Abstract
Fungal diseases caused by Fusarium spp. significantly threaten food security and sustainable agriculture. One of the traditional strategies for eradicating Fusarium spp. incidents is the use of chemical and synthetic fungicides. The excessive use of these products generates environmental damage and has negative effects on crop yield. It puts plants in stressful conditions, kills the natural soil microbiome, and makes phytopathogenic fungi resistant. Finally, it also causes health problems in farmers. This drives the search for and selection of natural alternatives, such as bio-fungicides. Among natural products, algae and cyanobacteria are promising sources of antifungal bio-compounds. These organisms can synthesize different bioactive molecules, such as fatty acids, phenolic acids, and some volatile organic compounds with antifungal activity, which can damage the fungal cell membrane that surrounds the hyphae and spores, either by solubilization or by making them porous and disrupted. Research in this area is still developing, but significant progress has been made in the identification of the compounds with potential for controlling this important pathogen. Therefore, this review focuses on the knowledge about the mechanisms of action of the fatty acids from macroalgae, microalgae, and cyanobacteria as principal biomolecules with antifungal activity, as well as on the benefits and challenges of applying these natural metabolites against Fusarium spp. to achieve sustainable agriculture.
1. Introduction
One of the objectives of the United Nations 2030 Sustainable Development Agenda is to preserve the life of terrestrial ecosystems and eradicate hunger; both objectives are related to new practices for more sustainable agriculture and more responsible food production. Phytopathogens inflict substantial damage, causing up to 30% of the diseases that affect more than 150 food crops, including cereals, vegetables, and fruits [1]. The global threat of fungal pathogens in crops results in a dramatic loss of yield, along with a significant impact on food quality, leading to multiple cascading effects and economic losses. The most prevalent phytopathogens that affect crops before and after harvest are Ustilago spp., Sclerotinia spp., Pestalotiopsis spp., Alternaria spp., Rhizoctonia sp., Colletotrichum sp., Botrytis sp., Phytophthora sp., and Fusarium spp. [2].
Fusarium is a genus comprising more than 100 species of filamentous fungi; some are considered the most severe crops phytopathogens. Fusarium has a wide host range and is difficult to control due to its ability to develop resistance, survive in the soil for long periods, persist within agricultural residues following harvest, and cause health damage with its toxins [3]. The main problems caused by Fusarium infection in plants are vascular wilting, root and stem rot, fruit rot, and mycotoxin contamination. Mycotoxins, which are secondary metabolites produced by Fusarium, such as trichothecenes, zearalenone, enniatins, deoxynivalenol, and fumonisins, pose a significant threat to health. Ingestion of these metabolites can harm both animals and humans through exposure to contaminated food [4,5].
Certain species of Fusarium have the highest incidence and impact on crops. They include Fusarium oxysporum, which causes vascular wilt in a wide range of crops [6], Fusarium graminearum, which primarily leads to discoloration of cereal ears and contamination with mycotoxins [7], and Fusarium solani, which is known for causing root and stem rot in crops such as potatoes, tomatoes, and beans [8]. Other species, such as Fusarium culmorum, Fusarium equiseti, Fusarium gallinaceum, and Fusarium chlamydosporum, are also commonly found in cereal crops, which cause considerable damage. Therefore, it is important to highlight that the incidence and impact of different Fusarium spp. vary depending on the type of crop, environmental conditions, and agricultural practices [9,10].
Implementing integrated control strategies against Fusarium spp. can help reduce the incidence and impact of this phytopathogen on agricultural crops. Traditionally, crop rotation, the use of resistant crop varieties, and effective soil management practices are employed to prevent or manage Fusarium spp. [11]. However, the use of synthetic fungicide products remains a quick and effective strategy. Some of the most used chemical fungicides against Fusarium spp. in crops include triazoles, strobilurins, carbendazim, mancozeb, benomyl, tebuconazole, and propiconazole [12]. Unfortunately, the excessive and indiscriminate use of these chemicals can lead to environmental and health problems, such as the development of resistance to fungicides in certain strains, increased carcinogenic effects in humans, and residual toxicity in the environment [13]. Concerns about the adverse effects of fungicides have prompted regulations that require compliance with specific production standards in the sector. This has motivated scientific researchers to utilize biotechnology in the search for promising, environmentally friendly natural alternatives.
The research studies point to the development of sustainable biological product technologies for control in agriculture. These products focus on biofungicides, biological bactericides, biostimulants, and other products derived from plant molecules and microorganisms. Biological control of phytopathogens involves identifying and utilizing various biological control agents with potential fungicidal activity. These agents include microorganisms such as bacteria and fungi, as well as plant-derived compounds from algae, lichens, and higher plants. Their antifungal effects operate through different mechanisms [14]. Furthermore, microorganisms act as antagonists to fungi by naturally competing for essential nutrients and space for colonization, thereby limiting the development of the involved phytopathogen [15]. Additionally, a study identified Bacillus strains that produced antifungal compounds, such as lipopeptides, as well as enzymes that inhibited the growth of F. equiseti [14]. Micoparasitism is another biofungicide mechanism in which microorganisms parasitize fungal hyphae to absorb their nutrients. In vitro tests showed that Trichoderma asperellum secretes cell wall-degrading enzymes, like chitinases and cellulases associated with mycoparasitic activity, against F. oxysporum in stevia plants, which reduces Fusarium wilt incidence by 80% [16].
An advantage of using microorganism biofungicides is that some biocontrol agents can promote plant defenses in addition to limiting the growth of phytopathogens. Bacillus subtilis has demonstrated an 85.3% inhibition ratio against Fusarium spp. in potatoes while also providing biostimulant properties [17]. A study published by Zhao et al. (2021) showed that it inhibited the mycelial growth of F. oxysporum by 81.3%. Furthermore, it was observed that Penicillium bilaiae significantly promoted the growth of sesame [18].
Some of the most effective control agents against Fusarium spp, with potential under greenhouse or field conditions, belong to the genera Trichoderma, Bacillus, Pseudomonas, and Streptomyces [19,20]. However, biomass production of these microorganisms in bioreactors and synthetic culture media can significantly increase costs, which represents a challenge for bioprocess scale-up.
An alternative is the antifungal activity of plant extracts or specific or secondary plant metabolites, which has also been studied. In vitro studies of these compounds are the first phase of bioprospecting, which is followed by the development of new fungicide products. These compounds are found in herbs and plants [21]. Some natural compounds that have shown efficacy against Fusarium in vitro include cinnamaldehyde and phenylpropanoids, which are present in cinnamon essential oil and have demonstrated 92.98% disease suppression [22]; the monoterpenes, which are present in oregano oil [23]; sulfur compounds, which are present in garlic extracts; and the oxygenated monoterpenes, which are present in turmeric oil [24]. However, cultivating terrestrial plants to extract antifungal compounds competes with the demand for food and, in turn, requires arable land and water, making it an unsustainable future strategy with environmental impact.
In this context, macroalgae, microalgae, and cyanobacteria, which produce bioactive metabolites, have shown promise as effective agents for the biological control of plant pathogens and the improvement of soil fertility but without the harmful effects or environmental impact of other methods. Algae and cyanobacteria are photosynthetic organisms capable of synthesizing bioactive compounds, such as peptides, fatty acids, polysaccharides, phenolic compounds, terpenes, alkaloids, and sulfur compounds, thus demonstrating antimicrobial and antifungal properties [25,26,27]. The easy cultivation of algae and cyanobacteria, which uses wastewater as an alternative culture medium and non-agricultural land for installing open reactors or the macroalgae collection from offshore, makes them viable candidates for various applications. In our case, we propose their use as a promising alternative for researching and producing biocontrol agents against Fusarium spp. In addition, in the cultivation of microalgae and cyanobacteria, abiotic stress induced by changes in pH, temperature, light intensity, and agitation in the photobioreactor influences the production of secondary metabolites, which may exhibit antifungal activity [28,29]. Several studies report antifungal activity in various macroalgae, microalgae, and cyanobacteria [30]. One study found that methanol extracts, which are rich in phenolic compounds, from Ulva lactuca, Sargassum dentifolium, and Gracilaria sp. effectively inhibited F. oxysporum growth in vitro. Additionally, a greenhouse trial with S. dentifolium showed a 40.8% reduction in Fusarium disease in tomatoes [31]. On the other hand, the microalgae Chlorella vulgaris, Dunaliella salina, Scenedesmus obliquus, Tetraselmis suecica, and Nannochloropsis oculata have also been studied for their in vitro antifungal activity against Fusarium spp. Regarding cyanobacteria, although they are the least reported, studies have found that conclude that the genera Nostoc, Spirulina, Anabaena, Westiellopsis, Hapalosiphon hibernicus, and Microcystis have antifungal activity [32,33].
Among algae and cyanobacterial metabolites with antifungal activity against Fusarium spp., fatty acids represent a promising area of study since their efficacy in inhibiting this pathogen has been demonstrated [34]. Algae and cyanobacteria synthesize various fatty acids, including polyunsaturated, medium-chain, and short-chain fatty acids. These molecules have been reported to possess antimicrobial, insecticidal, and antifungal properties, with a minimum inhibitory concentration (MIC) of 600 μg·mL−1 against C. albicans [35,36,37]. Studies indicate that fatty acids from algae and cyanobacteria can exert their antifungal activity against fungi and Fusarium spp. through several mechanisms, such as inhibition of fungal spore germination, destruction of the fungal cell wall, inhibition of lipid synthesis, induction of defense compounds in plants, and activation of elicitors and signaling compounds [38,39].
Despite extensive research on the antifungal potential of algae and cyanobacteria, there remains a critical gap in the literature regarding the specific efficacy of fatty acids, which are derived from these organisms, against the phytopathogenic fungus Fusarium spp. This review addresses that gap by comprehensively analyzing the antifungal effects of polar and non-polar algae extracts against Fusarium. The analysis will encompass macroalgae, microalgae, and cyanobacteria, with a particular emphasis on elucidating the role of fatty acids in this context and exploring whether the extracts exhibit biostimulant and/or fertilizing properties.
2. Materials and Methods
For this literature review, an exhaustive search was conducted in the PubMed (https://pubmed.ncbi.nlm.nih.gov), Crossref (https://www.crossref.org), and Scopus (https://www.scopus.com) databases before 29 November 2024. The initial selection of literature was based on the following keywords: #1: “Fusarium” or “phytopathogen”; #2: “macroalgae”, “microalgae”, “cyanobacteria*”, “natural extract”, or “antipathogen”; #3: “fatty acids”, “peptides”, or “metabolites”. The publication date ranged from 1 January 2014 to 29 November 2024. The search results were integrated using a Boolean search’s logical “AND” operator. Subsequently, the retrieved articles were filtered to exclude those of limited relevance, prioritizing studies specifically addressing the use of algal metabolites in developing valuable antifungal products, with a clear focus on Fusarium spp. and algae. This filtering process was conducted by analyzing each article’s title, keywords, and abstract.
3. Fusarium: Impact and Challenges in Agriculture
3.1. Morphology Characteristics
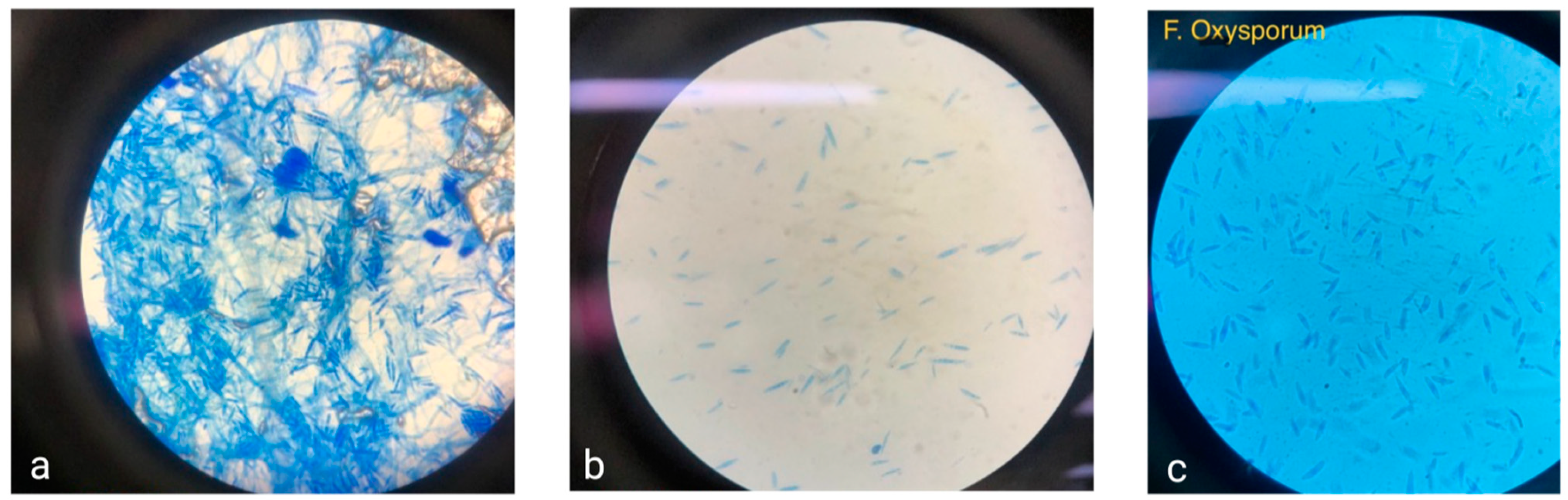
Fusarium is a genus of ascomycetous fungi notable for the loss of sexual reproduction. This genus includes around 70 recognized species, identified through a polyphasic approach, and around 300 putative species. Morphologically, Fusarium. are characterized by conidia formation in chains on different types of conidiophores. These include macroconidia (3–5 cells, curved) that are produced as asexual reproductive structures and microconidia (1–2 cells) of straight or slightly curved ovoid shape and chlamydospores [40]. Fusarium hyphae are hyaline and septate, with a diameter of 3 to 8 μm (Figure 1a,b). The Fusarium cell wall consists of an outer layer of glycoproteins, mannose, and melanin, while the inner layer is composed of β-(1-3)-glucans, β-(1-6)-glucans, and chitin [41]. The cellular membrane of Fusarium is primarily made up of phospholipids and ergosterol [42]. The Fusarium genus is distinguished by its cottony mycelium, often displaying characteristic pigmentation in shades of violet, pink, or yellow. This pigmentation varies among species; for instance, F. solani may exhibit red, pink, brown, or gray hues, Fusarium verticillioides typically appears white with yellow and purple tones, and F. oxysporum (Figure 1c) presents as cottony white with a yellow center [43].
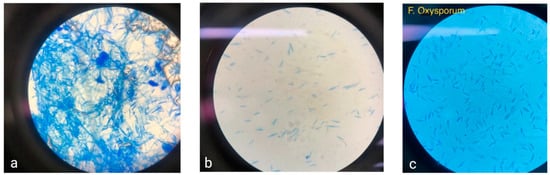
Figure 1.
(a,b) Microscopic views of Mexican corn isolates. (c) F. oxysporum at 40×.
Fusarium spp., in addition to its distinct morphological characteristics, exhibits remarkable adaptability to a wide range of environmental conditions. These fungi predominantly colonize warm, sandy soils where they can persist over long periods, with optimal growth occurring at temperatures between 20 and 32 °C. Fusarium spp. [44]. can be part of the typical soil crop microflora, contributing to a healthy ecosystem. However, some species act as plant pathogens, causing diseases in fruits and cereals crops, such as tomato, wheat, and corn. Fusarium spp. frequently inhabit roots and plant debris, acting as saprophytes [45]. Soil pH also plays a role in modulating Fusarium spp. growth; under stress conditions, they may produce various mycotoxins, such as fusaric acid and fumonisins, which serve as valuable identifiers for species differentiation and crop impact assessment [46]. Globally, Fusarium spp. ranks among the most critical phytopathogens, contributing to a wide array of diseases across both agricultural and horticultural crops.
3.2. General Description of Fusarium spp. Infection Mechanism as a Plant Pathogen
Fusarium spp. can infect plants at any stage of development, leading to seedling, root, crown rot, stem, and even tassel rot. The phytopathogenicity of Fusarium spp. is caused by a combination of mechanisms [47]. Among them, the fungus synthesizes enzymes like pectinase, cellulase, protease, and lipase, which degrade the plant cell walls to allow the fungus to invade [43]. Furthermore, Fusarium spp. produces toxins such as fusaric acid, fumonisins, and ochratoxin A. These toxins are highly toxic to both plants and animals, contributing to disease symptoms and potential health problems if consumed [4]. Additionally, Fusarium spp. utilizes G protein to regulate their response to environmental stress. These proteins allow the fungus to adapt to different crop conditions, making it a more persistent threat [48].
Fusarium infection develops in stages. In the first stage, the hyphae of the fungus adhere to the surface of the plant roots, then mechanically penetrate the plant tissue, thanks to the pressure of the hyphae and secreted enzymes, which degrade the defense barriers of the cell walls [49]. The fungus must tolerate the plant antifungal compounds and inhibit its defense response by secreting phytotoxins. Once the fungus colonizes the plant, its mycelium spreads intracellularly until it reaches the xylem of stems and leaves, absorbing the nutrients necessary for its development [50]. This fungal pathogen disrupts the plant vascular system, leading to wilting, chlorosis (yellowing), and ultimately plant death. Vascular wilt specifically targets the vascular system, hindering the transport of water and essential nutrients [51]. Colonization is rapid due to the formation of microconidia within the xylem, where the spores germinate, and the germ tubes pass through the xylem cells, subsequently forming hyphae, conidiophores, and conidia [40]. The mycelium of the fungus accumulates in the vascular tissues, while the parenchyma cells multiply as a plant defense response, secreting toxins that compress the vessels. The characteristic wilting of Fusarium spp. diseases results from water stress due to the limitation of the functioning of the vessels, which can generate low productivity or even the death of the plant. This invasion also causes root and stem rot [52].
3.3. Damage to Most Important Crops: Diseases Caused by Fusarium spp.
Fungal diseases caused by Fusarium species present a major global challenge to agriculture. Fusarium spp. infects a wide range of crops, impacting not only cereals and legumes but also causing root and stem rot in fruits and vegetables [53,54]. Crops like tomatoes, bananas, and cotton are particularly susceptible to vascular wilt. Similarly, maize is vulnerable to Fusarium spp.-mediated ear rot, which leads to substantial reductions in both grain yield and quality [6,55], thus compromising food production and quality. Two of the most common Fusarium-related diseases affecting cereal crops worldwide are Fusarium head blight (FHB) and Fusarium crown rot (FCR). FHB, which primarily targets wheat, corn, and barley, causes rotting of the grain head, leading to significant reductions in both grain yield and quality [56]. Additionally, infections by some Fusarium result in the production of fumonisins, a group of mycotoxins in grains that are highly toxic to humans and animals [4]. Table 1 provides an overview of Fusarium diseases, including FHB and FCR, and highlights their detection technique. In the United States, a leading global wheat producer, FHB can cause yield losses exceeding 30%, involving at least 17 Fusarium spp. Infected grains often wither and discolor prematurely, leading to spike death. Additionally, FHB contamination with mycotoxins presents significant storage risks as these toxins can persist for years, reducing grain market value. Recent U.S. market discounts have ranged from USD 1.84 to 3.67 per ton for every 0.5 ppm of mycotoxin detected in grains [57].
On the other hand, FCR can persist in wheat crops in arid and semi-arid regions, as fungal hyphae survive in plant residues for up to three years under suitable environmental conditions. The infection begins at the roots as the plant grows and subsequently spreads throughout the plant. FCR, caused by Fusarium pseudograminearum, F. oxysporum and F. culmorum, either individually or together, affects the crown and basal stem tissues, hindering water transport from the roots to the canopy [58]. Severe infection in basal tissues leads to premature senescence of the heads, known as whiteheads. FCR typically progresses from a chronic, low-impact disease to an acute, destructive one during drought stress around or after anthesis [59].
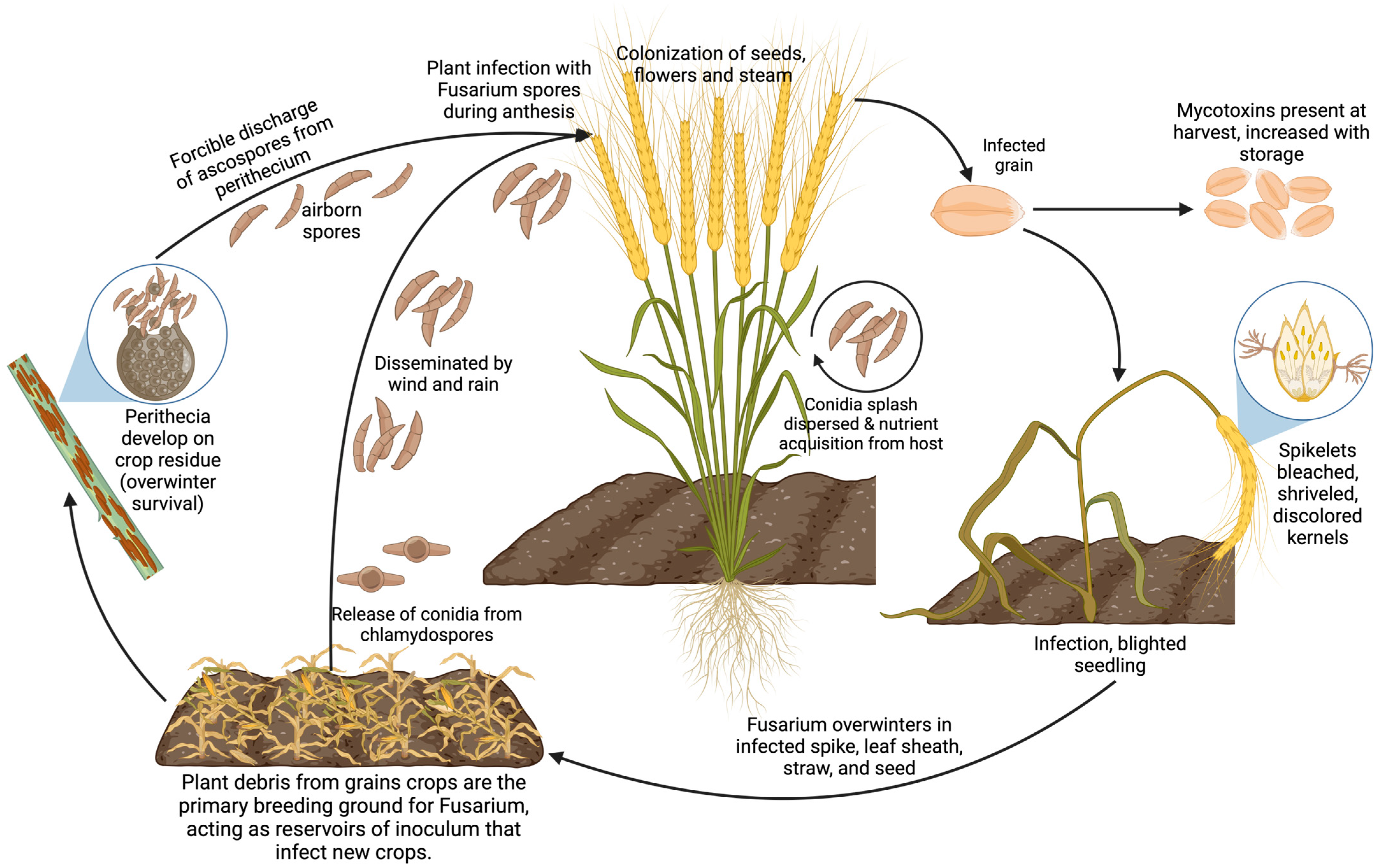
The development of Fusarium infections is often aggravated by agricultural practices such as inadequate tillage in intensive cropping systems and the improper application of fungicides, combined with climatic conditions that enhance the pathogen’s survival during its saprophytic phase in crop residues [60] (Figure 2). Moreover, the variability in pathogenicity, toxicity, and fungicide sensitivity across different Fusarium spp. strains poses significant challenges to controlling efforts, thereby fueling scientific interest in the discovery of broad-spectrum biocontrol agents [7].
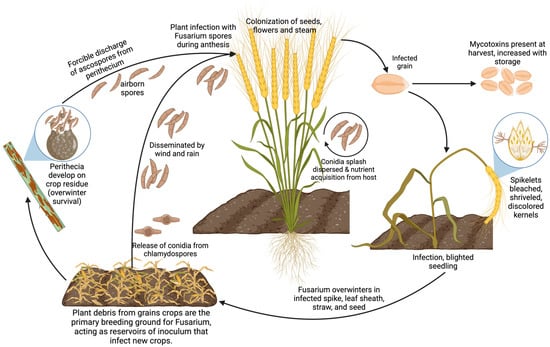
Figure 2.
Fusarium life cycle: from soil to plant wilting.

Table 1.
Pathogenic Fusarium species, their hosts, associated diseases, and detection methods.
Table 1.
Pathogenic Fusarium species, their hosts, associated diseases, and detection methods.
| Pathogen | Disease Caused | Host/Plant Part | Detection Technique | Reference |
|---|---|---|---|---|
| Fusarium spp. | Wheat crown rot | Wheat/Wheatears | PCR-based methods | [61] |
| F. verticillioides | Fumonisins | Corn | Immunoassay kits, PCR-ELISA | [62] |
| F. oxysporum Fusarium cubense | Panama disease (Fusarium wilt) | Banana/Roots | Raman spectroscopic fingerprints | [63] |
| F. solani | Coffee corky root | Coffee seedlings | Isolation and identification of fungal structures, species-specific PCR | [64] |
| F. oxysporum | Fusarium wilt | Melon | Loop-mediated isothermal amplification (LAMP), PCR | [65] |
| F. equiseti | Potato dry rot | Potato/Tubers | Isolation and identification of fungal structures, PCR | [66] |
| F. equiseti | Foliar disease | Lettuce plants | Real-time PCR, digital PCR | [67] |
| Fusarium proliferatum | Clove rot | Garlic | Near-infrared spectroscopy (NIRS) | [68] |
| Fusarium sambucinum | Fusarium canker | Humulus lupulus | Amplified polymorphic DNA PCR assay | [69] |
| Fusarium virguliforme | Soybean Sudden Death Syndrome | Soybean | TaqMan qPCR assay based on the ribosomal DNA (rDNA) intergenic spacer | [70] |
ELISA (Enzyme-Linked Immunosorbent Assay), DNA (Deoxyribonucleic Acid) analysis, TaqMan (Taq Polymerase-based Fluorescent Probe System), qPCR (Quantitative Polymerase Chain Reaction), rDNA (Recombinant DNA), PCR (Polymerase Chain Reaction), and LAMP (Loop-Mediated Isothermal Amplification).
3.4. Management Strategies for Diseases Caused by Fusarium spp.
Managing Fusarium spp. diseases requires a comprehensive approach that integrates multiple strategies. Implementing effective cultural practices can significantly reduce soil pathogen populations and minimize the risk of disease. Genetically resistant crops are a cornerstone of prevention [71], with the selection of varieties containing specific resistance genes against pathogen races being key to this approach. Additional cultural strategies include crop rotation with non-host species of Fusarium, proper tillage to promote residue decomposition, and the removal of infected plant material at the end of the harvest cycle. These practices are essential for effective disease management [72].
Nonetheless, the control of diseases caused by Fusarium spp. still relies heavily on chemical fungicides, which pose environmental and public health risks and contribute to fungal resistance. In this context, searching for natural alternatives and sustainable control methods is important. Biocontrol strategies against Fusarium spp. involve both direct and indirect mechanisms. Direct mechanisms include the production of antifungal compounds and secondary metabolites that inhibit the growth of the pathogen. In contrast, indirect mechanisms involve competition for nutrients and colonization space, which limits the pathogen’s ability to thrive. Understanding these metabolic pathways and biocontrol mechanisms provides valuable insights for the development of novel and more effective biocontrol agents [40]. The development of new biocontrol methods for combating diseases caused by Fusarium spp. has increasingly focused on obtaining antifungal compounds or secondary metabolites from plants and microorganisms. In this context, a study of 88 actinomycete isolates identified Streptomyces violaceusniger as a promising biocontrol agent. This strain demonstrated a potent inhibition efficiency of 60.46% against F. oxysporum by disrupting the membrane integrity and ultrastructure of fungal cells. Notably, its secondary metabolites, 5-hydroxymethyl-2-furancarboxaldehyde and n-hexadecenoic acid, have been identified as antifungal compounds. The former has been previously described as an intermediate in agricultural chemicals, while the latter exhibits cytotoxic properties, thus highlighting their potential for the effective management of diseases caused by Fusarium spp. [73]. Li et al. (2021) demonstrated that methanolic extracts of Streptomyces sp. exhibited antifungal activity against F. oxysporum, inhibiting 86.45% of mycelial growth and reducing the disease index in banana seedlings. This effect was linked to membrane disruption and ultrastructural damage in Fusarium sp. cells, which lead to nucleic acid release and increased extracellular conductivity, indicating plasma membrane damage [74].
A previous study identified several phenolic acids as bioactive compounds with antifungal properties achieving up to 88.9% of inhibition against F. oxysporum. Notable compounds included p-hydroxybenzoic acid, benzoic acid, gallic acid, rosmarinic acid, and o-coumaric acid, which were found in extracts of Conium maculatum (leaves), Acacia salign (bark), Schinus terebinthifolius (wood), and Ficus eriobotryoides (leaves) [75]. Alkaloids, including piperaduncin, asebogenin, and methyllinderatin, were found in Piper aduncum extract with a mycelial growth inhibition of 95.26%. Berberine has also been shown to exhibit antifungal properties by inducing the accumulation of reactive oxygen species, leading to cell death, with EC50 values of 0.065 μg·mL−1 against R. solani [76,77]. Among the metabolites that have shown excellent results are essential oils, such as tea tree oil, oregano oil, and garlic and turmeric extracts. However, the primary challenge in applying natural-origin compounds lies in scaling up their production to enable the development of large-scale methods for bioactive compound synthesis. A study examined the antifungal activities and fatty acid composition of Jatropha oils. The oils were tested at 1% and 2% concentrations against Fusarium solani strains isolated from tomato plants. The results showed that both concentrations inhibited fungal growth and reduced sporulation, with a maximum inhibition of 72.7%. Gas chromatography/mass spectrometry (GC-MS) analysis revealed that both oils were predominantly unsaturated, with the main fatty acids being vaccenic, nonadecanoic, linoleic, palmitic, myristic, and erucic acids [78]. In addition to essential oils, fatty acids in A. jensenii and L. rhamnosus (3-(4-hydroxyphenyl) propionic acid) also exhibit higher antifungal activity. Derivatives of hydroxydecanoic acid inhibit the growth of R. mucilaginosa at 10 μg·mL−1, contributing to antifungal activity. These findings suggest that the viscosity and reactivity of fatty acids facilitate their integration into fungal cell membranes, promoting membrane disruption through their lipolytic activity [79].
Certain microorganisms act as antagonists to Fusarium spp. through mechanisms that combine antifungal activity with plant growth promotion. A previous study showed that Aspergillus fumigatus and Rhizopus oryzae, both plant growth promoters, exhibited antifungal activity against F. oxysporum, the cause of Fusarium spp. wilt in tomatoes. The study reports that the MIC of R. oryzae extract was 0.5 m mL−1, while the MIC of A. fumigatus extract was 1 mg·mL−1. In vivo assays demonstrated disease severity reductions of 12.5% and 37.5% for A. fumigatus and R. oryzae, respectively, with protection levels of 86.35% and 59.06% [80]. Another study by Rojas et al. (2020) [81] reported that wheat endophytic fungi, such as Anthracocystis floccosa and Penicillium olsonii, exhibit potential as biocontrol agents against Fusarium spp. These fungi colonize plant tissues asymptomatically while occupying the same biological niche as the pathogens. Similarly, research by Kemp et al. (2020) [82] demonstrated that the endophytic fungus Sarocladium zeae stimulates the secretion of defense-related hormones in wheat plants, effectively activating their innate defense mechanisms. This enhanced defense response in seedlings was associated with a 57.9% reduction in disease severity and a 61.2% decrease in the accumulation of mycotoxins such as deoxynivalenol. Antagonistic microorganisms have been effectively utilized as a biological control strategy against Fusarium spp., which employs various mechanisms such as nutrient competition, production of antifungal metabolites, interaction through structural and biochemical mechanisms, and stimulation of plant defenses to enhance stress tolerance [83]. This approach represents a sustainable and environmentally friendly alternative to traditional Fusarium sp. management. Currently, a wide range of products is available on the market, most of which are derived from bacteria (Table 2) (all websites were accessed on 29 November 2024). Table 2 presents three commercial biofungicides containing Bacillus spp. (Gram-positive) as active ingredients: Serenade Garden Disease Control, Companion® Biofungicide, and Trilogy® Biofungicide. The mode of action involves plant root colonization and the production of antifungal compounds. While the specific antifungal metabolites are not listed, Bacillus spp. are known to produce volatile organic compounds (3-methyl-1-butanol, 2-methyl-1-butanol, butane-1-methoxy-3-methyl, 2-hydroxy-3-pentanone, 3-methyl-2-pentane, and methanethiol) [84], as well as lipopeptides like surfactin, iturin, and fengycin, which show antifungal activity against Fusarium spp. [85,86]

Table 2.
Overview and comparison of commercial biofungicides for sustainable agricultural practices.
Additionally, Table 2 lists RootShield® 10G Biofungicide, which contains Trichoderma harzianum as the active ingredient. While the specific antifungal metabolites are not specified, Trichoderma spp. are known to produce volatile organic compounds such as butenolides [87], phenolic compounds like flavonoids and catechins [88], and saturated and polyunsaturated fatty acids (pentadecanoic acid, palmitic acid, methyl linolelaidate, linoleic acid, and 9,17-octadecadienal) [89], all with antifungal activity against Fusarium spp.
Although microorganisms and terrestrial plant extracts have proven to be effective, antifungals, macroalgae, microalgae, and cyanobacteria emerge as even more promising organisms due to their abundance, rapid growth, and unique bioactive compounds. These include their ability to grow in wastewater or saline environments, their non-competition with arable land, their CO2 capture capabilities, and the lower cost of culture media. Additionally, their biochemical composition can be easily controlled by optimizing cultivation parameters. These organisms represent a valuable source of bioactive compounds and secondary metabolites with diverse biological activities, such as antifungal, antibacterial, antiviral, anticancer, and antioxidant properties [90,91]. Numerous studies highlight the potential of their extracts and compounds for the development of pharmaceutical, nutraceutical, and agrochemical products [92,93]. A key advantage of using these organisms is their biodiversity as each species possesses a unique metabolic profile, thus increasing the likelihood of discovering new compounds with antifungal activity. Furthermore, macroalgae, microalgae, and cyanobacteria can be easily cultivated with fewer resources than terrestrial plants, which makes them efficient producers of secondary metabolites at high rates [94]. Algal and cyanobacterial compounds have shown potential for controlling diseases like Fusarium spp. through both direct antagonistic activity and the induction of plant defense. Recent research suggests Ascophyllum nodosum extract use can reduce abiotic stress-induced toxicities in plants and offers an eco-friendly alternative to synthetic fungicides. However, further research is needed to optimize their use and effectiveness in real-world conditions, particularly in controlling fungal pathogens like Fusarium spp. under practical cultivation conditions [95].
4. Algae and Cyanobacteria as Sources of Antifungal Compounds
Algae refers to a large group of unicellular or multicellular plant-like organisms that range in size from 1 µm to the giant kelp, which can grow up to 60 m in length [96]. These organisms are aquatic, oxygenic, phototrophic, and photosynthetic [97]. There are over 55,000 species and around 10 million strains, which can be classified into nine main divisions based on their size, pigmentation, structure, development rate, and cell wall phytochemicals [97]. Macroalgae, commonly known as seaweed, are multicellular and macroscopic. They are plant-like organisms but lack functional tissues [96]. On the other hand, microalgae are microscopic, unicellular forms of algae that exist as individual cells or colonies in both natural and marine waters [98]. They typically store 40–80% lipids (dry weight) and 6–75% proteins (dry weight) [99]. Their simple unicellular structure allows for faster division, leading to high reproduction and growth rates. Cyanobacteria (often referred to as blue-green algae) are an ancient group of prokaryotic, single-celled organisms [100]. They can form colonies that appear as filaments, sheets, or short, slimy biofilms [101]. Algae represent a diverse group that includes macroalgae, microalgae, and cyanobacteria, with the main differences lying in their size and evolutionary adaptations.
Macroalgae, microalgae, and cyanobacteria all contain chlorophyll, which enables them to absorb sunlight and perform photosynthesis [97]. In macroalgae, photosynthesis occurs in the stroma of chloroplasts, while in cyanobacteria, it takes place within thylakoids [102]. During the light-dependent phase, photons are absorbed by pigments like phycobilins, passing through Photosystems II and I, which leads to the production of ATP and NADPH [102]. These molecules drive the Calvin–Benson–Bassham cycle, where carbon is fixed into glucose, which enters glycolysis [102]. Other pathways, such as the oxidative pentose phosphate pathway, contribute to secondary metabolism, ultimately producing bioactive compounds [97,102,103]. These bioactive compounds, including antifungal agents, make algae and cyanobacteria promising sources of natural antifungal alternatives.
Macroalgae, microalgae, and cyanobacteria, predominantly found in marine environments, are subject to various biotic and abiotic factors that influence their metabolism. Biotically, these organisms are susceptible to predators, which prompts them to synthesize and release extracellular molecules and secondary metabolites as a defense mechanism [104]. This phenomenon, known as allelopathy, involves the release of bioactive molecules for inhibiting the growth or activity of other organisms, including microorganisms, plants, and insects [105]. In algae, these allelochemicals include fatty acids and their derivatives, phenolic compounds, alkaloids, peptides, and volatile organic compounds, each synthesized via specific metabolic pathways. Fatty acids and their derivatives are produced from Acetyl-CoA through pathways that yield monounsaturated and polyunsaturated fatty acids, with derivatives formed via oxidative transformations [106]. Algae phenolic compounds, derived from the phenylpropanoid pathway using phenylalanine and tyrosine as precursors, include substances such as gallic acid, cinnamic acid, rosmarinic acid, and quercetin. These compounds modify the structure of the fungal cell membrane, resulting in the loss of K+, a reduction in ATP, and the disruption of essential enzymatic processes required for fungal survival [107]. Terpenoids, such as those in Dictyopteris polypodioides, induce stress in fungi by triggering calcium bursts within cells, damaging cell membranes, and exhibiting strong antifungal activity, particularly against the Fusarium species [95]. Alkaloids, characterized by nitrogen-containing heterocyclic structures, originate from amino acids [26]. Peptides are synthesized through non-ribosomal peptide synthesis [108]. Lastly, volatile organic compounds, such as terpenoids, hydrocarbons, and ketones, are released when algae are attacked by predators or undergo senescence [109].
The chemical and structural properties of these allelochemicals enable them to inhibit microorganisms, including fungi. These bioactive molecules from algae exhibit bio-fungistatic properties and, in some cases, bio-fungicidal activity against phytopathogenic fungi such as F. oxysporum, Phytophthora capsica, Alternaria alternata, Rhizoctonia, Rosellinia, and Sclerotinia. The antifungal activity of algal extracts shows a linear relationship with their concentration and evaluated parameters such as mycelial growth and dry weight [3]. Cyanobacteria such as Nostoc, Anabaena, Microcystis, and Scytonema have the unique ability to synthesize bioactive compounds with antifungal and antibacterial properties. These metabolites effectively control plant diseases caused by soil pathogens like Fusarium and Rhizoctonia in a highly selective manner, targeting pathogens without harming beneficial organisms in the ecosystem. Unlike conventional chemical pesticides, which can have broad, non-selective effects, these metabolites provide a more targeted and environmentally friendly approach to pest management. However, some cyanobacteria produce cytotoxic compounds that may adversely affect treated plants [110]. Cyanoabacteria produce a cyanotoxins as cytotoxins like cylindrospermopsin [111] can present possible antifungal activity against Fusarium spp. Considering that crop plants are vulnerable to over 10,000 species of phytopathogenic fungi, allelochemicals from algae represent a valuable source of antifungal compounds. Their mechanisms of action often involve targeting fungal cell walls and membranes. For example, fatty acids penetrate the chitin cell wall and interact with the ergosterol-rich cell membrane, increasing its fluidity and inducing conformational changes that lead to cell degradation [34]. Volatile organic compounds disrupt membrane integrity by peroxidizing membrane lipids [112]. Phenolic compounds interfere with cellular respiration by altering cytochrome pathways, while cyclic peptides form pores in the fungal membrane due to electrostatic interactions between their terminal amino acids and polysaccharides in the cell wall [113,114]. Alkaloids compromise fungal cell wall morphology and membrane integrity [115].
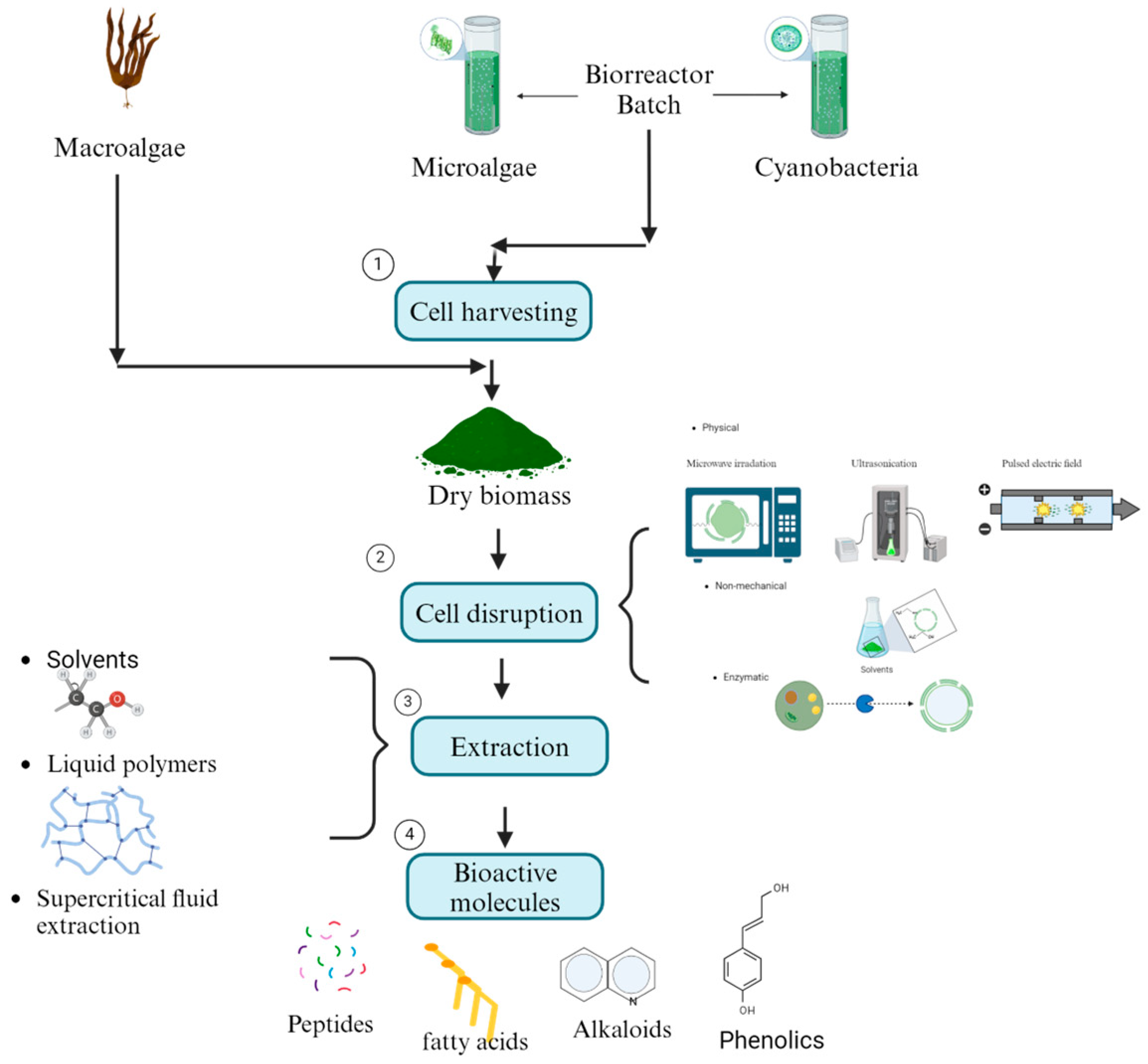
Algal bioactive molecules can be utilized in two primary ways. The first involves using living microalgae and cyanobacteria cells, which naturally release allelochemicals to inhibit phytopathogenic fungi [116]. However, this approach has been underexplored in soil environments, where the survival and efficacy of microalgae and cyanobacteria remain unclear [117]. The second, more commonly studied method involves lysing macroalgae, microalgae, and cyanobacteria cells to release their bioactive compounds. This is achieved using mechanical methods, such as microwave irradiation, ultrasonication, pulsed electric fields, and thermal treatments, or non-mechanical methods involving chemical solvents, acids, alkalis, detergents, and enzymatic treatments [118]. The bioactive molecules are then extracted using techniques such as solvent extraction with polar and non-polar solvents (e.g., diethyl ether, chloroform, ethyl acetate, acetone, ethanol, methanol, and water) or emerging bio-based solvents like ionic liquids and liquid polymers [119]. Supercritical fluid extraction (SFE) is another well-established technique that allows simultaneous extraction and separation by optimizing parameters such as temperature, pressure, flow rate, and processing time [120]. These extraction steps are essential for isolating bioactive molecules efficiently (Figure 3). Finally, the antifungal activity of crude or fractionated extracts is assessed using diffusion and dilution assays [121].
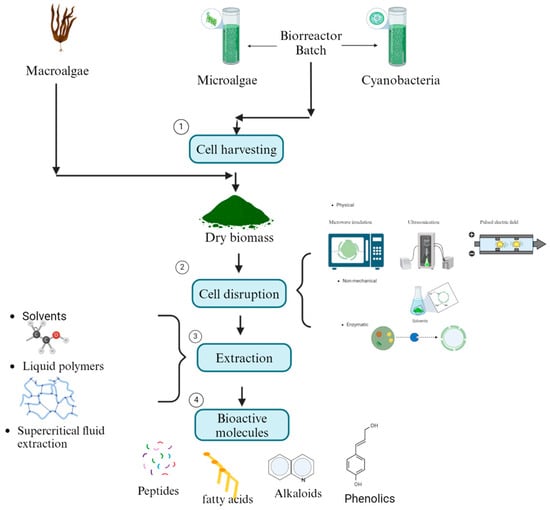
Figure 3.
Diagram with the steps of primary recovery of bioactive molecules of macro-, micro-, and cyanobacteria biomass.
Due to the focus of this review on the phytopathogen Fusarium spp., we summarize the research presented in Table 3, which reports on the antifungal activity of crude biomass extracts from macroalgae, microalgae, and cyanobacteria against Fusarium spp. Firstly, regarding cyanobacteria strains such as Spirulina sp., Scaglioni et al. (2019) [122] conducted an antifungal assay using Spirulina sp. aqueous extract by agar dilution at 25 °C for 7 days against F. graminearum and F. meridionale and they reported a mycelial growth rate as low as 0.21 cm·day−1, using water extracts. Arnhold et al. (2014) [123] used the same method and cyanobacterium but tested at 35–40 °C; the authors observed mycelial growth rates ranging from 0.1 cm·day−1 to 0.45 cm·day−1 for F. meridionale and F. graminearum using methanolic extracts. Both studies identified phenolic acids, including chlorogenic acid, protocatechuic acid, gallic acid, and caffeic acid, as the metabolites responsible for the antifungal activity. These phenolic compounds were found in both aqueous and methanolic extracts, with high-performance liquid chromatography coupled mass spectrometry (HPLC/MS) used to separate and identify them. Additionally, Nostoc calcicola and Nostoc carneum, as reported by El-Sheekh et al. (2020) [38] and Farghl and El-Sheekh (2019) [124], exhibited inhibition zones of 16 mm and 160 mm, respectively, when using methanolic and ethyl acetate extracts. GC-MS analysis identified aromatic carboxylic acids and terpenoids as the active metabolites in these extracts.

Table 3.
Antifungal metabolites extracted from biomass of macro-, micro-, and cyanobacteria.
In the case of macroalgae, Mostafa et al. (2022) reported a mycelial growth diameter of 41, 49, and 45 mm for the methanolic extracts of S. dentifolium, G. compressa, and U. lactuca, respectively, at a concentration of 20 µg·mL against F. oxysporum f. sp. lycopersici. The metabolites identified in the extract by high-performance liquid chromatography/ultraviolet-visible (HPLC/UV-Vis) were phenolics and included phloroglucinol, gallic acid, and vanillic acid [30]. On the other hand, Mohy El-Din and Mohyeldin (2018) reported inhibition zones of 19, 9, 19, and 11 mm against F. solani with the methanolic extracts of C. sinuosa, P. pavonia, C. barbata, and S. vulgare and with metabolites such as aromatic carboxylic acids and volatiles identified by GC/MS [126].
For microalgae, Senousy et al. (2022) and Scaglioni et al. (2019) identified phenolic acids such as chlorogenic acid, gallic acid, and protocatechuic acid as metabolites with antifungal activity in methanolic extracts of the biomass of Dunaliella sp., C. sorokiniana, and Nannochloropsis sp. In the case of Dunaliella sp. and C. sorokiniana, the extracts showed 82% and 40% inhibition, respectively, against F. solani. The methanolic extract of Nannochloropsis sp. exhibited a mycelial growth rate lower than 0.32 cm·day−1 against F. asiaticum, F. graminearum, and F. meridionale [122,127]. Additionally, Alallaf et al. (2021) reported that the hexane extract of N. arenaria biomass showed 22% inhibition at 6000 µg·mL−1, with a minimum inhibitory concentration (MIC) against F. oxysporum. GC-MS analysis identified aromatic carboxylic acids and sterols such as di-n-octyl phthalate and beta-sitosterol as the active metabolites [128].
Role of Algae Fatty Acids as Antifungal
The fatty acids from macroalgae, microalgae, and cyanobacteria receive special attention in this review because, when researchers analyze the metabolite profiles of bioactive molecules using GC-MS, esterified fatty acids, whether unsaturated, polyunsaturated, or a combination of both, are often among the most abundant compounds identified. This makes it interesting to discuss how these fatty acids are synthesized in macroalgae, microalgae, and cyanobacteria in general and how these biomolecules exhibit antifungal activity against Fusarium spp. This includes exploring the mode of antifungal action, which is discussed in the following section.
At the end of glycolysis, pyruvate is converted into Acetyl-CoA, which begins the synthesis of free fatty acids. Acetyl-CoA is first converted into Malonyl-CoA, which is then transferred to acyl carrier proteins (ACP) and undergoes multiple enzymatic reactions to form fatty-acyl-ACP [106]. Enzymes like β-ketoacyl-ACP synthase and β-ketoacyl-ACP reductase participate in elongation and desaturation cycles, producing fatty acids ranging from C4 to C18. Finally, Acyl-ACP thioesterase releases free fatty acids, which are essential for the production of bioactive compounds, including those with antifungal properties [129,130]. Polyunsaturated fatty acids (PUFAs), such as oleic acid (C18:1), linoleic acid (C18:2), and arachidonic acid (C20:4), are produced from C16–C18 fatty acids through desaturation by and desaturases [131,132]. Even though Acyl-ACP thioesterase is absent, the presence of free fatty acids in the cytoplasm of algae is due to a lipid remodeling or lipid degradation process in which free fatty acids are released from the membrane by lipase and are subsequently esterified with the thiol of acyl carrier protein by acyl–acyl carrier protein synthases [130]. These PUFAs, along with their derivatives, such as fatty alcohols and alkanes, have demonstrated antifungal activity. Notably, the extract exhibited an MIC50 of 50 µg·mL−1 against various pathogens, including Fusarium spp.
The fatty acids synthesized by microalgae and cyanobacteria can exhibit antifungal activity when released into the extracellular matrix through the action of lipase enzymes. Alternatively, free fatty acids can be extracted from biomass using cell lysis techniques and solvent extraction, with polar solvents like methanol and ethanol or non-polar solvents like chloroform [3]. In this review, we will focus on fatty acids extracted from dry biomass using polar solvents by specifically examining their effects on the mycelium inhibition zone (mm), percentage of inhibition, and minimal inhibitory concentration (µg mL−1).
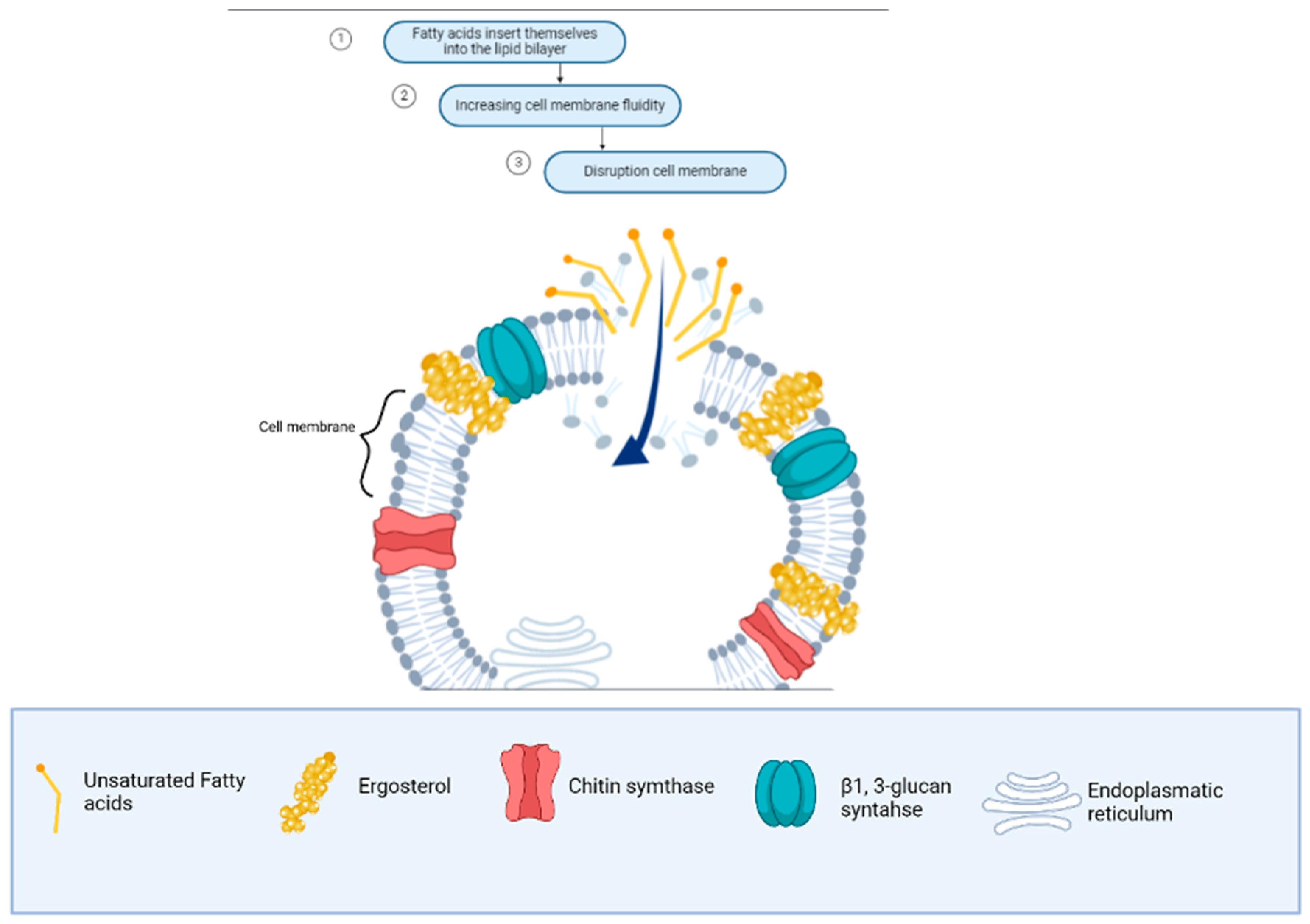
Firstly, it is important to understand the antifungal mechanism of fatty acids as carboxylic acid molecules. When these fatty acids pass through the cell wall, they interact with the ergosterol-rich membrane, disrupting its stability and cellular functions [133]. Figure 4 illustrates the sequential steps involved: increased membrane fluidity (dependent on sterol content) leads to membrane expansion, causes conformational changes in membrane proteins, and ultimately results in cytoplasmic disintegration. However, it is important to consider that the effectiveness of the antifungal activity of fatty acids depends on the sterol concentration in the fungal membrane. At low sterol concentrations, fatty acids are more effective, while at high sterol concentrations, fungi may exhibit resistance [34]. The structure of the fatty acids, including the presence of double bonds (saturated or polyunsaturated) or hydroxy groups, also influences their interaction with the cell membrane [134].
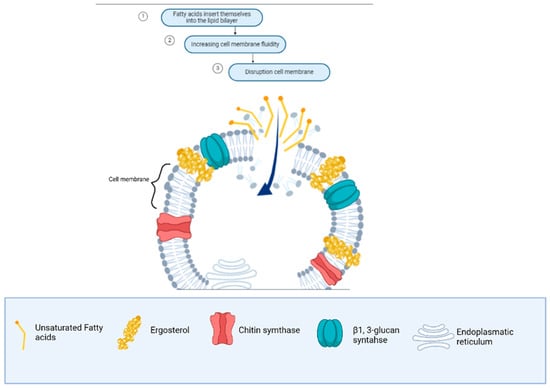
Figure 4.
Antifungal mechanism of unsaturated fatty acids from macro-, micro-, and cyanobacteria in the cell membrane of Fusarium spp.
The hydrophobic groups of saturated fatty acids are involved in antifungal activity by interacting with the cell membrane, which leads to its disintegration due to changes in intracellular hydrostatic turgor pressure [34]. For example, lauric acid (C12:0) at concentrations of 600–1200 mg·L−1 and capric acid (C10:0) at 300–1200 mg·L−1 exhibit antifungal effects [135], while Bhattacharyya et al. (2020) report that a 0.2% p/v concentration is effective in disrupting the granular cytoplasm [136]. Structurally, tanikoloide is a saturated fatty acid, with a lactone group attached to a hydroxy methylene group; it is isolated from the lipophilic extract of the cyanobacterium Lyngbya majuscula, which also demonstrates antifungal properties [137]. As shown in Table 4, El-Sheekh et al. (2022) reported the mass spectrum of two saturated fatty acids, 12,15-octadecadienoic acid methyl ester and palmitic acid, which are isolated from a methanolic extract of N. calcicola and exhibited a 16 mm inhibition zone against F. oxysporum [138]. Similarly, a study identified three saturated fatty acids—tetradecanoic acid methyl ester, hexadecanoic acid methyl ester, and eicosanoic acid methyl ester—from an ethyl acetate extract of the Nostoc carneum pellet, which showed a mycelial inhibition of 3–160 mm against F. oxysporum at a concentration of 300,000 µg·L−1 [124]. Additionally, Perveen and Alwathnani (2013) reported that compounds such as 3-chloropropionic acid, heptadecyl ester, 1,2-benzene dicarboxylic acid bis (2-ethoxyethyl) ester, and bromoacetic acid, pentadecyl ester—extracted from a methanol:acetone:diethyl ether extract of S. platensis biomass—demonstrated strong antifungal activity against F. Oxysporum [125].
As shown in Table 4, El-Sheekh et al. (2020) reported saturated fatty acids, including hexadecanoic acid methyl ester, 9-octadecenoic acid methyl ester, tetradecanoic acid methyl ester, and cis-11-eicosenoic acid methyl ester, from methanol and acetone extracts of Cystoseira myrica, Padina boergesenii, and Sargassum cinereum. These exhibited inhibition zones of 15 mm, 13 mm, and 14 mm, respectively, against F. oxysporum [38]. Similarly, Mohamed and Saber (2019) reported palmitic acid, myristic acid, and stearic acid as saturated fatty acids, with a MIC of 6 µg·mL−1 in chloroform extracts from Hemidiscus cuneiformis, showing a 16 mm inhibition zone against F. oxysporum [139]. Furthermore, Mohy El-Din and Mohyeldin (2018) identified palmitic acid and myristic acid in C. sinuosa, P. pavonia, C. barbata, and S. Vulgare methanol extracts, with inhibition zones of 19 mm, 9 mm, 19 mm, and 11 mm, respectively, against F. solani [126]. In another study, Perveen et al. (2022) reported hexadecanoic acid from diethyl ether extracts, which showed 73% growth inhibition of F. oxysporum and 50% growth inhibition of F. Solani [39].
The antifungal activity of unsaturated fatty acids, including monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), is higher than that of saturated fatty acids. This is due to the presence of double bonds, which increase the fluidity of the cell membrane. All structural aspects of PUFAs, including shape, chain length, and the position and orientation of double bonds, contribute to their antifungal activity. Cis double bonds are particularly effective, as they induce greater membrane deformation compared to trans bonds due to their lower thermodynamic stability [34,133]. Isonitrile mirabile, a polyunsaturated fatty acid with a nitrogen atom as a halogenated compound, was isolated from the lipophilic extract of the cyanobacterium Scytonema mirabile and exhibited antifungal activity. Additionally, majusculoic acid, a PUFA containing bromine, was isolated from marine cyanobacteria and demonstrated antifungal properties [137]. Furthermore, Farghl and El-Sheekh (2019) reported PUFAs, which included tetradecanoic acid 12-methyl ester, 9,12-octadecadienoic acid methyl ester, and 9-octadecenoic acid methyl ester with antifungal activity against F. oxysporum and showed a 160 mm inhibition zone [124].

Table 4.
Fatty acids from macro- and microalgae and cyanobacteria with antifungal activity against Fusarium spp.
Table 4.
Fatty acids from macro- and microalgae and cyanobacteria with antifungal activity against Fusarium spp.
| Specie | Organism | Fatty Acids | Growth/Conditions | Solvent Extraction | Technique | Positive Drug Control | Result | References |
|---|---|---|---|---|---|---|---|---|
| N. calcicola | Cyanobacteria | 9-octadecenoic acid Oleic acid 12,15-octadecadienoic acid methyl ester Palmitic acid | Batch 28 °C, 24 h light BG-11 medium | Methanol | GC/MS | NA | 16 mm of inhibition zone mycelium of F. oxysporum f. sp. lycopersici | [138] |
| N. carneum | Cynobacteria | Palmitic acid 9,12-Octadecadienoic acid Alpha-Linoleic acid | Batch 30 °C, 12 h light BG-11 medium | Ethyl-acetate Ethanol | GC/MS | NA | 3, 160 mm of inhibition zone mycelium of F. oxysporum | [124] |
| Lyngnbya wollei | Cyanobacteria | Gamma-Linoleic acid Palmitic acid 6,9,12,15-Octadecatetraenoicacid, methyl ester | Batch 28 °C 16 h light BG-11 medium | Methanol | GC/MS | NA | 46% inhibition observed against F. udum and 40% against F. oxysporum | [107] |
| Oscillatoria princeps | Cyanobacteria | Alpha-Linoleic acid 9-Octadecenoic acid Hexadecanoic acid 9,15-Octadecadienoic acid 11-Octadecenoic acid | BG-11 | Diethyl ether | GC/MS | Nystatin | Inhibition zone 14 mm F. verticelloides and 15 mm F. proleferatum | [140] |
| C. myrica. P. boergesenii S. cinereum | Macroalgae | Hexadecanoic acid, methyl ester 9-octadecenoic acid, methyl ester Tetradecanoic acid, methyl ester cis-11-Eicosenoic acid, methyl ester | NA | Methanol Acetone | GC/MS | Gentamycine and Ampicilline | Inhibition zone (mm) 15, 13, 14 by C. myrica, S. cinereum and P. boergesenii to F. oxyporum | [127] |
| H. cuneiformis | Macroalgae | Palmitic acid Myristic acid Stearic acid Oleic acid Palmitoleic acid | NA | Chloroform | GC/MS | Amphotericin B | 6 µg/mL as MIC and 16 mm of inhibition zone of F. oxysporum | [139] |
| C. sinuosa. P. pavonia. C. barbata. S.vulgare | Macroalgae | Palmitic acid Myristic acid Palmitoleic acid Oleic acid Linoleic acid | NA | Methanol | GC/MS | Miconazole Flucanozole Itraconzaole | Inhibition zone diameters 19 mm, 9 mm, 19 mm, 11 mm by C. sinuosa, P. pavonisa, C. barbata and S. vulgare, respectively, to F. solani | [126] |
| C. vulgaris | Microalgae | Hexadecanoic acid Octadecenoic acid, methyl ester. | 25 °C 24 h light BG-11 Medium | Diethyl ether | GC/MS | NA | Growth inhibition (%) 73 F. oxysporum 50 F. solani | [39] |
GC/MS (gas chromatography/mass spectrometry); MIC (minimum inhibitory concentration); NA (not applicable).
5. Future Directions and Challenges
Current research has highlighted the potential of algal and cyanobacterial extracts as valuable sources of antifungal bioactive molecules [3]. Although general antimicrobial properties are well documented [141], there is limited research on their antifungal effects [137], particularly against phytopathogenic fungi like Fusarium spp.
In vitro methods for evaluating antifungal activity, including percentage inhibition and inhibition zones (mm), are well-established for plant extract [142]. However, studies on algae crude extracts often lack MIC (µg mL−1) determination, focusing mainly on inhibition percentages. This gap highlights the need for a more thorough evaluation of the antifungal potential of algae metabolites, particularly with phytopathogenic fungi.
Crude extracts are typically characterized by GC/MS, but this technique only detects volatile compounds and some fatty acids, often without derivatization [143]. Therefore, combining GC/MS with HPLC/MS is recommended to capture a broader range of metabolites, as these techniques reveal significantly different metabolite concentrations [144]. This dual approach would provide a more comprehensive view of the metabolites in the crude extract [145].
However, further studies are necessary to better understand the mechanisms by which these bioactive molecules interact with the Fusarium spp. cell wall [146]. The cell wall of Fusarium spp. consists of a glycoprotein-rich outer layer and a chitin/glucan matrix, which is critical for developing selective fungicides [147,148]. Metabolites such as phenolic acids, aromatic compounds, volatile compounds, and bioactive molecules like fatty acids have been shown to interact with the cell membrane and disrupt the cell wall [26,149,150]. Several hypotheses exist regarding how these metabolites affect the cell wall. First, bioactive molecules may form complexes with cell wall polymers, leading to conformational changes [114]. Second, antifungal biomolecules may penetrate the cell wall polymers and act directly on the ergosterol membrane [147]. Finally, these molecules may inhibit chitin synthase and β(1,3)-D-glucan synthase through competitive or non-competitive inhibition [151]. These mechanisms explain how bioactive molecules can interact with the cell wall, reach the cell membrane, and exert antifungal activity [148]. In line with these findings, environmental impact assessments of cyanobacterial and microalgal cultivation technologies, as well as large-scale seaweed harvesting, should be conducted to ensure the sustainability of these methods [141,152]. Scalability challenges in microalgal biomass use stem from the complexity and cost of harvesting and extracting bioactive metabolites. A study on producing 22,000 L of amphidinol-based fungicide in pilot-scale reactors found that adopting a biorefinery approach, which included co-products such as fatty acids and carotenoids, resulted in 34.61 tons of CO2 emissions over 15 years. This approach reduced the Global Warming Potential by 82–98% and demonstrated lower toxicity compared to commercial fungicides. Key improvements for commercial viability include enhancing bioreactors, reducing carbon footprint, using alternative media like wastewater, and optimizing extraction processes [153]. Moreover, the extraction and purification of high-value lipids, such as PUFAs, remain expensive and technologically demanding. While algal lipids exhibit useful functional properties, their performance can be inferior to low-molecular-weight surfactants in specific applications. Genetic modification of algae presents a promising strategy to enhance light utilization efficiency and increase lipid yield [154,155].
Nevertheless, although research on bioactive molecules with antifungal activity against Fusarium is still limited, most studies have focused on crude extracts using organic solvents, which range from non-polar to polar. These studies generally identify the chromatographic profile of bioactive compounds based on their polarity [3,156]. Researchers commonly use GC/MS as a chromatographic technique due to its ability to separate volatile bioactive molecules or those that can be derivatized, facilitating the identification of their structure. Fatty acid methyl esters, aromatic carboxylic acids, and volatile compounds can be detected using GC/MS, which is often preferred over HPLC for its robustness and ability to detect non-volatile compounds through derivatization [157,158]. However, both chromatography techniques are essential for a comprehensive analysis of all bioactive molecules in crude extracts [156]. Crude extract analysis is not conducted in isolation; compounds are fractionated and analyzed individually, which is crucial for identifying specific antifungal compounds [159]. This specificity is key to identifying and characterizing effective antifungal molecules [160].
Cyanobacteria such as Nostoc spp. and Anabaena spp. are known to synthesize cyclic peptides and lipopeptides with antifungal activity, but these molecules have mainly been studied in relation to human fungal pathogens like Candida spp. [161,162]. In contrast, phenolic acids are the most reported antifungal metabolites in eukaryotic microalgae and macroalgae [163]. Despite the known antifungal properties of these bioactive molecules in biological control, research on their effectiveness against phytopathogenic fungi is limited [35]. Future studies should first evaluate the antifungal activity of crude extracts against phytopathogenic fungi like Fusarium spp., followed by a determination of the profile of active compounds in the extract [164]. While this experimental approach is valid, future research should prioritize evaluating purified molecules such as fatty acids, phenolic acids, and cyclic peptides against phytopathogenic fungi, rather than relying solely on crude extract activity [161,162,165]. In this context, allelopathy in cyanobacteria and algae plays a crucial role by releasing metabolites with antibacterial and antifungal effects, which can be studied through co-cultivation to activate silent genes under stress [105,166]. Additionally, algae excrete volatile organic compounds with antifungal potential, and while their isolation is challenging, methods like counter-current chromatography are gaining interest for their high recovery and cost-effectiveness [112,167].
Existing chromatographic and spectroscopic profiles from studies on antifungal activity against Fusarium spp. suggest that fats and volatile compounds such as alkanes and hydrocarbons are the most important components with antifungal properties [104,137]. Since fatty acids are the most predominant bioactive compounds against Fusarium spp., they can be purified from oil extracts and further evaluated for their antifungal activity [168]. If their antifungal activity proves promising, additional studies will be needed to assess their potential in planta or in crop assays infected with Fusarium spp. [169]. Practical implementation of sustainable farming practices, advanced analytical tools, fractionation of extracts, investigation of well-known classes of antifungals, and a deeper understanding of the specificity of fatty acids are key directions for fully utilizing this natural resource in sustainable and effective applications [35,168,170]. Building on sustainable farming practices and advanced analytical techniques, we conclude that marine organisms offer bioactive compounds with potential as biofungicides. Understanding their pharmacokinetics, including absorption, distribution, metabolism, and excretion, is essential for optimizing their application in crops for human consumption [171]. Studies on Schizochytrium sp. oil demonstrated over 90% cell viability in Caco-2 cells after 48 h of exposure to 200 µg/mL of DHA, and acute toxicity in vivo tests classified it as category 5 under OECD 423 guidelines, with the highest safety level at 2000 mg/kg [172]. Furthermore, nanoencapsulation of fucoxanthin from Phaeodactylum tricornutum significantly improved metabolite absorption, although challenges persist, such as the low oral bioavailability of compounds like astaxanthin (90.1% lower compared to intravenous administration) [173]. These findings suggest algal metabolites may improve the safety of marine-derived biofungicides in agriculture by ensuring low oral bioavailability. However, further studies on their pharmacology in animals are needed to ensure safe and effective use. In parallel, the integration of Artificial Intelligence (AI) in discovering antifungal metabolites, such as cyclic peptides and lipopeptides, is a growing trend [174]. AI techniques like Generative Adversarial Networks, Variational Autoencoders, Recurrent Neural Networks, and Graph Neural Networks can encode peptide prototypes and generate analogs through latent space sampling [175,176]. Additionally, AI-based databases such as GNPS, NPAtlas, and DNP help identify fragmentation patterns and unique compounds, streamlining the antifungal screening process by dereplicating known compounds and prioritizing novel antifungal metabolites [166].
6. Conclusions
Macroalgae, microalgae, and cyanobacteria are emerging as sustainable sources of natural antifungal agents, with crude polar extracts demonstrating notable antifungal activity against Fusarium spp. This is evidenced by metrics such as percentage inhibition, mycelial inhibition zones, and growth rates. Although the fungicidal effects have not been thoroughly investigated, these results present a valuable opportunity to fractionate the extracts, identify minimum inhibitory concentrations (MICs), and perform additional assays to gain a deeper understanding of the mechanisms involved. Investigating variations in ergosterol and chitosan levels could provide insights into whether these bioactive compounds influence fungal membranes and cell walls. Gas chromatography analysis has pinpointed fatty acids as significant bioactive metabolites, indicating their potential for purification and separate evaluation. Furthermore, phenolic compounds and other metabolites deserve more attention. The ability of algae and cyanobacteria to thrive in wastewater and saline conditions and to sequester CO2 enhances their appeal for biocontrol applications, while lower cultivation costs are required as compared to terrestrial plants. This review serves as the first extensive compilation of research on the antifungal properties of algae and cyanobacteria extracts, particularly against Fusarium spp., establishing a foundation for future studies that could transform agricultural practices by providing eco-friendly and cost-effective solutions for tackling fungal pathogens.
Author Contributions
Conceptualization, M.E.L.-A. and G.M.G.-M.; methodology, M.E.L.-A., G.M.G.-M., L.D.L.-P. and J.H.E.-L., writing—original draft preparation, M.E.L.-A. and G.M.G.-M.; resources, G.M.G.-M.; writing—review and editing, M.E.L.-A., G.M.G.-M., L.D.L.-P. and J.H.E.-L.; project administration, G.M.G.-M.; supervision, G.M.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tecnologico de Monterrey project entitled “Exploring and optimizing CO2 bio fixation process for microalgae lipids production to formulate green metalworking fluids—for cleaner manufacturing processes” (ID: I023—IAMSM002—C4-T2—E). Challenge-Based Research Funding Program call 2022.
Acknowledgments
Partial support for this work was provided by the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT), Mexico, under the Sistema Nacional de Investigadoras e Investigadores (SNII) program, awarded to Georgia María González Meza [CVU: 490688] and Joel H. Elizondo Luevano [CVU: 418935]. The authors also extend their gratitude to CONAHCYT for the Technology Scholarship awarded to Miguel E. Lopez Arellanes [CVU: 1064202].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agnolucci, P.; Rapti, C.; Alexander, P.; De Lipsis, V.; Holland, R.A.; Eigenbrod, F.; Ekins, P. Impacts of Rising Temperatures and Farm Management Practices on Global Yields of 18 Crops. Nat. Food 2020, 1, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Baek, K.-H. Control of Phytopathogens Using Sustainable Biogenic Nanomaterials: Recent Perspectives, Ecological Safety, and Challenging Gaps. J. Clean. Prod. 2022, 372, 133729. [Google Scholar] [CrossRef]
- Rampersad, S. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and Toxicity of a Fusarium Mycotoxin, Zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Ahmad, K.; Bashir Kutawa, A.; Siddiqui, Y.; Saad, N.; Geok Hun, T.; Hata, E.M.; Hossain, M.I. Biology, Diversity, Detection and Management of Fusarium oxysporum f. sp. Niveum Causing Vascular Wilt Disease of Watermelon (Citrullus lanatus): A Review. Agronomy 2021, 11, 1310. [Google Scholar] [CrossRef]
- de Chaves, M.A.; Reginatto, P.; da Costa, B.S.; de Paschoal, R.I.; Teixeira, M.L.; Fuentefria, A.M. Fungicide Resistance in Fusarium graminearum Species Complex. Curr. Microbiol. 2022, 79, 62. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J. The Fusarium solani Species Complex: Ubiquitous Pathogens of Agricultural Importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Singh, A.; Sirari, A.; Anupam; Kaur, K.; Kumar, A.; Lal, M.K.; Tiwari, R.K.; Kumar, R. Investigating the Impact of Fungicides and Mungbean Genotypes on the Management of Pod Rot Disease Caused by Fusarium equiseti and Fusarium chlamydosporum. Front. Plant Sci. 2023, 14, 1164245. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, X.; Cao, Y.; Song, Z.; Ma, K.; Fan, Y.; Ma, M. Fusarium culmorum and Fusarium equiseti Causing Root Rot Disease on Lycium barbarum (Goji Berry) in China. Plant Dis. 2020, 104, 3066. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P. Time for Re-Evaluating the Human Carcinogenicity of Ethylenedithiocarbamate Fungicides? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2632. [Google Scholar] [CrossRef] [PubMed]
- Subba, R.; Mathur, P. Functional Attributes of Microbial and Plant Based Biofungicides for the Defense Priming of Crop Plants. Theor. Exp. Plant Physiol. 2022, 34, 301–333. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, J.; Tan, T.; Xu, J.; Shen, A.; Yang, X.; Li, J.; Zeng, L.; Wei, L. Isolation and Characterization of Antagonistic Paenibacillus polymyxa HX-140 and Its Biocontrol Potential against Fusarium Wilt of Cucumber Seedlings. BMC Microbiol. 2021, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gutiérrez, C.; Arroyave, C.; Llugany, M.; Poschenrieder, C.; Martos, S.; Peláez, C. Trichoderma asperellum as a Preventive and Curative Agent to Control Fusarium Wilt in Stevia Rebaudiana. Biol. Control 2021, 155, 104537. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological Potential of Bacillus subtilis V26 for the Control of Fusarium Wilt and Tuber Dry Rot on Potato Caused by Fusarium Species and the Promotion of Plant Growth. Biol. Control 2021, 152, 104444. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhao, H.; Ni, Y.; Lian, Q.; Qian, H.; He, B.; Liu, H.; Ma, Q. Biological Control of Fusarium Wilt of Sesame by Penicillium bilaiae 47M-1. Biol. Control 2021, 158, 104601. [Google Scholar] [CrossRef]
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in Biological Control Strategies to Manage Microbial Plant Pathogens: A Review. Arch. Microbiol. 2022, 204, 666. [Google Scholar] [CrossRef]
- Simbo, D.; Elena, P.; Meisam, Z.; Dire, A.A.A.A.; Parpura, D.; Lapshin, G.; Abdullah, B. Yield Losses of Cereal Crops by Fusarium Link: A Review on the Perspective of Biological Control Practices. Res. Crops 2022, 23, 418–436. [Google Scholar] [CrossRef]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural Products from Medicinal Plants against Phytopathogenic Fusarium Species: Current Research Endeavours, Challenges and Prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Ma, J.; Liu, L.; Li, J.; Shen, T.; Tian, Y. The Major Component of Cinnamon Oil as a Natural Substitute against Fusarium solani on Astragalus membranaceus. J. Appl. Microbiol. 2022, 132, 3125–3141. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, A.; Ippolito, A.; Sanzani, S.M. First Report of Stemphylium eturmiunum Causing Postharvest Rot of Sweet Cherry in Italy. Crop Prot. 2020, 132, 105112. [Google Scholar] [CrossRef]
- Senouci, H.; Benyelles, N.G.; Dib, M.E.A.; Costa, J.; Muselli, A. Chemical Composition and Combinatory Antifungal Activities of Ammoides Verticillata, Allium Sativum and Curcuma Longa Essential Oils Against Four Fungi Responsible for Tomato Diseases. Comb. Chem. High Throughput Screen. 2020, 23, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.-L. Anticancer, Antiviral, Antibacterial, and Antifungal Properties in Microalgae. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–261. [Google Scholar]
- Afzal, S.; Yadav, A.K.; Poonia, A.K.; Choure, K.; Yadav, A.N.; Pandey, A. Antimicrobial Therapeutics Isolated from Algal Source: Retrospect and Prospect. Biologia 2022, 78, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Munaro, D.; Nunes, A.; Schmitz, C.; Bauer, C.; Coelho, D.S.; Oliveira, E.R.; Yunes, R.A.; Moura, S.; Maraschin, M. Metabolites Produced by Macro-and Microalgae as Plant Biostimulants. Stud. Nat. Prod. Chem. 2021, 71, 87–120. [Google Scholar]
- Elsheikh, S.; Eltanahy, E. Overview of Secondary Metabolites in Cyanobacteria: A Potential Source of Plant Growth-Promoting and Abiotic Stress Resistance. In Bacterial Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 29–57. [Google Scholar]
- Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Taher, M.A.; Baka, Z.A. In Vitro and In Vivo Biocontrol of Tomato Fusarium Wilt by Extracts from Brown, Red, and Green Macroalgae. Agriculture 2022, 12, 345. [Google Scholar] [CrossRef]
- Vicente, T.F.L.; Lemos, M.F.L.; Félix, R.; Valentão, P.; Félix, C. Marine Macroalgae, a Source of Natural Inhibitors of Fungal Phytopathogens. J. Fungi 2021, 7, 1006. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; van Staden, J. Bioprospecting for Bioactive Compounds in Microalgae: Antimicrobial Compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Asimakis, E.; Shehata, A.A.; Eisenreich, W.; Acheuk, F.; Lasram, S.; Basiouni, S.; Emekci, M.; Ntougias, S.; Taner, G.; May-Simera, H.; et al. Algae and Their Metabolites as Potential Bio-Pesticides. Microorganisms 2022, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Toshkova-Yotova, T.; Georgieva, A.; Iliev, I.; Alexandrov, S.; Ivanova, A.; Pilarski, P.; Toshkova, R. Antitumor and Antimicrobial Activity of Fatty Acids from Green Microalga Coelastrella sp. BGV. S. Afr. J. Bot. 2022, 151, 394–402. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Mousa, A.S.H.; Farghl, A.A.M. Biological Control of Fusarium Wilt Disease of Tomato Plants Using Seaweed Extracts. Arab. J. Sci. Eng. 2020, 45, 4557–4570. [Google Scholar] [CrossRef]
- Perveen, K.; Bukhari, N.A.; Al Masoudi, L.M.; Alqahtani, A.N.; Alruways, M.W.; Alkhattaf, F.S. Antifungal Potential, Chemical Composition of Chlorella vulgaris and SEM Analysis of Morphological Changes in Fusarium oxysporum. Saudi J. Biol. Sci. 2022, 29, 2501–2505. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Mehta, K.; Prajapati, J.; Shukla, A.; Parmar, P.; Goswami, D.; Saraf, M. An Anecdote of Mechanics for Fusarium Biocontrol by Plant Growth Promoting Microbes. Biol. Control 2022, 174, 105012. [Google Scholar] [CrossRef]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell Wall and Glycoproteins and Their Crucial Role in the Phytopathogenic Fungi Infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for Controlling the Sporulation in Fusarium spp. J. Fungi 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, D.A.; Ivanova, E.A.; Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Tkhakakhova, A.K.; Kholodov, V.A. Diversity, Ecological Characteristics and Identification of Some Problematic Phytopathogenic Fusarium in Soil: A Review. Diversity 2023, 15, 49. [Google Scholar] [CrossRef]
- Nag, P.; Paul, S.; Shriti, S.; Das, S. Defence Response in Plants and Animals against a Common Fungal Pathogen, Fusarium oxysporum. Curr. Res. Microb. Sci. 2022, 3, 100135. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Tahira, R.; Jamal, A. Fusarium spp. Mycotoxin Production, Diseases and Their Management: An Overview. Pak. J. Agric. Res. 2021, 34, 254–493. [Google Scholar] [CrossRef]
- Rauwane, M.E.; Ogugua, U.V.; Kalu, C.M.; Ledwaba, L.K.; Woldesemayat, A.A.; Ntushelo, K. Pathogenicity and Virulence Factors of Fusarium graminearum Including Factors Discovered Using Next Generation Sequencing Technologies and Proteomics. Microorganisms 2020, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Stępień, Ł.; Lalak-Kańczugowska, J. Signaling Pathways Involved in Virulence and Stress Response of Plant-Pathogenic Fusarium Species. Fungal Biol. Rev. 2021, 35, 27–39. [Google Scholar] [CrossRef]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Chang, J.; Yang, W. Infection Strategies and Pathogenicity of Biotrophic Plant Fungal Pathogens. Front. Microbiol. 2022, 13, 799396. [Google Scholar] [CrossRef]
- Husaini, A.M.; Sakina, A.; Cambay, S.R. Host–Pathogen Interaction in Fusarium oxysporum Infections: Where Do We Stand? Mol. Plant Microbe Interact. 2018, 31, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Lal, D.; Dev, D.; Kumari, S.; Pandey, S.; Aparna; Sharma, N.; Nandni, S.; Jha, R.K.; Singh, A. Fusarium Wilt Pandemic: Current Understanding and Molecular Perspectives. Funct. Integr. Genom. 2024, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking Intruders: Inducible Physico-Chemical Barriers against Plant Vascular Wilt Pathogens. J. Exp. Bot. 2021, 72, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Williamson-Benavides, B.A.; Dhingra, A. Understanding Root Rot Disease in Agricultural Crops. Horticulturae 2021, 7, 33. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Degani, O. A Review: Late Wilt of Maize—The Pathogen, the Disease, Current Status, and Future Perspective. J. Fungi 2021, 7, 989. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight From a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef] [PubMed]
- Dahl, B.; Wilson, W.W. Risk Premiums Due to Fusarium Head Blight (FHB) in Wheat and Barley. Agric. Syst. 2018, 162, 145–153. [Google Scholar] [CrossRef]
- Scott, C.; Punja, Z.K. Biological Control of Fusarium oxysporum Causing Damping-off and Pythium myriotylum Causing Root and Crown Rot on Cannabis (Cannabis sativa L.) Plants. Can. J. Plant Pathol. 2023, 45, 238–252. [Google Scholar] [CrossRef]
- Smiley, R.W.; Machado, S. Fusarium Crown Rot of Winter Wheat Influenced by Resource Competition Near a Tree Windbreak. Plant Dis. 2020, 104, 348–357. [Google Scholar] [CrossRef]
- Desai, S.; Dubey, S.C.; Prasad, R.D. Impacts of Climate Change on Fusarium Species Vis-à-Vis Adaptation Strategies. Indian Phytopathol. 2020, 73, 593–603. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Meng, C.; Zhang, D.; Xu, N.; Yu, J. Establishment and Application of a Multiplex PCR Assay for Detection of Rhizoctonia cerealis, Bipolaris sorokiniana, and Fusarium spp. in Winter Wheat. J. Plant Pathol. 2020, 102, 19–27. [Google Scholar] [CrossRef]
- Omori, A.M.; Ono, E.Y.S.; Bordini, J.G.; Hirozawa, M.T.; Fungaro, M.H.P.; Ono, M.A. Detection of Fusarium Verticillioides by PCR-ELISA Based on FUM21 Gene. Food Microbiol. 2018, 73, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Lin, H.-K.; Lin, Y.-H. Construction of Raman Spectroscopic Fingerprints for the Detection of Fusarium Wilt of Banana in Taiwan. PLoS ONE 2020, 15, e0230330. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Becerra, R.; López-Lima, D.; Villain, L.; Breitler, J.-C.; Carrión, G.; Desgarennes, D. Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease. J. Fungi 2021, 7, 253. [Google Scholar] [CrossRef]
- Almasi, M.A. Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Visual Detection of Fusarium oxysporum f.sp. Melonis. Hortic. Plant J. 2019, 5, 129–136. [Google Scholar] [CrossRef]
- Heltoft, P.; Brurberg, M.B.; Skogen, M.; Le, V.H.; Razzaghian, J.; Hermansen, A. Fusarium spp. Causing Dry Rot on Potatoes in Norway and Development of a Real-Time PCR Method for Detection of Fusarium Coeruleum. Potato Res. 2016, 59, 67–80. [Google Scholar] [CrossRef]
- Tziros, G.T.; Samaras, A.; Karaoglanidis, G.S. Fusarium Equiseti as an Emerging Foliar Pathogen of Lettuce in Greece: Identification and Development of a Real-Time PCR for Quantification of Inoculum in Soil Samples. Pathogens 2022, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Mamolini, E.; De Bastiani, M.; Marchetti, M. Quantitative Determination of Fusarium proliferatum Concentration in Intact Garlic Cloves Using Near-Infrared Spectroscopy. Sensors 2016, 16, 1099. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.J.; Borland, T.G.; Bergl, D.D.; Claassen, B.J.; Flodquist, T.A.; Montgomery, A.S.; Rivedal, H.M.; Woodhall, J.; Ocamb, C.M.; Gent, D.H. A Quantitative PCR Assay for Detection and Quantification of Fusarium sambucinum. Plant Dis. 2022, 106, 2601–2606. [Google Scholar] [CrossRef]
- Wang, J.; Jacobs, J.L.; Byrne, J.M.; Chilvers, M.I. Improved Diagnoses and Quantification of Fusarium virguliforme, Causal Agent of Soybean Sudden Death Syndrome. Phytopathology 2015, 105, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Höfle, L.; Werner, B.T.; Imani, J.; Schmidt, A.; Jelonek, L.; Kogel, K. SIGS vs HIGS: A Study on the Efficacy of Two DsRNA Delivery Strategies to Silence Fusarium FgCYP51 Genes in Infected Host and Non-host Plants. Mol. Plant Pathol. 2019, 20, 1636–1644. [Google Scholar] [CrossRef]
- Theron, J.S.; Johannes van Coller, G.; Rose, L.J.; Labuschagne, J.; Swanepoel, P.A. The Effect of Crop Rotation and Tillage Practice on Fusarium Crown Rot and Agronomic Parameters of Wheat in South Africa. Crop Prot. 2023, 166, 106175. [Google Scholar] [CrossRef]
- Jing, T.; Zhou, D.; Zhang, M.; Yun, T.; Qi, D.; Wei, Y.; Chen, Y.; Zang, X.; Wang, W.; Xie, J. Newly Isolated Streptomyces sp. JBS5-6 as a Potential Biocontrol Agent to Control Banana Fusarium Wilt: Genome Sequencing and Secondary Metabolite Cluster Profiles. Front. Microbiol. 2020, 11, 602591. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Zhou, D.; Zhang, M.; Qi, D.; Jing, T.; Zang, X.; Qi, C.; Wang, W.; Xie, J. Biological Control of Banana Wilt Disease Caused by Fusarium oxyspoum f. sp. Cubense Using Streptomyces sp. H4. Biol. Control 2021, 155, 104524. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mohamed, A.A.; Ali, H.M.; Al Farraj, D.A. Characterization of Phytoconstituents from Alcoholic Extracts of Four Woody Species and Their Potential Uses for Management of Six Fusarium Oxysporum Isolates Identified from Some Plant Hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Laverde, D.; Barbosa-Cornelio, R.; Coy-Barrera, E. Antifungal Activity against Fusarium Oxysporum of Botanical End-Products: An Integration of Chemical Composition and Antifungal Activity Datasets to Identify Antifungal Bioactives. Plants 2021, 10, 2563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, C.; Luo, X.; Li, A.; Zhang, S.; An, J.; Zhang, Z.; Ma, Y.; Zhang, B.; Liu, Y. Design, Synthesis, and Biological Evaluation of Novel Berberine Derivatives against Phytopathogenic Fungi. Pest Manag. Sci. 2022, 78, 4361–4376. [Google Scholar] [CrossRef] [PubMed]
- Sama, H.; Sombié, P.A.E.D.; Guenne, S.; Soura, H.B.; Hilou, A. Antifungal Potential and Fatty Acid Profile of Two Jatropha curcas (Euphorbiaceae) Oils. J. Agric. Food Res. 2021, 6, 100244. [Google Scholar] [CrossRef]
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.-B.; Jardin, J.; Coton, M.; Leyva Salas, M.; Coton, E.; Valence, F.; Mounier, J. Antifungal Activity of Fermented Dairy Ingredients: Identification of Antifungal Compounds. Int. J. Food Microbiol. 2020, 322, 108574. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; El-Wakil, D.A.; Hashem, A.H.; Abdelaziz, A.M. Antagonistic Effect of Plant Growth-Promoting Fungi Against Fusarium Wilt Disease in Tomato: In Vitro and In Vivo Study. Appl. Biochem. Biotechnol. 2022, 194, 5100–5118. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.L.; Latz, M.A.C.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of Fungal Endophytes with Biocontrol Potential against Fusarium Head Blight in Wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Kemp, N.D.; Vaughan, M.M.; McCormick, S.P.; Brown, J.A.; Bakker, M.G. Sarocladium zeae Is a Systemic Endophyte of Wheat and an Effective Biocontrol Agent against Fusarium Head Blight. Biol. Control 2020, 149, 104329. [Google Scholar] [CrossRef]
- Chacón-Cerdas, R.; Barboza-Barquero, L.; Albertazzi, F.J.; Rivera-Méndez, W. Transcription Factors Controlling Biotic Stress Response in Potato Plants. Physiol. Mol. Plant Pathol. 2020, 112, 101527. [Google Scholar] [CrossRef]
- Tuyen, D.T.; Trung, N.T.; Thao, N.T.; Le Thanh, N.S.; Dai Nguyen, N.P.; Anh Tuyet, N.T.; Cuong, N.T.; Chan, S.S.; Khoo, K.S.; Show, P.L. Antifungal Activity of Secondary Metabolites Purified from Bacillus subtilis Isolated in Vietnam and Evaluated on in Vitro and in Vivo Models. Int. Biodeterior. Biodegrad. 2023, 179, 105558. [Google Scholar] [CrossRef]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species Against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Luan, P.; Fan, M.; Wu, X.; Sun, Z.; Shang, Z.; Yang, Y.; Li, C. Antifungal Efficacy of Bacillus amyloliquefaciens ZK-9 against Fusarium graminearum and Analysis of the Potential Mechanism of Its Lipopeptides. Int. J. Food Microbiol. 2024, 422, 110821. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Ponce de la Cal, A.; Louis, Y.; Baró Robaina, Y.; Coll, Y.; Spengler, I.; Mirabal-Gallardo, Y. Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53). J. Fungi 2024, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Lakhdari, W.; Benyahia, I.; Bouhenna, M.M.; Bendif, H.; Khelafi, H.; Bachir, H.; Ladjal, A.; Hammi, H.; Mouhoubi, D.; Khelil, H.; et al. Exploration and Evaluation of Secondary Metabolites from Trichoderma harzianum: GC-MS Analysis, Phytochemical Profiling, Antifungal and Antioxidant Activity Assessment. Molecules 2023, 28, 5025. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.; Divyashree, M.; Mani, M.K.; Mamatha, B.S. Algae and Cyanobacteria as a Source of Novel Bioactive Compounds for Biomedical Applications. In Advances in Cyanobacterial Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–194. [Google Scholar]
- Singh, S.K.; Kaur, R.; Bansal, A.; Kapur, S.; Sundaram, S. Biotechnological Exploitation of Cyanobacteria and Microalgae for Bioactive Compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–259. [Google Scholar]
- Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms 2022, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Gupta, E.; Singh, P.; Prasad, R. Application of Microalgae Metabolites in Food and Pharmaceutical Industry. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 391–408. [Google Scholar]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef] [PubMed]
- Chanthini, K.M.-P.; Senthil-Nathan, S. Marine Weeds against Fungal Phytopathogens—Current Agronomical Implications and Intriguing Perspectives for a Sustainable Future. Physiol. Mol. Plant Pathol. 2024, 130, 102240. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Martins, A.P.; Colepicolo, P.; Yokoya, N.S. Concise Review on Seaweed Photosynthesis: From Physiological Bases to Biotechnological Applications. J. Photochem. Photobiol. 2023, 16, 100194. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from Microalgae and Cyanobacteria: Applications and Market Survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential Utilization of Bioproducts from Microalgae for the Quality Enhancement of Natural Products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Woodhouse, J.N. Defining Cyanobacterial Species: Diversity and Description Through Genomics. CRC Crit. Rev. Plant Sci. 2020, 39, 101–124. [Google Scholar] [CrossRef]
- Springstein, B.L.; Nürnberg, D.J.; Weiss, G.L.; Pilhofer, M.; Stucken, K. Structural Determinants and Their Role in Cyanobacterial Morphogenesis. Life 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Noreña-Caro, D.; Benton, M.G. Cyanobacteria as Photoautotrophic Biofactories of High-Value Chemicals. J. CO2 Util. 2018, 28, 335–366. [Google Scholar] [CrossRef]
- Tibocha-Bonilla, J.D.; Zuñiga, C.; Godoy-Silva, R.D.; Zengler, K. Advances in Metabolic Modeling of Oleaginous Microalgae. Biotechnol. Biofuels 2018, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; Macrae, A.; de Souza, J.E.; Neves Junior, A.; Vermelho, A.B. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants 2023, 12, 1896. [Google Scholar] [CrossRef]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and Allelochemicals from Microalgae: An Innovative Source for Bio-Herbicidal Compounds and Biocontrol Research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Choi, Y.-N.; Lee, J.W.; Kim, J.W.; Park, J.M. Acetyl-CoA-Derived Biofuel and Biochemical Production in Cyanobacteria: A Mini Review. J. Appl. Phycol. 2020, 32, 1643–1653. [Google Scholar] [CrossRef]
- Verma, S.; Thapa, S.; Siddiqui, N.; Chakdar, H. Cyanobacterial Secondary Metabolites towards Improved Commercial Significance through Multiomics Approaches. World J. Microbiol. Biotechnol. 2022, 38, 100. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Srivastava, A.; Young, J.D. Metabolic Flux Phenotyping of Secondary Metabolism in Cyanobacteria. Trends Microbiol. 2023, 31, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Vaz, M.G.M.V.; Genuário, D.B.; Fiore, M.F.; Debonsi, H.M. Volatile Compounds Produced by Cyanobacteria Isolated from Mangrove Environment. Curr. Microbiol. 2019, 76, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Basit, A.; Ullah, I.; Mohamed, H.I. Cyanobacteria and Algae as Biocontrol Agents Against Fungal and Bacterial Plant Pathogens. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–23. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial Volatile Organic Compounds: Antifungal Mechanisms, Applications, and Challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, D.; Gao, C.; Li, J.; Du, B.; Wang, Z.; Qian, S. Recent Advances for Alkaloids as Botanical Pesticides for Use in Organic Agriculture. Int. J. Pest Manag. 2023, 69, 288–298. [Google Scholar] [CrossRef]
- Albuquerque, M.V.d.C.; Ramos, R.d.O.; de Paula e Silva, M.C.C.; Rodrigues, R.M.M.; Leite, V.D.; Lopes, W.S. Allelopathic Effects of Cyanotoxins on the Physiological Responses of Chlorella vulgaris. Toxicon 2024, 248, 107847. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.; Singh, R.P.; Kumar, A. Cyanobacterial Biodiversity and Their Potential Application in Sustainable Agriculture. In Sustainable Agricultural Practices; Elsevier: Amsterdam, The Netherlands, 2024; pp. 209–222. [Google Scholar]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2020, 9, 10. [Google Scholar] [CrossRef]
- Doble, M.; Kruthiventi, A.K. Alternate Solvents. In Green Chemistry and Engineering; Elsevier: Amsterdam, The Netherlands, 2007; pp. 93–104. [Google Scholar]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding Pitfalls in Determining Antimicrobial Activity of Plant Extracts and Publishing the Results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Pagnussatt, F.A.; Lemos, A.C.; Nicolli, C.P.; Del Ponte, E.M.; Badiale-Furlong, E. Nannochloropsis sp. and Spirulina sp. as a Source of Antifungal Compounds to Mitigate Contamination by Fusarium graminearum Species Complex. Curr. Microbiol. 2019, 76, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Arnhold Pagnussatt, F.; Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Inhibition of Fusarium Graminearum Growth and Mycotoxin Production by Phenolic Extract from Spirulina sp. Pestic. Biochem. Physiol. 2014, 108, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Farghl, A.; El-Sheekh, M.M.; Mousa, A.S. Extraction and characterization of antimicrobial active substance from cyanobacteria Nostoc carneum and Anabaena circinalis. Fresenius Environ. Bull. 2019, 28, 5481–5490. [Google Scholar]
- Perveen, K.; Alwathnani, H.A. Antifungal Activity of Methanol, Acetone and Diethyl Ether Extracts of Cyanobacteria Against Plant Pathogenic Fungi. Asian J. Chem. 2013, 25, 7531–7534. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M.; Mohyeldin, M.M. Component Analysis and Antifungal Activity of the Compounds Extracted from Four Brown Seaweeds with Different Solvents at Different Seasons. J. Ocean. Univ. China 2018, 17, 1178–1188. [Google Scholar] [CrossRef]
- Senousy, H.H.; El-Sheekh, M.M.; Saber, A.A.; Khairy, H.M.; Said, H.A.; Alhoqail, W.A.; Abu-Elsaoud, A.M. Biochemical Analyses of Ten Cyanobacterial and Microalgal Strains Isolated from Egyptian Habitats, and Screening for Their Potential against Some Selected Phytopathogenic Fungal Strains. Agronomy 2022, 12, 1340. [Google Scholar] [CrossRef]
- Alallaf, A.; Kotab, M.; Shafik, H.; Elsayed, A. In Vitro Efficacy of Biologically Active Compounds Derived from Navicula Arenaria against Soil Borne Phytopathogenic Macrophomina phaseolina and Fusarium oxysporum. Alfarama J. Basic Appl. Sci. 2021, 2, 285–296. [Google Scholar] [CrossRef]
- Blasio, M.; Balzano, S. Fatty Acids Derivatives from Eukaryotic Microalgae, Pathways and Potential Applications. Front. Microbiol. 2021, 12, 718933. [Google Scholar] [CrossRef]
- Mund, N.K.; Liu, Y.; Chen, S. Advances in Metabolic Engineering of Cyanobacteria for Production of Biofuels. Fuel 2022, 322, 124117. [Google Scholar] [CrossRef]
- Cerone, M.; Smith, T.K. Desaturases: Structural and Mechanistic Insights into the Biosynthesis of Unsaturated Fatty Acids. IUBMB Life 2022, 74, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, Y.; Sun, L.; Deng, Q.; Zhao, J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins 2021, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Dacko, A.; Tan, A.K.; Xiang, S.; Curtis, J.M.; Gänzle, M.G. Structure-Function Relationships of Antifungal Monohydroxy Unsaturated Fatty Acids (HUFA) of Plant and Bacterial Origin. Food Res. Int. 2020, 134, 109237. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.L.S.; da Silva, A.F.; Carvalho, P.H.A.; Pacheco, B.S.; Pereira, C.M.P.; Lund, R.G. Aliphatic Fatty Acids and Esters: Inhibition of Growth and Exoenzyme Production of Candida, and Their Cytotoxicity in Vitro. Arch. Oral. Biol. 2014, 59, 880–886. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Sinha, M.; Singh, H.; Patel, R.S.; Ghosh, S.; Sardana, K.; Ghosh, S.; Sengupta, S. Mechanistic Insight Into the Antifungal Effects of a Fatty Acid Derivative Against Drug-Resistant Fungal Infections. Front. Microbiol. 2020, 11, 2116. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, S.; Xavier, L.; Vasconcelos, V.; Santos, A. Cyanobacteria: A Promising Source of Antifungal Metabolites. Mar. Drugs 2023, 21, 359. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Deyab, M.A.; Hasan, R.S.A.; Abu Ahmed, S.E.; Elsadany, A.Y. Biological Control of Fusarium Tomato-Wilt Disease by Cyanobacteria nostoc spp. Arch. Microbiol. 2022, 204, 116. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Saber, A. Antifungal Potential of the Bioactive Constituents in Extracts of the Mostly Untapped Brown Seaweed Hormophysa cuneiformis from The Egyptian Coastal Waters. Egypt. J. Bot. 2019, 59, 695–708. [Google Scholar] [CrossRef]
- Marrez, D.A.; Sultan, Y.Y.; Naguib, M.M.; Higazy, A.M. Antimicrobial Activity, Cytotoxicity and Chemical Constituents of the Freshwater Microalga Oscillatoria princeps. Biointerface Res. Appl. Chem. 2021, 12, 961–977. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial Potential of Macro and Microalgae against Pathogenic and Spoilage Microorganisms in Food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-H.; Kuo, C.-H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.L.; Pereira-Caro, G.; Ludwig, I.; Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Crozier, A.; Moreno-Rojas, J.M. A Critical Evaluation of the Use of Gas Chromatography- and High Performance Liquid Chromatography-Mass Spectrometry Techniques for the Analysis of Microbial Metabolites in Human Urine after Consumption of Orange Juice. J. Chromatogr. A 2018, 1575, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Anasane, N.; Gade, R.; Ingle, P. Effective Management of Soft Rot of Ginger Caused by Pythium spp. and Fusarium spp.: Emerging Role of Nanotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 6827–6839. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.Á.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C.G. Natural Products Targeting the Synthesis of β(1,3)-D-Glucan and Chitin of the Fungal Cell Wall. Existing Drugs and Recent Findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, I.; Steinberg, G.; Gurr, S. The Role of the Fungal Cell Wall in the Infection of Plants. Trends Microbiol. 2017, 25, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, K.L.; Senhorinho, G.N.A.; Laamanen, C.A.; Scott, J.A. A Review of Extremophilic Microalgae: Impacts of Experimental Cultivation Conditions for the Production of Antimicrobials. Algal Res. 2024, 78, 103427. [Google Scholar] [CrossRef]
- Kar, J.; Ramrao, D.P.; Zomuansangi, R.; Lalbiaktluangi, C.; Singh, S.M.; Joshi, N.C.; Kumar, A.; Kaushalendra; Mehta, S.; Yadav, M.K.; et al. Revisiting the Role of Cyanobacteria-Derived Metabolites as Antimicrobial Agent: A 21st Century Perspective. Front. Microbiol. 2022, 13, 1034471. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Mc Gee, D.; Archer, L.; Smyth, T.J.; Fleming, G.T.A.; Touzet, N. Bioprospecting and LED-Based Spectral Enhancement of Antimicrobial Activity of Microalgae Isolated from the West of Ireland. Algal Res. 2020, 45, 101704. [Google Scholar] [CrossRef]
- López-Herrada, E.; Gallardo-Rodríguez, J.J.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F. Life-Cycle Assessment of a Microalgae-Based Fungicide under a Biorefinery Approach. Bioresour. Technol. 2023, 383, 129244. [Google Scholar] [CrossRef] [PubMed]
- Avinash, A.; Sasikumar, P.; Pugazhendhi, A. Analysis of the Limiting Factors for Large Scale Microalgal Cultivation: A Promising Future for Renewable and Sustainable Biofuel Industry. Renew. Sustain. Energy Rev. 2020, 134, 110250. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae N-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Kiani, H.; Aznar, R.; Poojary, M.M.; Tiwari, B.K.; Halim, R. Chromatographic Techniques to Separate and Identify Bioactive Compounds in Microalgae. Front. Energy Res. 2022, 10, 904014. [Google Scholar] [CrossRef]
- Pérez, J.P.; Muñoz, A.A.; Figueroa, C.P.; Agurto-Muñoz, C. Current Analytical Techniques for the Characterization of Lipophilic Bioactive Compounds from Microalgae Extracts. Biomass Bioenergy 2021, 149, 106078. [Google Scholar] [CrossRef]
- Parkes, R.; McGee, D.; McDonnell, A.; Gillespie, E.; Touzet, N. Rapid Screening of Phenolic Compounds in Extracts of Photosynthetic Organisms Separated Using a C18 Monolithic Column Based HPLC-UV Method. J. Chromatogr. B 2022, 1213, 123521. [Google Scholar] [CrossRef] [PubMed]
- Schuelter, A.R.; Kroumov, A.D.; Hinterholz, C.L.; Fiorini, A.; Trigueros, D.E.G.; Vendruscolo, E.G.; Zaharieva, M.M.; Módenes, A.N. Isolation and Identification of New Microalgae Strains with Antibacterial Activity on Food-Borne Pathogens. Engineering Approach to Optimize Synthesis of Desired Metabolites. Biochem. Eng. J. 2019, 144, 28–39. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Soengas, R.; Probert, I.; Guilloud, E.; Gourvil, P.; Mehiri, M.; López, Y.; Cepas, V.; Gutiérrez-del-Río, I.; Redondo-Blanco, S.; et al. NMR Characterization and Evaluation of Antibacterial and Antiobiofilm Activity of Organic Extracts from Stationary Phase Batch Cultures of Five Marine Microalgae (Dunaliella sp., D. Salina, Chaetoceros calcitrans, C. gracilis and Tisochrysis lutea). Phytochemistry 2019, 164, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Vyas, D. Antimicrobial Effect of a Cyclic Peptide Nostophycin Isolated from Wastewater Cyanobacteria, Nostoc Calcicola. Curr. Bot. 2021, 12, 94–101. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Heinilä, L.; Aesoy, R.; Sivonen, K.; Galica, T.; Hrouzek, P.; Herfindal, L. Chemical Diversity and Cellular Effects of Antifungal Cyclic Lipopeptides from Cyanobacteria. Physiol. Plant 2021, 173, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Blandino, M.; Scarpino, V.; Giordano, D.; Testa, G.; Badiale-Furlong, E. Application of Fungicides and Microalgal Phenolic Extracts for the Direct Control of Fumonisin Contamination in Maize. J. Agric. Food Chem. 2018, 66, 4835–4841. [Google Scholar] [CrossRef]
- Toribio, A.J.; Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; López-González, J.A.; Moreno, J. Seed Biopriming with Cyanobacterial Extracts as an Eco-Friendly Strategy to Control Damping off Caused by Pythium ultimum in Seedbeds. Microbiol. Res. 2021, 248, 126766. [Google Scholar] [CrossRef]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of Microalgae and Cyanobacteria as Potential Sources of Antimicrobial Compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.A.; Alferova, V.A.; Korshun, V.A.; Tyurin, A.P. Modern Trends in Natural Antibiotic Discovery. Life 2023, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Monteiro, A.F.; Bertonha, A.F.; Bernardi, D.I.; Gubiani, J.R.; Slivinski, J.; Michaliski, L.F.; Tonon, L.A.C.; Venancio, V.A.; Freire, V.F. Approaches for the Isolation and Identification of Hydrophilic, Light-Sensitive, Volatile and Minor Natural Products. Nat. Prod. Rep. 2019, 36, 981–1004. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal Polyunsaturated Fatty Acids: Hotspots and Production Techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Cyanobacteria in Plant Health: Biological Strategy against Abiotic and Biotic Stresses. Crop Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the Interaction of the Antifungal and Cytotoxic Cyclic Glycolipopeptide Hassallidin with Sterol-Containing Lipid Membranes. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Singh, H.; Sharma, S.; Kaur, M.; Singh, A.; Kaur, A.; Jain, S.K. Pre-Clinical and Cellular Safety Assessment of Oral Administered DHA Rich Microalgae Oil from Schizochytrium sp. (Strain ATCC-20889): Acute, Sub-Chronic and Genotoxicity. Drug Chem. Toxicol. 2024, 47, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, K.T.; Hwang, S.; Choi, K.Y.; Park, Y.J.; Choi, J.-H.; Truong, T.Q.; Kim, S.M. Nanoencapsulation Enhances the Bioavailability of Fucoxanthin in Microalga Phaeodactylum tricornutum Extract. Food Chem. 2023, 403, 134348. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, P.; Szczurek, E. Artificial Intelligence-Driven Antimicrobial Peptide Discovery. Curr. Opin. Struct. Biol. 2023, 83, 102733. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Alvarez, J.A.E.; Zabetakis, D.; Walper, S.A.; Malanoski, A.P. PepVAE: Variational Autoencoder Framework for Antimicrobial Peptide Generation and Activity Prediction. Front. Microbiol. 2021, 12, 725727. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, A.; Cai, X.; Personne, H.; Köhler, T.; van Delden, C.; Reymond, J.-L. Machine Learning Designs Non-Hemolytic Antimicrobial Peptides. Chem. Sci. 2021, 12, 9221–9232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).