Virus Infection of a Freshwater Cyanobacterium Contributes Significantly to the Release of Toxins Through Cell Lysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Growth Conditions
2.2. Virus Propagation
2.3. Infectious Titre Quantification of Virus Lysate
2.4. Cell Density Measurements
2.5. Infection Experiments and Toxin Concentration Measurements
2.6. Calculated Cellular Microcystin-LR Release per Cell and Estimated Degradation Rate
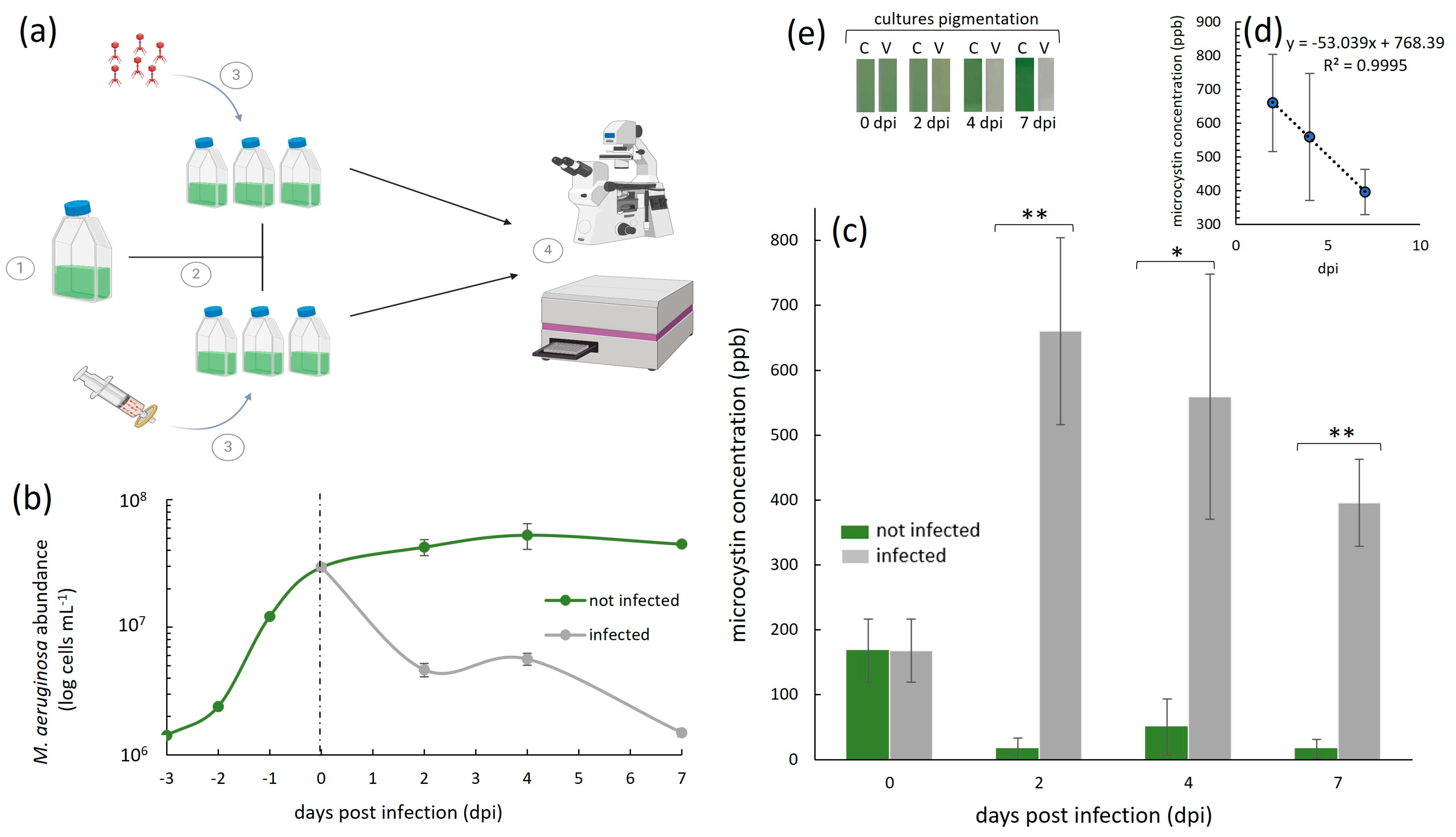
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Kummu, M.; De Moel, H.; Ward, P.J.; Varis, O. How close do we live to water? A global analysis of population distance to freshwater bodies. PLoS ONE 2011, 6, e20578. [Google Scholar] [CrossRef]
- Rinke, K.; Keller, P.S.; Kong, X.; Borchardt, D.; Weitere, M. Ecosystem services from inland waters and their aquatic ecosystems. In Atlas of Ecosystem Services: Drivers, Risks, and Societal Responses; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–195. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Lewandowska, A.; Sommer, U. Climate change and the spring bloom: A mesocosm study on the influence of light and temperature on phytoplankton and mesozooplankton. Mar. Ecol. Prog. Ser. 2010, 405, 101–111. [Google Scholar] [CrossRef]
- Sommer, U.; Lengfellner, K. Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Glob. Change Biol. 2008, 14, 1199–1208. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z. Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production. Green Process. Synth. 2022, 11, 64–70. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- EPA. The Effects: Dead Zones and Harmful Algal Blooms. In Nutrient Pollution; EPA: San Francisco, CA, USA, 2023. Available online: https://www.epa.gov/nutrientpollution/effects-dead-zones-and-harmful-algal-blooms (accessed on 10 October 2024).
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Herfindal, L.; Selheim, F. Microcystin produces disparate effects on liver cells in a dose dependent manner. Mini Rev. Med. Chem. 2006, 6, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Clifford, J.A.; Aldulescu, M.; Frenkel, J.A.; Holland, M.A.; Hall, M.L.; Glaser, K.B.; Berry, J. Cyanobacterial Microcystis aeruginosa lipopolysaccharide elicits release of superoxide anion, thromboxane B2, cytokines, chemokines, and matrix metalloproteinase-9 by rat microglia. Toxicol. Sci. 2011, 121, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.R.; Sklenar, K.S.; Blake, L.J. A costly endeavor: Addressing algae problems in a water supply. J.-Am. Water Work. Assoc. 2015, 107, E255–E262. [Google Scholar] [CrossRef]
- Bižić, M.; Klintzsch, T.; Ionescu, D.; Hindiyeh, M.Y.; Günthel, M.; Muro-Pastor, A.M.; Eckert, W.; Urich, T.; Keppler, F.; Grossart, H.P. Aquatic and terrestrial cyanobacteria produce methane. Sci. Adv. 2020, 6, eaax5343. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mou, X.; Cao, H.; Struewing, I.; Allen, J.; Lu, J. Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environ. Pollut. 2021, 288, 117682. [Google Scholar] [CrossRef]
- Wang, K.; Mou, X. Coordinated diel gene expression of cyanobacteria and their microbiome. Microorganisms 2021, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Aranda, Y.N.; Bhatt, P.; Ates, N.; Engel, B.A.; Simsek, H. Cyanophage-cyanobacterial interactions for sustainable aquatic environment. Environ. Res. 2023, 229, 115728. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, D.; Pan, L.; Li, M.; Tong, Y. Cyanobacteria-cyanophage interactions between freshwater and marine ecosystems based on large-scale cyanophage genomic analysis. Sci. Total Environ. 2024, 950, 175201. [Google Scholar] [CrossRef]
- Šulčius, S.; Mazur-Marzec, H.; Vitonytė, I.; Kvederavičiūtė, K.; Kuznecova, J.; Šimoliūnas, E.; Holmfeldt, K. Insights into cyanophage-mediated dynamics of nodularin and other non-ribosomal peptides in Nodularia spumigena. Harmful Algae 2018, 78, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Steffen, M.M.; Davis, T.W.; McKay, R.M.L.; Bullerjahn, G.S.; Krausfeldt, L.E.; Stough, J.M.; Neitzey, M.L.; Gilbert, N.E.; Boyer, G.L.; Johengen, T.H.; et al. Ecophysiological examination of the Lake Erie Microcystis bloom in 2014: Linkages between biology and the water supply shutdown of Toledo, OH. Environ. Sci. Technol. 2017, 51, 6745–6755. [Google Scholar] [CrossRef] [PubMed]
- McKindles, K.M.; Manes, M.A.; DeMarco, J.R.; Andrew, M.; Michael, M.R.; Davis, T.W.; Bullerjahn, G.S.; Schaffner, D.W. Dissolved microcystin release coincident with lysis of a bloom dominated by Microcystis spp. in Western Lake Erie attributed to a novel cyanophage. Appl. Environ. Microbiol. 2020, 86, e01397-20. [Google Scholar] [CrossRef]
- Bergh, Ø.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Sime-Ngando, T. Environmental bacteriophages: Viruses of microbes in aquatic ecosystems. Front. Microbiol. 2014, 5, 355. [Google Scholar] [CrossRef]
- Mateus, M.D. Bridging the gap between knowing and modeling viruses in marine systems—An upcoming frontier. Front. Mar. Sci. 2017, 3, 284. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, T.; Chen, Q.; Chen, T.; Hu, J.; Sun, L.; Wang, B.; Li, W.; Ni, J. Unveiling the unknown viral world in groundwater. Nat. Commun. 2024, 15, 6788. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Miki, T.; Noble, R.; Peduzzi, P.; Wilhelm, S. Viruses in aquatic ecosystems: Important advancements of the last 20 years and prospects for the future in the field of microbial oceanography and limnology. Adv. Oceanogr. Limnol. 2010, 1, 97–141. [Google Scholar] [CrossRef]
- Yoshida, T.; Nagasaki, K.; Takashima, Y.; Shirai, Y.; Tomaru, Y.; Takao, Y.; Sakamoto, S.; Hiroishi, S.; Ogata, H. Ma-LMM01 infecting toxic Microcystis aeruginosa illuminates diverse cyanophage genome strategies. J. Bacteriol. 2008, 190, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Clarke, J.D. Microcystin toxicokinetics, molecular toxicology, and pathophysiology in preclinical rodent models and humans. Toxins 2021, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cyanobacterial toxins: Microcystins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; WHO/HEP/ECH/WSH/2020.6. [Google Scholar]
- Yoshida, T.; Takashima, Y.; Tomaru, Y.; Shirai, Y.; Takao, Y.; Hiroishi, S.; Nagasaki, K. Isolation and characterization of a cyanophage infecting the toxic cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 2006, 72, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Handbook of Methods in Aquatic Microbial Ecology, 1st ed.; Kemp, P.F., Cole, J.J., Sherr, B.F., Sherr, E.B., Eds.; Lewis Publishers: Chicago, IL, USA, 1993; pp. 121–134. [Google Scholar]
- Almuhtaram, H.; Wang, C.; Hofmann, R. The importance of measuring ultraviolet fluence accurately: A review of microcystin-LR removal by direct photolysis. Environ. Sci. Technol. Lett. 2021, 8, 199–205. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ismail, A.A.; Kowalska, E.; Bahnemann, D.W. A comparative study of microcystin-LR degradation by UV-A, solar and visible light irradiation using bare and C/N/S-modified titania. Catalysts 2019, 9, 877. [Google Scholar] [CrossRef]
- Walker, D.; Fathabad, S.G.; Tabatabai, B.; Jafar, S.; Sitther, V. Microcystin Levels in Selected Cyanobacteria Exposed to Varying Salinity. J. Water Resour. Prot. 2019, 11, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Du, Y.; Wang, L.; Qian, J.; Chen, J.; Wu, Q.; Hu, X. Toxin release of cyanobacterium Microcystis aeruginosa after exposure to typical tetracycline antibiotic contaminants. Toxins 2017, 9, 53. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Westrick, J.A.; Furr, E.; Birbeck, J.A.; Reitz, L.A.; Stanislawczyk, K.; Li, W.; Weber, P.K.; Bridgeman, T.B.; Davis, T.W.; et al. Quantification of microcystin production and biodegradation rates in the western basin of Lake Erie. Limnol. Oceanogr. 2022, 67, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Fang, W.; Ma, M.; Xu, W.; Ye, J. Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water. Water 2023, 15, 3591. [Google Scholar] [CrossRef]
- Kormas, K.A.; Lymperopoulou, D.S. Cyanobacterial toxin degrading bacteria: Who are they? BioMed Res. Int. 2013, 2013, 463894. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F. A mini review on microcystins and bacterial degradation. Toxins 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, W.; Xu, Q.; Liu, Z.; Teng, J.; Yan, H.; Liu, X. Microcystins in water: Detection, microbial degradation strategies, and mechanisms. Int. J. Environ. Res. Public Health 2022, 19, 13175. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E. Understanding and predicting harmful algal blooms in a changing climate: A trait-based framework. Limnol. Oceanogr. Lett. 2023, 8, 229–246. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian Recreational Water Quality: Cyanobacteria and Their Toxins; Health Canada: Toronto, ON, Canada, 2022; ISBN 978-0-660-41541-3. Pub 210507. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, V.; Meza-Padilla, I.; Nissimov, J.I. Virus Infection of a Freshwater Cyanobacterium Contributes Significantly to the Release of Toxins Through Cell Lysis. Microorganisms 2025, 13, 486. https://doi.org/10.3390/microorganisms13030486
Lee V, Meza-Padilla I, Nissimov JI. Virus Infection of a Freshwater Cyanobacterium Contributes Significantly to the Release of Toxins Through Cell Lysis. Microorganisms. 2025; 13(3):486. https://doi.org/10.3390/microorganisms13030486
Chicago/Turabian StyleLee, Victoria, Isaac Meza-Padilla, and Jozef I. Nissimov. 2025. "Virus Infection of a Freshwater Cyanobacterium Contributes Significantly to the Release of Toxins Through Cell Lysis" Microorganisms 13, no. 3: 486. https://doi.org/10.3390/microorganisms13030486
APA StyleLee, V., Meza-Padilla, I., & Nissimov, J. I. (2025). Virus Infection of a Freshwater Cyanobacterium Contributes Significantly to the Release of Toxins Through Cell Lysis. Microorganisms, 13(3), 486. https://doi.org/10.3390/microorganisms13030486







