HNP-1: From Structure to Application Thanks to Multifaceted Functions
Abstract
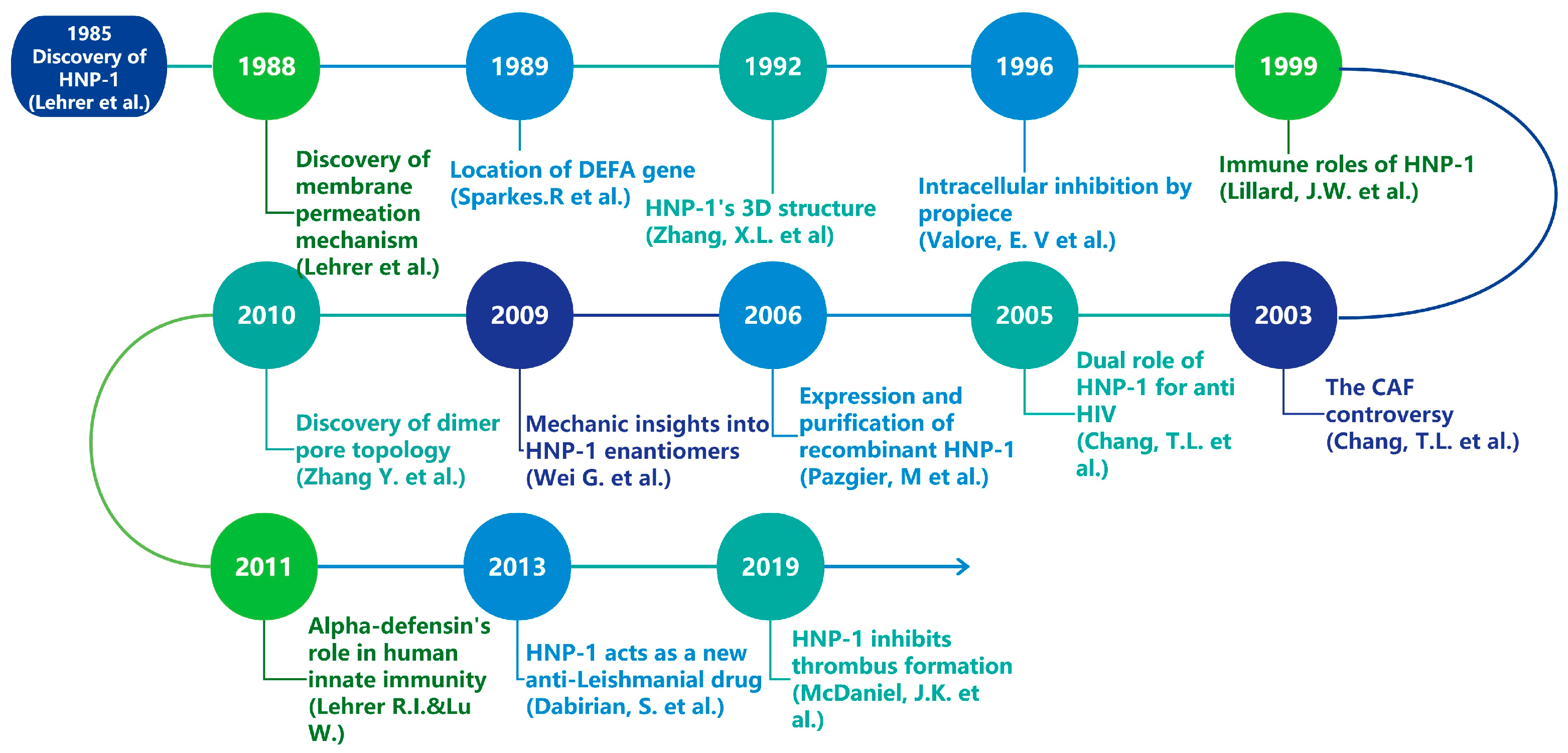
1. Introduction
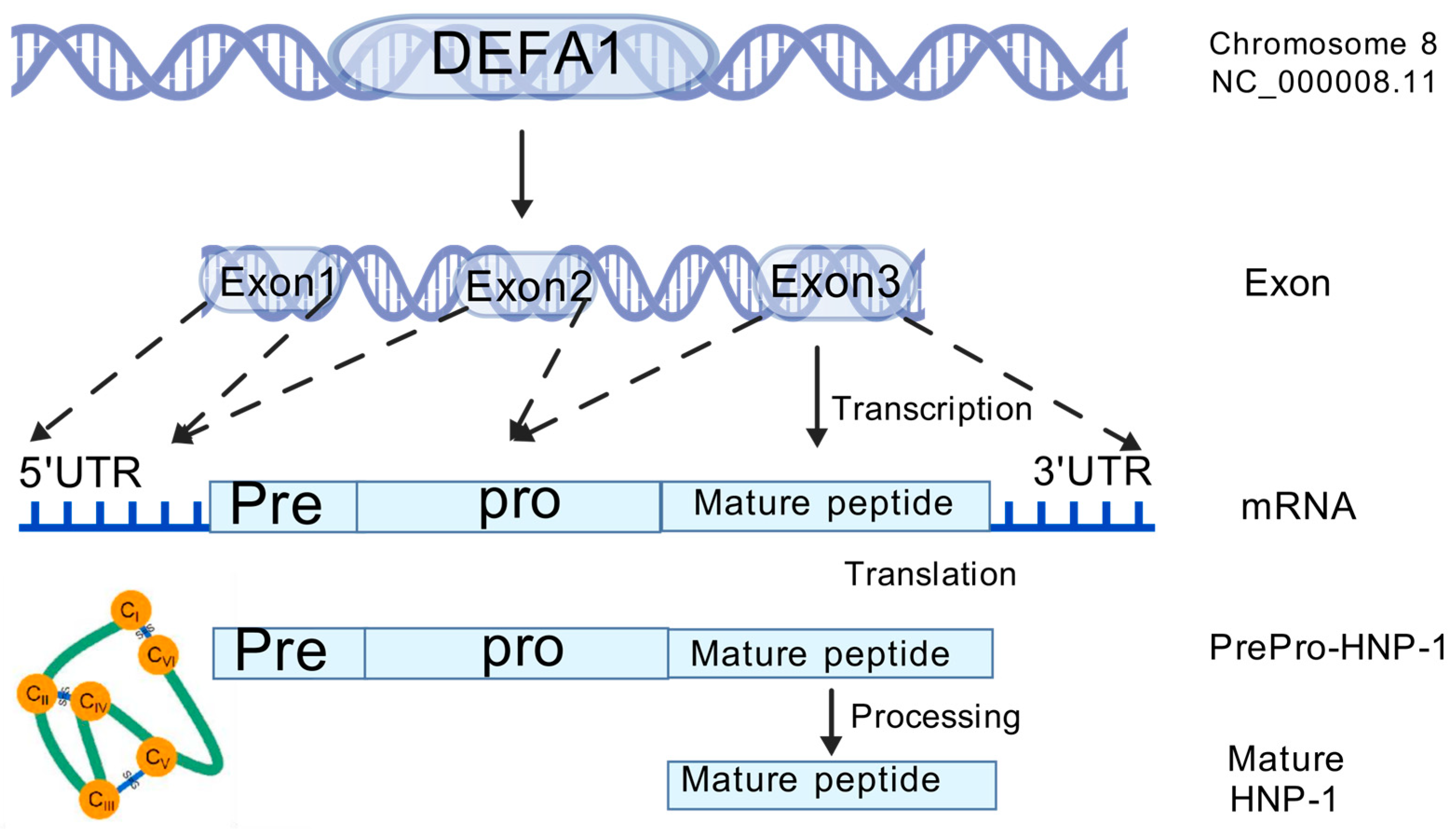
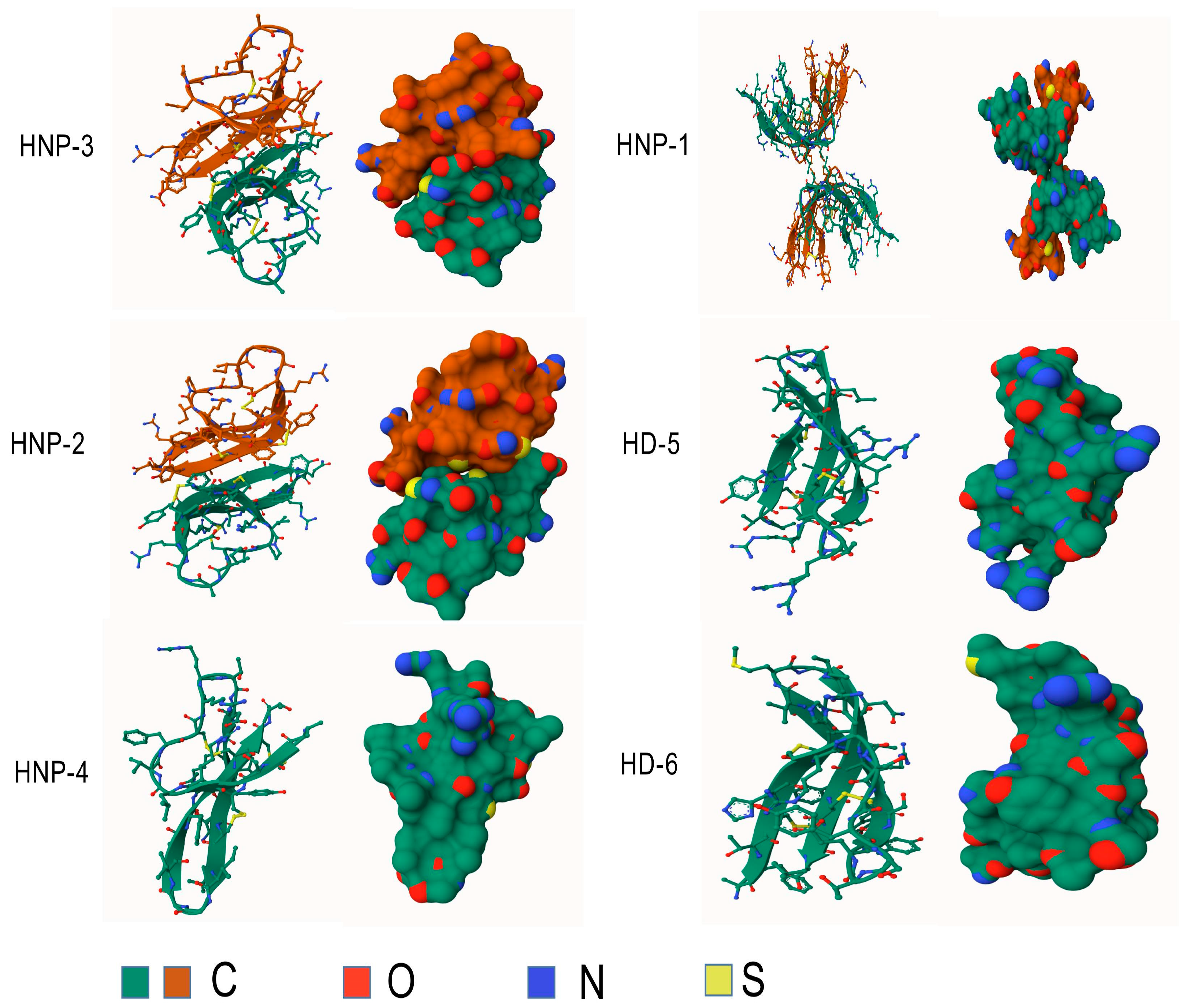
2. Gene, Structure and Distribution
3. Synthesis and Release
4. Immune Functions
4.1. Inflammatory Effects
4.2. Anti-Inflammatory Effects
| Function | Target Cell | Effect/Signaling | Reference |
|---|---|---|---|
| cytokine induction | LPS-activated monocytes monocytes activated by S. aureus or PMA mononuclear cell lines airway epithelial cells | blocked the release of IL-1 beta upregulated the expression of TNF-alpha and IL-1beta while downregulating IL-10 release of IL-1 beta upregulate the synthesis of IL-8 | [92] [92] [92] [79] |
| chemotactic effect | human monocytes platelet human mast cells macrophages CD4+/CD45RA+ naive CD8+ T cells immature human dendritic cells immature murine dendritic cells | adhesion CCR5 chemotactic agent chemotactic agent migration migration TNF-alpha TNF-alpha | [71] [72,73] [74] [74] [81] [81] [81] [82] |
| tumor cell lysis | murine teratocarcinoma | abrogates its oncogenicity in vivo | [94] |
| mouse 4T1 breast cancer | increased the tumor’s susceptibility to doxorubicin (Dox) | [95] | |
| A549 lung cancer | increased the tumor’s susceptibility to doxorubicin (Dox) | [95] |
4.3. Anticancer Effects
5. Antimicrobial Functions and Underlying Mechanisms
5.1. Anti-Viral Effects
5.1.1. Anti-HIV Activity
5.1.2. Other Viruses
5.2. Antibacterial Effects
| Bacteria | Bactericidal Mechanism | Resistant Mechanism | Reference |
|---|---|---|---|
| Histoplasma capsulatum | HNP-1 restricts intracellular growth | - | [114] |
| Escherichia coli | Permeabilizes membranes | - | [115,116] |
| Escherichia coli | Inhibits DNA/RNA/protein synthesis | [118] | |
| Salmonella typhimurium | Forms channels in membranes | PhoP and PhoQ dependent defensin resistance | [121,124] |
| Klebsiella pneumoniae | Reduces LPS and outer membrane proteins | - | [124] |
| Staphylococcus aureus | Targets lipid II; disrupts membrane | Dlt operon reduces HNP-1 binding | [136,139] |
| Streptococcus pyogenes | Targets ExPortal for secretion | - | [125] |
| Vibrio cholerae | - | Modifies HNP-1 | [126] |
| Fusobacterium nucleatum | - | Inhibition of membrane permeability | [129,130] |
| Pseudomonas aeruginosa | Inactivates exotoxin A | - | [131] |
| Shigella | Facilitates adhesion and invasion | - | [132] |
| Mycobacterium tuberculosis | - | MprF reduces negative charge on surface | [137,138] |
| Enterococcus faecalis | - | MprF-related resistance | [137] |
6. Biosynthesis and Mass Production
7. Future Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamas, N.; Kariuki, J.; Balasa, E.; Kinamon, T.; Liauw, F.; Charles, S.; Jaka, B.; Purdie, R.; Chavan, B.; Oluku Lawal, M.; et al. Antimicrobial Resistance Survivors: Calling the World to Action. Lancet 2024, 403, 2355–2357. [Google Scholar] [CrossRef]
- UNRIC. The Global Threat of Antimicrobial Resistance, a Silent Pandemic”, unric.org. Available online: https://unric.org/en/the-global-threat-of-antimicrobial-resistance-a-silent-pandemic/ (accessed on 26 September 2024).
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Escobar-Salom, M.; Torrens, G.; Jordana-Lluch, E.; Oliver, A.; Juan, C. Mammals’ Humoral Immune Proteins and Peptides Targeting the Bacterial Envelope: From Natural Protection to Therapeutic Applications against Multidrug-resistant Gram-negatives. Biol. Rev. 2022, 97, 1005–1037. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian Defensins in Immunity: More than Just Microbicidal. Trends Immunol. 2002, 23, 291–296. [Google Scholar] [CrossRef]
- Kagan, B.L.; Ganz, T.; Lehrer, R.I. Defensins: A Family of Antimicrobial and Cytotoxic Peptides. Toxicology 1994, 87, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian Defensins in the Antimicrobial Immune Response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef]
- Selsted, M.E.; Harwig, S.S.; Ganz, T.; Schilling, J.W.; Lehrer, R.I. Primary Structures of Three Human Neutrophil Defensins. J. Clin. Investig. 1985, 76, 1436–1439. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lu, W. A-Defensins in Human Innate Immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef]
- SPARKES, R. Assignment of Defensin Gene(s) to Human Chromosome 8p23. Genomics 1989, 5, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Selsted, M.E.; Pardi, A. NMR Studies of Defensin Antimicrobial Peptides. 1. Resonance Assignment and Secondary Structure Determination of Rabbit NP-2 and Human HNP-1. Biochemistry 1992, 31, 11348–11356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Doherty, T.; Li, J.; Lu, W.; Barinka, C.; Lubkowski, J.; Hong, M. Resonance Assignment and Three-Dimensional Structure Determination of a Human α-Defensin, HNP-1, by Solid-State NMR. J. Mol. Biol. 2010, 397, 408–422. [Google Scholar] [CrossRef]
- Valore, E.V.; Martin, E.; Harwig, S.S.; Ganz, T. Intramolecular Inhibition of Human Defensin HNP-1 by Its Propiece. J. Clin. Investig. 1996, 97, 1624–1629. [Google Scholar] [CrossRef]
- Lillard, J.W.; Boyaka, P.N.; Chertov, O.; Oppenheim, J.J.; McGhee, J.R. Mechanisms for Induction of Acquired Host Immunity by Neutrophil Peptide Defensins. Proc. Natl. Acad. Sci. USA 1999, 96, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Vargas, J.; DelPortillo, A.; Klotman, M.E. Dual Role of α-Defensin-1 in Anti–HIV-1 Innate Immunity. J. Clin. Investig. 2005, 115, 765–773. [Google Scholar] [CrossRef]
- Chang, T.L.-Y.; François, F.; Mosoian, A.; Klotman, M.E. CAF-Mediated Human Immunodeficiency Virus (HIV) Type 1 Transcriptional Inhibition Is Distinct from α-Defensin-1 HIV Inhibition. J. Virol. 2003, 77, 6777–6784. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Barton, A.; Ganz, T. Concurrent Assessment of Inner and Outer Membrane Permeabilization and Bacteriolysis in E. coli by Multiple-Wavelength Spectrophotometry. J. Immunol. Methods 1988, 108, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; de Leeuw, E.; Pazgier, M.; Yuan, W.; Zou, G.; Wang, J.; Ericksen, B.; Lu, W.-Y.; Lehrer, R.I.; Lu, W. Through the Looking Glass, Mechanistic Insights from Enantiomeric Human Defensins. J. Biol. Chem. 2009, 284, 29180–29192. [Google Scholar] [CrossRef]
- Dabirian, S.; Taslimi, Y.; Zahedifard, F.; Gholami, E.; Doustdari, F.; Motamedirad, M.; Khatami, S.; Azadmanesh, K.; Nylen, S.; Rafati, S. Human Neutrophil Peptide-1 (HNP-1): A New Anti-Leishmanial Drug Candidate. PLoS Negl. Trop. Dis. 2013, 7, e2491. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M.; Lubkowski, J. Expression and Purification of Recombinant Human α-Defensins in Escherichia coli. Protein Expr. Purif. 2006, 49, 1–8. [Google Scholar] [CrossRef]
- Rice, W.G.; Ganz, T.; Kinkade, J.M.; Selsted, M.E.; Lehrer, R.I.; Parmley, R.T. Defensin-Rich Dense Granules of Human Neutrophils. Blood 1987, 70, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Aldred, P.M.R.; Hollox, E.J.; Armour, J.A.L. Copy Number Polymorphism and Expression Level Variation of the Human α-Defensin Genes DEFA1 and DEFA3. Hum. Mol. Genet. 2005, 14, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Jespersgaard, C.; Fode, P.; Dybdahl, M.; Vind, I.; Nielsen, O.H.; Csillag, C.; Munkholm, P.; Vainer, B.; Riis, L.; Elkjaer, M.; et al. Alpha-Defensin DEFA1A3 Gene Copy Number Elevation in Danish Crohn’s Disease Patients. Dig. Dis. Sci. 2011, 56, 3517–3524. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Hou, J.; Shu, Q.; Yin, Y.; Fu, W.; Han, F.; Hou, T.; Zeng, C.; Nemeth, E.; et al. Increased Gene Copy Number of DEFA1/DEFA3 Worsens Sepsis by Inducing Endothelial Pyroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 3161–3170. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Heng, H.H.Q.; Ganz, T. The Human β-Defensin-1 and α-Defensins Are Encoded by Adjacent Genes: Two Peptide Families with Differing Disulfide Topology Share a Common Ancestry. Genomics 1997, 43, 316–320. [Google Scholar] [CrossRef]
- Valore, E.V.; Ganz, T. Posttranslational Processing of Defensins in Immature Human Myeloid Cells. Blood 1992, 79, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Pardi, A.; Zhang, X.L.; Selsted, M.E.; Skalicky, J.J.; Yip, P.F. NMR Studies of Defensin Antimicrobial Peptides. 2. Three-Dimensional Structures of Rabbit NP-2 and Human HNP-1. Biochemistry 1992, 31, 11357–11364. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Pazgier, M.; de Leeuw, E.; Rajabi, M.; Li, J.; Zou, G.; Jung, G.; Yuan, W.; Lu, W.-Y.; Lehrer, R.I.; et al. Trp-26 Imparts Functional Versatility to Human α-Defensin HNP1. J. Biol. Chem. 2010, 285, 16275–16285. [Google Scholar] [CrossRef]
- Pazgier, M.; Wei, G.; Ericksen, B.; Jung, G.; Wu, Z.; de Leeuw, E.; Yuan, W.; Szmacinski, H.; Lu, W.Y.; Lubkowski, J.; et al. Sometimes it takes two to tango: Contributions of dimerization to functions of human α-defensin HNP1 peptide. J. Biol. Chem. 2012, 287, 8944–8953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maemoto, A.; Qu, X.; Rosengren, K.J.; Tanabe, H.; Henschen-Edman, A.; Craik, D.J.; Ouellette, A.J. Functional Analysis of the α-Defensin Disulfide Array in Mouse Cryptdin-4. J. Biol. Chem. 2004, 279, 44188–44196. [Google Scholar] [CrossRef]
- Tanabe, H.; Ayabe, T.; Maemoto, A.; Ishikawa, C.; Inaba, Y.; Sato, R.; Moriichi, K.; Okamoto, K.; Watari, J.; Kono, T.; et al. Denatured Human α-Defensin Attenuates the Bactericidal Activity and the Stability against Enzymatic Digestion. Biochem. Biophys. Res. Commun. 2007, 358, 349–355. [Google Scholar] [CrossRef]
- Rajabi, M.; De Leeuw, E.; Pazgier, M.; Li, J.; Lubkowski, J.; Lu, W. The Conserved Salt Bridge in Human α-Defensin 5 Is Required for Its Precursor Processing and Proteolytic Stability. J. Biol. Chem. 2008, 283, 21509–21518. [Google Scholar] [CrossRef]
- Chan, A.W.E.; Hutchinson, E.G.; Harris, D.; Thornton, J.M. Identification, Classification, and Analysis of Beta-bulges in Proteins. Protein Sci. 1993, 2, 1574–1590. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Guo, Q. PAR-4 Is Involved in Regulation of β-Secretase Cleavage of the Alzheimer Amyloid Precursor Protein. J. Biol. Chem. 2005, 280, 13824–13832. [Google Scholar] [CrossRef]
- Zhao, L.; Ericksen, B.; Wu, X.; Zhan, C.; Yuan, W.; Li, X.; Pazgier, M.; Lu, W. Invariant Gly Residue Is Important for α-Defensin Folding, Dimerization, and Function. J. Biol. Chem. 2012, 287, 18900–18912. [Google Scholar] [CrossRef] [PubMed]
- Daher, K.A.; Selsted, M.E.; Lehrer, R.I. Direct Inactivation of Viruses by Human Granulocyte Defensins. J. Virol. 1986, 60, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Jung, G.; Ruchala, P.; Wang, W.; Micewicz, E.D.; Waring, A.J.; Gillespie, E.J.; Bradley, K.A.; Ratner, A.J.; Rest, R.F.; et al. Human α-Defensins Inhibit Hemolysis Mediated by Cholesterol-Dependent Cytolysins. Infect. Immun. 2009, 77, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gajendran, N.; Mittrücker, H.-W.; Weiwad, M.; Song, Y.-H.; Hurwitz, R.; Wilmanns, M.; Fischer, G.; Kaufmann, S.H.E. Human α-Defensins Neutralize Anthrax Lethal Toxin and Protect against Its Fatal Consequences. Proc. Natl. Acad. Sci. USA 2005, 102, 4830–4835. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and Regulation of Defensins in Host Defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Mandal, M.; Nagaraj, R. Antibacterial Activities and Conformations of Synthetic A-defensin HNP-1 and Analogs with One, Two and Three Disulfide Bridges. J. Pept. Res. 2002, 59, 95–104. [Google Scholar] [CrossRef]
- Moazzezy, N.; Asadi Karam, M.R.; Rafati, S.; Bouzari, S.; Oloomi, M. Inhibition and Eradication Activity of Truncated α-Defensin Analogs against Multidrug Resistant Uropathogenic Escherichia Coli Biofilm. PLoS ONE 2020, 15, e0235892. [Google Scholar] [CrossRef]
- Moazzezy, N.; Asadi Karam, M.R.; Rafati, S.; Bouzari, S.; Oloomi, M. A Synthetic Peptide 2Abz23S29 Reduces Bacterial Titer and Induces Pro-Inflammatory Cytokines in a Murine Model of Urinary Tract Infection. Drug Des. Devel Ther. 2020, 14, 2797–2807. [Google Scholar] [CrossRef]
- Moazzezy, N.; Rismani, E.; Rezaei, M.; Karam, M.R.A.; Rafati, S.; Bouzari, S.; Oloomi, M. Computational Evaluation of Modified Peptides from Human Neutrophil Peptide 1 (HNP-1). J. Biomol. Struct. Dyn. 2022, 40, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Powell, R.; Lu, W. Productive Folding of Human Neutrophil α-Defensins in Vitro without the pro-Peptide. J. Am. Chem. Soc. 2003, 125, 2402–2403. [Google Scholar] [CrossRef]
- Wei, J.; Huang, Y.; Zhong, D.; Kang, L.; Ishag, H.; Mao, X.; Cao, R.; Zhou, B.; Chen, P. Design and Evaluation of a Multi-Epitope Peptide against Japanese Encephalitis Virus Infection in BALB/c Mice. Biochem. Biophys. Res. Commun. 2010, 396, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Pantelis, A.; Kraus, D.; Reckenbeil, J.; Reich, R.; Jepsen, S.; Fischer, H.P.; Allam, J.P.; Novak, N.; Wenghoefer, M. Human α-Defensin (DEFA) Gene Expression Helps to Characterise Benign and Malignant Salivary Gland Tumours. BMC Cancer 2012, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Mü, C.A.; Markovic-Lipkovski, J.; Klatt, T.; Gamper, J.; Schwarz, G.; Beck, H.; Deeg, M.; Kalbacher, H.; Widmann, S.; Wessels, J.T.; et al. Human-Defensins HNPs-1,-2, and-3 in Renal Cell Carcinoma Influences on Tumor Cell Proliferation. Am. J. Pathol. 2002, 160, 1311–1324. [Google Scholar] [CrossRef]
- Melle, C.; Ernst, G.; Schimmel, B.; Bleul, A.; Thieme, H.; Kaufmann, R.; Mothes, H.; Settmacher, U.; Claussen, U.; Halbhuber, K.J.; et al. Discovery and Identification of α-Defensins as Low Abundant, Tumor-Derived Serum Markers in Colorectal Cancer. Gastroenterology 2005, 129, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ferdowsi, S.; Pourfathollah, A.A.; Amiri, F.; Rafiee, M.H.; Aghaei, A. Evaluation of Anticancer Activity of α-Defensins Purified from Neutrophils Trapped in Leukoreduction Filters. Life Sci. 2019, 224, 249–254. [Google Scholar] [CrossRef]
- Hanaoka, Y.; Yamaguchi, Y.; Yamamoto, H.; Ishii, M.; Nagase, T.; Kurihara, H.; Akishita, M.; Ouchi, Y. In Vitro and In Vivo Anticancer Activity of Human β-Defensin-3 and Its Mouse Homolog. Anticancer Res. 2016, 36, 5999–6004. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Álvarez, L.; Cabañas, R.; Fiandor, A.; Díaz, R.; Escamochero, S.; Prior, N.; Blanca, M.; Bellón, T. Expression of α-Defensin 1-3 in T Cells from Severe Cutaneous Drug-Induced Hypersensitivity Reactions. Allergy 2011, 66, 360–367. [Google Scholar] [CrossRef]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jö, H.; Wigzell, H.; Gudmundsson, G.H. The Human Antimicrobial and Chemotactic Peptides LL-37 and-Defensins Are Expressed by Specific Lymphocyte and Monocyte Populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Chalifour, A.; Jeannin, P.; Gauchat, J.-F.; Blaecke, A.; Malissard, M.; N’Guyen, T.; Thieblemont, N.; Delneste, Y. Direct Bacterial Protein PAMP Recognition by Human NK Cells Involves TLRs and Triggers α-Defensin Production. Blood 2004, 104, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Meinken, C.; Bastian, M.; Bruns, H.; Legaspi, A.; Ochoa, M.T.; Krutzik, S.R.; Bloom, B.R.; Ganz, T.; Modlin, R.L.; et al. Macrophages Acquire Neutrophil Granules for Antimicrobial Activity against Intracellular Pathogens. J. Immunol. 2006, 177, 1864–1871. [Google Scholar] [CrossRef]
- Wu, Z.; Cocchi, F.; Gentles, D.; Ericksen, B.; Lubkowski, J.; DeVico, A.; Lehrer, R.I.; Lu, W. Human Neutrophil A-defensin 4 Inhibits HIV-1 Infection in Vitro. FEBS Lett. 2005, 579, 162–166. [Google Scholar] [CrossRef]
- Welkos, S.; Cote, C.K.; Hahn, U.; Shastak, O.; Jedermann, J.; Bozue, J.; Jung, G.; Ruchala, P.; Pratikhya, P.; Tang, T.; et al. Humanized θ-Defensins (Retrocyclins) Enhance Macrophage Performance and Protect Mice from Experimental Anthrax Infections. Antimicrob. Agents Chemother. 2011, 55, 4238–4250. [Google Scholar] [CrossRef] [PubMed]
- Esmaielbeiki, R.; Naughton, D.P.; Nebel, J.-C. Structure Prediction of LDLR-HNP1 Complex Based on Docking Enhanced by LDLR Binding 3D Motif. Protein Pept. Lett. 2012, 19, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Daher, K.A.; Lehrer, R.I.; Ganz, T.; Kronenberg, M. Isolation and Characterization of Human Defensin CDNA Clones. Proc. Natl. Acad. Sci. USA 1988, 85, 7327–7331. [Google Scholar] [CrossRef]
- Michaelson, D.; Rayner, J.; Couto, M.; Ganz, T. Cationic Defensins Arise from Charge-Neutralized Propeptides: A Mechanism for Avoiding Leukocyte Autocytotoxicity? J. Leukoc. Biol. 1992, 51, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, G.I.; Ganz, T. Defensins Mediate the Microbicidal Activity of Human Neutrophil Granule Extract against Acinetobacter Calcoaceticus. Infect. Immun. 1987, 55, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Borregaard, N. The Individual Regulation of Granule Protein MRNA Levels during Neutrophil Maturation Explains the Heterogeneity of Neutrophil Granules. J. Leukoc. Biol. 1999, 66, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Nordenfelt, P.; Tapper, H. Phagosome Dynamics during Phagocytosis by Neutrophils. J. Leukoc. Biol. 2011, 90, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Joiner, K.A.; Ganz, T.; Albert, J.; Rotrosen, D. The Opsonizing Ligand on Salmonella Typhimurium Influences Incorporation of Specific, but Not Azurophil, Granule Constituents into Neutrophil Phagosomes. J. Cell Biol. 1989, 109, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Leikina, E.; Delanoe-Ayari, H.; Melikov, K.; Cho, M.-S.; Chen, A.; Waring, A.J.; Wang, W.; Xie, Y.; Loo, J.A.; Lehrer, R.I.; et al. Carbohydrate-Binding Molecules Inhibit Viral Fusion and Entry by Crosslinking Membrane Glycoproteins. Nat. Immunol. 2005, 6, 995–1001. [Google Scholar] [CrossRef]
- Wang, W.; Owen, S.M.; Rudolph, D.L.; Cole, A.M.; Hong, T.; Waring, A.J.; Lal, R.B.; Lehrer, R.I. Activity of α- and θ-Defensins against Primary Isolates of HIV-1. J. Immunol. 2004, 173, 515–520. [Google Scholar] [CrossRef]
- Brook, M.; Tomlinson, G.H.; Miles, K.; Smith, R.W.P.; Rossi, A.G.; Hiemstra, P.S.; van ’t Wout, E.F.A.; Dean, J.L.E.; Gray, N.K.; Lu, W.; et al. Neutrophil-Derived Alpha Defensins Control Inflammation by Inhibiting Macrophage MRNA Translation. Proc. Natl. Acad. Sci. USA 2016, 113, 4350–4355. [Google Scholar] [CrossRef] [PubMed]
- Mallow, E.B.; Harris, A.; Salzman, N.; Russell, J.P.; DeBerardinis, R.J.; Ruchelli, E.; Bevins, C.L. Human Enteric Defensins. J. Biol. Chem. 1996, 271, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Su, Q.; Tempst, P. Differentiation-Stimulated Activity Binds an ETS-like, Essential Regulatory Element in the Human Promyelocytic Defensin-1Promoter. J. Biol. Chem. 1998, 273, 8727–8740. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi-Ishii, Y.; Hasebe, T.; Nagaoka, I. Role of CCAAT/Enhancer-Binding Protein Site in Transcription of Human Neutrophil Peptide-1 and -3 Defensin Genes. J. Immunol. 2000, 164, 3264–3273. [Google Scholar] [CrossRef]
- Ashitani, J.; Nakazato, M.; Mukae, H.; Taniguchi, H.; Date, Y.; Matsukura, S. Recombinant Granulocyte Colony-Stimulating Factor Induces Production of Human Neutrophil Peptides in Lung Cancer Patients with Neutropenia. Regul. Pept. 2000, 95, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Territo, M.C.; Ganz, T.; Selsted, M.E.; Lehrer, R. Monocyte-Chemotactic Activity of Defensins from Human Neutrophils. J. Clin. Investig. 1989, 84, 2017–2020. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.-E.; Ortega-Gomez, A.; Wichapong, K.; Bongiovanni, D.; Horckmans, M.; Megens, R.T.A.; Leoni, G.; Ferraro, B.; Rossaint, J.; Paulin, N.; et al. Recruitment of Classical Monocytes Can Be Inhibited by Disturbing Heteromers of Neutrophil HNP1 and Platelet CCL5. Sci. Transl. Med. 2015, 7, 317ra196. [Google Scholar] [CrossRef] [PubMed]
- Wichapong, K.; Alard, J.-E.; Ortega-Gomez, A.; Weber, C.; Hackeng, T.M.; Soehnlein, O.; Nicolaes, G.A.F. Structure-Based Design of Peptidic Inhibitors of the Interaction between CC Chemokine Ligand 5 (CCL5) and Human Neutrophil Peptides 1 (HNP1). J. Med. Chem. 2016, 59, 4289–4301. [Google Scholar] [CrossRef] [PubMed]
- Grigat, J.; Soruri, A.; Forssmann, U.; Riggert, J.; Zwirner, J. Chemoattraction of Macrophages, T Lymphocytes, and Mast Cells Is Evolutionarily Conserved within the Human α-Defensin Family. J. Immunol. 2007, 179, 3958–3965. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human Neutrophil Defensins Selectively Chemoattract Naive T and Immature Dendritic Cells. J. Leukoc. Biol. 2000, 68, 9–14. [Google Scholar] [CrossRef]
- Tecle, T.; White, M.R.; Gantz, D.; Crouch, E.C.; Hartshorn, K.L. Human Neutrophil Defensins Increase Neutrophil Uptake of Influenza A Virus and Bacteria and Modify Virus-Induced Respiratory Burst Responses. J. Immunol. 2007, 178, 8046–8052. [Google Scholar] [CrossRef] [PubMed]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil Alpha-Defensin Human Neutrophil Peptide Modulates Cytokine Production in Human Monocytes and Adhesion Molecule Expression in Endothelial Cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA–Peptide Complexes in Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, J.; Yang, W.; Xiang, F.; Yang, F.; Zhao, Y.; Cao, Z.; Li, W.; Chen, Z.; Wu, Y. Human A-defensins Are Immune-related Kv1.3 Channel Inhibitors: New Support for Their Roles in Adaptive Immunity. FASEB J. 2015, 29, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.G.; Bao, J.; Zander, C.B.; McDaniel, J.K.; Chetty, P.S.; Seeholzer, S.H.; Bdeir, K.; Cines, D.B.; Zheng, X.L. Human Neutrophil Peptides Inhibit Cleavage of von Willebrand Factor by ADAMTS13: A Potential Link of Inflammation to TTP. Blood 2016, 128, 110–119. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.K.; Abdelgawwad, M.S.; Hargett, A.; Renfrow, M.B.; Bdeir, K.; Cao, W.; Cines, D.B.; Zheng, X.L. Human Neutrophil Peptide-1 Inhibits Thrombus Formation under Arterial Flow via Its Terminal Free Cysteine Thiols. J. Thromb. Haemost. 2019, 17, 596–606. [Google Scholar] [CrossRef]
- Higazi, M.; Abdeen, S.; Abu-Fanne, R.; Heyman, S.N.; Masarwy, A.; Bdeir, K.; Maraga, E.; Cines, D.B.; Higazi, A.A.-R. Opposing Effects of HNP1 (α-Defensin-1) on Plasma Cholesterol and Atherogenesis. PLoS ONE 2020, 15, e0231582. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Y.; Zhang, K.; Li, H.; Chen, W.; Meng, G.; Fang, X. Alarmin HNP-1 Promotes Pyroptosis and IL-1β Release through Different Roles of NLRP3 Inflammasome via P2X7 in LPS-Primed Macrophages. Innate Immun. 2014, 20, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Ibusuki, K.; Sakiyama, T.; Kanmura, S.; Maeda, T.; Iwashita, Y.; Nasu, Y.; Sasaki, F.; Taguchi, H.; Hashimoto, S.; Numata, M.; et al. Human Neutrophil Peptides Induce Interleukin-8 in Intestinal Epithelial Cells through the P2 Receptor and ERK1/2 Signaling Pathways. Int. J. Mol. Med. 2015, 35, 1603–1609. [Google Scholar] [CrossRef]
- Khine, A.A.; Del Sorbo, L.; Vaschetto, R.; Voglis, S.; Tullis, E.; Slutsky, A.S.; Downey, G.P.; Zhang, H. Human Neutrophil Peptides Induce Interleukin-8 Production through the P2Y6 Signaling Pathway. Blood 2006, 107, 2936–2942. [Google Scholar] [CrossRef]
- Syeda, F.; Liu, H.; Tullis, E.; Liu, M.; Slutsky, A.S.; Zhang, H. Differential Signaling Mechanisms of HNP-induced IL-8 Production in Human Lung Epithelial Cells and Monocytes. J. Cell Physiol. 2008, 214, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, T.W.L.; Ramwadhdoebé, T.H.; Trouw, L.A.; van den Ham, D.L.; van der Borden, V.; Drijfhout, J.W.; Hiemstra, P.S.; Daha, M.R.; Roos, A. Human Neutrophil Peptide-1 Inhibits Both the Classical and the Lectin Pathway of Complement Activation. Mol. Immunol. 2007, 44, 3608–3614. [Google Scholar] [CrossRef]
- Masera, R.G.; Bateman, A.; Muscettola, M.; Solomon, S.; Angeli, A. Corticostatins/Defensins Inhibit in Vitro NK Activity and Cytokine Production by Human Peripheral Blood Mononuclear Cells. Regul. Pept. 1996, 62, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Grutkoski, P.S.; Graeber, C.T.; Lim, Y.P.; Ayala, A.; Simms, H.H. α-Defensin 1 (Human Neutrophil Protein 1) as an Antichemotactic Agent for Human Polymorphonuclear Leukocytes. Antimicrob. Agents Chemother. 2003, 47, 2666–2668. [Google Scholar] [CrossRef]
- Shi, J.; Aono, S.; Lu, W.; Ouellette, A.J.; Hu, X.; Ji, Y.; Wang, L.; Lenz, S.; van Ginkel, F.W.; Liles, M.; et al. A Novel Role for Defensins in Intestinal Homeostasis: Regulation of IL-1β Secretion. J. Immunol. 2007, 179, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.; Clarke, D.J.; Lu, W.; Sibinska, Z.; Beaumont, P.E.; Davidson, D.J.; Barr, T.A.; Campopiano, D.J.; Gray, M. Dying and Necrotic Neutrophils Are Anti-Inflammatory Secondary to the Release of α-Defensins. J. Immunol. 2009, 183, 2122–2132. [Google Scholar] [CrossRef]
- Lichtenstein, A.; Ganz, T.; Selsted, M.E.; Lehrer, R.I. In Vitro Tumor Cell Cytolysis Mediated by Peptide Defensins of Human and Rabbit Granulocytes. Blood 1986, 68, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- LI, Y.; MA, C.; SHI, X.; WEN, Z.; LI, D.; SUN, M.; DING, H. Effect of Nitric Oxide Synthase on Multiple Drug Resistance Is Related to Wnt Signaling in Non-Small Cell Lung Cancer. Oncol. Rep. 2014, 32, 1703–1708. [Google Scholar] [CrossRef]
- Gaspar, D.; Freire, J.M.; Pacheco, T.R.; Barata, J.T.; Castanho, M.A.R.B. Apoptotic Human Neutrophil Peptide-1 Anti-Tumor Activity Revealed by Cellular Biomechanics. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2015, 1853, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, F.; Chen, K.; Yang, X.; Wang, Y. Preclinical Safety Evaluation of a Recombinant Plasmid Vector Encoding Mature Human Neutrophil Peptide-1 by Repeated Local Administrations in Nonhuman Primates. Hum. Gene Ther. 2021, 32, 1382–1389. [Google Scholar] [CrossRef]
- Ganz, T. Extracellular Release of Antimicrobial Defensins by Human Polymorphonuclear Leukocytes. Infect. Immun. 1987, 55, 568–571. [Google Scholar] [CrossRef]
- McKay, M.S.; Olson, E.; Hesla, M.A.; Panyutich, A.; Ganz, T.; Perkins, S.; Rossomando, E.F. Immunomagnetic Recovery of Human Neutrophil Defensins from the Human Gingival Crevice. Oral. Microbiol. Immunol. 1999, 14, 190–193. [Google Scholar] [CrossRef]
- Clark, R.A.; Page, R.C.; Wilde, G. Defective Neutrophil Chemotaxis in Juvenile Periodontitis. Infect. Immun. 1977, 18, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Panyutich, A.V.; Szold, O.; Poon, P.H.; Tseng, Y.; Ganz, T. Identification of defensin binding to C1 complement. FEBS Lett. 1994, 356, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, A.; Balducci, E.; Pistolesi, S.; Pogni, R. The Defensin–Lipid Interaction: Insights on the Binding States of the Human Antimicrobial Peptide HNP-1 to Model Bacterial Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2013, 1828, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Monkey Puzzles. Science 2002, 296, 2325–2326. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.; Ye, Y.; de Armas, L.R.; Soto, M.; Yarosh, W.; Marcsisin, R.A.; Tran, D.; Selsted, M.E.; Camerini, D. Cyclic and Acyclic Defensins Inhibit Human Immunodeficiency Virus Type-1 Replication by Different Mechanisms. PLoS ONE 2010, 5, e9737. [Google Scholar] [CrossRef]
- Demirkhanyan, L.H.; Marin, M.; Padilla-Parra, S.; Zhan, C.; Miyauchi, K.; Jean-Baptiste, M.; Novitskiy, G.; Lu, W.; Melikyan, G.B. Multifaceted Mechanisms of HIV-1 Entry Inhibition by Human α-Defensin. J. Biol. Chem. 2012, 287, 28821–28838. [Google Scholar] [CrossRef] [PubMed]
- Levinson, P.; Choi, R.Y.; Cole, A.L.; Hirbod, T.; Rhedin, S.; Payne, B.; Guthrie, B.L.; Bosire, R.; Cole, A.M.; Farquhar, C.; et al. HIV-Neutralizing Activity of Cationic Polypeptides in Cervicovaginal Secretions of Women in HIV-Serodiscordant Relationships. PLoS ONE 2012, 7, e31996. [Google Scholar] [CrossRef]
- Demirkhanyan, L.; Marin, M.; Lu, W.; Melikyan, G.B. Sub-Inhibitory Concentrations of Human α-Defensin Potentiate Neutralizing Antibodies against HIV-1 Gp41 Pre-Hairpin Intermediates in the Presence of Serum. PLoS Pathog. 2013, 9, e1003431. [Google Scholar] [CrossRef]
- Levinson, P.; Kaul, R.; Kimani, J.; Ngugi, E.; Moses, S.; MacDonald, K.S.; Broliden, K.; Hirbod, T. Levels of Innate Immune Factors in Genital Fluids: Association of Alpha Defensins and LL-37 with Genital Infections and Increased HIV Acquisition. AIDS 2009, 23, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Valere, K.; Rapista, A.; Eugenin, E.; Lu, W.; Chang, T.L. Human Alpha-Defensin HNP1 Increases HIV Traversal of the Epithelial Barrier: A Potential Role in STI-Mediated Enhancement of HIV Transmission. Viral Immunol. 2015, 28, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Daher, K.; Ganz, T.; Selsted, M.E. Direct Inactivation of Viruses by MCP-1 and MCP-2, Natural Peptide Antibiotics from Rabbit Leukocytes. J. Virol. 1985, 54, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.; García-Sastre, A.; Ruchala, P.; Lehrer, R.I.; Chang, T.; Klotman, M.E. A-Defensin Inhibits Influenza Virus Replication by Cell-Mediated Mechanism(s). J. Infect. Dis. 2007, 196, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, B.; Gastaldelli, M.; Kälin, S.; Imelli, N.; Boucke, K.; Wandeler, E.; Mercer, J.; Hemmi, S.; Greber, U.F. Subversion of CtBP1-Controlled Macropinocytosis by Human Adenovirus Serotype 3. EMBO J. 2008, 27, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Kälin, S.; Amstutz, B.; Gastaldelli, M.; Wolfrum, N.; Boucke, K.; Havenga, M.; DiGennaro, F.; Liska, N.; Hemmi, S.; Greber, U.F. Macropinocytotic Uptake and Infection of Human Epithelial Cells with Species B2 Adenovirus Type 35. J. Virol. 2010, 84, 5336–5350. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; White, M.R.; Tecle, T.; Gantz, D.; Crouch, E.C.; Jung, G.; Ruchala, P.; Waring, A.J.; Lehrer, R.I.; Hartshorn, K.L. Interactions of α-, β-, and θ-Defensins with Influenza A Virus and Surfactant Protein D. J. Immunol. 2009, 182, 7878–7887. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, E.; Koneru, P.C.; Kvaratskhelia, M.; Strömstedt, A.A.; Lu, W.; Kudryashov, D.S. Thermodynamic Instability of Viral Proteins Is a Pathogen-Associated Molecular Pattern Targeted by Human Defensins. Sci. Rep. 2016, 6, 32499. [Google Scholar] [CrossRef]
- Falco, A.; Mas, V.; Tafalla, C.; Perez, L.; Coll, J.M.; Estepa, A. Dual Antiviral Activity of Human Alpha-Defensin-1 against Viral Haemorrhagic Septicaemia Rhabdovirus (VHSV): Inactivation of Virus Particles and Induction of a Type I Interferon-Related Response. Antiviral Res. 2007, 76, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, A.; Marin, M.; Honnen, W.; Ramasamy, S.; Porter, E.; Subbian, S.; Pinter, A.; Melikyan, G.B.; Lu, W.; et al. Human Defensins Inhibit SARS-CoV-2 Infection by Blocking Viral Entry. Viruses 2021, 13, 1246. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, E.; Zani, A.; Vilmen, G.; Sharma, A.; Lu, W.; Yount, J.S.; Kudryashov, D.S. Inhibition of SARS-CoV-2 Infection by Human Defensin HNP1 and Retrocyclin RC-101. J. Mol. Biol. 2022, 434, 167225. [Google Scholar] [CrossRef]
- Couto, M.A.; Liu, L.; Lehrer, R.I.; Ganz, T. Inhibition of Intracellular Histoplasma Capsulatum Replication by Murine Macrophages That Produce Human Defensin. Infect. Immun. 1994, 62, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Barton, A.; Daher, K.A.; Harwig, S.S.; Ganz, T.; Selsted, M.E. Interaction of Human Defensins with Escherichia Coli. Mechanism of Bactericidal Activity. J. Clin. Investig. 1989, 84, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, Y.; Zhang, M.; Wu, S.; Wei, W.; Xiao, W.; Wang, Y.; Zhao, J.; Liu, N.; Jin, Y.; et al. Recombinant HNP-1 Produced by Escherichia Coli Triggers Bacterial Apoptosis and Exhibits Antibacterial Activity against Drug-Resistant Bacteria. Microbiol. Spectr. 2022, 10, e00860-21. [Google Scholar] [CrossRef]
- Kokryakov, V.N.; Harwig, S.S.L.; Panyutich, E.A.; Shevchenko, A.A.; Aleshina, G.M.; Shamova, O.V.; Korneva, H.A.; Lehrer, R.I. Protegrins: Leukocyte Antimicrobial Peptides That Combine Features of Corticostatic Defensins and Tachyplesins. FEBS Lett. 1993, 327, 231–236. [Google Scholar] [CrossRef]
- Kagan, B.L.; Selsted, M.E.; Ganz, T.; Lehrer, R.I. Antimicrobial Defensin Peptides Form Voltage-Dependent Ion-Permeable Channels in Planar Lipid Bilayer Membranes. Proc. Natl. Acad. Sci. USA 1990, 87, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, W.; Hong, M. The Membrane-Bound Structure and Topology of a Human α-Defensin Indicate a Dimer Pore Mechanism for Membrane Disruption. Biochemistry 2010, 49, 9770–9782. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Pulkkinen, W.S.; Selsted, M.E.; Mekalanos, J.J. Characterization of Defensin Resistance Phenotypes Associated with Mutations in the PhoP Virulence Regulon of Salmonella Typhimurium. Infect. Immun. 1990, 58, 3706–3710. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Yan, Z.; Tu, F.; He, T.; Gopinath, S.C.B.; Rui, X.; Cao, F. Human Neutrophil Peptide 1 Promotes Immune Sterilization in Vivo by Reducing the Virulence of Multidrug-resistant Klebsiella pneumoniae and Increasing the Ability of Macrophages. Biotechnol. Appl. Biochem. 2022, 69, 2091–2101. [Google Scholar] [CrossRef]
- Vega, L.A.; Caparon, M.G. Cationic Antimicrobial Peptides Disrupt the Streptococcus pyogenes ExPortal. Mol. Microbiol. 2012, 85, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Castagnini, M.; Picchianti, M.; Talluri, E.; Biagini, M.; Del Vecchio, M.; Di Procolo, P.; Norais, N.; Nardi-Dei, V.; Balducci, E. Arginine-Specific Mono ADP-Ribosylation In Vitro of Antimicrobial Peptides by ADP-Ribosylating Toxins. PLoS ONE 2012, 7, e41417. [Google Scholar] [CrossRef] [PubMed]
- Oguri, T.; Yeo, W.-S.; Bae, T.; Lee, H. Identification of EnvC and Its Cognate Amidases as Novel Determinants of Intrinsic Resistance to Cationic Antimicrobial Peptides. Antimicrob. Agents Chemother. 2016, 60, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, E.; Quintyn, R.; Seveau, S.; Lu, W.; Wysocki, V.H.; Kudryashov, D.S. Human Defensins Facilitate Local Unfolding of Thermodynamically Unstable Regions of Bacterial Protein Toxins. Immunity 2014, 41, 709–721. [Google Scholar] [CrossRef]
- Keskin, M.; Könönen, E.; Söderling, E.; Isik, G.; Firatli, E.; Uitto, V.-J.; Gürsoy, U.K. Increased Proliferation and Decreased Membrane Permeability as Defense Mechanisms of Fusobacterium Nucleatum against Human Neutrophilic Peptide-1. Anaerobe 2014, 30, 35–40. [Google Scholar] [CrossRef]
- Musrati, A.A.; Fteita, D.; Paranko, J.; Könönen, E.; Gürsoy, U.K. Morphological and Functional Adaptations of Fusobacterium Nucleatum Exposed to Human Neutrophil Peptide-1. Anaerobe 2016, 39, 31–38. [Google Scholar] [CrossRef]
- Zou, G.; de Leeuw, E. Neutralization of Pseudomonas Auruginosa Exotoxin A by Human Neutrophil Peptide 1. Biochem. Biophys. Res. Commun. 2018, 501, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Fang, K.; Xiao, J.; Zhang, W.; Zhang, B.; Yuan, W.; Lu, W.; Xu, D. Critical Determinants of Human Neutrophil Peptide 1 for Enhancing Host Epithelial Adhesion of Shigella flexneri. Cell Microbiol. 2019, 21, e13069. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; Wiedemann, I.; van Kraaij, C.; Kuipers, O.P.; Sahl, H.-G.; de Kruijff, B. Use of the Cell Wall Precursor Lipid II by a Pore-Forming Peptide Antibiotic. Science 1999, 286, 2361–2364. [Google Scholar] [CrossRef]
- Sass, V.; Schneider, T.; Wilmes, M.; Körner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.-G. Human β-Defensin 3 Inhibits Cell Wall Biosynthesis in Staphylococci. Infect. Immun. 2010, 78, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus Resistance to Human Defensins and Evasion of Neutrophil Killing via the Novel Virulence Factor Mprf Is Based on Modification of Membrane Lipids with L-Lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef]
- Ernst, C.M.; Staubitz, P.; Mishra, N.N.; Yang, S.-J.; Hornig, G.; Kalbacher, H.; Bayer, A.S.; Kraus, D.; Peschel, A. The Bacterial Defensin Resistance Protein MprF Consists of Separable Domains for Lipid Lysinylation and Antimicrobial Peptide Repulsion. PLoS Pathog. 2009, 5, e1000660. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.V.; Kristian, S.A.; Weidenmaier, C.; Faigle, M.; van Kessel, K.P.M.; van Strijp, J.A.G.; Götz, F.; Neumeister, B.; Peschel, A. Staphylococcus aureus Strains Lacking D-Alanine Modifications of Teichoic Acids Are Highly Susceptible to Human Neutrophil Killing and Are Virulence Attenuated in Mice. J. Infect. Dis. 2002, 186, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Bayer, A.S.; Mishra, N.N.; Meehl, M.; Ledala, N.; Yeaman, M.R.; Xiong, Y.Q.; Cheung, A.L. The Staphylococcus Aureus Two-Component Regulatory System, GraRS, Senses and Confers Resistance to Selected Cationic Antimicrobial Peptides. Infect. Immun. 2012, 80, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Bayer, A.S.; Yeaman, M.R.; Xiong, Y.Q.; Waring, A.J.; Memmi, G.; Donegan, N.; Chaili, S.; Yang, S.-J. Site-Specific Mutation of the Sensor Kinase GraS in Staphylococcus Aureus Alters the Adaptive Response to Distinct Cationic Antimicrobial Peptides. Infect. Immun. 2014, 82, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Bokarewa, M.; Foster, T.; Mitchell, J.; Higgins, J.; Tarkowski, A. Staphylococcus aureus Resists Human Defensins by Production of Staphylokinase, a Novel Bacterial Evasion Mechanism. J. Immunol. 2004, 172, 1169–1176. [Google Scholar] [CrossRef]
- Cardot-Martin, E.; Casalegno, J.S.; Badiou, C.; Dauwalder, O.; Keller, D.; Prévost, G.; Rieg, S.; Kern, W.V.; Cuerq, C.; Etienne, J.; et al. α-Defensins Partially Protect Human Neutrophils against Panton-Valentine Leukocidin Produced by Staphylococcus aureus. Lett. Appl. Microbiol. 2015, 61, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Abdossamadi, Z.; Seyed, N.; Zahedifard, F.; Taheri, T.; Taslimi, Y.; Montakhab-Yeganeh, H.; Badirzadeh, A.; Vasei, M.; Gharibzadeh, S.; Rafati, S. Human Neutrophil Peptide 1 as Immunotherapeutic Agent against Leishmania Infected BALB/c Mice. PLoS Negl. Trop. Dis. 2017, 11, e0006123. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, O.; Pathirana, R.U.; Kay, J.G.; Edgerton, M. Candida Albicans Ras1 Inactivation Increases Resistance to Phagosomal Killing by Human Neutrophils. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Piers, K.L.; Brown, M.H.; Hancock, R.E.W. Recombinant DNA Procedures for Producing Small Antimicrobial Cationic Peptides in Bacteria. Gene 1993, 134, 7–13. [Google Scholar] [CrossRef]
- Li, D.; Guo, R.; Chen, F.; Wang, J.; Wang, F.; Wan, Y. Genetically Engineered Goats as Efficient Mammary Gland Bioreactors for Production of Recombinant Human Neutrophil Peptide 1 Using CRISPR/Cas9. Biology 2024, 13, 367. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, A.; Qi, B.; Yu, H.; Xiong, Y.; Zhou, G.; Qin, M.; Dou, J.; Wang, J. Secretion Expression of Human Neutrophil Peptide 1 (HNP1) in Pichia Pastoris and Its Functional Analysis against Antibiotic-Resistant Helicobacter Pylori. Appl. Microbiol. Biotechnol. 2018, 102, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Prahl, A.; Powell, R.; Ericksen, B.; Lubkowski, J.; Lu, W. From pro Defensins to Defensins: Synthesis and Characterization of Human Neutrophil pro A-defensin-1 and Its Mature Domain. J. Pept. Res. 2003, 62, 53–62. [Google Scholar] [CrossRef]
- Baumgarten, T.; Ytterberg, A.J.; Zubarev, R.A.; de Gier, J.-W. Optimizing Recombinant Protein Production in the Escherichia Coli Periplasm Alleviates Stress. Appl. Environ. Microbiol. 2018, 84, e00270-18. [Google Scholar] [CrossRef] [PubMed]
- Frain, K.M.; Robinson, C.; van Dijl, J.M. Transport of Folded Proteins by the Tat System. Protein J. 2019, 38, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Low, K.O.; Muhammad Mahadi, N.; Md. Illias, R. Optimisation of Signal Peptide for Recombinant Protein Secretion in Bacterial Hosts. Appl. Microbiol. Biotechnol. 2013, 97, 3811–3826. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotaki, A.; De Geyter, J.; Šoštaric’, N.; Economou, A.; Karamanou, S. Protein Export through the Bacterial Sec Pathway. Nat. Rev. Microbiol. 2017, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Erental, A.; Sharon, I.; Engelberg-Kulka, H. Two Programmed Cell Death Systems in Escherichia Coli: An Apoptotic-Like Death Is Inhibited by the MazEF-Mediated Death Pathway. PLoS Biol. 2012, 10, e1001281. [Google Scholar] [CrossRef]
- Pahil, K.S.; Gilman, M.S.A.; Baidin, V.; Clairfeuille, T.; Mattei, P.; Bieniossek, C.; Dey, F.; Muri, D.; Baettig, R.; Lobritz, M.; et al. A New Antibiotic Traps Lipopolysaccharide in Its Intermembrane Transporter. Nature 2024, 625, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Zampaloni, C.; Mattei, P.; Bleicher, K.; Winther, L.; Thäte, C.; Bucher, C.; Adam, J.-M.; Alanine, A.; Amrein, K.E.; Baidin, V.; et al. A Novel Antibiotic Class Targeting the Lipopolysaccharide Transporter. Nature 2024, 625, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Tongaonkar, P.; Golji, A.E.; Tran, P.; Ouellette, A.J.; Selsted, M.E. High Fidelity Processing and Activation of the Human α-Defensin HNP1 Precursor by Neutrophil Elastase and Proteinase 3. PLoS ONE 2012, 7, e32469. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.; Courbet, A.; de Haas, R.J.; Bethel, N.; et al. Robust deep learning–based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F.; et al. De novo design of protein structure and function with RFdiffusion. Nature 2023, 620, 1089–1100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Z.; Zhou, Z.; Huang, Z.; Yang, Y.; Wu, J.; Liu, Y. HNP-1: From Structure to Application Thanks to Multifaceted Functions. Microorganisms 2025, 13, 458. https://doi.org/10.3390/microorganisms13020458
Zhang J, Liu Z, Zhou Z, Huang Z, Yang Y, Wu J, Liu Y. HNP-1: From Structure to Application Thanks to Multifaceted Functions. Microorganisms. 2025; 13(2):458. https://doi.org/10.3390/microorganisms13020458
Chicago/Turabian StyleZhang, Jiaqi, Zhaoke Liu, Zhihao Zhou, Zile Huang, Yifan Yang, Junzhu Wu, and Yanhong Liu. 2025. "HNP-1: From Structure to Application Thanks to Multifaceted Functions" Microorganisms 13, no. 2: 458. https://doi.org/10.3390/microorganisms13020458
APA StyleZhang, J., Liu, Z., Zhou, Z., Huang, Z., Yang, Y., Wu, J., & Liu, Y. (2025). HNP-1: From Structure to Application Thanks to Multifaceted Functions. Microorganisms, 13(2), 458. https://doi.org/10.3390/microorganisms13020458




