SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Searches
2.2. Types of Included Studies
2.3. Data Selection
2.4. Evaluation of Quality
2.5. Comparison of the Results
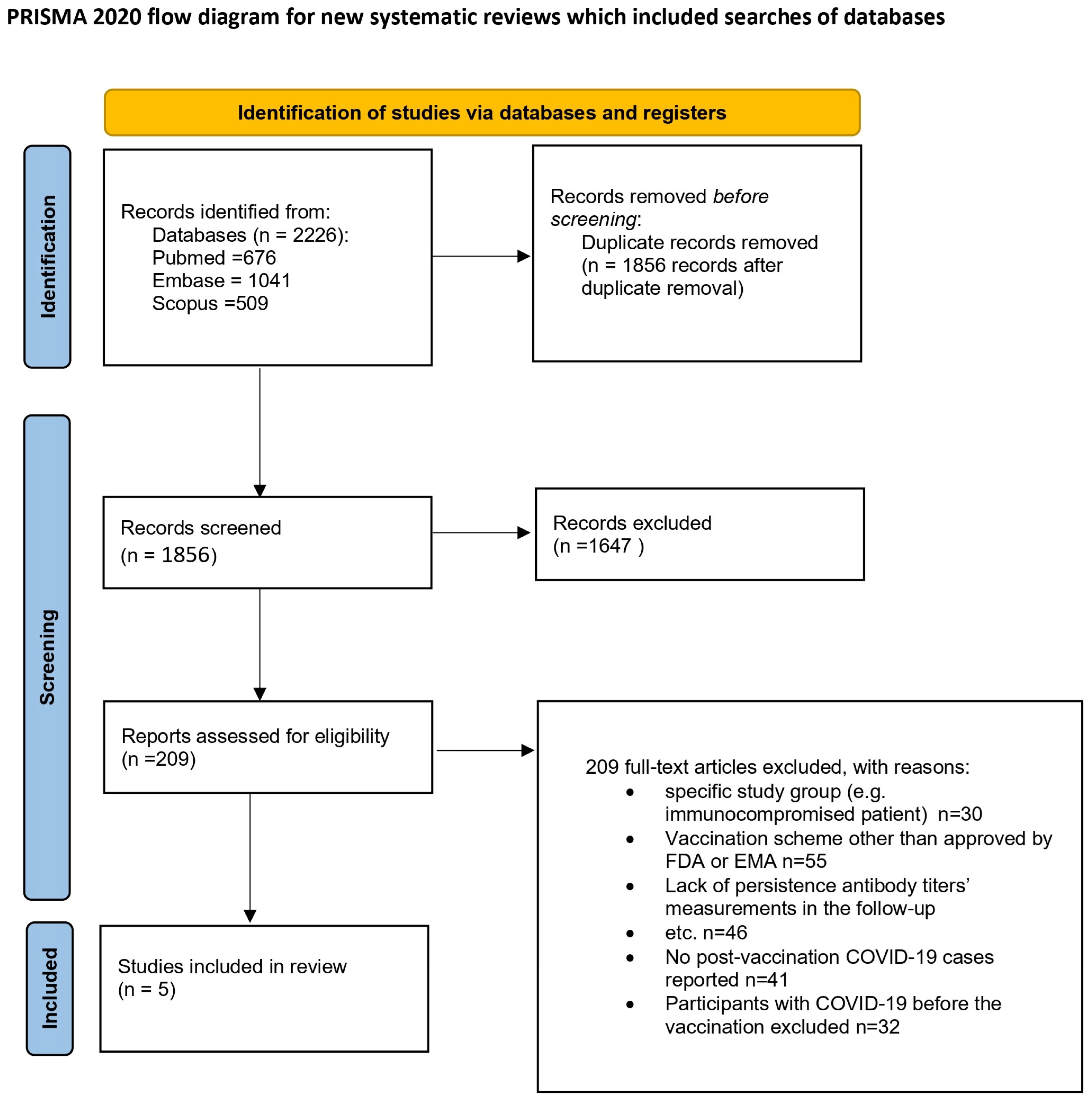
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Björkman, A.; Gisslén, M.; Gullberg, M.; Ludvigsson, J. The Swedish COVID-19 approach: A scientific dialogue on mitigation policies. Front. Public Health 2023, 11, 1206732. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control—Historical Data (to 20 June 2022) on the Weekly Number of New Reported COVID-19 Cases and Deaths Worldwide. Available online: https://www.ecdc.europa.eu/en/publications-data/download-historical-data-20-june-2022-weekly-number-new-reported-covid-19-cases (accessed on 12 December 2024).
- Demographic Situation in Poland Up to 2020 Death and Mortality. Available online: https://stat.gov.pl/en/topics/population/population/demographic-situation-in-poland-up-to-2020-deaths-and-mortality,15,1.html (accessed on 12 December 2024).
- Population Statistics 2021–2024 (Month) and 1998–2023 (Year) from Sweden. Available online: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population-and-living-conditions/population-composition-and-development/population-statistics/ (accessed on 12 December 2024).
- Khanijahani, A.; Iezadi, S.; Gholipour, K.; Azami-Aghdash, S.; Naghibi, D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int. J. Equity Health 2021, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Lam, C.Y.; Tan, P.H.; Tsang, H.F.; Wong, S.C.C. Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 8155. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef] [PubMed]
- COVID-19-Medicines. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 12 December 2024).
- WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/vaccines?m49=001&n=c (accessed on 12 December 2024).
- Movsisyan, M.; Truzyan, N.; Kasparova, I.; Chopikyan, A.; Sawaqed, R.; Bedross, A.; Sukiasyan, M.; Dilbaryan, K.; Shariff, S.; Kantawala, B.; et al. Tracking the evolution of anti-SARS-CoV-2 antibodies and long-term humoral immunity within 2 years after COVID-19 infection. Sci. Rep. 2024, 14, 13417. [Google Scholar] [CrossRef] [PubMed]
- Scourfield, D.O.; Reed, S.G.; Quastel, M.; Alderson, J.; Bart, V.M.T.; Teijeira Crespo, A.; Jones, R.; Pring, E.; Richter, F.C.; Burnell, S.E.A. The role and uses of antibodies in COVID-19 infections: A living review. Oxf. Open Immunol. 2021, 2, iqab003. [Google Scholar] [CrossRef]
- WHO COVID-19 Cases. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 12 December 2024).
- Owsianka, I.; Jachowicz-Matczak, E.; Brodowicz, J.; Sułkowska, J.; Heczko, P.; Wójkowska-Mach, J.; Bociąga-Jasik, M. Protective Antibody Levels Against SARS-CoV-2 After Vaccination in the Adult Immunocompetent Population. 2022, pp. 1–5. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=383367 (accessed on 12 December 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cadima.info/index.php/area/evidenceSynthesisDatabase (accessed on 12 December 2024).
- SARS-CoV-2 IgG. Available online: https://www.fda.gov/media/137383/download (accessed on 14 December 2024).
- Ferrari, D.; Ambrosi, A.; Di Resta, C.; Tomaiuolo, R.; Locatelli, M.; Banfi, G. Evaluation of antibody titer kinetics and SARS-CoV-2 infections in a large cohort of healthcare professionals ten months after administration of the BNT162b2 vaccine. J. Immunol. Methods 2022, 506, 113293. [Google Scholar] [CrossRef] [PubMed]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef]
- Vietri, M.T.; Albanese, L.; Passariello, L.; D’Elia, G.; Caliendo, G.; Molinari, A.M.; Angelillo, I.F. Evaluation of neutralizing antibodies after vaccine BNT162b2: Preliminary data. J. Clin. Virol. 2022, 146, 105057. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Ceci, S.; Patano, A.; Corriero, A.; Azzollini, D.; Marinelli, G.; Coloccia, G.; Piras, F.; Barile, G.; et al. Antispike Immunoglobulin-G (IgG) Titer Response of SARS-CoV-2 mRNA-Vaccine (BNT162b2): A Monitoring Study on Healthcare Workers. Biomedicines 2022, 10, 2402. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Yi, J.; Chen, Y.; Liang, J.; Wang, L.; Ma, J.; Zhu, R.; Zhang, X.; Hu, D.; et al. Synergistic evolution: The dynamic adaptation of SARS-CoV-2 and human protective immunity in the real world. J. Infect. 2024, 89, 106310. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The end of the COVID-19 pandemic. Eur. J. Clin. Investig. 2022, 52, e13782. [Google Scholar] [CrossRef] [PubMed]
- Mahilkar, S.; Agrawal, S.; Chaudhary, S.; Parikh, S.; Sonkar, S.C.; Verma, D.; Chitalia, V.; Mehta, D.; Koner, B.; Vijay, N.; et al. SARS-CoV-2 variants: Impact on biological and clinical outcome. Front. Med. 2022, 9, 995960. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Elahi, R.; Esmaeilzadeh, A. OMICRON: Virology, immunopathogenesis, and laboratory diagnosis. J. Gene Med. 2022, 24, e3435. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef]
- COVID-19 Results Briefing the European Region. Available online: https://www.healthdata.org/sites/default/files/files/Projects/COVID/2021/44566_briefing_European_Region_2.pdf (accessed on 14 December 2024).
- COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/covid-19/vaccine-tracker.html#uptake-tab (accessed on 14 December 2024).
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.-Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef]
- Naleway, A.L.; Groom, H.C.; Crawford, P.M.; Salas, S.B.; Henninger, M.L.; Donald, J.L.; Smith, N.; Thompson, M.G.; Blanton, L.H.; Bozio, C.H.; et al. Incidence of SARS-CoV-2 Infection, Emergency Department Visits, and Hospitalizations Because of COVID-19 Among Persons Aged ≥ 12 Years, by COVID-19 Vaccination Status—Oregon and Washington, July 4–September 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]

| Database | Search Terms | Date Searched Through | Number of Articles |
|---|---|---|---|
| PubMed | (((“COVID-19”[Mesh]) OR (“SARS-CoV-2”[Mesh])) AND ((“Immunogenicity, Vaccine”[Mesh]) OR (“Immunoglobulins, Intravenous”[Mesh]))) AND ((“Vaccination”[Mesh]) OR (“Vaccines”[Mesh])) NOT SYSTEMATIC REVIEW, NOT REVIEW | 04 January 2023 | 676 |
| Embase | 50 AND ‘Article’/it AND (2020:py OR 2021:py OR 2022:py OR 2023:py) AND (‘clinical article’/de OR ‘controlled clinical trial’/de OR ‘controlled study’/de OR ‘diagnostic test accuracy study’/de OR ‘human’/de OR ‘major clinical study’/de) | 04 January 2023 | 1041 |
| Scopus | ((TITLE-ABS-KEY (COVID-19)) OR (TITLE-ABS-KEY (Sars-CoV-2))) AND ((TITLE-ABS-KEY (immunogenicity)) OR (TITLE-ABS-KEY (“antibody levels”)))) AND ((TITLE-ABS-KEY (vaccination)) OR (TITLE-ABS-KEY (vaccine))) not “SYSTEMATIC REVIEW” AND (EXCLUDE (DOCTYPE, “re”)) | 04 January 2023 | 509 |
| Level of Antibodies Against SARS-CoV-2 | [17] | [18] | [19] | [20] | [21] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| The first period of time: 42–51 days after first vaccination | IgA + IgG + IgM COV+ (n = 98) Me | 2500 * | IgG n = 475 Me (IQR) | 1314.1 (0.3–5712) | IgG n = 45 M (95% CI) | 1901.8 (1698.4–2105.1) | Not estimated | COV + Median (IQR) | 1436.3 (915.1–2225.1) | |
| IgA + IgG + IgM COV− (n = 1074) Me (IQR) | 1659.0 (1611.5) | IgA n = 475 Me (IQR) | 22.1 (0–64.6) | COV− Median (IQR) | 2577.1 (1554.2–3200.6) | |||||
| The second period of time: 81–90 days after first vaccination | Not estimated | IgG n = 377 Me (IQR) | 306.8 (0–5712) | IgG n = 45 M (95% CI) | 1244.9 (1067.3–1422.5) | IgG M (SD) | 8413 (9510) | |||
| IgA n = 377 Me (IQR) | 7.9 (0–43.4) | |||||||||
| The third period of time: 158–180 days after first vaccination | IgA + IgG + IgM COV+ (n = 91) Me | 2500 * | IgG n = 322 Me (IQR) | 96.46 (0–5712) | Not estimated | IgG M (SD) | 3.880 (5.156) | |||
| IgA + IgG + IgM COV− (n = 1037) Me (IQR) | 584.0 (607.0) | IgA | Not estimated | |||||||
| The fourth period of time: 288–302 days after first vaccination | IgA + IgG + IgM COV+ (n = 69) Me (IQR) | 2308.0 (1345.5) | Not estimated | Not estimated | IgG M (SD) | 1.473 (1.818) | ||||
| IgA + IgG + IgM COV− (n = 753) Me (IQR) | 419.0 (526.5) | |||||||||
| Ref | Date of First Blood Collection | Country | Study Sample | Age [Years] | Vaccine Product | Diagnostic Tests Which Were Used to Measure Antibody Levels | |
|---|---|---|---|---|---|---|---|
| [17] | 01.2021–02.2021 | Italy | 1172 | 766 females Mean (SD) | 49.0 ± 16.7 | BNT162b2 mRNA | ECLIA |
| 406 males Mean (SD) | 52.0 ± 20.0 | ||||||
| [18] | 12.2020–06.2021 | Greece | 511 | Me (range) | 54.0 (19–105) | BNT162b2 mRNA | CMIA, ELISA |
| [19] | 01.2021 | Italy | 52 | Range | 25–70 | BNT162b2 mRNA | CLIA |
| [20] | 01.2021–10.2021 | Italy | 230 | 20–70 | BNT162b2 mRNA | ELISA | |
| [21] | 12.2020–01.2021 | United Kingdom | 4315 | BNT162b2 Me (IQR) | 41 (30.51) | BNT162b2 mRNA or ChAdOx1-S | ELISA |
| ChAdOx1-S Me (IQR) | 42 (30.52) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bylica, I.; Jachowicz-Matczak, E.; Brodowicz, J.; Sułkowska, J.; Bociąga-Jasik, M.; Heczko, P.; Gagatek, S.; Bylica, J.; Wójkowska-Mach, J. SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review. Microorganisms 2025, 13, 535. https://doi.org/10.3390/microorganisms13030535
Bylica I, Jachowicz-Matczak E, Brodowicz J, Sułkowska J, Bociąga-Jasik M, Heczko P, Gagatek S, Bylica J, Wójkowska-Mach J. SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review. Microorganisms. 2025; 13(3):535. https://doi.org/10.3390/microorganisms13030535
Chicago/Turabian StyleBylica, Izabella, Estera Jachowicz-Matczak, Justyna Brodowicz, Joanna Sułkowska, Monika Bociąga-Jasik, Piotr Heczko, Sebastian Gagatek, Jan Bylica, and Jadwiga Wójkowska-Mach. 2025. "SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review" Microorganisms 13, no. 3: 535. https://doi.org/10.3390/microorganisms13030535
APA StyleBylica, I., Jachowicz-Matczak, E., Brodowicz, J., Sułkowska, J., Bociąga-Jasik, M., Heczko, P., Gagatek, S., Bylica, J., & Wójkowska-Mach, J. (2025). SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review. Microorganisms, 13(3), 535. https://doi.org/10.3390/microorganisms13030535






