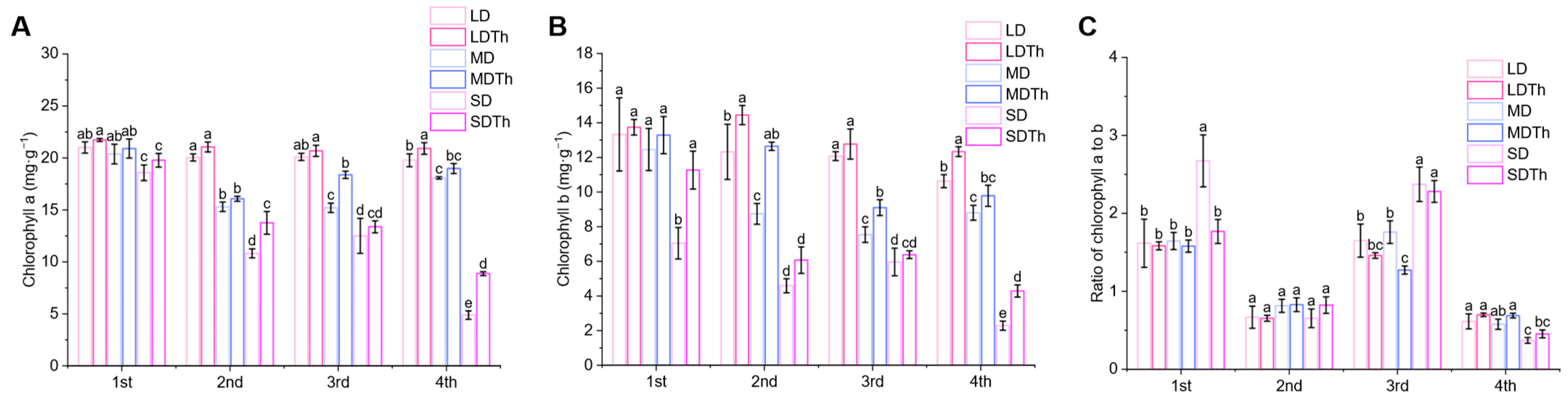
3.5. Photosynthetic Pigments of N. tabacum L. After T. harzianum Inoculation During DS Treatment
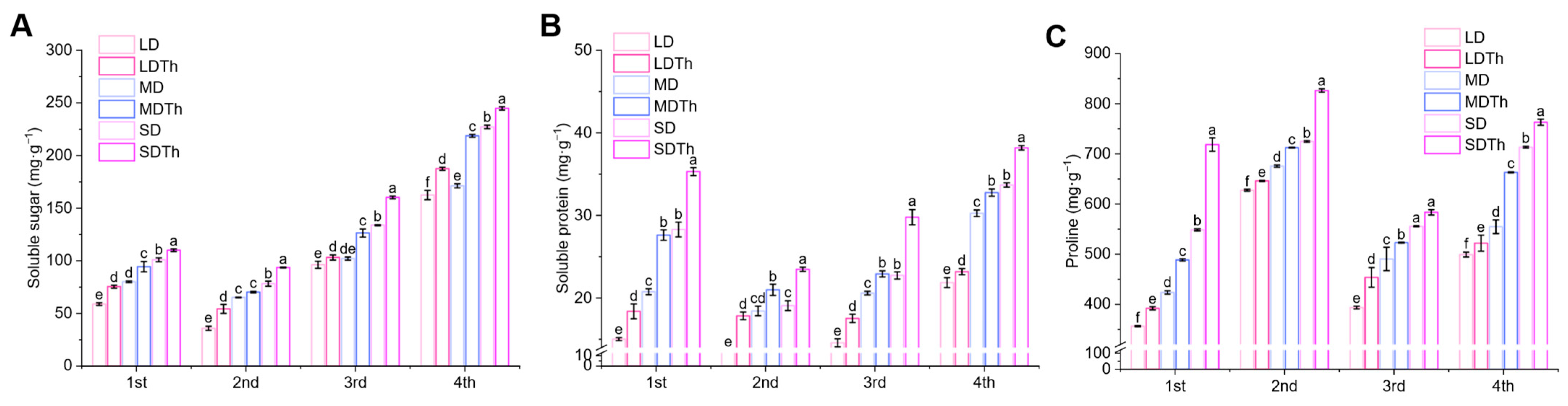
3.6. Osmoprotectants of N. tabacum L. After T. harzianum Inoculation During DS Treatment
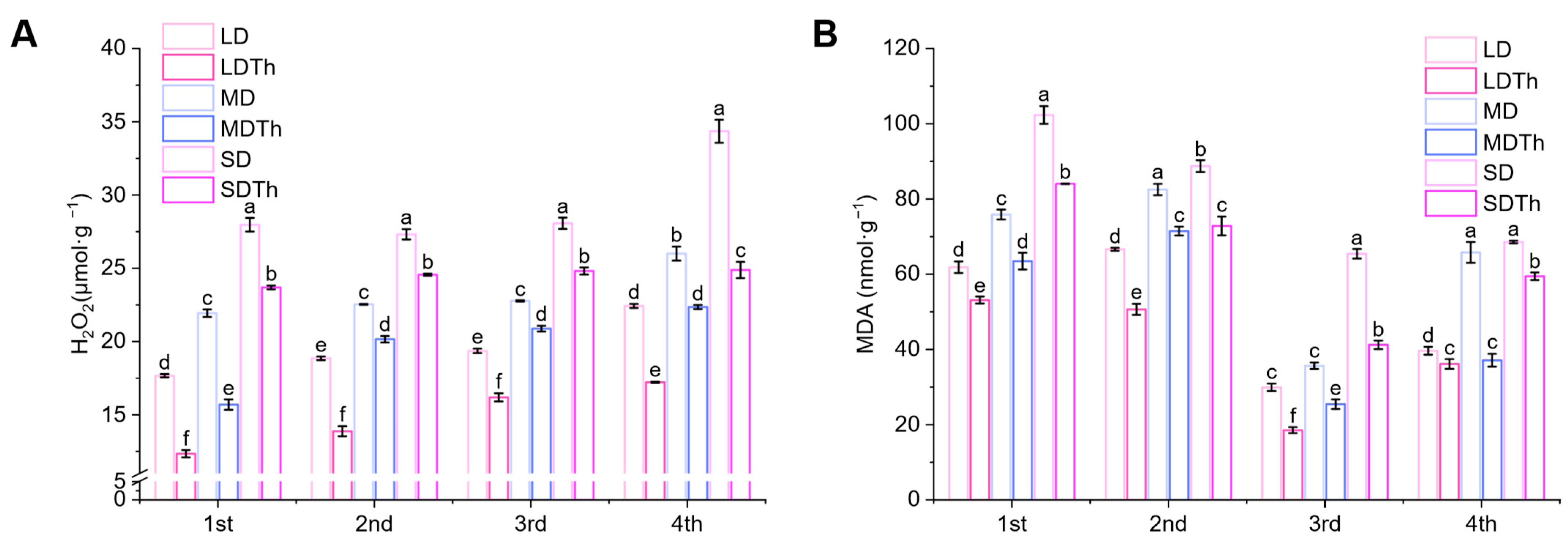
3.7. Membrane Lipid Peroxidation Index of N. tabacum L. After T. harzianum Inoculation During DS Treatment
3.8. Endogenous Protective Enzymes’ Activity of N. tabacum L. After T. harzianum Inoculation During DS Treatment
3.9. Nitrate Reductase Activity of N. tabacum L. After T. harzianum Inoculation During DS Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mora, C.; Spirandelli, D.; Franklin, E.C.; Lynham, J.; Kantar, M.B.; Miles, W.; Smith, C.Z.; Freel, K.; Moy, J.; Louis, L.V.; et al. Broad threat to humanity from cumulative climate hazards intensified by greenhouse gas emissions. Nat. Clim. Change 2018, 8, 1062–1071. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jia, Z.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.; Qian, J.; Liu, X.; Zhang, J.; et al. Meta-analysis reveals the effects of microbial inoculants on the biomass and diversity of soil microbial communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar] [CrossRef]
- Cunha, I.d.C.M.d.; Silva, A.V.R.d.; Boleta, E.H.M.; Pellegrinetti, T.A.; Zagatto, L.F.G.; Zagatto, S.d.S.S.; Chaves, M.G.d.; Mendes, R.; Patreze, C.M.; Tsai, S.M.; et al. The interplay between the inoculation of plant growth-promoting rhizobacteria and the rhizosphere microbiome and their impact on plant phenotype. Microbiol. Res. 2024, 283, 127706. [Google Scholar] [CrossRef]
- Li, P.; Dini-Andreote, F.; Jiang, J. Exploiting microbial competition to promote plant health. Trends Plant Sci. 2024, 29, 1056–1058. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Liu, Y.; Zhang, J.; Jiang, M.; Nong, C.; Chen, J.; Hou, K.; Chen, Y.; Wu, W. Plant Growth-Promoting Rhizobacteria Are Key to Promoting the Growth and Furanocoumarin Synthesis of Angelica dahurica var. formosana under Low-Nitrogen Conditions. J. Agric. Food Chem. 2024, 72, 6964–6978. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Z.; Gao, X.; Long, J.; Wang, Y.; Wang, W.; Li, C.; He, Y.; Wu, Z. Combined control of plant diseases by Bacillus subtilis SL44 and Enterobacter hormaechei Wu15. Sci. Total Environ. 2024, 934, 173297. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, X.; Shu, D.; Siddique, K.H.M.; Song, X.; Wu, P.; Li, C.; Zhao, X. Enhancing soil health and nutrient cycling through soil amendments: Improving the synergy of bacteria and fungi. Sci. Total Environ. 2024, 923, 171332. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Zhao, J.; Zhang, A.; Pang, Q. Enhancement of sulfur metabolism and antioxidant machinery confers Bacillus sp. Jrh14-10-induced alkaline stress tolerance in plant. Plant Physiol. Biochem. 2023, 203, 108063. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Zhu, Y.; Khan, A.; Zhao, L.; Yang, Y.-M.; Wang, N.; Hao, M.; Ma, Y.; Nepal, J.; Ullah, F.; et al. Above-and below-ground feedback loop of maize is jointly enhanced by plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in drier soil. Sci. Total Environ. 2024, 917, 170417. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Ainalidou, A.; Mellidou, I.; Karamanoli, K. Metabolome and transcriptome reprogramming underlying tomato drought resistance triggered by a Pseudomonas strain. Plant Physiol. Biochem. 2023, 203, 108080. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Z.; Sun, H.; Guo, H.; Song, Y.; Zhang, H.; Ruan, Y.; Xu, Q.; Huang, Q.; Shen, Q.; et al. Synthetic community derived from grafted watermelon rhizosphere provides protection for ungrafted watermelon against Fusarium oxysporum via microbial synergistic effects. Microbiome 2024, 12, 101. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O. Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth. J. Fungi 2023, 9, 239. [Google Scholar] [CrossRef]
- Mohammadi, E.; Fattahi, M.; Barin, M.; Ashrafi-Saeidlou, S. Arbuscular mycorrhiza and vermicompost alleviate drought stress and enhance yield, total flavonoid concentration, rutin content, and antioxidant activity of buckwheat (Fagopyrum esculentum Moench). S. Afr. J. Bot. 2022, 148, 588–600. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Ruíz-Herrera, L.F.; Pelagio-Flores, R.; Macías-Rodríguez, L.I.; Martínez-Trujillo, M.; López-Coria, M.; Sánchez-Nieto, S.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma atroviride-emitted volatiles improve growth of Arabidopsis seedlings through modulation of sucrose transport and metabolism. Plant Cell Environ. 2021, 44, 1961–1976. [Google Scholar] [CrossRef]
- Rebolledo-Prudencio, O.G.; Estrada-Rivera, M.; Dautt-Castro, M.; Arteaga-Vazquez, M.A.; Arenas-Huertero, C.; Rosendo-Vargas, M.M.; Jin, H.; Casas-Flores, S. The small RNA-mediated gene silencing machinery is required in Arabidopsis for stimulation of growth, systemic disease resistance, and suppression of the nitrile-specifier gene NSP4 by Trichoderma atroviride. Plant J. 2022, 109, 873–890. [Google Scholar] [CrossRef]
- Yin, Q.; Feng, Z.; Ren, Z.; Wang, H.; Wu, D.; Jaisi, A.; Yang, M. Utilizing transcriptomics and metabolomics reveal drought tolerance mechanism in Nicotiana tabacum. bioRxiv 2024. [Google Scholar] [CrossRef]
- Molina-Hidalgo, F.J.; Vazquez-Vilar, M.; D’Andrea, L.; Demurtas, O.C.; Fraser, P.; Giuliano, G.; Bock, R.; Orzaez, D.; Goossens, A. Engineering Metabolism in Nicotiana Species: A Promising Future. Trends Biotechnol. 2021, 39, 901–913. [Google Scholar] [CrossRef]
- Mateos-Fernández, R.; Vacas, S.; Navarro-Fuertes, I.; Navarro-Llopis, V.; Orzáez, D.; Gianoglio, S. Assessment of tobacco (Nicotiana tabacum) and N. benthamiana as biofactories of irregular monoterpenes for sustainable crop protection. Ind. Crops Prod. 2023, 206, 117634. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, L.Y.; Kong, J.; Huang, Z.H.; Gao, Y.D.; Zhang, Z.X.; Zhou, Y.J.; Wu, R.Y.; Xu, H.J.; An, S.J. Expression of recombinant human Apolipoprotein A-I(Milano) in Nicotiana tabacum. Bioresour. Bioprocess. 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Saenz-de la, O.D.; Morales, L.O.; Strid, A.; Torres-Pacheco, I.; Guevara-Gonzalez, R.G. Ultraviolet-B exposure and exogenous hydrogen peroxide application lead to cross-tolerance toward drought in Nicotiana tabacum L. Physiol. Plant 2021, 173, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.Y.; Jia, W.; Chen, Z.; Xu, Z.C. Chloride salinity in a chloride-sensitive plant: Focusing on photosynthesis, hormone synthesis and transduction in tobacco. Plant Physiol. Biochem. 2020, 153, 119–130. [Google Scholar] [CrossRef]
- Shang, X.; Hui, L.; Jianlong, Z.; Hao, Z.; Cao, C.; Le, H.; Weimin, Z.; Yang, L.; Gao, Y.; Hou, X. The application of plant growth-promoting rhizobacteria enhances the tolerance of tobacco seedling to salt stress. Ecotoxicol. Environ. Saf. 2023, 265, 115512. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Li, Q.; Zhang, J.; Ji, C.; Sui, J.; Liu, Z.; Song, X.; Liu, X. Isolation and characterization of antagonistic bacteria with the potential for biocontrol of soil-borne wheat diseases. J. Appl. Microbiol. 2018, 125, 1868–1880. [Google Scholar] [CrossRef]
- Dai, J.; Wen, D.; Li, H.; Yang, J.; Rao, X.; Yang, Y.; Yang, J.; Yang, C.; Yu, J. Effect of hydrogen sulfide (H(2)S) on the growth and development of tobacco seedlings in absence of stress. BMC Plant Biol. 2024, 24, 162. [Google Scholar] [CrossRef]
- Weatherley, P.E. A Convenient Volumenometer for Biological Work. J. Exp. Bot. 1950, 1, 244–248. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Zheng, Q.; Yang, Q.; Wang, J.; Zhu, J.; Zhan, X. Toxicity of photoaged polyvinyl chloride microplastics to wheat seedling roots. J. Hazard. Mater. 2024, 463, 132816. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Kaur, R.; Saxena, S. Penicillium citrinum, a Drought-Tolerant Endophytic Fungus Isolated from Wheat (Triticum aestivum L.) Leaves with Plant Growth-Promoting Abilities. Curr. Microbiol. 2023, 80, 184. [Google Scholar] [CrossRef] [PubMed]
- Tarroum, M.; Romdhane, W.B.; Al-Qurainy, F.; Ali, A.A.M.; Al-Doss, A.; Fki, L.; Hassairi, A. A novel PGPF Penicillium olsonii isolated from the rhizosphere of Aeluropus littoralis promotes plant growth, enhances salt stress tolerance, and reduces chemical fertilizers inputs in hydroponic system. Front. Microbiol. 2022, 13, 996054. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Han, F.; Yang, X.; Chu, H.; Luo, C.; Tan, S.; Lv, S.; Qin, M.; Xie, G. Endophytic Penicillium oxalicum CX-1 prevented Phytophthora cactorum blight on Salvia miltiorrhiza and promoted plant growth. J. Appl. Microbiol. 2023, 134, lxad010. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urias, P. Could Trichoderma Be a Plant Pathogen? Successful Root Colonization. In Trichoderma: Host Pathogen Interactions and Applications; Sharma, A.K., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 35–59. [Google Scholar]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Konstantinou, A.; Folina, A.; Konstantas, A.; et al. Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Carro-Huerga, G.; Compant, S.; Gorfer, M.; Cardoza, R.E.; Schmoll, M.; Gutierrez, S.; Casquero, P.A. Colonization of Vitis vinifera L. by the Endophyte Trichoderma sp. Strain T154: Biocontrol Activity Against Phaeoacremonium minimum. Front. Plant Sci. 2020, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Glucosinolates profile of Arabidopsis thaliana modified root colonization of Trichoderma species. Biol. Control 2021, 155, 104522. [Google Scholar] [CrossRef]
- Vadez, V.; Grondin, A.; Chenu, K.; Henry, A.; Laplaze, L.; Millet, E.J.; Carminati, A. Crop traits and production under drought. Nat. Rev. Earth Environ. 2024, 5, 211–225. [Google Scholar] [CrossRef]
- Gui, Z.-Q.; Yuan, X.-L.; Yang, J.; Du, Y.-M.; Zhang, P. An updated review on chemical constituents from Nicotiana tabacum L.: Chemical diversity and pharmacological properties. Ind. Crops Prod. 2024, 214, 118497. [Google Scholar] [CrossRef]
- Gossart, N.; Berhin, A.; Sergeant, K.; Alam, I.; Andre, C.; Hausman, J.F.; Boutry, M.; Hachez, C. Engineering Nicotiana tabacum trichomes for triterpenic acid production. Plant Sci. 2023, 328, 111573. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Zhao, Q.; Huang, A.C. Making small molecules in plants: A chassis for synthetic biology-based production of plant natural products. J. Integr. Plant Biol. 2023, 65, 417–443. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress. 2023, 10, 100296. [Google Scholar] [CrossRef]
- Hu, W.; Tian, S.B.; Di, Q.; Duan, S.H.; Dai, K. Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum Linn.) under drought stress. Photosynthetica 2018, 56, 1204–1211. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; Niu, L.; Wang, G.; Yin, J.; Song, X.; Ottosen, C.O.; Rosenqvist, E.; Mittler, R.; Wu, Z.; et al. Synergistic regulation at physiological, transcriptional and metabolic levels in tomato plants subjected to a combination of salt and heat stress. Plant J. 2024, 117, 1656–1675. [Google Scholar] [CrossRef]
- Imtiaz, H.; Mir, A.R.; Corpas, F.J.; Hayat, S. Impact of potassium starvation on the uptake, transportation, photosynthesis, and abiotic stress tolerance. Plant Growth Regul. 2023, 99, 429–448. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Zeidi, M.A.; Siddique, K.H.M.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A.; Kamal, A. Osmoprotective Role of Sugar in Mitigating Abiotic Stress in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 53–70. [Google Scholar]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Zhang, Z.-W.; Yang, X.-Y.; Wang, C.-Q.; Lan, T.; Tang, X.-Y.; Chen, G.-D.; Zeng, J.; Yuan, S. Nitrate reductase is a key enzyme responsible for nitrogen-regulated auxin accumulation in Arabidopsis roots. Biochem. Biophys. Res. Commun. 2020, 532, 633–639. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Q.; Feng, Z.; Ren, Z.; Li, A.; Jaisi, A.; Yang, M. Rhizosphere Growth-Promoting Fungi of Healthy Nicotiana tabacum L.: A Systematic Approach to Boosting Plant Growth and Drought Resistance. Microorganisms 2025, 13, 543. https://doi.org/10.3390/microorganisms13030543
Yin Q, Feng Z, Ren Z, Li A, Jaisi A, Yang M. Rhizosphere Growth-Promoting Fungi of Healthy Nicotiana tabacum L.: A Systematic Approach to Boosting Plant Growth and Drought Resistance. Microorganisms. 2025; 13(3):543. https://doi.org/10.3390/microorganisms13030543
Chicago/Turabian StyleYin, Quanyu, Zhao Feng, Zhichao Ren, Ao Li, Amit Jaisi, and Mengquan Yang. 2025. "Rhizosphere Growth-Promoting Fungi of Healthy Nicotiana tabacum L.: A Systematic Approach to Boosting Plant Growth and Drought Resistance" Microorganisms 13, no. 3: 543. https://doi.org/10.3390/microorganisms13030543
APA StyleYin, Q., Feng, Z., Ren, Z., Li, A., Jaisi, A., & Yang, M. (2025). Rhizosphere Growth-Promoting Fungi of Healthy Nicotiana tabacum L.: A Systematic Approach to Boosting Plant Growth and Drought Resistance. Microorganisms, 13(3), 543. https://doi.org/10.3390/microorganisms13030543





