Abstract
Most research on the vaginal microbiome has focused on bacterial communities (the bacteriome), but viruses, including eukaryotic viruses and bacteriophages, are also important players in vaginal health and disease states. In this review, we will briefly discuss the bacterial microbiome, delve into what is known about the vaginal virome and its impact on women’s health, and finish with novel vaginal microbial or microbial-derived therapeutics on the horizon. More studies on the vaginal virome and its impact on women’s health are needed to better prevent and treat gynecological, reproductive, and neonatal diseases.
1. Introduction
The vaginal mucosa, similar to other mucosal sites, is the first barrier of protection against invading pathogens. The vaginal microbiome is an important component of this defense and carries out functions that help to protect against a number of urogenital diseases, including yeast infections, bacterial vaginosis, urinary tract infections, HIV, and other sexually transmitted infections [1]. The vaginal microflora, in turn, are impacted by host factors including gestational status, menstrual cycle, sexual activity, age, contraceptive use, diet, lifestyle factors such as tobacco use, immune status, race/ethnicity, and environment [1,2,3,4]. The vaginal microbiome is composed of a variety of bacteria, yeast, viruses, and protozoa that play a role in women’s health, where disruptions in a woman’s normal vaginal microbiome contribute to vaginal disease states through transkingdom interactions, including with the host mucosa and immune system. In this review, we will dive deeper into understanding the relationship between the vaginal microbiome, in particular the vaginal viral microbiome (virome), and disease, as well as discuss new and emerging microbiome-targeted treatments for these disease states.
In the last three years, there has been a doubling of the manuscripts detailing the vaginal virome and its impact on women’s health using next-generation sequencing [5,6,7,8,9,10,11,12,13,14] compared to the decade prior [15,16,17,18,19]. The last few years have also seen the development of promising new microbial-based therapies, highlighting the growing importance of a better understanding of all members of the microbiota. The present review summarizes knowledge on the vaginal virome’s composition, interaction with other microbes in its environment, and its contribution to women’s health. We searched for and included all research manuscripts published in English within the last 10 years and indexed within the PubMed database that detail the vaginal virome through next-generation sequencing using mesh terms including “vaginal virome”, “female genital tract virome”, and “vaginal virus”. For transkingdom interactions and microbial-based therapeutics summation, we searched all types of papers, including reviews, retrospective analyses, and research studies, within the last decade, prioritizing the last 5 years and using mesh terms such as “vaginal microbiome”, “current treatments for bacterial vaginosis”, and “vaginal microbiome transplantation”.
2. The Vaginal Bacteriome
The vaginal bacterial microbiome (bacteriome) is the best-studied component of the vaginal microbiome and is generally divided into lower-diversity Lactobacillus-dominant (LD) bacteriomes versus higher-diversity non-LD (NLD) bacteriomes dominated by facultative and obligate anaerobic flora. These distinctions have clinical and biological relevance. LD bacteriomes tend to show lower levels of localized inflammation, whereas higher diversity NLD bacteriomes are associated with vaginal disease states, including BV, preterm birth, infertility, higher transmission and acquisition of sexually transmitted infections (STIs) including HPV and HIV, and increased risk of cervical cancer [2,3,4,15,20,21]. The most prominent Lactobacillus species present in the vaginal bacteriome include L. crispatus, L. gasseri, L. jensenii, and L. iners [1,3]. Lactobacilli are known producers of lactic acid, hydrogen peroxide, and other antimicrobial and antifungal products that prevent the overgrowth of pathogenic bacteria and fungi [3,22].
2.1. Bacteriome Communities
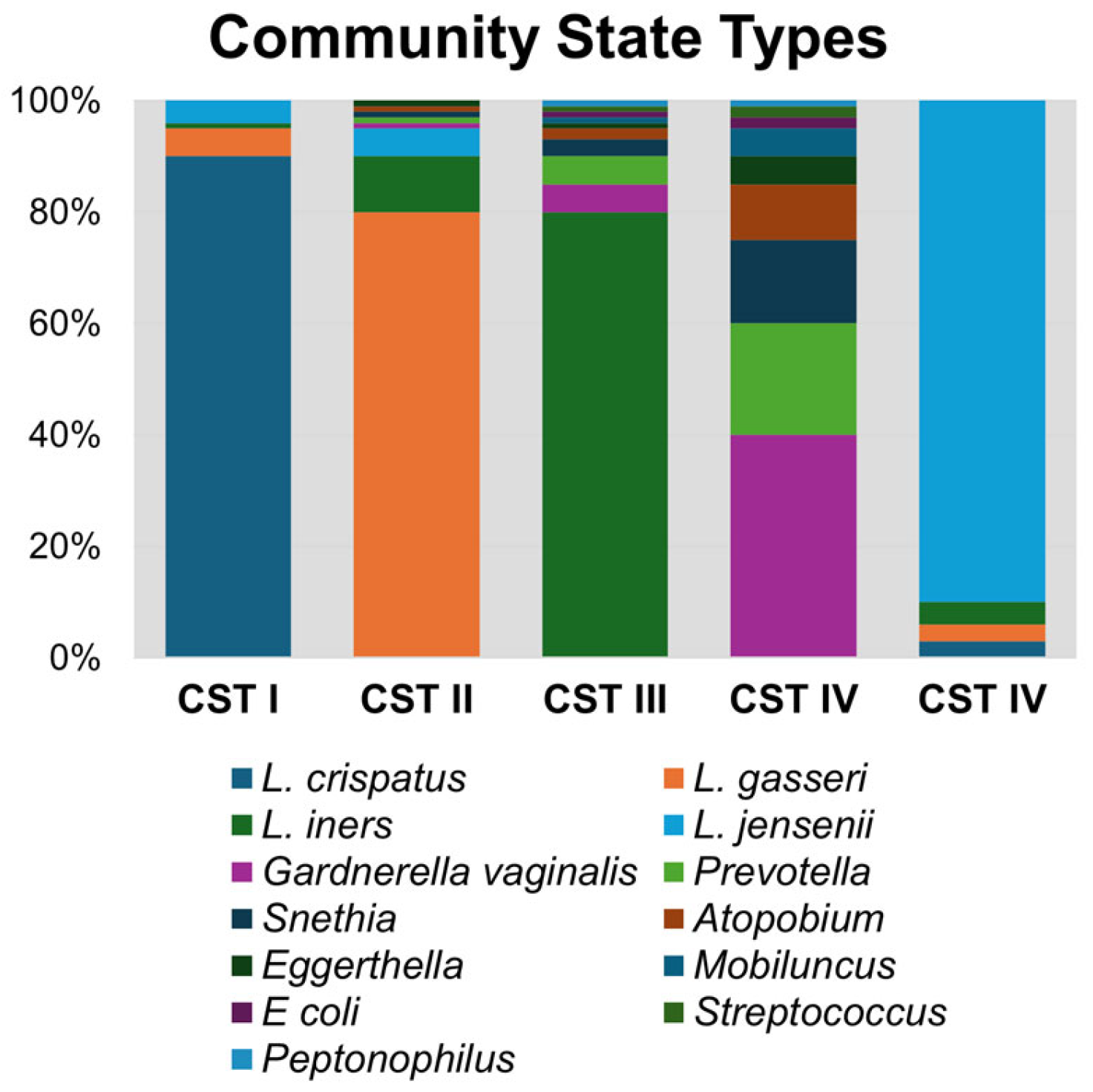
Unlike in other biological niches, it is well established that vaginal bacteria form defined community groupings that are similar across populations. The most widely used classification method for vaginal bacteriomes is known as community state types (CSTs; Figure 1), which are numbered according to the dominant species of that group. CST I, II, III, IV, and V correspond with bacteriome domination by L. crispatus, L. gasseri, L. iners, anaerobic bacteria, and L. jensenii, respectively [3]. CST IV is notable for having diverse anaerobic bacteria, including Gardnerella vaginalis, Prevotella bivia, Sneathia sanguinis, Mobiluncus mulieris, and Eggerthella lenta, among others [3,23,24]. Lactobacilli, in particular L. crispatus, are known to reduce the response and activity of inflammatory compounds, whereas microbial communities with a predominance of Gardnerella or other anaerobes are known to produce pro-inflammatory cytokines, such as IL-6, IL-8, and TNF-α [15]. Bacteriomes dominated by anaerobic bacteria (CST IV) or L. iners (CST III) are associated with higher rates of reproductive tract inflammation and pathologies, such as bacterial vaginosis (BV) and HIV acquisition and transmission [1,3,22]. On the other hand, CST I, II, and V are associated with decreased vaginal inflammation and improved health outcomes and are protective against urogenital diseases.
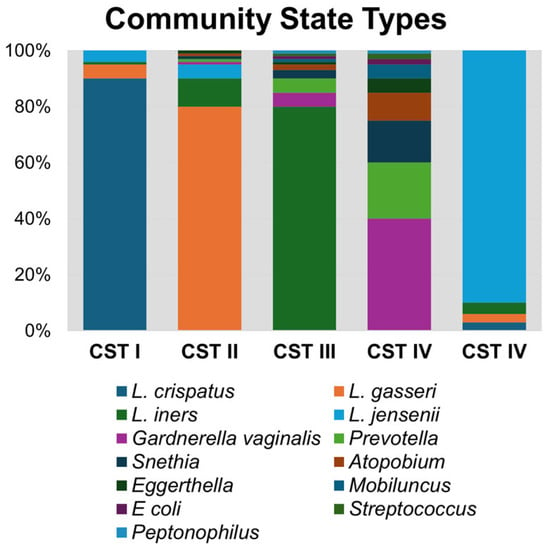
Figure 1.
Community state type (CST) bacterial taxa composition. A representative synthetic bacteriome is graphed illustrating relative abundance of bacterial genera within each CST group.
Baseline vaginal bacteriome composition differs by geographic area, ethnicity, and race. About 90% of Caucasian women in North America have LD bacteriome communities, while only 60–65% of Black women in the US and 37–62% of Black women in Sub-Saharan Africa have LD bacteriomes. Black and Hispanic women are more likely to have higher diversity vaginal bacteriomes reflective of CST III and IV, hence the prevalence of conditions such as BV is significantly greater in Black and Hispanic women, at rates of 51.6% and 32.1% respectively, compared to Caucasian women, who have a prevalence rate of 23.2% [3,25].
2.2. Impact of the Bacteriome on HIV Acquisition and Pathogenesis
High diversity bacteriomes are associated with an increased rate of transmission and acquisition of STIs, including HIV. In 2023, 1.3 million people contracted HIV globally, with Sub-Saharan Africa disproportionately affected, including women and girls who accounted for 62% of all new HIV infections in this region [26]. Baseline vaginal bacteriomes contribute to the high rate of HIV acquisition in this population. In a prospective study to ascertain risk factors for HIV acquisition, vaginal swab samples were collected and analyzed from 236 HIV-negative women at risk for HIV acquisition [15]. Women with L. crispatus-dominated vaginal bacteriomes did not acquire HIV during their observation period. On the other hand, women with intermediate diversity L. iners-dominant vaginal communities and NLD bacteriomes acquired HIV at greatly increased rates compared to L. crispatus-dominated vaginal bacteriomes. The authors concluded that non-iners LD vaginal bacterial communities were protective against HIV acquisition and other pathogenic microorganisms, and those with L. iners-dominated communities and high diversity NLD bacteriomes were more susceptible to contracting HIV [15]. The mechanism behind this is two-fold. Inflammatory cytokines produced by L. iners-dominant and NLD bacteriomes cause not only increased permeability of the vaginal epithelium and cervical cell layers to HIV but also recruit CD4+ HIV target cells to the vaginal mucosa [15,27]. The underlying difference in mucosal permeability relies on the levels of lactic acid (LA) isomers. Cervicovaginal mucus with high levels of D-LA isomer produced by L. crispatus yields a low pH, which promotes carboxyl group hydrogen bonding between HIV virion glycans and surface mucins. This bonding causes HIV virion immobilization and prevention of HIV invasion into the genital mucosa [28]. Therefore L. crispatus-dominant bacteriomes have multiple mechanisms to protect against HIV infection, demonstrating the complex interplay between the vaginal bacteriome, host, and viral populations.
2.3. Postmenopausal Bacteriome
The decline in estrogen levels after menopause significantly impacts the vaginal microbiota, usually resulting in a reduced quantity of lactobacilli and other lactic acid-producing bacteria [29,30]. There is a transition to fewer LD bacteriomes and increased bacteriome diversity (CST IV) in the postmenopausal period, including increased abundance of Prevotella, Streptococcus, Gardnerella, and Anaerococcus [29,30,31]. CST IV, linked with poor reproductive health outcomes, has also been linked with a greater incidence of vaginal atrophy and dryness after menopause [29]. No studies to date have investigated the vaginal virome of postmenopausal women, but we would expect the virome to follow a similar trend as the bacteriome with increased diversity during the postmenopausal period based on the virome studies of BV detailed below [5].
3. The Vaginal Virome
The vaginal virome is comprised of eukaryotic viruses that infect human cells and bacteriophages, which infect the bacteriome. The majority of vaginal virome studies have focused on eukaryotic DNA viruses, such as Papillomaviridae, Herpesviridae, Anelloviridae, Polyomaviridae, Poxviridae, and Adenoviridae, through PCR-based techniques [32]. However, eukaryotic viruses usually comprise less than 5% of viral sequences found in the human vagina. More recent studies have attempted unbiased surveys of vaginal viruses (see Table 1), though RNA viruses are still understudied.

Table 1.
Vaginal virome manuscripts examining both the eukaryotic virome and bacteriophages.
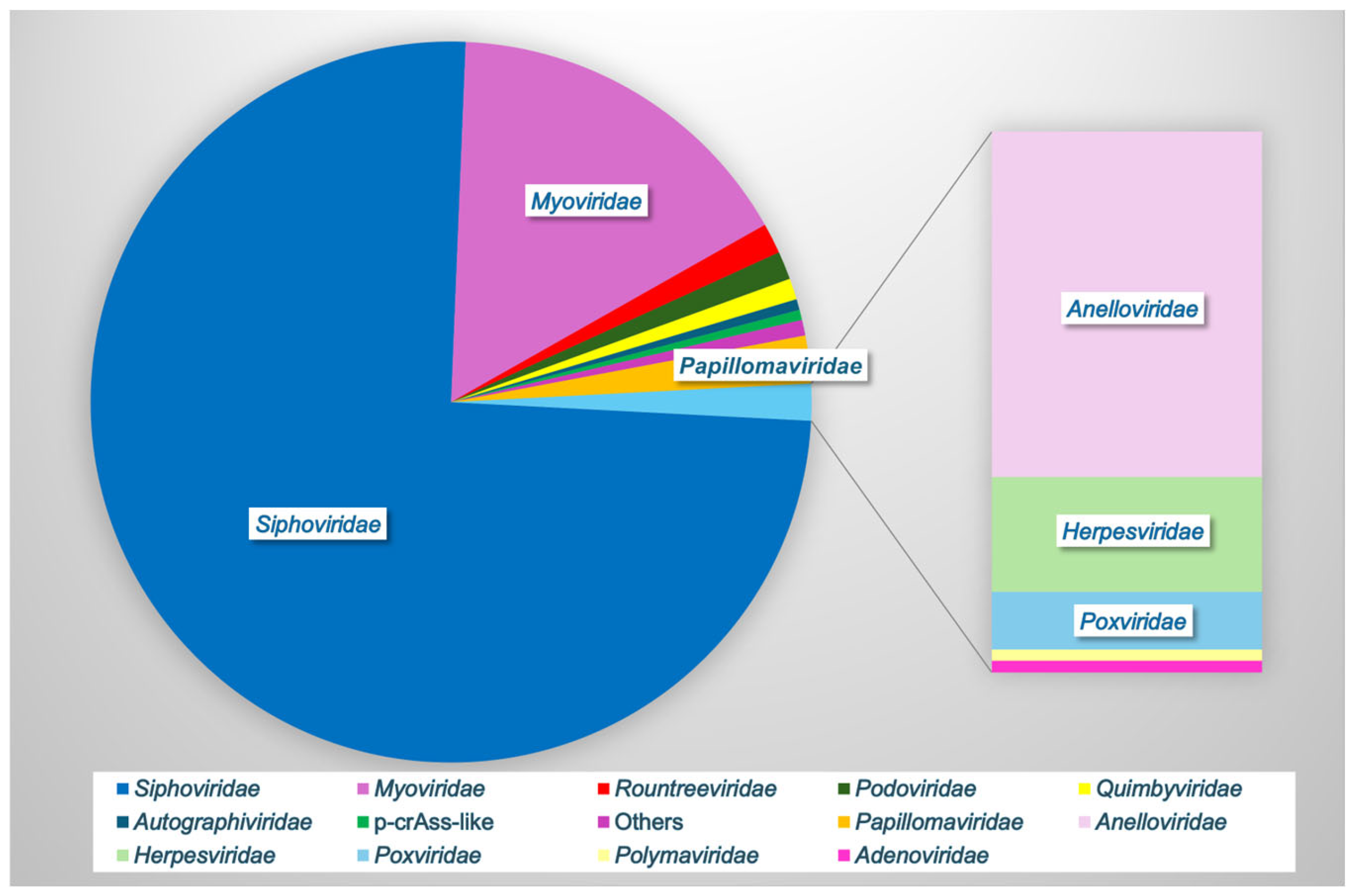
In one of the largest analyses to date, Huang et al., 2024, combined vaginal metagenomic sequencing data from 32 studies to compile a reference database of over 33,000 microbial genomes, including 4263 species-level viral operational taxonomic units (vOTUs) [13]. Rarefaction showed that these viral sequences did not reach a plateau, suggesting that other undiscovered viruses may be present in the vaginal vault [13]. Furthermore, 85.5% of the vOTUs were not found in other virome databases, indicating the significant unexplored viral diversity in this unique niche [13]. Only 66% of the vOTUs could be assigned to a known viral family, with the most frequently observed families being Siphoviridae (76% of known families), Myoviridae (16%), Papillomaviridae (2%), Rountreeviridae (1.4%), Podoviridae (1.3%), Quimbyviridae (<1%), Autographiviridae, crAss-like, and others (Figure 2). Apart from Papillomaviridae, the other top families (98%) were all bacteriophages [13]. Only 70 of the 4263 vOTUs were assigned to eukaryotic viruses, highlighting the importance of bacteriophages within this environment.
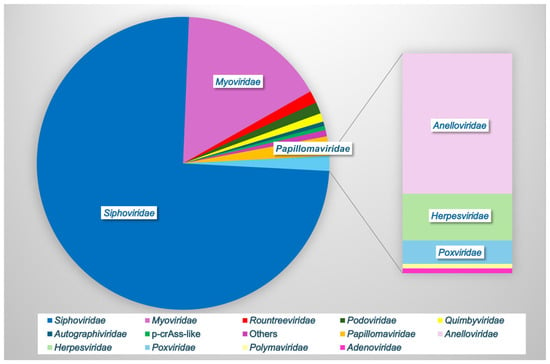
Figure 2.
Vaginal viral family relative abundance. Bacteriophages comprise the majority of the vaginal virome (Siphoviridae, Myoviridae, Rountreeviridae, Podoviridae, Quimbyviridae, Autographiviridae, and p-crAss-like phage), while eukaryotic viruses (Papillomaviridae, Anelloviridae, Herpesviridae, Poxviridae, Polyomaviridae, and Adenoviridae) comprise a small minority. Data based on [5,13].
3.1. Vaginal Eukaryotic Viruses
Eukaryotic viral STIs have been well-studied through PCR-based techniques and are known contributors to vaginal disease [32]. The vaginal vault represents a physical and immunological barrier against the invasion of pathogenic organisms, often through the activation of pattern recognition receptors to trigger localized inflammation and viral clearance. Some DNA eukaryotic viruses, such as anelloviruses, are ubiquitous and considered commensals, but eukaryotic viral STIs, such as members of the Polyomaviridae, Herpesviridae, and Poxviridae families, are associated with increased vaginal inflammation, vaginal mucosa barrier degradation and adverse reproductive health and gynecological outcomes, including preterm birth, bacterial vaginosis (BV), and ulcerogenital disease [16,33,34,35,36,37,38,39]. Zika virus can be sexually transmitted and has been found in the female reproductive tract, which may have profound implications for vertical transmission in pregnant women and risk of birth defects [40]. However, it has not been as well-studied in women as in men, and the clinical relevance of vaginal shedding is unclear. Coinfection with multiple viruses can act synergistically to increase mucosal damage. HIV is associated with increased risk of urogenital herpes simplex virus (HSV) lesions and human papillomavirus (HPV) persistence [41,42,43], while other viral STIs can increase the risk of HIV transmission and shedding [44,45,46,47]. These examples illustrate the complex interplay of the vaginal eukaryotic virome in vaginal health.
3.2. The Vaginal Phageome
Bacteriophages are the most abundant vaginal viral group (Figure 2) and can directly interact with the host to elicit a humoral immune response. Bacteriophage DNA can also be recognized by cellular pattern recognition receptors, including toll-like receptor (TLR)-9, to induce an inflammatory type-1 interferon (IFN) response [48]. Bacteriophages can indirectly stimulate the host immune response through the regulation of bacterial populations and the release of bacterial products such as endotoxin during bacterial lysis [32]. Functional annotation of the vaginal virome shows about 20% of bacteriophage genes identified were viral auxiliary metabolic genes, including numerous peptidoglycan lyase/hydrolase-related genes, which may facilitate bacterial cell wall degradation during phage infection [13].
Bacteriophages may exhibit one of two different life cycles: lysogenic or lytic. Lytic bacteriophages enter and hijack the bacterial replication machinery to produce often thousands of progeny viruses, resulting in lysis and death of the host cell. Lysogenic bacteriophages, on the other hand, integrate into the host genome, replicating when the host replicates. Lysogenic bacteriophages can carry virulence factors that may provide survival advantages to the bacterial host, including conferring tolerance to ecological stressors, superinfection immunity, increased pathogenicity, and antibiotic resistance [49,50]. However, when the environment becomes unfavorable, lysogenic phages can rapidly switch life cycles and become lytic, thus subsuming bacterial functions and ultimately causing host bacterial death [51]. This life cycle switch can be triggered through bacteriophage quorum sensing mechanisms [51,52]. Prior work has shown an increased prevalence of lysogenic phages in women with BV, which may provide survival benefits to their hosts to improve persistence [5]. Ex vivo studies showed lysogeny is also common in L. crispatus, at rates up to 77% of clinical isolates [53]. Further, host ranges of clinical Lactobacillus phages were broad and included multiple Lactobacillus species, and because phages that are lysogenic in one species can be lytic in other species, this may be one way lactobacilli maintain the stability of the vaginal microbiome [54].
3.3. Transkingdom Dynamics of the Vaginal Microbiome
Bacteriome shifts from LD to non-LD can occur rapidly. While factors that trigger shifts, such as menstrual cycle and sexual activity, are known, the mechanisms and dynamics of these shifts (i.e., loss of Lactobacillus vs. overgrowth of other bacteria causing suppression of Lactobacillus) and relation to other factors (phage blooms, genetics, and environmental changes) are unclear. Commercially available yogurts containing Lactobacillus strains have inducible phages that can inhibit vaginal lactobacilli [55], suggesting that exogenous introduction may play a role in bacteriome shifts.
Recently, Hugerth et al. used metagenomic sequencing (MGS) to characterize daily vaginal swabs from 49 healthy reproductive-age women to assess the dynamics of the vaginal bacteriome and virome [11]. They found the bacterial communities fluctuated considerably in many individual women over time (6/49 varied with menstruation and 11/49 were unstable throughout the cycle). Women with stable non-iners LD bacteriomes exhibited 1 log higher levels of bacteriophage counts, with bacteriophage spikes noted with the bacteriome shifts. Higher levels of bacteriophage reads were also noted during menstruation in women whose bacteriome shifted during menstruation, suggesting that bacteriophages may contribute to the resolution of the dysbiosis in these women. While phages are believed to play a role in the stability of the vaginal bacteriome, even daily sampling was insufficient to ascertain if phage blooms precede and, hence, may cause bacteriome shifts [11]. Further studies would be needed to justify phage therapy for the treatment of BV or promoting LD bacteriomes for vaginal health.
3.4. Benefits and Limitations to Metagenomic Sequencing Studies
The majority of viruses are unculturable, making traditional methods to identify novel viruses impossible. Viruses also lack a universal sequencing marker, such as the bacterial 16S ribosomal RNA gene widely used for bacteriome research, negating the possibility of lower-cost sequence amplification techniques [56,57]. Over the last two decades, MGS has exponentially expanded the number of known viruses, creating a new field of study, the virome [58]. However, there are limitations and biases associated with virome MGS. MGS is expensive, which until recently has resulted in small sample sizes for most studies, limiting statistical power to determine associations with disease. MGS also has lower sensitivity than targeted PCR-based methods but is the only method currently available to identify unculturable, novel viruses [57]. RNA viruses in particular continue to be understudied due to decreased sequencing yield with combined DNA/RNA viral amplification techniques and poor identification in taxonomic pipelines due to divergent genomes [59].
Viruses overall represent a minute fraction of the total biomass of any sample, often requiring special techniques to amplify viral sequences sufficiently for detection by MGS [60]. Unfortunately, there are no standardized nucleic acid extraction protocols, amplification protocols, or taxonomic identification pipelines for virome MGS, and many techniques introduce bias [56,57]. For instance, nucleic acid extraction methods that involve filtration for viral sequence enrichment can filter out larger virions, including crAssphage. Commonly used amplification techniques to increase viral yield, such as rolling circle amplification, bias toward small circular DNA viral genomes [57]. Perhaps most importantly, viral identification is biased toward known viruses, as these are present in viral taxonomic databases. The majority of sequences in virome-sequencing datasets cannot be mapped to known viruses, and have been dubbed the “viral dark matter” [61]. Current bioinformatics tools have difficulty identifying highly divergent viruses, but deep artificial neural networks have been used to “learn” viral features and more successfully predict viral sequences with higher accuracy [61], including the recent identification of over 160,000 new putative RNA viruses using a deep learning algorithm [59]. While the field faces challenges, the evolving methods and bioinformatics tools will continue to ensure new findings for many decades to come.
4. The Contribution of the Vaginal Virome to Women’s Health
The following examples illustrate the well-documented role the vaginal virome plays in vaginal health and disease states (Table 2).

Table 2.
Vaginal virome disease associations.
4.1. Cervical Cancer
HPV, the causative agent of cervical cancer, is the most prevalent vaginal eukaryotic virus worldwide, and the most common sexually transmitted virus [62]. HPV is a small DNA virus that infects squamous epithelial cells but is often cleared within 6-18 months after acquisition. However, persistent infection by certain HPV genotypes is associated with a high risk of progression to anogenital and oropharyngeal cancers (HR-HPV) or low risk of progression (LR-HPV), the latter of which cause anogenital warts [20]. The link between HPV and cervical cancer is better reviewed elsewhere, including another review in this special issue [63]. Co-infection with other STIs, including HIV, HSV, and Epstein–Barr virus (EBV), along with high diversity bacteriomes, are associated with decreased vaginal HPV clearance and persistent infection with HR-HPV (Figure 3), thus increasing the risk of HPV-related malignancies (Table 2) [64]. Cervical cancer is the most common AIDS-defining cancer in women, underscoring the interactions between HIV and HPV [62]. Co-infection with HPV and HSV also increases the risk of cervical squamous cell carcinoma or adenocarcinoma by 2- to 9-fold more than HPV infection alone [64]. Further, the presence of HPV may alter female genital tract eukaryotic virome diversity [65], suggesting a complex interaction between virome components.
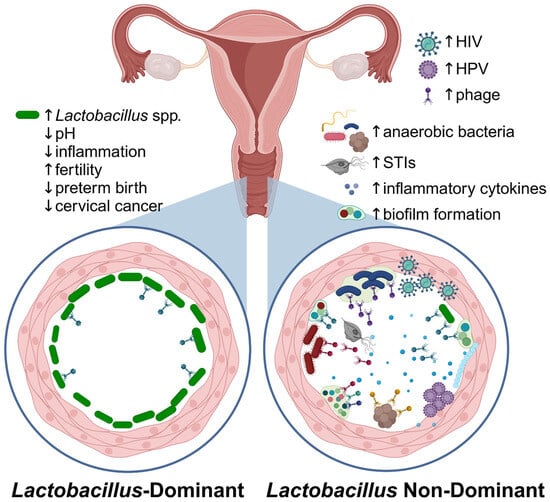
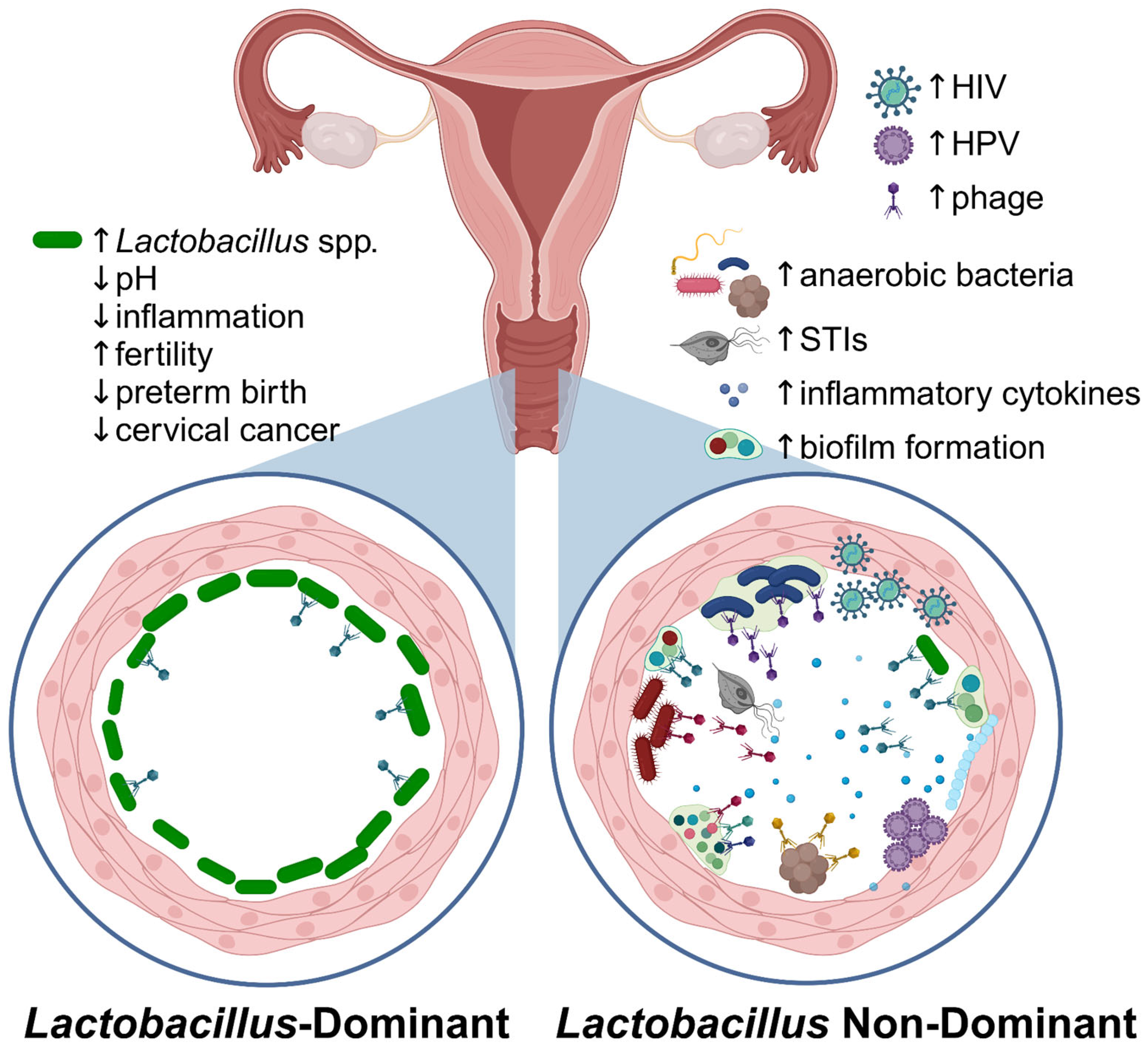
Figure 3.
The vaginal microbiome in health and disease. Callouts illustrate differences in the microenvironment in Lactobacillus-dominant versus non-Lactobacillus-dominant (i.e., BV) vaginal bacteriomes. Figure created in Biorender.
NLD and BV-associated bacteriomes increased HPV persistence and carcinogenic progression (Table 2) [6,62,66,67]. Recent work has shown that the HPV E7 oncoprotein downregulates several host antimicrobial defense peptides that are normally secreted in response to bacterial LPS [67]. HPV colonization may, therefore, allow for the overgrowth of more pathogenic bacteria through the downregulation of host defense mechanisms. Lactobacillus spp., however, were immune to the antibacterial effects of these peptides, instead cleaving them for use as an amino acid source for growth [67]. L. crispatus and L. gasseri were also shown in vitro to downregulate the expression of HPV E6 and E7 oncoproteins [68], and therefore, these lactobacilli may play a direct role in preventing HPV virulence.
4.2. Bacterial Vaginosis
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in reproductive-age women. This clinical disease occurs when disruption of the baseline vaginal bacteriome occurs, shifting away from a low-diversity LD bacteriome toward more diverse, NLD bacteriomes populated largely by anaerobes, including Gardnerella vaginalis, Prevotella spp., and Fannyhessea vaginae (previously known as Atopobium vaginae). Clinical symptoms manifest as vaginal malodor, discharge, itchiness, and vaginal inflammation [20]. More concerningly, BV is associated with numerous adverse health outcomes, including preterm labor and preterm birth, preterm rupture of membranes, chorioamnionitis, endometriosis [69], infertility [70], and an increased risk of STIs, including HR-HPV [62] and HIV [20,32]. Interestingly, smoking is a risk factor for BV, and prior work has shown that cigarette smoke chemicals are found in vaginal secretions and can induce lytic conversion of Lactobacillus bacteriophages [71], potentially explaining this association with BV.
Vaginal phageome communities differ between women with and without BV [5,16], with decreased Lactobacillus phages [16] and higher levels of bacillus and Escherichia phages [5]. Further, similar to vaginal bacterial community state types, vaginal bacteriophages form community groups dubbed viral state types (VSTs), which differ in diversity and are correlated with BV/LD status (Figure 3 and Table 2) [5,6]. Groups differ on the impact of BV/NLD on other eukaryotic viruses, such as anelloviruses, where some find an increased eukaryotic virome diversity in more diverse vaginal bacteriomes [6,8], while others found no difference [5]. This difference in phageomes indicates that phage populations may play a significant role in shaping vaginal bacterial communities and may represent a novel alternative to antibiotics for the treatment of BV.
4.3. Infertility and Preterm Birth
A few studies have examined the vaginal virome in IVF and infertility. A small study (n = 26) examining women undergoing in vitro fertilization (IVF) found a trend toward higher eukaryotic DNA virome diversity in women in whom IVF failed (did not achieve clinical pregnancy) [72]. Among women randomized to receive azithromycin antibiotic prophylaxis prior to embryo transfer, they found a higher diversity of herpesviruses and papillomaviruses [72]. However, the azithromycin group also received a different stimulation protocol due to significantly lower anti-Müllerian hormone (AMH) levels, a marker of ovarian reserve that correlates with IVF success rates. While, overall, there was no difference in pregnancy rates between prophylactic azithromycin administration groups, these protocol differences or underlying vaginal mucosal differences (as AMH tends to decrease as women approach menopause) may have impacted virome diversity, and this was not addressed. Another small study of 46 women showed a higher prevalence of a specific anellovirus, Torquetenovirus (TTV), in women with infertility, regardless of male or female factor infertility cause [10]. Anelloviruses are small circular DNA viruses that are often markers of immunosuppressed status but are not known to cause any disease and are often thought to be commensal viruses. These authors found no difference in L. crispatus dominance in the bacteriome in infertility, but individuals with TTV were less likely to have L. crispatus-dominant bacteriomes [10]. More research is needed in this area to better ascertain the impact the vaginal virome may play on infertility.
Prior older prospective studies in mice and humans have shown that increased vaginal eukaryotic DNA virome diversity increases the risk of preterm birth [33,73,74]. However, the phageome was not assessed in these studies. Since increased vaginal bacteriome diversity has also been strongly correlated with preterm birth [32], examination of the vaginal phageome would be of interest.
5. Vaginal Therapeutics
While antibiotic resistance and relapse are increasing, several promising microbially targeted or microbially derived products are in pre-clinical or clinical trials to improve women’s health.
5.1. Probiotics and Anti-Biofilm Agents
Treatment for BV has remained largely unchanged for almost half a century, with the first-line treatment being the antibiotic metronidazole [20,24]. While initial cure rates can approach 80%, more than half of women will experience recurrent BV within 6 months of therapy and up to 80% by one year [75,76]. This is partly due to the formation of biofilms by BV-associated bacteria, including Gardnerella and Prevotella, among others [77]. Biofilms provide a protective microenvironment for the growth of biofilm-forming organisms and are difficult to penetrate by antibiotics and host antimicrobial compounds. Ineffective penetration of antibiotics makes bacterial eradication difficult, allowing bacterial regrowth after the cessation of antibiotics. BV may also be re-introduced during sexual intercourse, and while it is not considered an STI, its prevalence is associated with sexual activity [20]. In vitro and in vivo studies have tested single-agent compounds on biofilm reduction. There was no overall efficacy found with antibiotics alone. However, promising results were noted with antiseptics, most notably a 4-log reduction in colony-forming units with iron sulfide in vitro, and octenidine in vivo, though resistance developed with repeated exposure to the latter. In vitro biofilm formation studies also noted promising results with the use of catatonic peptides, the synthetic phage endolysin enzyme PM-477, and plant extracts including Thymbra capitata essential oil, including in polymicrobial biofilms and ex vivo biofilms [77,78].
In a systematic review of treatment modalities for BV, both vaginally applied sucrose and probiotics resulted in higher clinical responses than metronidazole treatment [79]. These compounds show promise, especially if used in combination with standard of care or other novel agents to help prevent recurrence. A novel Lactobacillus crispatus probiotic (CTV-05) was studied in a randomized trial of weekly applications for 2 weeks after an initial metronidazole gel course, finding that colonization occurred in 61% of individuals at day 10, but waned once the applications were stopped [80]. Further supporting this, a recent systematic review of 11 clinical trials involving 1493 BV patients suggests lower recurrence rates at 3 months, and longer cure rates at 24 weeks in those given antibiotics followed by intravaginal probiotics versus those treated with antibiotics alone [81]. Another systematic review of 35 randomized controlled trials comprising 3751 subjects treated with probiotics also found a significant increase in BV cure rates and a significant reduction in BV recurrence rates [82].
Probiotics have also been trialed to help in the eradication of female genital tract HR-HPV infection and, thus, decrease progression to neoplasia. A placebo-controlled trial randomized 100 women with HR-HPV infection to receive either intravaginal L. crispatus strain chen-01 for 5 months or a placebo [83]. Six months after intravaginal L. crispatus chen-01 transplantation, women had significantly reduced HPV viral loads and decreased cytological transformation and vaginal inflammation, along with higher L. crispatus carriage and overall lower bacteriome diversity, similar to HPV-uninfected subjects [83]. These examples illustrate the need for further well-designed large-scale clinical trials to validate the impact of adjunctive intravaginal therapies to restore vaginal health.
5.2. Vaginal Microbiota Transplant
Another proposed therapeutic for recurrent BV is vaginal microbiota transplantation (VMT), which involves transplanting donor vaginal fluid rich in low diversity L. crispatus, L. jensenii, or L. gasseri (CSTs I, II, and V) transvaginally into recipients with BV or other vaginal disease states [84]. One study has demonstrated success with VMT, with four out of five recipients achieving remission of their BV during follow-up periods with no adverse effects. However, the participants received antibiotics for 2 years along with small amounts of fresh VMT, which also could have influenced the participants’ ability to achieve remission [85]. These studies offer additional insight into the connection between the vaginal ecosystem, the development of disease, and the possibility of preventing disease with the transplantation of vaginal microbial communities that are known to be optimal for vaginal health [84,85].
Although VMT may be a promising solution to BV, there are some drawbacks including the possibility of transferring pathogens, such as resistant fungi, or non-optimal vaginal communities [86,87]. Bacteriophages may be transplanted with donor VMT, which could result in transfer to undesirable genes including antimicrobial resistance (AMR), such as has been documented with fecal microbiota transfer (FMT) [88] and has led to bacteremia with fatal consequences [89]. While VMT would be less likely to result in bacteremia than FMT due to decreased mucosal surface area interaction, consideration should be given to similar screening protocols as with FMT. A prior study examining Lactobacillaceae genomes from commercial strains found that around half of the isolates harbored an integrated prophage, most often consisting of Siphoviridae or Myoviridae families, with at least four potential AMR genes encoded within the phage genomes [90]. This further highlights the importance of including AMR screening in VMT, which is not currently included in published screening protocols [84]. VMT may also be prohibitively expensive to lower income patients due to the procedure itself, and the need for donors to undergo rigorous screening to minimize adverse effects and ensure the safety of the recipients. Larger clinical trials are needed to evaluate the safety and efficacy of VMT for BV and its efficacy for other vaginal disease states.
5.3. Oleic Acid Treatment
Unsaturated long-chain fatty acids (uLCFAs) have shown antimicrobial activity against gram-positive organisms, and using a targeted approach, Zhu et al., 2024 investigated the impact of uLCFAs to modulate vaginal lactobacilli composition [21]. Oleic acid (OA) and similar long-chain fatty acids selectively inhibited L. iners, including metronidazole-resistant strains, while promoting the growth of non-iners lactobacilli [21]. The mechanism behind this was OA-induced upregulation of gene products in L. crispatus, L. jensenii, and L. gasseri, including a conserved putative fatty acid efflux pump and oleate hydrolase, that are missing from L. iners and BV-associated bacteria, making the latter susceptible to OA [21]. This study demonstrates the therapeutic potential of OA in the treatment of BV and preventing recurrence.
5.4. Phage Lysins
Phage lysins are bacteriophage lytic enzymes produced to cleave bacterial cell wall peptidoglycan for phage progeny release. They are effective for controlling bacterial populations on mucosal surfaces, rapidly lysing target gram-positive bacteria and resulting in bacterial death. They have been shown to act synergistically with antibiotics, and have been tested in a variety of diseases [91]. Group B streptococcus (GBS) often colonizes the urogenital tract and is the leading cause of neonatal meningitis. Women are screened prior to delivery and treated with antibiotics if vaginally colonized. Clinically pathogenic strains carry prophages whose genes enhance bacterial growth and biofilm formation [92], which may help GBS adapt to the vaginal environment and increase pathogenicity in neonates. However, a new therapeutic recombinant endolysin derived from a GBS prophage, EN534-C, was shown to lyse clinical GBS isolates but not beneficial vaginal lactobacilli in vitro [93]. This targeting of a specific bacterial pathogen is one example of the potential use of these novel antimicrobial agents in improving women’s health outcomes.
6. Conclusions and Future Research Directions
The complex interplay between the vaginal bacteriome, virome, and host immune defenses that impacts the development and persistence of female genital tract diseases is just beginning to be better understood, allowing for the development of novel, non-antibiotic antimicrobial compounds that show great promise in improving women’s health. However, much more research is needed. More research and standardization of virome protocols, from nucleic acid extraction to amplification and taxonomic identification pipelines, are needed to allow for cross-cohort comparisons to better differentiate the biological differences implicated with disease from procedural biases or population differences. Additionally, the implications of the virome in key areas of women’s health have yet to be fully investigated. Few studies have focused on the post-menopausal vaginal bacteriome and its impact on gynecological health [69], and the postmenopausal virome is completely unexplored. The RNA vaginal virome is also understudied. We know HIV and Zika virus are vaginally shed, which can have serious adverse effects on neonatal health [40,94,95], but the impact of other RNA viruses is unknown. Large-scale clinical trials are needed to test novel vaginal microbiota-based or targeted compounds to ensure safety and efficacy. A better understanding of the genetic bases and molecular mechanisms the virome uses to impact disease could lead to improved health outcomes in cervical cancer, preterm birth, infertility, BV, and HIV disease.
Author Contributions
Conceptualization, C.L.M.; methodology, C.L.M.; writing—original draft preparation, C.L.M. and K.l.O.; writing—review and editing, C.L.M. and K.l.O.; visualization, C.L.M.; supervision, C.L.M.; funding acquisition, C.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
C.L.M. was funded by NIH NIAID K08 grant number AI181643, and K.O. was funded by the HIV Vaccine Trials Network Research and Mentorship Program (RAMP).
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IVF | in vitro fertilization |
| CST | Community state type |
| WGS | whole genome sequencing |
| VLP | virus-like particle |
| LD | Lactobacillus-dominant |
| NLD | non-Lactobacillus-dominant |
| CVL | cervicovaginal lavage |
| WLWH | women living with HIV |
| ART | antiretroviral therapy |
| IFN | Interferon |
| OTU | operational taxonomic unit |
| TTV | Torquetenovirus |
| MGS | metagenomic sequencing |
| STI | sexually transmitted infections |
| TLR | toll-like receptor |
| AMH | anti-Müllerian hormone |
| VST | viral state types |
| VMT | vaginal microbiota transplantation |
| FMT | fecal microbiota transfer |
| uLCFAs | unsaturated long-chain fatty acids |
| OA | oleic acid |
| GBS | Group B streptococcus |
References
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, e471.e1–e471.e9. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Saraf, V.S.; Sheikh, S.A.; Ahmad, A.; Gillevet, P.M.; Bokhari, H.; Javed, S. Vaginal microbiome: Normalcy vs dysbiosis. Arch. Microbiol. 2021, 203, 3793–3802. [Google Scholar] [CrossRef]
- Madere, F.S.; Sohn, M.; Winbush, A.K.; Barr, B.; Grier, A.; Palumbo, C.; Java, J.; Meiring, T.; Williamson, A.L.; Bekker, L.G.; et al. Transkingdom Analysis of the Female Reproductive Tract Reveals Bacteriophages form Communities. Viruses 2022, 14, 430. [Google Scholar] [CrossRef]
- Kaelin, E.A.; Skidmore, P.T.; Laniewski, P.; Holland, L.A.; Chase, D.M.; Herbst-Kralovetz, M.M.; Lim, E.S. Cervicovaginal DNA Virome Alterations Are Associated with Genital Inflammation and Microbiota Composition. mSystems 2022, 7, e0006422. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, L.; Xiao, B.; Zhang, B.; Zuo, Z.; Ji, P.; Zheng, J.; Li, X.; Zhao, F. Maternal and neonatal viromes indicate the risk of offspring’s gastrointestinal tract exposure to pathogenic viruses of vaginal origin during delivery. mLife 2022, 1, 303–310. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Han, X.; Ma, Y.; Liu, Y.; Gao, S.; Zhang, C. Altered vaginal eukaryotic virome is associated with different cervical disease status. Virol. Sin. 2023, 38, 184–197. [Google Scholar] [CrossRef]
- Britto, A.M.A.; Siqueira, J.D.; Curty, G.; Goes, L.R.; Policarpo, C.; Meyrelles, A.R.; Furtado, Y.; Almeida, G.; Giannini, A.L.M.; Machado, E.S.; et al. Microbiome analysis of Brazilian women cervix reveals specific bacterial abundance correlation to RIG-like receptor gene expression. Front. Immunol. 2023, 14, 1147950. [Google Scholar] [CrossRef]
- Da Costa, A.C.; Bortoletto, P.; Spandorfer, S.D.; Tozetto-Mendoza, T.R.; Linhares, I.M.; Mendes-Correa, M.C.; Witkin, S.S. Association between torquetenovirus in vaginal secretions and infertility: An exploratory metagenomic analysis. Am. J. Reprod. Immunol. 2023, 90, e13788. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Krog, M.C.; Vomstein, K.; Du, J.; Bashir, Z.; Kaldhusdal, V.; Fransson, E.; Engstrand, L.; Nielsen, H.S.; Schuppe-Koistinen, I. Defining Vaginal Community Dynamics: Daily microbiome transitions, the role of menstruation, bacteriophages, and bacterial genes. Microbiome 2024, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, S.; Lv, O.; Wang, G.; Zhang, Y.; Li, S.; Zhang, W.; Long, F.; Shen, Z.; Bai, S.; et al. Comparative analysis of the vaginal bacteriome and virome in healthy women living in high-altitude and sea-level areas. Eur. J. Med. Res. 2024, 29, 157. [Google Scholar] [CrossRef]
- Huang, L.; Guo, R.; Li, S.; Wu, X.; Zhang, Y.; Guo, S.; Lv, Y.; Xiao, Z.; Kang, J.; Meng, J.; et al. A multi-kingdom collection of 33,804 reference genomes for the human vaginal microbiome. Nat. Microbiol. 2024, 9, 2185–2200. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, E.A.; Mitchell, C.; Soria, J.; Rosa, A.; Ticona, E.; Coombs, R.W.; Frenkel, L.M.; Bull, M.E.; Lim, E.S. Longitudinal cervicovaginal microbiome and virome alterations during ART and discordant shedding in women living with HIV. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef]
- Jakobsen, R.R.; Haahr, T.; Humaidan, P.; Jensen, J.S.; Kot, W.P.; Castro-Mejia, J.L.; Deng, L.; Leser, T.D.; Nielsen, D.S. Characterization of the Vaginal DNA Virome in Health and Dysbiosis. Viruses 2020, 12, 1143. [Google Scholar] [CrossRef]
- Da Costa, A.C.; Moron, A.F.; Forney, L.J.; Linhares, I.M.; Sabino, E.; Costa, S.F.; Mendes-Correa, M.C.; Witkin, S.S. Identification of bacteriophages in the vagina of pregnant women: A descriptive study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 976–982. [Google Scholar] [CrossRef]
- Zhang, H.T.; Wang, H.; Wu, H.S.; Zeng, J.; Yang, Y. Comparison of viromes in vaginal secretion from pregnant women with and without vaginitis. Virol. J. 2021, 18, 11. [Google Scholar] [CrossRef]
- Happel, A.U.; Balle, C.; Maust, B.S.; Konstantinus, I.N.; Gill, K.; Bekker, L.G.; Froissart, R.; Passmore, J.A.; Karaoz, U.; Varsani, A.; et al. Presence and Persistence of Putative Lytic and Temperate Bacteriophages in Vaginal Metagenomes from South African Adolescents. Viruses 2021, 13, 2341. [Google Scholar] [CrossRef]
- WHO (Ed.) Recommendations for the Treatment of Trichomonas vaginalis, Mycoplasma genitalium, Candida albicans, Bacterial Vaginosis and Human Papillomavirus (Anogenital Warts); WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Zhu, M.; Frank, M.W.; Radka, C.D.; Jeanfavre, S.; Xu, J.; Tse, M.W.; Pacheco, J.A.; Kim, J.S.; Pierce, K.; Deik, A.; et al. Vaginal Lactobacillus fatty acid response mechanisms reveal a metabolite-targeted strategy for bacterial vaginosis treatment. Cell 2024, 187, 5413–5430.e29. [Google Scholar] [CrossRef]
- Farcasanu, M.; Kwon, D.S. The Influence of Cervicovaginal Microbiota on Mucosal Immunity and Prophylaxis in the Battle against HIV. Curr. HIV/AIDS Rep. 2018, 15, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.S.; Li, L. Quantifying the human vaginal community state types (CSTs) with the species specificity index. PeerJ 2017, 5, e3366. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Laniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, D.J. Evaluation of Health Disparity in Bacterial Vaginosis and the Implications for HIV-1 Acquisition in African American Women. Am. J. Reprod. Immunol. 2016, 76, 99–107. [Google Scholar] [CrossRef]
- UNAIDS. FACT SHEET 2024: Global HIV Statistics; UNAIDS: Geneva, Switzerland, 2024. [Google Scholar]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Nunn, K.L.; Wang, Y.Y.; Harit, D.; Humphrys, M.S.; Ma, B.; Cone, R.; Ravel, J.; Lai, S.K. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6, e01084-15. [Google Scholar] [CrossRef]
- De Oliveira, N.S.; de Lima, A.B.F.; de Brito, J.C.R.; Sarmento, A.C.A.; Goncalves, A.K.S.; Eleuterio, J., Jr. Postmenopausal Vaginal Microbiome and Microbiota. Front. Reprod. Health 2021, 3, 780931. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Han, Q.; Chu, W.; Lu, G.; Chan, W.Y.; Qin, Y.; Du, Y. Changes in the vaginal microbiota associated with primary ovarian failure. BMC Microbiol. 2020, 20, 230. [Google Scholar] [CrossRef]
- Daniel, G.A.; Hu, Y.; Tsementzi, D.; Jhaney, C.I.; Hu, Y.J.; Yeager, K.A.; Bai, J.; Dolan, M.; Bruner, D.W. Exploring the Vaginal Microbiome and Intravaginal Practices in Postmenopausal Women. Nurs. Res. 2021, 70, 405–411. [Google Scholar] [CrossRef]
- Madere, F.S.; Monaco, C.L. The female reproductive tract virome: Understanding the dynamic role of viruses in gynecological health and disease. Curr. Opin. Virol. 2022, 52, 15–23. [Google Scholar] [CrossRef]
- Wylie, K.M.; Wylie, T.N.; Cahill, A.G.; Macones, G.A.; Tuuli, M.G.; Stout, M.J. The vaginal eukaryotic DNA virome and preterm birth. Am. J. Obstet. Gynecol. 2018, 219, 189.e1–189.e12. [Google Scholar] [CrossRef]
- Siqueira, J.D.; Curty, G.; Xutao, D.; Hofer, C.B.; Machado, E.S.; Seuánez, H.N.; Soares, M.A.; Delwart, E.; Soares, E.A. Composite Analysis of the Virome and Bacteriome of HIV/HPV Co-Infected Women Reveals Proxies for Immunodeficiency. Viruses 2019, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.J.; Plummer, F.A. The Microbiological Context of HIV Resistance: Vaginal Microbiota and Mucosal Inflammation at the Viral Point of Entry. Int. J. Inflam. 2012, 2012, 131243. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S. HIV and sexually transmitted diseases: Lethal synergy. Top. HIV Med. 2004, 12, 104–107. [Google Scholar]
- Mayer, K.H.; Venkatesh, K.K. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am. J. Reprod. Immunol. 2011, 65, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.J.; Quinn, T.C. Developments in STD/HIV interactions: The intertwining epidemics of HIV and HSV-2. Infect. Dis. Clin. N. Am. 2005, 19, 415–425. [Google Scholar] [CrossRef]
- Mwatelah, R.; McKinnon, L.R.; Baxter, C.; Abdool Karim, Q.; Abdool Karim, S.S. Mechanisms of sexually transmitted infection-induced inflammation in women: Implications for HIV risk. J. Int. AIDS Soc. 2019, 22 (Suppl. 6), e25346. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Chen, Z.; Ye, F.; Sun, Z. The Potential Role of Zika and Dengue Virus Infection in the Urogenital System Disorders: An Overview. Rev. Med. Virol. 2025, 35, e70010. [Google Scholar] [CrossRef]
- Strick, L.B.; Wald, A.; Celum, C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin. Infect. Dis. 2006, 43, 347–356. [Google Scholar] [CrossRef]
- Maiman, M.; Fruchter, R.G.; Clark, M.; Arrastia, C.D.; Matthews, R.; Gates, E.J. Cervical cancer as an AIDS-defining illness. Obstet. Gynecol. 1997, 89, 76–80. [Google Scholar] [CrossRef]
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Gao, W.; Weng, J.; Gao, Y.; Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect. Dis. 2013, 13, 271. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Krass, L.; Landay, A.; Spear, G.T. The role of bacterial vaginosis and trichomonas in HIV transmission across the female genital tract. Curr. HIV Res. 2012, 10, 202–210. [Google Scholar] [CrossRef]
- Moi, H. Prevalence of bacterial vaginosis and its association with genital infections, inflammation, and contraceptive methods in women attending sexually transmitted disease and primary health clinics. Int. J. STD AIDS 1990, 1, 86–94. [Google Scholar] [CrossRef]
- Reimers, L.L.; Mehta, S.D.; Massad, L.S.; Burk, R.D.; Xie, X.; Ravel, J.; Cohen, M.H.; Palefsky, J.M.; Weber, K.M.; Xue, X.; et al. The Cervicovaginal Microbiota and Its Associations with Human Papillomavirus Detection in HIV-Infected and HIV-Uninfected Women. J. Infect. Dis. 2016, 214, 1361–1369. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef]
- Waldor, M.K. Bacteriophage biology and bacterial virulence. Trends Microbiol. 1998, 6, 295–297. [Google Scholar] [CrossRef]
- Sausset, R.; Petit, M.A.; Gaboriau-Routhiau, V.; De Paepe, M. New insights into intestinal phages. Mucosal Immunol. 2020, 13, 205–215. [Google Scholar] [CrossRef]
- Leon-Felix, J.; Villicana, C. The impact of quorum sensing on the modulation of phage-host interactions. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis-lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef]
- Damelin, L.H.; Paximadis, M.; Mavri-Damelin, D.; Birkhead, M.; Lewis, D.A.; Tiemessen, C.T. Identification of predominant culturable vaginal Lactobacillus species and associated bacteriophages from women with and without vaginal discharge syndrome in South Africa. J. Med. Microbiol. 2011, 60, 180–183. [Google Scholar] [CrossRef]
- Kiliç, A.O.; Pavlova, S.I.; Alpay, S.; Kiliç, S.S.; Tao, L. Comparative study of vaginal Lactobacillus phages isolated from women in the United States and Turkey: Prevalence, morphology, host range, and DNA homology. Clin. Diagn. Lab. Immunol. 2001, 8, 31–39. [Google Scholar] [CrossRef]
- Tao, L.; Pavlova, S.I.; Mou, S.M.; Ma, W.G.; Kilic, A.O. Analysis of lactobacillus products for phages and bacteriocins that inhibit vaginal lactobacilli. Infect. Dis. Obstet. Gynecol. 1997, 5, 244–251. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, Y. Ten computational challenges in human virome studies. Virol. Sin. 2024, 39, 845–850. [Google Scholar] [CrossRef]
- Khan Mirzaei, M.; Xue, J.; Costa, R.; Ru, J.; Schulz, S.; Taranu, Z.E.; Deng, L. Challenges of Studying the Human Virome—Relevant Emerging Technologies. Trends Microbiol. 2021, 29, 171–181. [Google Scholar] [CrossRef]
- Chang, W.S.; Harvey, E.; Mahar, J.E.; Firth, C.; Shi, M.; Simon-Loriere, E.; Geoghegan, J.L.; Wille, M. Improving the reporting of metagenomic virome-scale data. Commun. Biol. 2024, 7, 1687. [Google Scholar] [CrossRef]
- Hou, X.; He, Y.; Fang, P.; Mei, S.Q.; Xu, Z.; Wu, W.C.; Tian, J.H.; Zhang, S.; Zeng, Z.Y.; Gou, Q.Y.; et al. Using artificial intelligence to document the hidden RNA virosphere. Cell 2024, 187, 6929–6942.e6916. [Google Scholar] [CrossRef]
- Monaco, C.L.; Kwon, D.S. Next-generation Sequencing of the DNA Virome from Fecal Samples. Bio Protoc. 2017, 7, e2159. [Google Scholar] [CrossRef]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Unraveling the viral dark matter through viral metagenomics. Front. Immunol. 2022, 13, 1005107. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Tosado-Rodriguez, E.; Alvarado-Velez, I.; Romaguera, J.; Godoy-Vitorino, F. Vaginal Microbiota and HPV in Latin America: A Narrative Review. Microorganisms 2024, 12, 619. [Google Scholar] [CrossRef]
- Akbari, E.; Milani, A.; Seyedinkhorasani, M.; Bolhassani, A. HPV co-infections with other pathogens in cancer development: A comprehensive review. J. Med. Virol. 2023, 95, e29236. [Google Scholar] [CrossRef]
- Sasivimolrattana, T.; Chantratita, W.; Sensorn, I.; Chaiwongkot, A.; Oranratanaphan, S.; Bhattarakosol, P. Human Virome in Cervix Controlled by the Domination of Human Papillomavirus. Viruses 2022, 14, 2066. [Google Scholar] [CrossRef]
- Happel, A.U.; Balle, C.; Havyarimana, E.; Brown, B.; Maust, B.S.; Feng, C.; Yi, B.H.; Gill, K.; Bekker, L.G.; Passmore, J.S.; et al. Cervicovaginal Human Papillomavirus Genomes, Microbiota Composition and Cytokine Concentrations in South African Adolescents. Viruses 2023, 15, 758. [Google Scholar] [CrossRef]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef]
- Nicolo, S.; Antonelli, A.; Tanturli, M.; Baccani, I.; Bonaiuto, C.; Castronovo, G.; Rossolini, G.M.; Mattiuz, G.; Torcia, M.G. Bacterial Species from Vaginal Microbiota Differently Affect the Production of the E6 and E7 Oncoproteins and of p53 and p-Rb Oncosuppressors in HPV16-Infected Cells. Int. J. Mol. Sci. 2023, 24, 7173. [Google Scholar] [CrossRef]
- Stewart, L.L.; Vodstrcil, L.A.; Coombe, J.; Bradshaw, C.S.; Hocking, J.S. Prevalence of bacterial vaginosis in postmenopausal women: A systematic review and meta-analysis. Sex Health 2022, 19, 17–26. [Google Scholar] [CrossRef]
- Cocomazzi, G.; De Stefani, S.; Del Pup, L.; Palini, S.; Buccheri, M.; Primiterra, M.; Scianname, N.; Faioli, R.; Maglione, A.; Baldini, G.M.; et al. The Impact of the Female Genital Microbiota on the Outcome of Assisted Reproduction Treatments. Microorganisms 2023, 11, 1443. [Google Scholar] [CrossRef]
- Pavlova, S.I.; Tao, L. Induction of vaginal Lactobacillus phages by the cigarette smoke chemical benzo[a]pyrene diol epoxide. Mutat. Res. 2000, 466, 57–62. [Google Scholar] [CrossRef]
- Eskew, A.M.; Stout, M.J.; Bedrick, B.S.; Riley, J.K.; Omurtag, K.R.; Jimenez, P.T.; Odem, R.R.; Ratts, V.S.; Keller, S.L.; Jungheim, E.S.; et al. Association of the eukaryotic vaginal virome with prophylactic antibiotic exposure and reproductive outcomes in a subfertile population undergoing in vitro fertilisation: A prospective exploratory study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 208–216. [Google Scholar] [CrossRef]
- Racicot, K.; Cardenas, I.; Wünsche, V.; Aldo, P.; Guller, S.; Means, R.E.; Romero, R.; Mor, G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J. Immunol. 2013, 191, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, I.; Means, R.E.; Aldo, P.; Koga, K.; Lang, S.M.; Booth, C.J.; Booth, C.; Manzur, A.; Oyarzun, E.; Romero, R.; et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J. Immunol. 2010, 185, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.; Wooten, D.; Annepally, S.; Burke, L.; Edi, R.; Morris, S.R. Impact of (recurrent) bacterial vaginosis on quality of life and the need for accessible alternative treatments. BMC Womens Health 2023, 23, 112. [Google Scholar] [CrossRef]
- Javed, A.; Parvaiz, F.; Manzoor, S. Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb. Pathog. 2019, 127, 21–30. [Google Scholar] [CrossRef]
- Gao, M.; Manos, J.; Whiteley, G.; Zablotska-Manos, I. Antibiofilm Agents for the Treatment and Prevention of Bacterial Vaginosis: A Systematic Narrative Review. J. Infect. Dis. 2024, 230, e508–e517. [Google Scholar] [CrossRef]
- Castro, J.; Sousa, L.G.V.; Franca, A.; Podpera Tisakova, L.; Corsini, L.; Cerca, N. Exploiting the Anti-Biofilm Effect of the Engineered Phage Endolysin PM-477 to Disrupt In Vitro Single- and Dual-Species Biofilms of Vaginal Pathogens Associated with Bacterial Vaginosis. Antibiotics 2022, 11, 558. [Google Scholar] [CrossRef]
- Fan, Y.; Gu, Y.; Xian, Y.; Li, Q.; He, Y.; Chen, K.; Yu, H.; Deng, H.; Xiong, L.; Cui, Z.; et al. Efficacy and safety of different drugs for the treatment of bacterial vaginosis: A systematic review and network meta-analysis. Front. Cell. Infect. Microbiol. 2024, 14, 1402346. [Google Scholar] [CrossRef]
- Ngugi, B.M.; Hemmerling, A.; Bukusi, E.A.; Kikuvi, G.; Gikunju, J.; Shiboski, S.; Fredricks, D.N.; Cohen, C.R. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm. Dis. 2011, 38, 1020–1027. [Google Scholar] [CrossRef]
- Ma, S.; Wang, W.; Su, Y.; Sun, W.; Ma, L. Antibiotics therapy combined with probiotics administered intravaginally for the treatment of bacterial vaginosis: A systematic review and meta-analysis. Open Med. 2023, 18, 20230644. [Google Scholar] [CrossRef]
- Abavisani, M.; Sahebi, S.; Dadgar, F.; Peikfalak, F.; Keikha, M. The role of probiotics as adjunct treatment in the prevention and management of gynecological infections: An updated meta-analysis of 35 RCT studies. Taiwan J. Obstet. Gynecol. 2024, 63, 357–368. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Hussain, F.A.; Bergerat, A.; Reissis, A.; Worrall, D.; Xu, J.; Gomez, I.; Bloom, S.M.; Mafunda, N.A.; Kelly, J.; et al. Screening and characterization of vaginal fluid donations for vaginal microbiota transplantation. Sci. Rep. 2022, 12, 17948. [Google Scholar] [CrossRef] [PubMed]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Zhang, G. Vaginal microbiota transplantation is a truly opulent and promising edge: Fully grasp its potential. Front. Cell. Infect. Microbiol. 2024, 14, 1280636. [Google Scholar] [CrossRef] [PubMed]
- Tuniyazi, M.; Zhang, N. Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation. Microorganisms 2023, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Vincent, C.; Edens, T.J.; Miller, M.; Manges, A.R. Antimicrobial Resistance Gene Acquisition and Depletion Following Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2018, 66, 456–457. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Happel, A.U.; Kullin, B.R.; Gamieldien, H.; Jaspan, H.B.; Varsani, A.; Martin, D.; Passmore, J.S.; Froissart, R. In Silico Characterisation of Putative Prophages in Lactobacillaceae Used in Probiotics for Vaginal Health. Microorganisms 2022, 10, 214. [Google Scholar] [CrossRef]
- Fischetti, V.A. Novel method to control pathogenic bacteria on human mucous membranes. Ann. N. Y. Acad. Sci. 2003, 987, 207–214. [Google Scholar] [CrossRef]
- Renard, A.; Diene, S.M.; Courtier-Martinez, L.; Gaillard, J.B.; Gbaguidi-Haore, H.; Mereghetti, L.; Quentin, R.; Francois, P.; Van Der Mee-Marquet, N. 12/111phiA Prophage Domestication Is Associated with Autoaggregation and Increased Ability to Produce Biofilm in Streptococcus agalactiae. Microorganisms 2021, 9, 1112. [Google Scholar] [CrossRef]
- Bocanova, L.; Psenko, M.; Barak, I.; Halgasova, N.; Drahovska, H.; Bukovska, G. A novel phage-encoded endolysin EN534-C active against clinical strain Streptococcus agalactiae GBS. J. Biotechnol. 2022, 359, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Nagot, N.; Ouedraogo, A.; Weiss, H.A.; Konate, I.; Sanon, A.; Defer, M.C.; Sawadogo, A.; Andonaba, J.B.; Vallo, R.; Becquart, P.; et al. Longitudinal effect following initiation of highly active antiretroviral therapy on plasma and cervico-vaginal HIV-1 RNA among women in Burkina Faso. Sex Transm. Infect. 2008, 84, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Souza, R.P.; Cruz, T.E.D.; Damke, G.; Damke, E.; Suehiro, T.T.; Silva, V.; Consolaro, M.E.L. Zika virus infection in the genital tract of non-pregnant females: A systematic review. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e16. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).