Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies
Abstract
1. Introduction
2. Characteristics of M. abscessus subsp. massiliense
3. Virulence Factors
4. Biofilm Formation
5. Immune Response
6. Therapeutic Strategies
6.1. Conventional Approaches
6.2. Emerging Therapeutic Strategies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cao, D.; Yuan, X.; Jiang, X.; Wu, T.; Xiang, Y.; Ji, Z.; Liu, J.; Dong, X.; Bi, K.; Tønjum, T.; et al. Antimicrobial and Antibiofilm Effects of Bithionol against Mycobacterium abscessus. Antibiotics 2024, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Lanni, A.; Borroni, E.; Iacobino, A.; Russo, C.; Gentile, L.; Fattorini, L.; Giannoni, F. Activity of Drug Combinations against Mycobacterium abscessus Grown in Aerobic and Hypoxic Conditions. Microorganisms 2022, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Jensen, P.Ø.; Qvist, T.; Kragh, K.N.; Ravnholt, C.; Fritz, B.G.; Johansen, U.R.; Bjarnsholt, T.; Hoiby, N. Biofilms of Mycobacterium abscessus Complex Can Be Sensitized to Antibiotics by Disaggregation and Oxygenation. Antimicrob. Agents Chemother. 2019, 64, e01212-19. [Google Scholar] [CrossRef]
- De, K.; Belardinelli, J.M.; Pandurangan, A.P.; Ehianeta, T.; Lian, E.; Palčeková, Z.; Lam, H.; Gonzalez-Juarrero, M.; Bryant, J.M.; Blundell, T.L.; et al. Lipoarabinomannan modification as a source of phenotypic heterogeneity in host-adapted Mycobacterium abscessus isolates. Proc. Natl. Acad. Sci. USA 2024, 121, e2403206121. [Google Scholar] [CrossRef]
- Mauch, R.M.; Jensen, P.Ø.; Qvist, T.; Kolpen, M.; Moser, C.; Pressler, T.; da Silva, M.T.N.; Høiby, N. Adaptive Immune Response to Mycobacterium abscessus Complex (MABSC) in Cystic Fibrosis and the Implications of Cross-Reactivity. Front. Cell. Infect. Microbiol. 2022, 12, 858398. [Google Scholar] [CrossRef]
- Vang, C.K.; Dawrs, S.N.; Oberlag, N.M.; Gilmore, A.E.; Hasan, N.A.; Honda, J.R. Comparative survival of environmental and clinical Mycobacterium abscessus isolates in a variety of diverse host cells. J. Appl. Microbiol. 2022, 132, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- Hunt-Serracin, A.C.; Parks, B.J.; Boll, J.; Boutte, C.C. Mycobacterium abscessus Cells Have Altered Antibiotic Tolerance and Surface Glycolipids in Artificial Cystic Fibrosis Sputum Medium. Antimicrob. Agents Chemother. 2019, 63, e02488-18. [Google Scholar] [CrossRef] [PubMed]
- López-Medrano, R.; Retuerto-Guerrero, M.; Blanco-Conde, S.; Morán-Fernández, M.B.; Rivero-Lezcano, O.M. Formation of Mycobacterium abscessus colonies in cellular culture in an in vitro infection model. MethodsX 2024, 12, 102667. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.Y.; Sun, M.F.; Li, T.C.; Lin, C.T. Effect of Coptis chinensis on Biofilm Formation and Antibiotic Susceptibility in Mycobacterium abscessus. Evid.-Based Complement. Altern. Med. 2020, 2020, 9754357. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Krishnamurthy, R.V.; Shandil, R.K.; Mohan, R.; Narayanan, S. A Novel Inhibitor against the Biofilms of Non-Tuberculous Mycobacteria. Pathogens 2024, 13, 40. [Google Scholar] [CrossRef]
- Kurbatfinski, N.; Hill, P.J.; Tobin, N.; Kramer, C.N.; Wickham, J.; Goodman, S.D.; Hall-Stoodley, L.; Bakaletz, L.O. Disruption of nontuberculous mycobacteria biofilms induces a highly vulnerable to antibiotic killing phenotype. Biofilm 2023, 6, 100166. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.L.; Kremer, L. Glycopeptidolipids, a double-edged sword of the Mycobacterium abscessus complex. Front. Microbiol. 2018, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Clary, G.; Sasindran, S.J.; Nesbitt, N.; Mason, L.; Cole, S.; Azad, A.; McCoy, K.; Schlesinger, L.S.; Hall-Stoodley, L. Mycobacterium abscessus Smooth and Rough Morphotypes Form Antimicrobial-Tolerant Biofilm Phenotypes but Are Killed by Acetic Acid [Internet]. Antimicrob. Agents Chemother. 2018, 62, e01782-17. [Google Scholar] [CrossRef]
- Gloag, E.S.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Mycobacterium abscessus biofilms have viscoelastic properties which may contribute to their recalcitrance in chronic pulmonary. Sci. Rep. 2021, 11, 5020. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, K.; Munshi, T.; López-Pérez, P.M.; Sequeira-Garcia, J.; Hofmann, S.; Bull, T.J. Discovery of Antimicrobial Peptides That Can Accelerate Culture Diagnostics of Slow-Growing Mycobacteria Including Mycobacterium tuberculosis. Microorganisms 2023, 11, 2225. [Google Scholar] [CrossRef]
- Idosa, A.W.; Wozniak, D.J.; Hall-Stoodley, L. Surface Dependent Inhibition of Mycobacterium abscessus by Diverse Pseudomonas aeruginosa Strains. Microbiol. Spectr. 2022, 10, e0247122. [Google Scholar] [CrossRef]
- Bonez, P.C.; Agertt, V.A.; Rossi, G.G.; Siqueira, F.d.S.; Siqueira, J.D.; Marques, L.L.; de Oliveira, G.N.M.; Santos, R.C.V.; de Campos, M.M.A. Sulfonamides complexed with metals as mycobacterial biofilms inhibitors. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100217. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.L.; Viljoen, A.; Ban, A.; Simeone, R.; Bernut, A.; Laencina, L.; Deramaudt, T.; Rottman, M.; Gaillard, J.L.; Majlessi, L.; et al. Mycobacterium abscessus smooth and rough variants differentially modulate the TLR2-dependent TNF production in macrophages. PLoS ONE 2016, 11, e0166265. [Google Scholar] [CrossRef]
- Guerrero-Bustamante, C.A.; Gengenbacher, M. Host-directed therapies for Mycobacterium abscessus infections: Targeting the Achilles’ heel. Front. Cell. Infect. Microbiol. 2022, 12, 867315. [Google Scholar] [CrossRef]
- Halloum, I.; Carrère-Kremer, S.; Blaise, M.; Viljoen, A.; Bernut, A.; Moigne, V.; Vilchéze, C.; Guérardel, Y.; Luftalla., G.; Hermann, J.L.; et al. Deletion of a single gene encoding a putative arabinosyltransferase abolishes cord formation and virulence of Mycobacterium abscessus. Proc. Natl. Acad. Sci. USA 2016, 113, 8807–8812. [Google Scholar]
- Owens, B.R.; Filloux, A. New anti-biofilm approaches for Mycobacterium abscessus lung disease. Curr. Opin. Pharmacol. 2019, 47, 101–106. [Google Scholar]
- Esteban, J.; García-Coca, M. Mycobacterium biofilms. Front. Microbiol. 2018, 8, 2651. [Google Scholar] [CrossRef] [PubMed]
- Degiacomi, G.; Sammartino, J.C.; Chiarelli, L.R.; Riabova, O.; Makarov, V.; Pasca, M.R. Mycobacterium abscessus, an emerging and worrisome pathogen among cystic fibrosis patients. Int. J. Mol. Sci. 2019, 20, 5868. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Lemmer, Y.; Beyers, N. Cystic fibrosis: A battleground for Mycobacterium abscessus persistence. Front. Microbiol. 2017, 8, 1906. [Google Scholar]
- Kidd, T.J.; Floto, R.A. Mycobacterium abscessus lung disease. Curr. Opin. Infect. Dis. 2018, 31, 157–163. [Google Scholar]
- Bryant, J.M.; Grogono, D.M.; Rodriguez-Rincon, D.; Karen, I.E.; Everall, I.; Moreno, P.; Verma, D.; Hill, E.; Drijkoningen, J.; Gilligan, P.; et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016, 354, 751–757. [Google Scholar] [CrossRef]
- Faboro, T.; Daniel, J. Biofilm formation and polar lipid biosynthesis in Mycobacterium abscessus are inhibited by naphthylmethylpiperazine. PLoS ONE 2024, 19, e0311669. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.H.; Rose, S.J.; Bai, X.; Lee, R.E.; Barry, C.E. Tween 80 potentiates the in vitro activity of antibiotics against Mycobacterium abscessus. J. Antimicrob. Chemother. 2016, 71, 3264–3267. [Google Scholar]
- Ferrell, K.C.; Johansen, M.D.; Triccas, J.A.; Counoupas, C. Virulence Mechanisms of Mycobacterium abscessus: Current Knowledge and Implications for Vaccine Design. Front. Microbiol. 2022, 13, 842017. [Google Scholar] [CrossRef]
- Aung, T.T.; Yam, J.K.; Lin, S.; Salleh, S.M.; Givskov, M.; Liu, S.; Lwin, N.C.; Yang, L.; Beuerman, R.W. Biofilms of Pathogenic Nontuberculous Mycobacteria Targeted by New Therapeutic Approaches. Antimicrob. Agents Chemother. 2015, 60, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Martins de Sousa, E.; Bonfim de Bortoli, F.; Amaral, E.P.; Batista, U.M.C.; Liberman Kipnis, E.; Marques Cardoso, U.M.; Quipnis, U.M.; Junqueira-Kipnis, A.P. Acute immune response to Mycobacterium massiliense in C57BL/6 and BALB/c mice. Infect. Immun. 2010, 78, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Lima Viana, J.; Zagmignan, A.; Lima Lobato, L.F.; Gomes Abreu, A., Jr.; da Silva, L.C.N.; de Sá, J.C.; Monteiro, C.A.; Lago, J.H.G.; Gonçalves, L.M.; Carvalho, R.C.; et al. Hydroalcoholic Extract and Ethyl Acetate Fraction of Bixa orellana Leaves Decrease the Inflammatory Response to Mycobacterium abscessus subsp. massiliense. Evid.-Based Complement. Altern. Med. ECAM 2018, 2018, 6091934. [Google Scholar] [CrossRef]
- Byrd, T.F.; Lyons, C.R. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 1999, 67, 4700–4707. [Google Scholar] [CrossRef] [PubMed]
- Bernut, A.; Herrmann, J.L.; Kissa, K.; Dubremetz, J.F.; Gaillard, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.D.; Elias, V.; Cipolla, D.; Gonda, I.; Bermudez, L.E. Effective Treatment of Mycobacterium avium subsp. hominissuis and Mycobacterium abscessus Species Infections in Macrophages, Biofilm, and Mice by Using Liposomal Ciprofloxacin [nternet]. Antimicrob. Agents Chemother. 2018, 62, e00440-18. [Google Scholar] [CrossRef] [PubMed]
- Senhaji-Kacha, A.; Esteban, J.; Garcia-Quintanilla, M. Considerations for Phage Therapy Against Mycobacterium abscessus. Front. Microbiol. 2021, 11, 609017. [Google Scholar] [CrossRef] [PubMed]
- Conyers, L.E.; Saunders, B.M. Treatment for non-tuberculous mycobacteria: Challenges and prospects. Front. Microbiol. 2024, 15, 1394220. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus infection. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Vandenheuvel, D.; Lavigne, R.; Brys, L. Bacteriophage therapy: Advances in formulation strategies and human clinical trials. Annu. Rev. Virol. 2015, 2, 599–626. [Google Scholar] [CrossRef]
- Foreman, M.; Kolodkin-Gal, I.; Barkan, D. A Pivotal Role for Mycobactin/mbtE in Growth and Adaptation of Mycobacterium abscessus. Microbiol. Spectr. 2022, 10, e0262322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Adhikrao, P.A. Targeting Mycobacterium tuberculosis iron-scavenging tools: A recent update on siderophores inhibitors. RSC Med. Chem. 2023, 14, 1885–1913. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Daley, C.L. Treatment of Mycobacterium abscessus PulmonaryMDisease. Chest 2022, 161, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Hugonnet, J.E.; Tremblay, L.W.; Boshoff, H.I.; Barry, C.E., 3rd; Blanchard, J.S. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 2009, 323, 1215–1218. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Tetz, V.V.; Tetz, G.V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob. Agents Chemother. 2010, 54, 1204–1209. [Google Scholar] [CrossRef]
- Dzalamidze, E.; Gorzynski, M.; Vande Voorde, R.; Nelson, D.; Danelishvili, L. Discovery of biofilm-inhibiting compounds to increase the effectiveness of antibiotics against M. abscessus infections. Pharmaceuticals 2025, 18, 225. [Google Scholar] [CrossRef]
- Weng, Y.-W.; Huang, C.-K.; Sy, C.-L.; Wu, K.-S.; Tsai, H.-C.; Lee, S.S.-J. Treatment for Mycobacterium abscessus complex–lung disease. J. Formos. Med. Assoc. 2020, 119, S58–S66. [Google Scholar] [CrossRef] [PubMed]
- Kleinboelting, S.; Ramos-Espiritu, L.; Buck, H.; Colis, L.; van den Heuvel, J.; Glickman, J.F.; Levin, L.R.; Buck, J.; Steegborn, C. Bithionol Potently Inhibits Human Soluble Adenylyl Cyclase through Binding to the Allosteric Activator Site. J. Biol. Chem. 2016, 291, 9776–9784. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Ojano-Dirain, C.; Yang, Q.; Liu, L.; Lu, L.; Progulske-Fox, A.; Wang, G.P.; Antonelli, P.; Schultz, G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016, 193, 692–693. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic Dispersion of Biofilms: An Emerging Biocatalytic Avenue to Combat Biofilm-Mediated Microbial Infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mishra, P. Bacitracin and Isothiocyanate Functionalized Silver Nanoparticles for Synergistic and Broad Spectrum Antibacterial and Antibiofilm Activity with Selective Toxicity to Bacteria over Mammalian Cells. Biomater. Adv. 2022, 133, 112649. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, A.L.; Dubée, V.; Guilloux, V.; Hugonnet, J.E.; Dubost, L.; Barbier, M.; Herrmann, J.L.; Kremer, L.; Arthur, M. Pharmacokinetic/pharmacodynamic studies of the beta-lactamase inhibitor avibactam combined with imipenem against Mycobacterium abscessus in vitro and in a mouse. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M. Role of bacterial biofilms in chronic infections. APMIS 2010, 118, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Blanchard, J.; Gonda, I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics 2016, 8, 6. [Google Scholar] [CrossRef]
- Boudehen, Y.; Tasrini, Y.; Aguilera-Correa, J.J.; Alcaraz, M.; Kremer, L. Silencing essential gene expression in Mycobacterium abscessus during infection. Microbiol. Spectr. 2023, 11, e02836-23. [Google Scholar] [CrossRef] [PubMed]
- Bernut, A.; Viljoen, A.; Dupont, C.; Sapriel, G.; Chevalier, A.; Malgrange, V.L.; de Chastellier, C.; Herrmann, J.L.; Kremer, L. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue in the MmpL4 protein conserved in all mycobacteria. Mol. Microbiol. 2014, 93, 1196–1210. [Google Scholar] [CrossRef]
- Chiron, R.; Hoefsloot, W.; Van Ingen, J.; Marchandin, H.; Kremer, L.; Morisse-Pradier, H.; Charriot, J.; Mallet, J.P.; Herrmann, J.L.; Caimmi, D.; et al. Amikacin Liposomal Inhalation Suspension in the Treatment of Mycobacterium abscessus Lung Infection: A French Observational Experience. Open Forum Infect. Dis. 2022, 9, ofac465. [Google Scholar] [CrossRef] [PubMed]
- Leon-Icaza, S.A.; Bagayoko, S.; Vergé, R.; Iakobachvili, N.; Ferrand, C.; Aydogan, T.; Bernard, C.; Dafun, A.S.; Murris-Espin, M.; Mazières, J.; et al. Druggable redox pathways against Mycobacterium abscessus in cystic fibrosis patient-derived airway organoids. PLoS Pathog. 2023, 19, e1011559. [Google Scholar] [CrossRef] [PubMed]
- Meliefste, H.M.; Mudde, S.E.; Ammerman, N.C.; de Steenwinkel, J.E.M.; Bax, H.I. A laboratory perspective on Mycobacterium abscessus biofilm culture, characterization and drug activity testing. Front. Microbiol. 2024, 15, 1392606. [Google Scholar] [CrossRef] [PubMed]
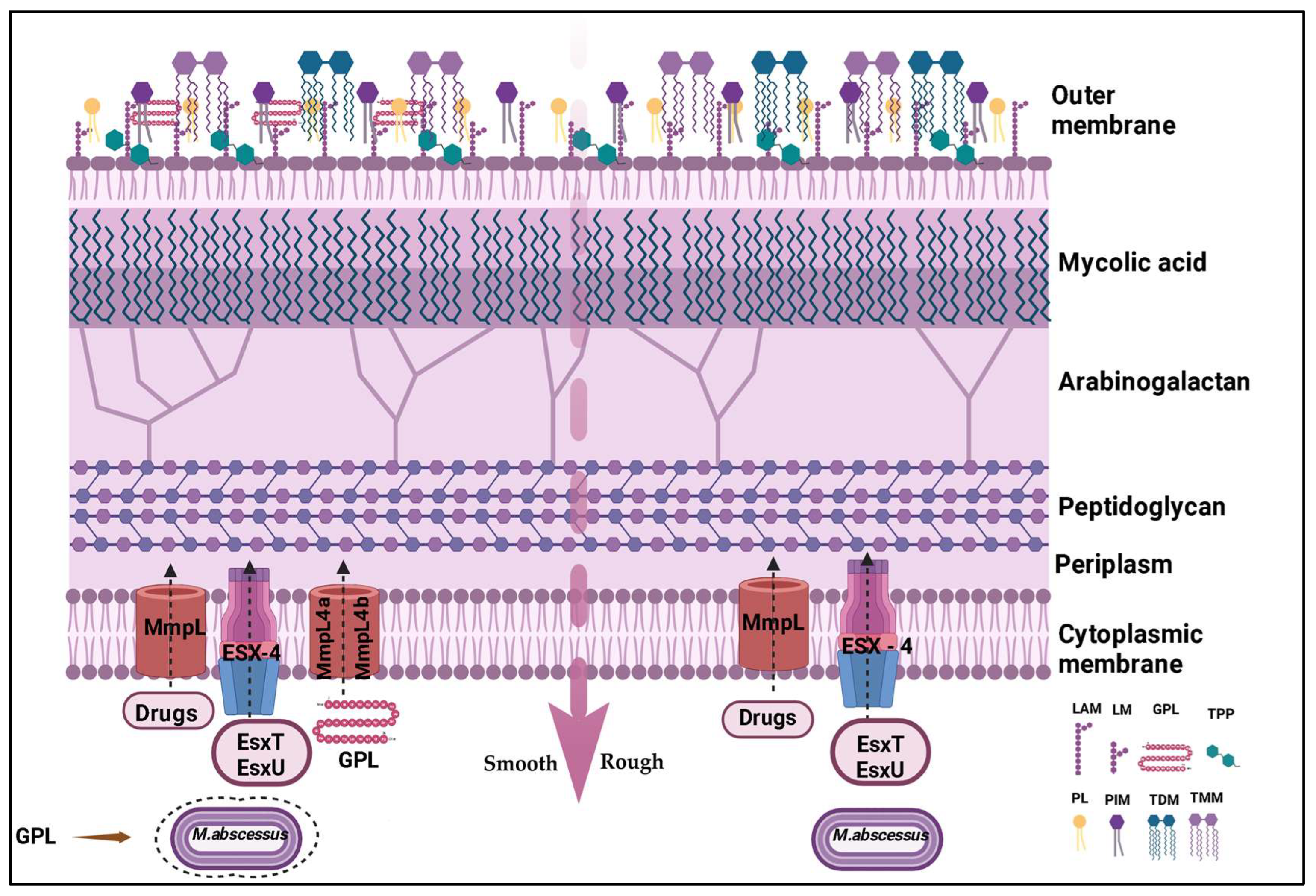

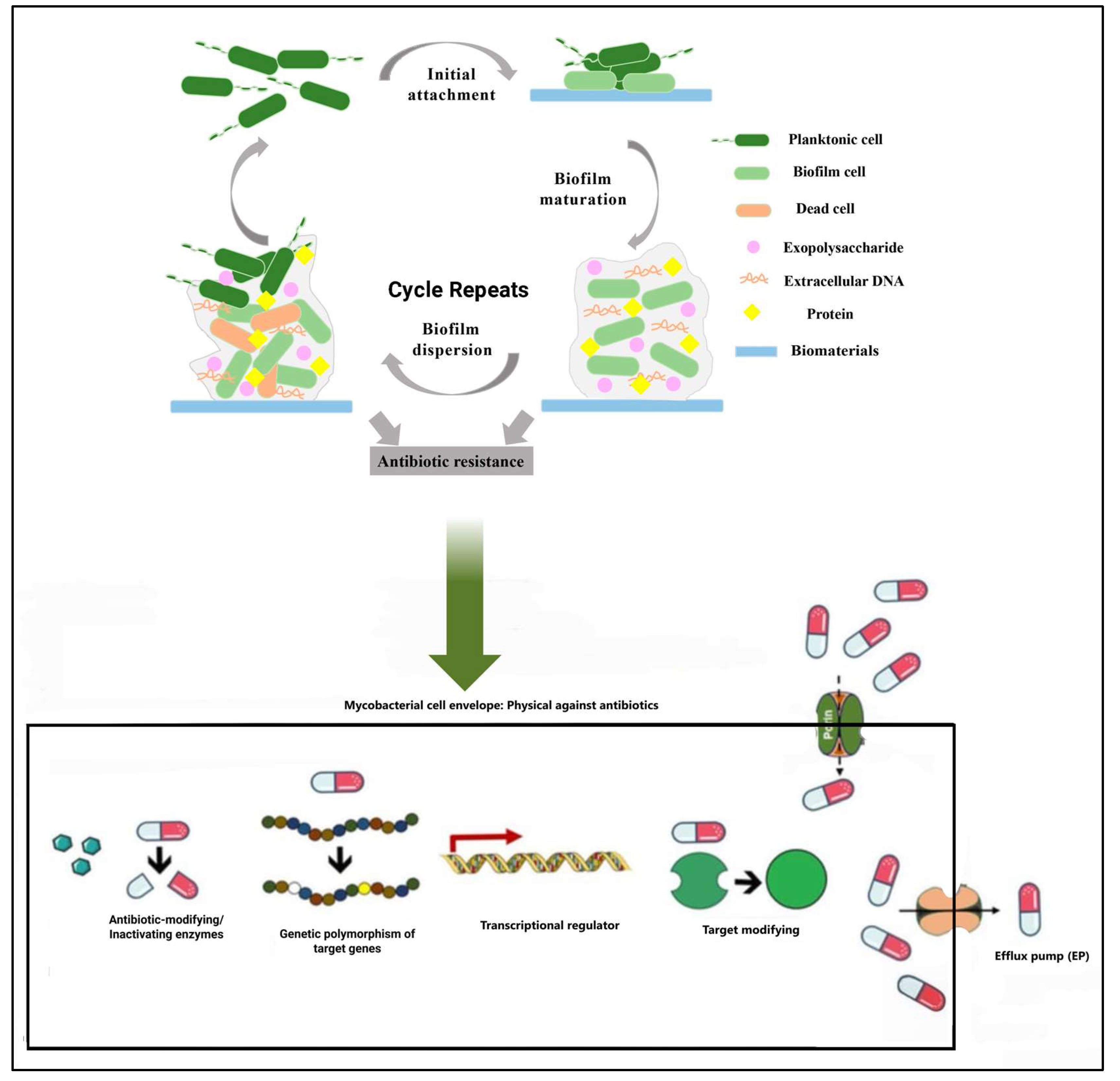
| Virulence Factor | Description | Role in Virulence | References |
|---|---|---|---|
| Morphotypes | Smooth [MaSm]: rich in GPLs, forms viscous biofilms, evades the immune system. Rough [MaRg]: fewer GPLs, more invasive, forms resistant aggregates. | Adaptation to the host environment, immune evasion, resistance to adverse conditions. | [18,19] |
| Glycopeptidolipids [GPLs] | Abundant cell wall components in the smooth morphotype, surface lipids that affect biofilm structure and modulate the immune response. | Adhesion, biofilm formation, immune response modulation [inhibits TLR2 and TNF-α], infection persistence. | [10,18] |
| Trehalose dimycolate [TDM] | Glycolipid present in both morphotypes. | Contributes to virulence, particularly in biofilms formed in the lungs of cystic fibrosis patients. | [19] |
| Lipoarabinomannan [LAM] | Lipoglycan present in the cell wall. | Modulates the immune response. | [19,20] |
| Surface proteins | Adhesins, porins, etc. | Host cell adhesion, nutrient uptake, antibiotic resistance. | [19,20,21] |
| Biofilms | Complex structures that protect bacteria from antibiotics and the host immune system, associated with infection persistence and recurrence. | Confer antibiotic resistance, shield bacteria from the immune system, facilitate bacterial communication and infection persistence. | [19,20,21] |
| Extracellular matrix [ECM] | Composed of polysaccharides, proteins, eDNA, and lipids. | Provides antibiotic resistance, protects bacteria from the immune system. | [20,21] |
| Quorum sensing | Bacterial communication system. | Influences biofilm formation and development. | [22] |
| Antibiotic Class | Medication | Route of Administration | Recommended Dose in Adults BTS, 2017 * (90) | Observations |
|---|---|---|---|---|
| Macrolides | Clarithromycin | Oral | 500 mg BID | Frequently used in infections caused by M. abscessus subsp. massiliense, which is generally sensitive to this antibiotic class [9,10]. |
| Aminoglycosides | Amikacin | Intravenous | 10–15 mg/kg QD | Commonly included in therapeutic regimens. Can also be administered via inhalation [Liposomal Amikacin for Inhalation] [3]. |
| Beta-lactams | Cefoxitin | Intravenous | 12 g daily in divided doses | Used in combination with other agents. Cefoxitin is one of the two beta-lactams commonly used to treat M. abscessus infections, along with imipenem [9,10]. |
| Other | Linezolid | Oral | 600 mg QD or BID | Used depending on the strain’s sensitivity profile and the patient’s tolerance [25]. |
| Clofazimine | Oral | 50–100 mg QD | Used depending on the strain’s sensitivity profile and the patient’s tolerance [2]. | |
| Quinolones | Oral | 1 mg/kg QD | Occasionally used, depending on the strain’s sensitivity profile and the patient’s tolerance. Ciprofloxacin is a commonly used quinolone [22]. |
| Therapeutic Approach | Description | Observations | References |
|---|---|---|---|
| New antibiotics | Bedaquiline: Inhibits mycobacterial ATP synthase. Tedizolid: Oxazolidinone antibiotic with activity against various non-tuberculous mycobacteria. Tiopetide (NF10011): produced by Streptomyces sp. | Bedaquiline has been approved for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. Tedizolid has demonstrated in vitro activity against M. abscessus. NF1001 exhibits activity against both planktonic forms (free cells) and biofilms of NTM, showing a reduction in bacterial load. | [8,10,12,14,17,34] |
| Antiparasitic agent | Bithionol: This antiparasitic agent has shown significant antimicrobial activity against M. abscessus, including the ability to eliminate biofilms. | Bithionol’s biofilm-eradicating efficacy is notable, achieving up to 99.9% elimination of biofilm bacteria at appropriate concentrations. | [48,50] |
| Phage therapy | Use of bacteriophages to infect and destroy M. abscessus bacteria. | Phages may be used in combination with antibiotics. Case studies have reported promising results in treating M. abscessus infections. | [2,37,40] |
| Bacteriophage | Modulation of the host immune response to enhance the ability to combat M. abscessus infection. | Research in this area is still preliminary. Potential strategies include the use of cytokines or other immunomodulatory molecules. | [5,10,40,41] |
| Beta-lactamase inhibitors | Avibactam: Inhibits the beta-lactamase Bla_Mab, which is responsible for beta-lactam resistance in M. abscessus. | Adding avibactam to therapeutic regimens containing beta-lactams may improve their efficacy. | [23,24,25,48] |
| Biofilm-targeted approaches | Strategies to inhibit biofilm formation, disperse existing biofilms, or increase the susceptibility of bacteria in biofilms to antibiotics. | Tween 80: A detergent that can disaggregate M. abscessus biofilms, making the bacteria more susceptible to antibiotics. Oxygenation: Increasing oxygen availability can enhance the activity of some antibiotics against M. abscessus. Iron chelator: The bacteria have mechanisms to survive in environments with low iron levels. | [6,20,29,41,42,43,44] |
| Enzymes | Enzymes that degrade the components of the extracellular matrix of the M. abscessus biofilm. | Enzymes with degradative potential include phospholipases, carbohydrases, proteases, and DNases. | [11,34,46,47,51,52] |
| Genetic editing and nanotechnology | Genetic editing and nanotechnology emerge as promising tools in the fight against M. abscessus infections. | Genetic editing allows for the manipulation of genes essential to the bacteria’s virulence and resistance, while nanotechnology provides solutions for drug delivery, biofilm destabilization, and infection monitoring. | [51,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira, R.L.d.S.; Nogueira, F.A.B.; Campos, R.d.F.P.d.C.; Ferreira, D.R.M.; Roxo, P.L.B.T.; de Azevedo, C.C.S.; Gimenes, E.C.M.; Bastos, R.L.C.; Nascimento, C.E.C.; Nunes, F.D.O.; et al. Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies. Microorganisms 2025, 13, 447. https://doi.org/10.3390/microorganisms13020447
Lira RLdS, Nogueira FAB, Campos RdFPdC, Ferreira DRM, Roxo PLBT, de Azevedo CCS, Gimenes ECM, Bastos RLC, Nascimento CEC, Nunes FDO, et al. Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies. Microorganisms. 2025; 13(2):447. https://doi.org/10.3390/microorganisms13020447
Chicago/Turabian StyleLira, Roseane Lustosa de Santana, Flávio Augusto Barros Nogueira, Rosália de Fátima Penna de Carvalho Campos, Dayenne Regina Mota Ferreira, Pedro Lucas Brito Tromps Roxo, Caio César Santana de Azevedo, Eleonôra Costa Monteiro Gimenes, Ruan Lucas Costa Bastos, Camila Evangelista Carnib Nascimento, Flávia Danyelle Oliveira Nunes, and et al. 2025. "Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies" Microorganisms 13, no. 2: 447. https://doi.org/10.3390/microorganisms13020447
APA StyleLira, R. L. d. S., Nogueira, F. A. B., Campos, R. d. F. P. d. C., Ferreira, D. R. M., Roxo, P. L. B. T., de Azevedo, C. C. S., Gimenes, E. C. M., Bastos, R. L. C., Nascimento, C. E. C., Nunes, F. D. O., Marques, M. C. P., Campos, C. D. L., Martinez, C. G., Zagmignan, A., Silva, L. C. N., Ribeiro, R. M., de Azevedo dos Santos, A. P. S., Carvalho, R. C., & de Sousa, E. M. (2025). Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies. Microorganisms, 13(2), 447. https://doi.org/10.3390/microorganisms13020447











