Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp.
Abstract
1. Introduction
2. Materials and Methods
3. Fusarium: Impact and Challenges in Agriculture
3.1. Morphology Characteristics
3.2. General Description of Fusarium spp. Infection Mechanism as a Plant Pathogen
3.3. Damage to Most Important Crops: Diseases Caused by Fusarium spp.
| Pathogen | Disease Caused | Host/Plant Part | Detection Technique | Reference |
|---|---|---|---|---|
| Fusarium spp. | Wheat crown rot | Wheat/Wheatears | PCR-based methods | [61] |
| F. verticillioides | Fumonisins | Corn | Immunoassay kits, PCR-ELISA | [62] |
| F. oxysporum Fusarium cubense | Panama disease (Fusarium wilt) | Banana/Roots | Raman spectroscopic fingerprints | [63] |
| F. solani | Coffee corky root | Coffee seedlings | Isolation and identification of fungal structures, species-specific PCR | [64] |
| F. oxysporum | Fusarium wilt | Melon | Loop-mediated isothermal amplification (LAMP), PCR | [65] |
| F. equiseti | Potato dry rot | Potato/Tubers | Isolation and identification of fungal structures, PCR | [66] |
| F. equiseti | Foliar disease | Lettuce plants | Real-time PCR, digital PCR | [67] |
| Fusarium proliferatum | Clove rot | Garlic | Near-infrared spectroscopy (NIRS) | [68] |
| Fusarium sambucinum | Fusarium canker | Humulus lupulus | Amplified polymorphic DNA PCR assay | [69] |
| Fusarium virguliforme | Soybean Sudden Death Syndrome | Soybean | TaqMan qPCR assay based on the ribosomal DNA (rDNA) intergenic spacer | [70] |
3.4. Management Strategies for Diseases Caused by Fusarium spp.
4. Algae and Cyanobacteria as Sources of Antifungal Compounds
Role of Algae Fatty Acids as Antifungal
| Specie | Organism | Fatty Acids | Growth/Conditions | Solvent Extraction | Technique | Positive Drug Control | Result | References |
|---|---|---|---|---|---|---|---|---|
| N. calcicola | Cyanobacteria | 9-octadecenoic acid Oleic acid 12,15-octadecadienoic acid methyl ester Palmitic acid | Batch 28 °C, 24 h light BG-11 medium | Methanol | GC/MS | NA | 16 mm of inhibition zone mycelium of F. oxysporum f. sp. lycopersici | [138] |
| N. carneum | Cynobacteria | Palmitic acid 9,12-Octadecadienoic acid Alpha-Linoleic acid | Batch 30 °C, 12 h light BG-11 medium | Ethyl-acetate Ethanol | GC/MS | NA | 3, 160 mm of inhibition zone mycelium of F. oxysporum | [124] |
| Lyngnbya wollei | Cyanobacteria | Gamma-Linoleic acid Palmitic acid 6,9,12,15-Octadecatetraenoicacid, methyl ester | Batch 28 °C 16 h light BG-11 medium | Methanol | GC/MS | NA | 46% inhibition observed against F. udum and 40% against F. oxysporum | [107] |
| Oscillatoria princeps | Cyanobacteria | Alpha-Linoleic acid 9-Octadecenoic acid Hexadecanoic acid 9,15-Octadecadienoic acid 11-Octadecenoic acid | BG-11 | Diethyl ether | GC/MS | Nystatin | Inhibition zone 14 mm F. verticelloides and 15 mm F. proleferatum | [140] |
| C. myrica. P. boergesenii S. cinereum | Macroalgae | Hexadecanoic acid, methyl ester 9-octadecenoic acid, methyl ester Tetradecanoic acid, methyl ester cis-11-Eicosenoic acid, methyl ester | NA | Methanol Acetone | GC/MS | Gentamycine and Ampicilline | Inhibition zone (mm) 15, 13, 14 by C. myrica, S. cinereum and P. boergesenii to F. oxyporum | [127] |
| H. cuneiformis | Macroalgae | Palmitic acid Myristic acid Stearic acid Oleic acid Palmitoleic acid | NA | Chloroform | GC/MS | Amphotericin B | 6 µg/mL as MIC and 16 mm of inhibition zone of F. oxysporum | [139] |
| C. sinuosa. P. pavonia. C. barbata. S.vulgare | Macroalgae | Palmitic acid Myristic acid Palmitoleic acid Oleic acid Linoleic acid | NA | Methanol | GC/MS | Miconazole Flucanozole Itraconzaole | Inhibition zone diameters 19 mm, 9 mm, 19 mm, 11 mm by C. sinuosa, P. pavonisa, C. barbata and S. vulgare, respectively, to F. solani | [126] |
| C. vulgaris | Microalgae | Hexadecanoic acid Octadecenoic acid, methyl ester. | 25 °C 24 h light BG-11 Medium | Diethyl ether | GC/MS | NA | Growth inhibition (%) 73 F. oxysporum 50 F. solani | [39] |
5. Future Directions and Challenges
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agnolucci, P.; Rapti, C.; Alexander, P.; De Lipsis, V.; Holland, R.A.; Eigenbrod, F.; Ekins, P. Impacts of Rising Temperatures and Farm Management Practices on Global Yields of 18 Crops. Nat. Food 2020, 1, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Baek, K.-H. Control of Phytopathogens Using Sustainable Biogenic Nanomaterials: Recent Perspectives, Ecological Safety, and Challenging Gaps. J. Clean. Prod. 2022, 372, 133729. [Google Scholar] [CrossRef]
- Rampersad, S. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and Toxicity of a Fusarium Mycotoxin, Zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Ahmad, K.; Bashir Kutawa, A.; Siddiqui, Y.; Saad, N.; Geok Hun, T.; Hata, E.M.; Hossain, M.I. Biology, Diversity, Detection and Management of Fusarium oxysporum f. sp. Niveum Causing Vascular Wilt Disease of Watermelon (Citrullus lanatus): A Review. Agronomy 2021, 11, 1310. [Google Scholar] [CrossRef]
- de Chaves, M.A.; Reginatto, P.; da Costa, B.S.; de Paschoal, R.I.; Teixeira, M.L.; Fuentefria, A.M. Fungicide Resistance in Fusarium graminearum Species Complex. Curr. Microbiol. 2022, 79, 62. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J. The Fusarium solani Species Complex: Ubiquitous Pathogens of Agricultural Importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Singh, A.; Sirari, A.; Anupam; Kaur, K.; Kumar, A.; Lal, M.K.; Tiwari, R.K.; Kumar, R. Investigating the Impact of Fungicides and Mungbean Genotypes on the Management of Pod Rot Disease Caused by Fusarium equiseti and Fusarium chlamydosporum. Front. Plant Sci. 2023, 14, 1164245. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, X.; Cao, Y.; Song, Z.; Ma, K.; Fan, Y.; Ma, M. Fusarium culmorum and Fusarium equiseti Causing Root Rot Disease on Lycium barbarum (Goji Berry) in China. Plant Dis. 2020, 104, 3066. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P. Time for Re-Evaluating the Human Carcinogenicity of Ethylenedithiocarbamate Fungicides? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2632. [Google Scholar] [CrossRef] [PubMed]
- Subba, R.; Mathur, P. Functional Attributes of Microbial and Plant Based Biofungicides for the Defense Priming of Crop Plants. Theor. Exp. Plant Physiol. 2022, 34, 301–333. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, J.; Tan, T.; Xu, J.; Shen, A.; Yang, X.; Li, J.; Zeng, L.; Wei, L. Isolation and Characterization of Antagonistic Paenibacillus polymyxa HX-140 and Its Biocontrol Potential against Fusarium Wilt of Cucumber Seedlings. BMC Microbiol. 2021, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gutiérrez, C.; Arroyave, C.; Llugany, M.; Poschenrieder, C.; Martos, S.; Peláez, C. Trichoderma asperellum as a Preventive and Curative Agent to Control Fusarium Wilt in Stevia Rebaudiana. Biol. Control 2021, 155, 104537. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological Potential of Bacillus subtilis V26 for the Control of Fusarium Wilt and Tuber Dry Rot on Potato Caused by Fusarium Species and the Promotion of Plant Growth. Biol. Control 2021, 152, 104444. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhao, H.; Ni, Y.; Lian, Q.; Qian, H.; He, B.; Liu, H.; Ma, Q. Biological Control of Fusarium Wilt of Sesame by Penicillium bilaiae 47M-1. Biol. Control 2021, 158, 104601. [Google Scholar] [CrossRef]
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in Biological Control Strategies to Manage Microbial Plant Pathogens: A Review. Arch. Microbiol. 2022, 204, 666. [Google Scholar] [CrossRef]
- Simbo, D.; Elena, P.; Meisam, Z.; Dire, A.A.A.A.; Parpura, D.; Lapshin, G.; Abdullah, B. Yield Losses of Cereal Crops by Fusarium Link: A Review on the Perspective of Biological Control Practices. Res. Crops 2022, 23, 418–436. [Google Scholar] [CrossRef]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural Products from Medicinal Plants against Phytopathogenic Fusarium Species: Current Research Endeavours, Challenges and Prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Ma, J.; Liu, L.; Li, J.; Shen, T.; Tian, Y. The Major Component of Cinnamon Oil as a Natural Substitute against Fusarium solani on Astragalus membranaceus. J. Appl. Microbiol. 2022, 132, 3125–3141. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, A.; Ippolito, A.; Sanzani, S.M. First Report of Stemphylium eturmiunum Causing Postharvest Rot of Sweet Cherry in Italy. Crop Prot. 2020, 132, 105112. [Google Scholar] [CrossRef]
- Senouci, H.; Benyelles, N.G.; Dib, M.E.A.; Costa, J.; Muselli, A. Chemical Composition and Combinatory Antifungal Activities of Ammoides Verticillata, Allium Sativum and Curcuma Longa Essential Oils Against Four Fungi Responsible for Tomato Diseases. Comb. Chem. High Throughput Screen. 2020, 23, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.-L. Anticancer, Antiviral, Antibacterial, and Antifungal Properties in Microalgae. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–261. [Google Scholar]
- Afzal, S.; Yadav, A.K.; Poonia, A.K.; Choure, K.; Yadav, A.N.; Pandey, A. Antimicrobial Therapeutics Isolated from Algal Source: Retrospect and Prospect. Biologia 2022, 78, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Munaro, D.; Nunes, A.; Schmitz, C.; Bauer, C.; Coelho, D.S.; Oliveira, E.R.; Yunes, R.A.; Moura, S.; Maraschin, M. Metabolites Produced by Macro-and Microalgae as Plant Biostimulants. Stud. Nat. Prod. Chem. 2021, 71, 87–120. [Google Scholar]
- Elsheikh, S.; Eltanahy, E. Overview of Secondary Metabolites in Cyanobacteria: A Potential Source of Plant Growth-Promoting and Abiotic Stress Resistance. In Bacterial Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 29–57. [Google Scholar]
- Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Taher, M.A.; Baka, Z.A. In Vitro and In Vivo Biocontrol of Tomato Fusarium Wilt by Extracts from Brown, Red, and Green Macroalgae. Agriculture 2022, 12, 345. [Google Scholar] [CrossRef]
- Vicente, T.F.L.; Lemos, M.F.L.; Félix, R.; Valentão, P.; Félix, C. Marine Macroalgae, a Source of Natural Inhibitors of Fungal Phytopathogens. J. Fungi 2021, 7, 1006. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; van Staden, J. Bioprospecting for Bioactive Compounds in Microalgae: Antimicrobial Compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Asimakis, E.; Shehata, A.A.; Eisenreich, W.; Acheuk, F.; Lasram, S.; Basiouni, S.; Emekci, M.; Ntougias, S.; Taner, G.; May-Simera, H.; et al. Algae and Their Metabolites as Potential Bio-Pesticides. Microorganisms 2022, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Toshkova-Yotova, T.; Georgieva, A.; Iliev, I.; Alexandrov, S.; Ivanova, A.; Pilarski, P.; Toshkova, R. Antitumor and Antimicrobial Activity of Fatty Acids from Green Microalga Coelastrella sp. BGV. S. Afr. J. Bot. 2022, 151, 394–402. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Mousa, A.S.H.; Farghl, A.A.M. Biological Control of Fusarium Wilt Disease of Tomato Plants Using Seaweed Extracts. Arab. J. Sci. Eng. 2020, 45, 4557–4570. [Google Scholar] [CrossRef]
- Perveen, K.; Bukhari, N.A.; Al Masoudi, L.M.; Alqahtani, A.N.; Alruways, M.W.; Alkhattaf, F.S. Antifungal Potential, Chemical Composition of Chlorella vulgaris and SEM Analysis of Morphological Changes in Fusarium oxysporum. Saudi J. Biol. Sci. 2022, 29, 2501–2505. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Mehta, K.; Prajapati, J.; Shukla, A.; Parmar, P.; Goswami, D.; Saraf, M. An Anecdote of Mechanics for Fusarium Biocontrol by Plant Growth Promoting Microbes. Biol. Control 2022, 174, 105012. [Google Scholar] [CrossRef]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell Wall and Glycoproteins and Their Crucial Role in the Phytopathogenic Fungi Infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for Controlling the Sporulation in Fusarium spp. J. Fungi 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, D.A.; Ivanova, E.A.; Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Tkhakakhova, A.K.; Kholodov, V.A. Diversity, Ecological Characteristics and Identification of Some Problematic Phytopathogenic Fusarium in Soil: A Review. Diversity 2023, 15, 49. [Google Scholar] [CrossRef]
- Nag, P.; Paul, S.; Shriti, S.; Das, S. Defence Response in Plants and Animals against a Common Fungal Pathogen, Fusarium oxysporum. Curr. Res. Microb. Sci. 2022, 3, 100135. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Tahira, R.; Jamal, A. Fusarium spp. Mycotoxin Production, Diseases and Their Management: An Overview. Pak. J. Agric. Res. 2021, 34, 254–493. [Google Scholar] [CrossRef]
- Rauwane, M.E.; Ogugua, U.V.; Kalu, C.M.; Ledwaba, L.K.; Woldesemayat, A.A.; Ntushelo, K. Pathogenicity and Virulence Factors of Fusarium graminearum Including Factors Discovered Using Next Generation Sequencing Technologies and Proteomics. Microorganisms 2020, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Stępień, Ł.; Lalak-Kańczugowska, J. Signaling Pathways Involved in Virulence and Stress Response of Plant-Pathogenic Fusarium Species. Fungal Biol. Rev. 2021, 35, 27–39. [Google Scholar] [CrossRef]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Chang, J.; Yang, W. Infection Strategies and Pathogenicity of Biotrophic Plant Fungal Pathogens. Front. Microbiol. 2022, 13, 799396. [Google Scholar] [CrossRef]
- Husaini, A.M.; Sakina, A.; Cambay, S.R. Host–Pathogen Interaction in Fusarium oxysporum Infections: Where Do We Stand? Mol. Plant Microbe Interact. 2018, 31, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Lal, D.; Dev, D.; Kumari, S.; Pandey, S.; Aparna; Sharma, N.; Nandni, S.; Jha, R.K.; Singh, A. Fusarium Wilt Pandemic: Current Understanding and Molecular Perspectives. Funct. Integr. Genom. 2024, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking Intruders: Inducible Physico-Chemical Barriers against Plant Vascular Wilt Pathogens. J. Exp. Bot. 2021, 72, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Williamson-Benavides, B.A.; Dhingra, A. Understanding Root Rot Disease in Agricultural Crops. Horticulturae 2021, 7, 33. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Degani, O. A Review: Late Wilt of Maize—The Pathogen, the Disease, Current Status, and Future Perspective. J. Fungi 2021, 7, 989. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight From a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef] [PubMed]
- Dahl, B.; Wilson, W.W. Risk Premiums Due to Fusarium Head Blight (FHB) in Wheat and Barley. Agric. Syst. 2018, 162, 145–153. [Google Scholar] [CrossRef]
- Scott, C.; Punja, Z.K. Biological Control of Fusarium oxysporum Causing Damping-off and Pythium myriotylum Causing Root and Crown Rot on Cannabis (Cannabis sativa L.) Plants. Can. J. Plant Pathol. 2023, 45, 238–252. [Google Scholar] [CrossRef]
- Smiley, R.W.; Machado, S. Fusarium Crown Rot of Winter Wheat Influenced by Resource Competition Near a Tree Windbreak. Plant Dis. 2020, 104, 348–357. [Google Scholar] [CrossRef]
- Desai, S.; Dubey, S.C.; Prasad, R.D. Impacts of Climate Change on Fusarium Species Vis-à-Vis Adaptation Strategies. Indian Phytopathol. 2020, 73, 593–603. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Meng, C.; Zhang, D.; Xu, N.; Yu, J. Establishment and Application of a Multiplex PCR Assay for Detection of Rhizoctonia cerealis, Bipolaris sorokiniana, and Fusarium spp. in Winter Wheat. J. Plant Pathol. 2020, 102, 19–27. [Google Scholar] [CrossRef]
- Omori, A.M.; Ono, E.Y.S.; Bordini, J.G.; Hirozawa, M.T.; Fungaro, M.H.P.; Ono, M.A. Detection of Fusarium Verticillioides by PCR-ELISA Based on FUM21 Gene. Food Microbiol. 2018, 73, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Lin, H.-K.; Lin, Y.-H. Construction of Raman Spectroscopic Fingerprints for the Detection of Fusarium Wilt of Banana in Taiwan. PLoS ONE 2020, 15, e0230330. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Becerra, R.; López-Lima, D.; Villain, L.; Breitler, J.-C.; Carrión, G.; Desgarennes, D. Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease. J. Fungi 2021, 7, 253. [Google Scholar] [CrossRef]
- Almasi, M.A. Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Visual Detection of Fusarium oxysporum f.sp. Melonis. Hortic. Plant J. 2019, 5, 129–136. [Google Scholar] [CrossRef]
- Heltoft, P.; Brurberg, M.B.; Skogen, M.; Le, V.H.; Razzaghian, J.; Hermansen, A. Fusarium spp. Causing Dry Rot on Potatoes in Norway and Development of a Real-Time PCR Method for Detection of Fusarium Coeruleum. Potato Res. 2016, 59, 67–80. [Google Scholar] [CrossRef]
- Tziros, G.T.; Samaras, A.; Karaoglanidis, G.S. Fusarium Equiseti as an Emerging Foliar Pathogen of Lettuce in Greece: Identification and Development of a Real-Time PCR for Quantification of Inoculum in Soil Samples. Pathogens 2022, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Mamolini, E.; De Bastiani, M.; Marchetti, M. Quantitative Determination of Fusarium proliferatum Concentration in Intact Garlic Cloves Using Near-Infrared Spectroscopy. Sensors 2016, 16, 1099. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.J.; Borland, T.G.; Bergl, D.D.; Claassen, B.J.; Flodquist, T.A.; Montgomery, A.S.; Rivedal, H.M.; Woodhall, J.; Ocamb, C.M.; Gent, D.H. A Quantitative PCR Assay for Detection and Quantification of Fusarium sambucinum. Plant Dis. 2022, 106, 2601–2606. [Google Scholar] [CrossRef]
- Wang, J.; Jacobs, J.L.; Byrne, J.M.; Chilvers, M.I. Improved Diagnoses and Quantification of Fusarium virguliforme, Causal Agent of Soybean Sudden Death Syndrome. Phytopathology 2015, 105, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Höfle, L.; Werner, B.T.; Imani, J.; Schmidt, A.; Jelonek, L.; Kogel, K. SIGS vs HIGS: A Study on the Efficacy of Two DsRNA Delivery Strategies to Silence Fusarium FgCYP51 Genes in Infected Host and Non-host Plants. Mol. Plant Pathol. 2019, 20, 1636–1644. [Google Scholar] [CrossRef]
- Theron, J.S.; Johannes van Coller, G.; Rose, L.J.; Labuschagne, J.; Swanepoel, P.A. The Effect of Crop Rotation and Tillage Practice on Fusarium Crown Rot and Agronomic Parameters of Wheat in South Africa. Crop Prot. 2023, 166, 106175. [Google Scholar] [CrossRef]
- Jing, T.; Zhou, D.; Zhang, M.; Yun, T.; Qi, D.; Wei, Y.; Chen, Y.; Zang, X.; Wang, W.; Xie, J. Newly Isolated Streptomyces sp. JBS5-6 as a Potential Biocontrol Agent to Control Banana Fusarium Wilt: Genome Sequencing and Secondary Metabolite Cluster Profiles. Front. Microbiol. 2020, 11, 602591. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Zhou, D.; Zhang, M.; Qi, D.; Jing, T.; Zang, X.; Qi, C.; Wang, W.; Xie, J. Biological Control of Banana Wilt Disease Caused by Fusarium oxyspoum f. sp. Cubense Using Streptomyces sp. H4. Biol. Control 2021, 155, 104524. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mohamed, A.A.; Ali, H.M.; Al Farraj, D.A. Characterization of Phytoconstituents from Alcoholic Extracts of Four Woody Species and Their Potential Uses for Management of Six Fusarium Oxysporum Isolates Identified from Some Plant Hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Laverde, D.; Barbosa-Cornelio, R.; Coy-Barrera, E. Antifungal Activity against Fusarium Oxysporum of Botanical End-Products: An Integration of Chemical Composition and Antifungal Activity Datasets to Identify Antifungal Bioactives. Plants 2021, 10, 2563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, C.; Luo, X.; Li, A.; Zhang, S.; An, J.; Zhang, Z.; Ma, Y.; Zhang, B.; Liu, Y. Design, Synthesis, and Biological Evaluation of Novel Berberine Derivatives against Phytopathogenic Fungi. Pest Manag. Sci. 2022, 78, 4361–4376. [Google Scholar] [CrossRef] [PubMed]
- Sama, H.; Sombié, P.A.E.D.; Guenne, S.; Soura, H.B.; Hilou, A. Antifungal Potential and Fatty Acid Profile of Two Jatropha curcas (Euphorbiaceae) Oils. J. Agric. Food Res. 2021, 6, 100244. [Google Scholar] [CrossRef]
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.-B.; Jardin, J.; Coton, M.; Leyva Salas, M.; Coton, E.; Valence, F.; Mounier, J. Antifungal Activity of Fermented Dairy Ingredients: Identification of Antifungal Compounds. Int. J. Food Microbiol. 2020, 322, 108574. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; El-Wakil, D.A.; Hashem, A.H.; Abdelaziz, A.M. Antagonistic Effect of Plant Growth-Promoting Fungi Against Fusarium Wilt Disease in Tomato: In Vitro and In Vivo Study. Appl. Biochem. Biotechnol. 2022, 194, 5100–5118. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.L.; Latz, M.A.C.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of Fungal Endophytes with Biocontrol Potential against Fusarium Head Blight in Wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Kemp, N.D.; Vaughan, M.M.; McCormick, S.P.; Brown, J.A.; Bakker, M.G. Sarocladium zeae Is a Systemic Endophyte of Wheat and an Effective Biocontrol Agent against Fusarium Head Blight. Biol. Control 2020, 149, 104329. [Google Scholar] [CrossRef]
- Chacón-Cerdas, R.; Barboza-Barquero, L.; Albertazzi, F.J.; Rivera-Méndez, W. Transcription Factors Controlling Biotic Stress Response in Potato Plants. Physiol. Mol. Plant Pathol. 2020, 112, 101527. [Google Scholar] [CrossRef]
- Tuyen, D.T.; Trung, N.T.; Thao, N.T.; Le Thanh, N.S.; Dai Nguyen, N.P.; Anh Tuyet, N.T.; Cuong, N.T.; Chan, S.S.; Khoo, K.S.; Show, P.L. Antifungal Activity of Secondary Metabolites Purified from Bacillus subtilis Isolated in Vietnam and Evaluated on in Vitro and in Vivo Models. Int. Biodeterior. Biodegrad. 2023, 179, 105558. [Google Scholar] [CrossRef]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species Against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Luan, P.; Fan, M.; Wu, X.; Sun, Z.; Shang, Z.; Yang, Y.; Li, C. Antifungal Efficacy of Bacillus amyloliquefaciens ZK-9 against Fusarium graminearum and Analysis of the Potential Mechanism of Its Lipopeptides. Int. J. Food Microbiol. 2024, 422, 110821. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Ponce de la Cal, A.; Louis, Y.; Baró Robaina, Y.; Coll, Y.; Spengler, I.; Mirabal-Gallardo, Y. Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53). J. Fungi 2024, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Lakhdari, W.; Benyahia, I.; Bouhenna, M.M.; Bendif, H.; Khelafi, H.; Bachir, H.; Ladjal, A.; Hammi, H.; Mouhoubi, D.; Khelil, H.; et al. Exploration and Evaluation of Secondary Metabolites from Trichoderma harzianum: GC-MS Analysis, Phytochemical Profiling, Antifungal and Antioxidant Activity Assessment. Molecules 2023, 28, 5025. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.; Divyashree, M.; Mani, M.K.; Mamatha, B.S. Algae and Cyanobacteria as a Source of Novel Bioactive Compounds for Biomedical Applications. In Advances in Cyanobacterial Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–194. [Google Scholar]
- Singh, S.K.; Kaur, R.; Bansal, A.; Kapur, S.; Sundaram, S. Biotechnological Exploitation of Cyanobacteria and Microalgae for Bioactive Compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–259. [Google Scholar]
- Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms 2022, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Gupta, E.; Singh, P.; Prasad, R. Application of Microalgae Metabolites in Food and Pharmaceutical Industry. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 391–408. [Google Scholar]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef] [PubMed]
- Chanthini, K.M.-P.; Senthil-Nathan, S. Marine Weeds against Fungal Phytopathogens—Current Agronomical Implications and Intriguing Perspectives for a Sustainable Future. Physiol. Mol. Plant Pathol. 2024, 130, 102240. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Martins, A.P.; Colepicolo, P.; Yokoya, N.S. Concise Review on Seaweed Photosynthesis: From Physiological Bases to Biotechnological Applications. J. Photochem. Photobiol. 2023, 16, 100194. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from Microalgae and Cyanobacteria: Applications and Market Survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential Utilization of Bioproducts from Microalgae for the Quality Enhancement of Natural Products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Woodhouse, J.N. Defining Cyanobacterial Species: Diversity and Description Through Genomics. CRC Crit. Rev. Plant Sci. 2020, 39, 101–124. [Google Scholar] [CrossRef]
- Springstein, B.L.; Nürnberg, D.J.; Weiss, G.L.; Pilhofer, M.; Stucken, K. Structural Determinants and Their Role in Cyanobacterial Morphogenesis. Life 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Noreña-Caro, D.; Benton, M.G. Cyanobacteria as Photoautotrophic Biofactories of High-Value Chemicals. J. CO2 Util. 2018, 28, 335–366. [Google Scholar] [CrossRef]
- Tibocha-Bonilla, J.D.; Zuñiga, C.; Godoy-Silva, R.D.; Zengler, K. Advances in Metabolic Modeling of Oleaginous Microalgae. Biotechnol. Biofuels 2018, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; Macrae, A.; de Souza, J.E.; Neves Junior, A.; Vermelho, A.B. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants 2023, 12, 1896. [Google Scholar] [CrossRef]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and Allelochemicals from Microalgae: An Innovative Source for Bio-Herbicidal Compounds and Biocontrol Research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Choi, Y.-N.; Lee, J.W.; Kim, J.W.; Park, J.M. Acetyl-CoA-Derived Biofuel and Biochemical Production in Cyanobacteria: A Mini Review. J. Appl. Phycol. 2020, 32, 1643–1653. [Google Scholar] [CrossRef]
- Verma, S.; Thapa, S.; Siddiqui, N.; Chakdar, H. Cyanobacterial Secondary Metabolites towards Improved Commercial Significance through Multiomics Approaches. World J. Microbiol. Biotechnol. 2022, 38, 100. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Srivastava, A.; Young, J.D. Metabolic Flux Phenotyping of Secondary Metabolism in Cyanobacteria. Trends Microbiol. 2023, 31, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Vaz, M.G.M.V.; Genuário, D.B.; Fiore, M.F.; Debonsi, H.M. Volatile Compounds Produced by Cyanobacteria Isolated from Mangrove Environment. Curr. Microbiol. 2019, 76, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Basit, A.; Ullah, I.; Mohamed, H.I. Cyanobacteria and Algae as Biocontrol Agents Against Fungal and Bacterial Plant Pathogens. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–23. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial Volatile Organic Compounds: Antifungal Mechanisms, Applications, and Challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, D.; Gao, C.; Li, J.; Du, B.; Wang, Z.; Qian, S. Recent Advances for Alkaloids as Botanical Pesticides for Use in Organic Agriculture. Int. J. Pest Manag. 2023, 69, 288–298. [Google Scholar] [CrossRef]
- Albuquerque, M.V.d.C.; Ramos, R.d.O.; de Paula e Silva, M.C.C.; Rodrigues, R.M.M.; Leite, V.D.; Lopes, W.S. Allelopathic Effects of Cyanotoxins on the Physiological Responses of Chlorella vulgaris. Toxicon 2024, 248, 107847. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.; Singh, R.P.; Kumar, A. Cyanobacterial Biodiversity and Their Potential Application in Sustainable Agriculture. In Sustainable Agricultural Practices; Elsevier: Amsterdam, The Netherlands, 2024; pp. 209–222. [Google Scholar]
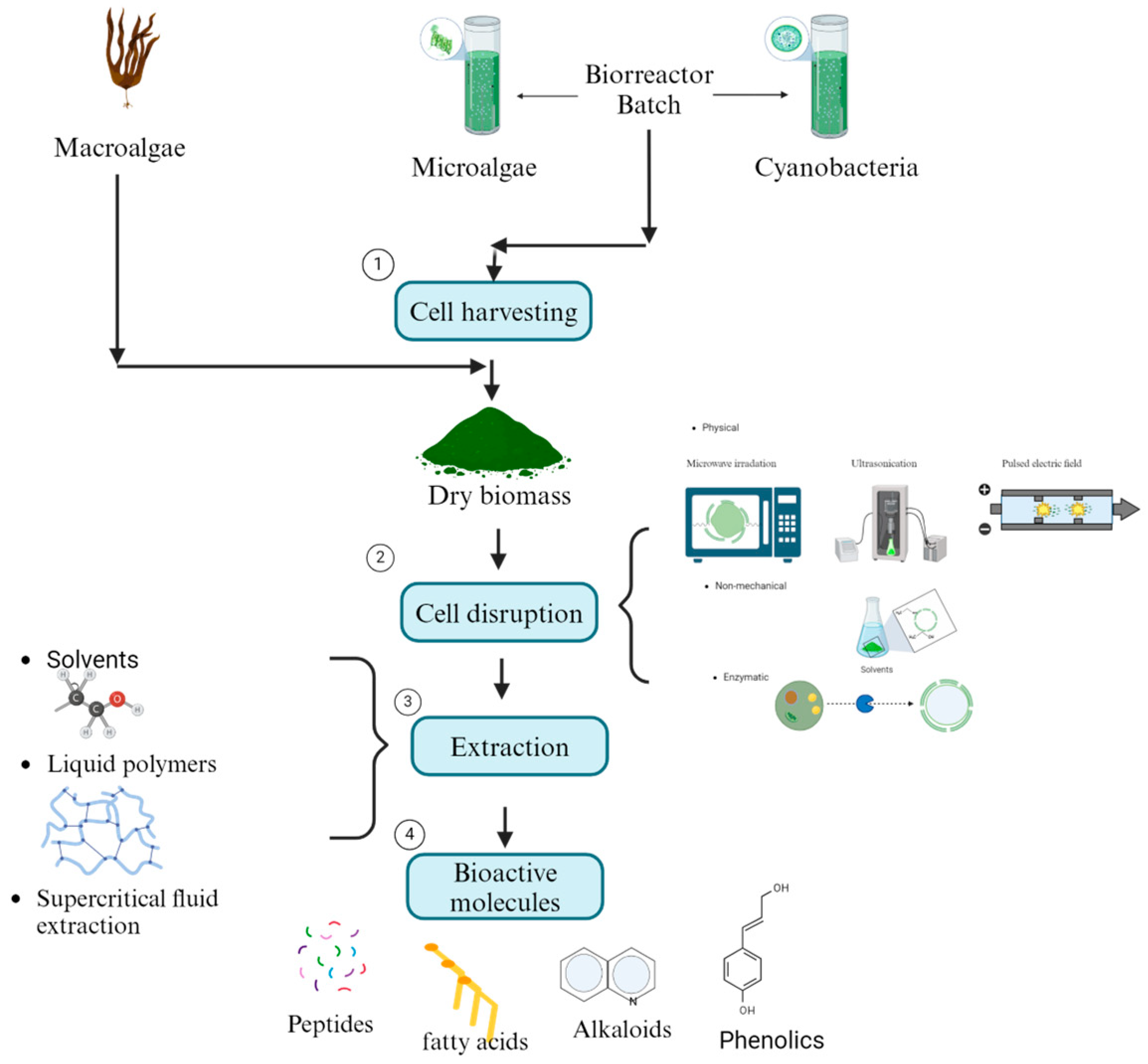
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2020, 9, 10. [Google Scholar] [CrossRef]
- Doble, M.; Kruthiventi, A.K. Alternate Solvents. In Green Chemistry and Engineering; Elsevier: Amsterdam, The Netherlands, 2007; pp. 93–104. [Google Scholar]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding Pitfalls in Determining Antimicrobial Activity of Plant Extracts and Publishing the Results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Pagnussatt, F.A.; Lemos, A.C.; Nicolli, C.P.; Del Ponte, E.M.; Badiale-Furlong, E. Nannochloropsis sp. and Spirulina sp. as a Source of Antifungal Compounds to Mitigate Contamination by Fusarium graminearum Species Complex. Curr. Microbiol. 2019, 76, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Arnhold Pagnussatt, F.; Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Inhibition of Fusarium Graminearum Growth and Mycotoxin Production by Phenolic Extract from Spirulina sp. Pestic. Biochem. Physiol. 2014, 108, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Farghl, A.; El-Sheekh, M.M.; Mousa, A.S. Extraction and characterization of antimicrobial active substance from cyanobacteria Nostoc carneum and Anabaena circinalis. Fresenius Environ. Bull. 2019, 28, 5481–5490. [Google Scholar]
- Perveen, K.; Alwathnani, H.A. Antifungal Activity of Methanol, Acetone and Diethyl Ether Extracts of Cyanobacteria Against Plant Pathogenic Fungi. Asian J. Chem. 2013, 25, 7531–7534. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M.; Mohyeldin, M.M. Component Analysis and Antifungal Activity of the Compounds Extracted from Four Brown Seaweeds with Different Solvents at Different Seasons. J. Ocean. Univ. China 2018, 17, 1178–1188. [Google Scholar] [CrossRef]
- Senousy, H.H.; El-Sheekh, M.M.; Saber, A.A.; Khairy, H.M.; Said, H.A.; Alhoqail, W.A.; Abu-Elsaoud, A.M. Biochemical Analyses of Ten Cyanobacterial and Microalgal Strains Isolated from Egyptian Habitats, and Screening for Their Potential against Some Selected Phytopathogenic Fungal Strains. Agronomy 2022, 12, 1340. [Google Scholar] [CrossRef]
- Alallaf, A.; Kotab, M.; Shafik, H.; Elsayed, A. In Vitro Efficacy of Biologically Active Compounds Derived from Navicula Arenaria against Soil Borne Phytopathogenic Macrophomina phaseolina and Fusarium oxysporum. Alfarama J. Basic Appl. Sci. 2021, 2, 285–296. [Google Scholar] [CrossRef]
- Blasio, M.; Balzano, S. Fatty Acids Derivatives from Eukaryotic Microalgae, Pathways and Potential Applications. Front. Microbiol. 2021, 12, 718933. [Google Scholar] [CrossRef]
- Mund, N.K.; Liu, Y.; Chen, S. Advances in Metabolic Engineering of Cyanobacteria for Production of Biofuels. Fuel 2022, 322, 124117. [Google Scholar] [CrossRef]
- Cerone, M.; Smith, T.K. Desaturases: Structural and Mechanistic Insights into the Biosynthesis of Unsaturated Fatty Acids. IUBMB Life 2022, 74, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef] [PubMed]
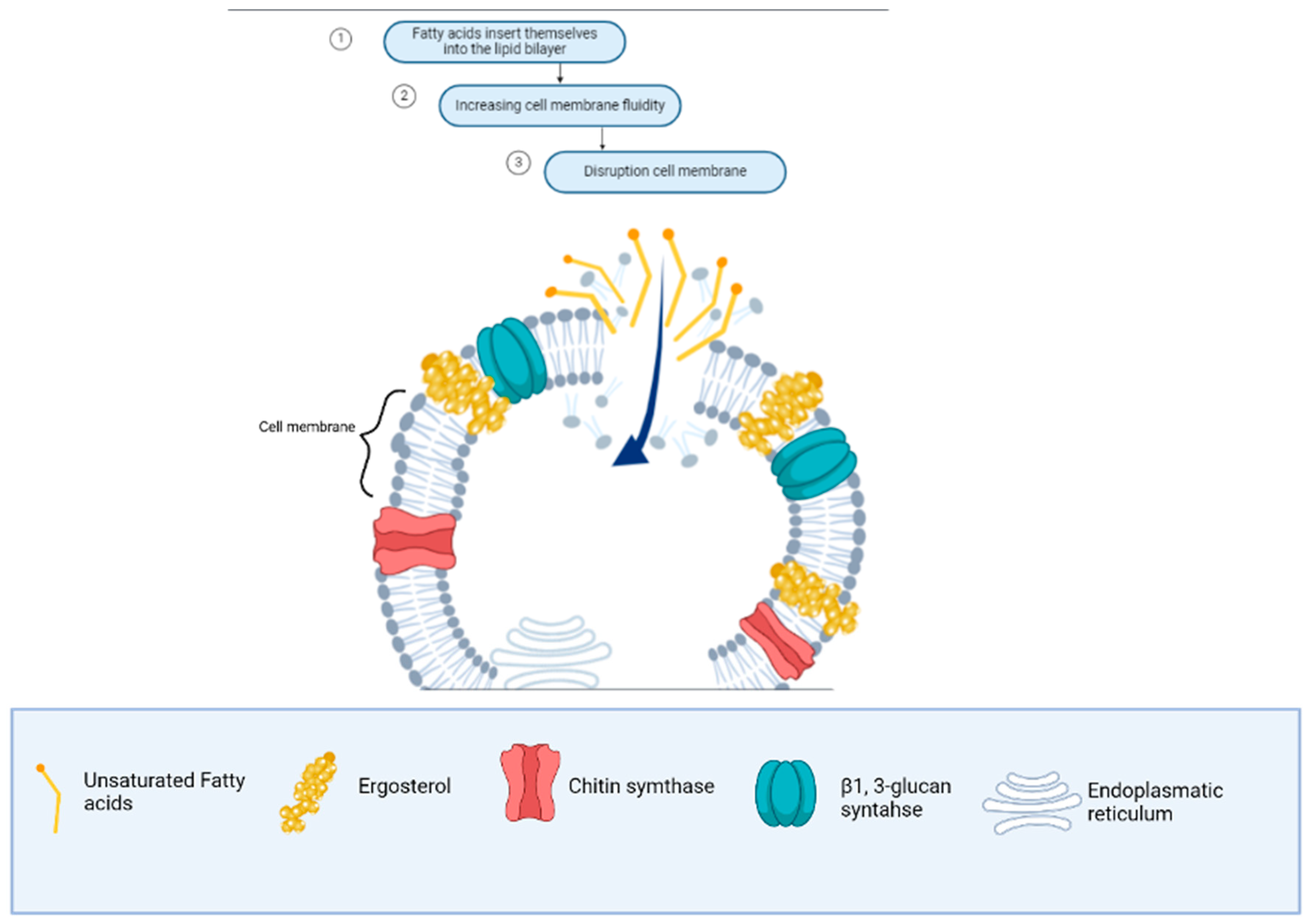
- Qiu, M.; Wang, Y.; Sun, L.; Deng, Q.; Zhao, J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins 2021, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Dacko, A.; Tan, A.K.; Xiang, S.; Curtis, J.M.; Gänzle, M.G. Structure-Function Relationships of Antifungal Monohydroxy Unsaturated Fatty Acids (HUFA) of Plant and Bacterial Origin. Food Res. Int. 2020, 134, 109237. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.L.S.; da Silva, A.F.; Carvalho, P.H.A.; Pacheco, B.S.; Pereira, C.M.P.; Lund, R.G. Aliphatic Fatty Acids and Esters: Inhibition of Growth and Exoenzyme Production of Candida, and Their Cytotoxicity in Vitro. Arch. Oral. Biol. 2014, 59, 880–886. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Sinha, M.; Singh, H.; Patel, R.S.; Ghosh, S.; Sardana, K.; Ghosh, S.; Sengupta, S. Mechanistic Insight Into the Antifungal Effects of a Fatty Acid Derivative Against Drug-Resistant Fungal Infections. Front. Microbiol. 2020, 11, 2116. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, S.; Xavier, L.; Vasconcelos, V.; Santos, A. Cyanobacteria: A Promising Source of Antifungal Metabolites. Mar. Drugs 2023, 21, 359. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Deyab, M.A.; Hasan, R.S.A.; Abu Ahmed, S.E.; Elsadany, A.Y. Biological Control of Fusarium Tomato-Wilt Disease by Cyanobacteria nostoc spp. Arch. Microbiol. 2022, 204, 116. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Saber, A. Antifungal Potential of the Bioactive Constituents in Extracts of the Mostly Untapped Brown Seaweed Hormophysa cuneiformis from The Egyptian Coastal Waters. Egypt. J. Bot. 2019, 59, 695–708. [Google Scholar] [CrossRef]
- Marrez, D.A.; Sultan, Y.Y.; Naguib, M.M.; Higazy, A.M. Antimicrobial Activity, Cytotoxicity and Chemical Constituents of the Freshwater Microalga Oscillatoria princeps. Biointerface Res. Appl. Chem. 2021, 12, 961–977. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial Potential of Macro and Microalgae against Pathogenic and Spoilage Microorganisms in Food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-H.; Kuo, C.-H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.L.; Pereira-Caro, G.; Ludwig, I.; Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Crozier, A.; Moreno-Rojas, J.M. A Critical Evaluation of the Use of Gas Chromatography- and High Performance Liquid Chromatography-Mass Spectrometry Techniques for the Analysis of Microbial Metabolites in Human Urine after Consumption of Orange Juice. J. Chromatogr. A 2018, 1575, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Anasane, N.; Gade, R.; Ingle, P. Effective Management of Soft Rot of Ginger Caused by Pythium spp. and Fusarium spp.: Emerging Role of Nanotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 6827–6839. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.Á.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C.G. Natural Products Targeting the Synthesis of β(1,3)-D-Glucan and Chitin of the Fungal Cell Wall. Existing Drugs and Recent Findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, I.; Steinberg, G.; Gurr, S. The Role of the Fungal Cell Wall in the Infection of Plants. Trends Microbiol. 2017, 25, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, K.L.; Senhorinho, G.N.A.; Laamanen, C.A.; Scott, J.A. A Review of Extremophilic Microalgae: Impacts of Experimental Cultivation Conditions for the Production of Antimicrobials. Algal Res. 2024, 78, 103427. [Google Scholar] [CrossRef]
- Kar, J.; Ramrao, D.P.; Zomuansangi, R.; Lalbiaktluangi, C.; Singh, S.M.; Joshi, N.C.; Kumar, A.; Kaushalendra; Mehta, S.; Yadav, M.K.; et al. Revisiting the Role of Cyanobacteria-Derived Metabolites as Antimicrobial Agent: A 21st Century Perspective. Front. Microbiol. 2022, 13, 1034471. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Mc Gee, D.; Archer, L.; Smyth, T.J.; Fleming, G.T.A.; Touzet, N. Bioprospecting and LED-Based Spectral Enhancement of Antimicrobial Activity of Microalgae Isolated from the West of Ireland. Algal Res. 2020, 45, 101704. [Google Scholar] [CrossRef]
- López-Herrada, E.; Gallardo-Rodríguez, J.J.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F. Life-Cycle Assessment of a Microalgae-Based Fungicide under a Biorefinery Approach. Bioresour. Technol. 2023, 383, 129244. [Google Scholar] [CrossRef] [PubMed]
- Avinash, A.; Sasikumar, P.; Pugazhendhi, A. Analysis of the Limiting Factors for Large Scale Microalgal Cultivation: A Promising Future for Renewable and Sustainable Biofuel Industry. Renew. Sustain. Energy Rev. 2020, 134, 110250. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae N-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Kiani, H.; Aznar, R.; Poojary, M.M.; Tiwari, B.K.; Halim, R. Chromatographic Techniques to Separate and Identify Bioactive Compounds in Microalgae. Front. Energy Res. 2022, 10, 904014. [Google Scholar] [CrossRef]
- Pérez, J.P.; Muñoz, A.A.; Figueroa, C.P.; Agurto-Muñoz, C. Current Analytical Techniques for the Characterization of Lipophilic Bioactive Compounds from Microalgae Extracts. Biomass Bioenergy 2021, 149, 106078. [Google Scholar] [CrossRef]
- Parkes, R.; McGee, D.; McDonnell, A.; Gillespie, E.; Touzet, N. Rapid Screening of Phenolic Compounds in Extracts of Photosynthetic Organisms Separated Using a C18 Monolithic Column Based HPLC-UV Method. J. Chromatogr. B 2022, 1213, 123521. [Google Scholar] [CrossRef] [PubMed]
- Schuelter, A.R.; Kroumov, A.D.; Hinterholz, C.L.; Fiorini, A.; Trigueros, D.E.G.; Vendruscolo, E.G.; Zaharieva, M.M.; Módenes, A.N. Isolation and Identification of New Microalgae Strains with Antibacterial Activity on Food-Borne Pathogens. Engineering Approach to Optimize Synthesis of Desired Metabolites. Biochem. Eng. J. 2019, 144, 28–39. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Soengas, R.; Probert, I.; Guilloud, E.; Gourvil, P.; Mehiri, M.; López, Y.; Cepas, V.; Gutiérrez-del-Río, I.; Redondo-Blanco, S.; et al. NMR Characterization and Evaluation of Antibacterial and Antiobiofilm Activity of Organic Extracts from Stationary Phase Batch Cultures of Five Marine Microalgae (Dunaliella sp., D. Salina, Chaetoceros calcitrans, C. gracilis and Tisochrysis lutea). Phytochemistry 2019, 164, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Vyas, D. Antimicrobial Effect of a Cyclic Peptide Nostophycin Isolated from Wastewater Cyanobacteria, Nostoc Calcicola. Curr. Bot. 2021, 12, 94–101. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Heinilä, L.; Aesoy, R.; Sivonen, K.; Galica, T.; Hrouzek, P.; Herfindal, L. Chemical Diversity and Cellular Effects of Antifungal Cyclic Lipopeptides from Cyanobacteria. Physiol. Plant 2021, 173, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Blandino, M.; Scarpino, V.; Giordano, D.; Testa, G.; Badiale-Furlong, E. Application of Fungicides and Microalgal Phenolic Extracts for the Direct Control of Fumonisin Contamination in Maize. J. Agric. Food Chem. 2018, 66, 4835–4841. [Google Scholar] [CrossRef]
- Toribio, A.J.; Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; López-González, J.A.; Moreno, J. Seed Biopriming with Cyanobacterial Extracts as an Eco-Friendly Strategy to Control Damping off Caused by Pythium ultimum in Seedbeds. Microbiol. Res. 2021, 248, 126766. [Google Scholar] [CrossRef]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of Microalgae and Cyanobacteria as Potential Sources of Antimicrobial Compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.A.; Alferova, V.A.; Korshun, V.A.; Tyurin, A.P. Modern Trends in Natural Antibiotic Discovery. Life 2023, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Monteiro, A.F.; Bertonha, A.F.; Bernardi, D.I.; Gubiani, J.R.; Slivinski, J.; Michaliski, L.F.; Tonon, L.A.C.; Venancio, V.A.; Freire, V.F. Approaches for the Isolation and Identification of Hydrophilic, Light-Sensitive, Volatile and Minor Natural Products. Nat. Prod. Rep. 2019, 36, 981–1004. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal Polyunsaturated Fatty Acids: Hotspots and Production Techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Cyanobacteria in Plant Health: Biological Strategy against Abiotic and Biotic Stresses. Crop Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the Interaction of the Antifungal and Cytotoxic Cyclic Glycolipopeptide Hassallidin with Sterol-Containing Lipid Membranes. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Singh, H.; Sharma, S.; Kaur, M.; Singh, A.; Kaur, A.; Jain, S.K. Pre-Clinical and Cellular Safety Assessment of Oral Administered DHA Rich Microalgae Oil from Schizochytrium sp. (Strain ATCC-20889): Acute, Sub-Chronic and Genotoxicity. Drug Chem. Toxicol. 2024, 47, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, K.T.; Hwang, S.; Choi, K.Y.; Park, Y.J.; Choi, J.-H.; Truong, T.Q.; Kim, S.M. Nanoencapsulation Enhances the Bioavailability of Fucoxanthin in Microalga Phaeodactylum tricornutum Extract. Food Chem. 2023, 403, 134348. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, P.; Szczurek, E. Artificial Intelligence-Driven Antimicrobial Peptide Discovery. Curr. Opin. Struct. Biol. 2023, 83, 102733. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Alvarez, J.A.E.; Zabetakis, D.; Walper, S.A.; Malanoski, A.P. PepVAE: Variational Autoencoder Framework for Antimicrobial Peptide Generation and Activity Prediction. Front. Microbiol. 2021, 12, 725727. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, A.; Cai, X.; Personne, H.; Köhler, T.; van Delden, C.; Reymond, J.-L. Machine Learning Designs Non-Hemolytic Antimicrobial Peptides. Chem. Sci. 2021, 12, 9221–9232. [Google Scholar] [CrossRef] [PubMed]


| Commercial Product | Active Ingredients | Category | Claimed Mode of Action | WEB Page |
|---|---|---|---|---|
| Serenade Garden Disease Control | B. subtilis strain QST 713 | BCAs | Colonization of plant roots and production of antifungal compounds | 1 |
| Companion® Biofungicide | Bacillus amyloliquefaciens strain D747 | BCAs | Production of antifungal metabolites | 2 |
| Trilogy® Biofungicide | B. subtilis strain QST 713 and Pseudomonas fluorescens strain Pf-L13 | BCAs | Colonization of plant roots and production of antifungal compounds | 3 |
| RootShield® 10G Biofungicide | Trichoderma harzianum strain 1295-22 | BCAs | Parasitism of fungal pathogens and production of antifungal metabolites | 4 |
| Actinovate® SP | Streptomyces lydicus strain WRLDac951 | BCAs | Production of multiple antifungal compounds | 5 |
| Prothio T ® | Prothioconazole | Chemical Fungicides | Inhibition of ergosterol biosynthesis in fungal cell membranes | 6 |
| Headline® | Strobilurin (multiple formulations) | Chemical Fungicides | Inhibition of mitochondrial electron transport in fungal cells | 7 |
| Switch® | Fludioxonil | Chemical Fungicides | Disruption of fungal cell wall biosynthesis | 8 |
| Tebuconazole 25 EW | Tebuconazol | Chemical Fungicides | Inhibition of ergosterol biosynthesis in fungal cell membranes | 9 |
| Phyton-27® | Laminaria digitata extract | BCAs | Disruption of fungal cell membranes and suppression of spore germination | 10 |
| Kocide® 2000 | Copper oxychloride | Broad spectrum, including fungal spores and mycelia | Disruption of fungal cell membranes and enzyme inhibition | 11 |
| Species | Organism | Compound | Structure /Type | Solvent | Method | Positive Drug Control | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Spirulina sp. | Cyanobacteria | Chlorogenic acid Protocatechuic acid Gallic acids | Phenolics | Water | LC/ESI/MS/MS | Synthetic acid phenolic standard | Mycelial growth rate was lowest as 0.21 cm·day−1 F. asiaticum, F. graminearum and F. meridionale | [122] |
| Spirulina sp. | Cyanobacteria | Gallic acid Caffeic acid Salicylic acid | Phenolics mixture | Methanol | HPLC-UV | NA | Mycelial growth rates ranged from 0.01 cm to 0.45 cm·day−1 for F. meridionale and F. graminearum | [123] |
| N. calcicola | Cyanobacteria | Phthalic acid Xylene | Aromatic carboxylic acids and aromatics | Methanol | GC/MS | NA | 16 mm of inhibition zone mycelium of F. oxysporum and F. lycopersici | [38] |
| N. carneum | Cyanobacteria | Phytol | Terpenoids | Ethyl acetate | GC/MS | Chloramphenicol | 3, 160 mm of inhibition zone mycelium of F. oxysporum | [124] |
| Spirulina platensis | Cyanobacteria | 2-Hexyl-1-nitrocyclohexane Bromoacetic acid | Nitroalkane bromine compounds | Methanol:acetone:diethyl eter (5:2:1) | GC/MS | NA | Strong antifungal activity for F. oxysporum at 50,000 µg/mL concentration of extract. | [125] |
| S.dentifolium G. compressa U. lactuca | Macroalgae | Phloroglucinol Gallic acid Vanillic acid | Phenolics | Methanol | HPLC | NA | Diameter of mycelial growth (mm) 41, 49, 45 at 20 µg/mL extract respectively to against F. oxysporim f sp. lycopersici | [30] |
| Colpomenia sinuosa. Padina pavonia. Cystoseira barbata. Sargassum vulgare. | Macroalgae | Hexadecanol Heptacosane diisooctyl phthalate Butyl octyl phthalate | Aromatic carboxylic acids and Volatiles | Methanol | GC/MS | Miconazole Flucanozole Itraconzaole | Inhibition zone diameters 19 mm, 9 mm, 19 mm, and 11 mm, respectively, against F. solani | [126] |
| Dunaliella sp. Chlorella sorokiniana | Microalgae | Gallic acid Quercetine Equivalents | Phenolics and alkaloids | Methanol | UV/VIS | Rhizolex-T | Inhibition (%) 82, 40 by Dunaliella sp and C. sorokiniana to F. solani | [127] |
| Navicula arenaria | Microalage | di-n-octyl phthalate Beta-Sitosterol | Aromatic carboxylic acids, sterols | Hexane | GC/MS | Miconazole And Nystatin | Mycelium growth inhibition 22% at 6000 µg/mL of hexane extract to F. oxysporum | [128] |
| Nannochlorosis sp. | Microalage | Chlorogenic acid Gallic acid Protocatechuic | Phenolics acids | Methanol | HPLC/MS | Synthetic acid phenolic standard | Mycelium Growth rate lowest < 0.32 cm·day−1 for F. asiaticum, F. graminearum, F. meridionale. | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Arellanes, M.E.; López-Pacheco, L.D.; Elizondo-Luevano, J.H.; González-Meza, G.M. Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp. Microorganisms 2025, 13, 439. https://doi.org/10.3390/microorganisms13020439
López-Arellanes ME, López-Pacheco LD, Elizondo-Luevano JH, González-Meza GM. Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp. Microorganisms. 2025; 13(2):439. https://doi.org/10.3390/microorganisms13020439
Chicago/Turabian StyleLópez-Arellanes, Miguel E., Lizbeth Denisse López-Pacheco, Joel H. Elizondo-Luevano, and Georgia María González-Meza. 2025. "Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp." Microorganisms 13, no. 2: 439. https://doi.org/10.3390/microorganisms13020439
APA StyleLópez-Arellanes, M. E., López-Pacheco, L. D., Elizondo-Luevano, J. H., & González-Meza, G. M. (2025). Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp. Microorganisms, 13(2), 439. https://doi.org/10.3390/microorganisms13020439









