The Role of Active Packaging in the Defense Against Foodborne Pathogens with Particular Attention to Bacteriophages
Abstract
1. Introduction
2. Antimicrobials in Food Packaging
3. Bacteriophages
3.1. History of Bacteriophages
3.2. Classification of Phages
3.3. Virus Replication
4. Application of Multifunctional Bacteriophages
4.1. Healthcare
4.2. Rapid Bacterial Detection
4.3. Bacteriophages as Antimicrobial Agents in Active Food Packaging
4.4. Commercially Available Bacteriophages
4.5. Challenges and Defects in the Application of Bacteriophage in Packaging Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onyeaka, H.; Ghosh, S.; Obileke, K.; Miri, T.; Odeyemi, O.A.; Nwaiwu, O.; Tamasiga, P. Preventing Chemical Contaminants in Food: Challenges and Prospects for Safe and Sustainable Food Production. Food Control 2024, 155, 110040. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Tauxe, R.V. Emerging Foodborne Pathogens. Int. J. Food Microbiol. 2002, 78, 31–41. [Google Scholar] [CrossRef]
- Hoffmann, S.; White, A.E.; McQueen, R.B.; Ahn, J.-W.; Gunn-Sandell, L.B.; Scallan Walter, E.J. Economic Burden of Foodborne Illnesses Acquired in the United States. Foodborne Pathog. Dis. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wittler, R.R. Foodborne and Waterborne Illness. Pediatr. Rev. 2023, 44, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- Panwar, S.; Duggirala, K.S.; Yadav, P.; Debnath, N.; Yadav, A.K.; Kumar, A. Advanced Diagnostic Methods for Identification of Bacterial Foodborne Pathogens: Contemporary and Upcoming Challenges. Crit. Rev. Biotechnol. 2023, 43, 982–1000. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- CDC Four Steps to Food Safety-CDC. Available online: https://www.cdc.gov/food-safety/prevention/index.html (accessed on 1 December 2024).
- Wagh, R.V.; Ezati, P.; Khan, A.; Priyadarshi, R.; Rhim, J. Vaccinium corymbosum—Derived Carbon Dots and Anthocyanin-Infused Gelatin Multifunctional Films for Smart Packaging Applications. Packag. Technol. Sci. 2024, 37, 1049–1064. [Google Scholar] [CrossRef]
- Rindhe, S.; Khan, A.; Priyadarshi, R.; Chatli, M.; Wagh, R.V.; Kumbhar, V.; Wankar, A.; Rhim, J. Application of Bacteriophages in Biopolymer-based Functional Food Packaging Films. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13333. [Google Scholar] [CrossRef] [PubMed]
- Wagh, R.V.; Priyadarshi, R.; Rhim, J.-W. Novel Bacteriophage-Based Food Packaging: An Innovative Food Safety Approach. Coatings 2023, 13, 609. [Google Scholar] [CrossRef]
- Wagh, R.V.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Carrageenan-Based Functional Films Hybridized with Carbon Dots and Anthocyanins from Rose Petals for Smart Food Packaging Applications. Int. J. Biol. Macromol. 2024, 272, 132817. [Google Scholar] [CrossRef]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial Food Packaging: Potential and Pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Orlova, E.V. Bacteriophages and Their Structural Organisation; IntechOpen: Lomdon, UK, 2012; ISBN 978-953-51-0272-4. [Google Scholar]
- Hameed, F.; Bandral, J.D.; Gupta, N.; Nayik, G.A.; Sood, M.; Rahman, R. Use of Bacteriophages as a Target Specific Therapy against Food-Borne Pathogens in Food Industry-a Review: Bacteriophage. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e2949. [Google Scholar] [CrossRef]
- Letarov, A.V. History of Early Bacteriophage Research and Emergence of Key Concepts in Virology. Biochemistry 2020, 85, 1093–1112. [Google Scholar] [CrossRef]
- Brüssow, H.; Hendrix, R.W. Phage Genomics: Small Is Beautiful. Cell 2002, 108, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 690706. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Molaei, R.; Kousheh, S.A.; Guimarães, J.T.; McClements, D.J. Carbon Dots Synthesized from Microorganisms and Food By-Products: Active and Smart Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2021, 63, 1943–1959. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Adv. Colloid. Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioproc Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Shakeel Ahmed, S.K. Handbook of Bionanocomposites, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2018; ISBN 9781351170680. [Google Scholar]
- Riahi, Z.; Rhim, J.-W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl Cellulose-Based Functional Film Integrated with Chitosan-Based Carbon Quantum Dots for Active Food Packaging Applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Antimicrobial Poly(Lactic Acid)/Cellulose Bionanocomposite for Food Packaging Application: A Review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan Films Incorporated with Apricot (Prunus Armeniaca) Kernel Essential Oil as Active Food Packaging Material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.; Osaili, T.; Sawalha, A.; Olaimat, A.N.; Albiss, B.A.; Mehyar, G.; Ayyash, M.; Holley, R. Antimicrobial Activity of Chitosan Coating Containing ZnO Nanoparticles against E. coli O157:H7 on the Surface of White Brined Cheese. Int. J. Food Microbiol. 2020, 334, 108838. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc Oxide Nanorods/Clove Essential Oil Incorporated Type B Gelatin Composite Films and Its Applicability for Shrimp Packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K. Flexible Chitosan-Nano ZnO Antimicrobial Pouches as a New Material for Extending the Shelf Life of Raw Meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging Chitosan-Essential Oil Films and Coatings for Food Preservation—A Review of Advances and Applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W.; Nobile, A.D.; Conte, A. Development of Multifunctional Pullulan/Chitosan-Based Composite Films Reinforced with ZnO Nanoparticles and Propolis for Meat Packaging Applications. Foods 2021, 10, 2789. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Moghaddas Kia, E.; Ghasempour, Z.; Ehsani, A. Preparation of Active Nanocomposite Film Consisting of Sodium Caseinate, ZnO Nanoparticles and Rosemary Essential Oil for Food Packaging Applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Bruna, J.E.; Galotto, M.J.; Guarda, A.; Rodríguez, F. A Novel Polymer Based on MtCu2+/Cellulose Acetate with Antimicrobial Activity. Carbohydr. Polym. 2014, 102, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Cellulose Nanofiber-Based Multifunctional Films Integrated with Carbon Dots and Anthocyanins from Brassica Oleracea for Active and Intelligent Food Packaging Applications. Int. J. Biol. Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Priyadarshi, R.; Han, S. Cellulose Nanofiber-Based Coating Film Integrated with Nitrogen-Functionalized Carbon Dots for Active Packaging Applications of Fresh Fruit. Postharvest Biol. Technol. 2022, 186, 111845. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Carboxymethyl Cellulose-Based Multifunctional Film Combined with Zinc Oxide Nanoparticles and Grape Seed Extract for the Preservation of High-Fat Meat Products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Shankar, S.; Tanomrod, N.; Rawdkuen, S.; Rhim, J.-W. Preparation of Pectin/Silver Nanoparticles Composite Films with UV-Light Barrier and Properties. Int. J. Biol. Macromol. 2016, 92, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Aleksanyan, K.V.; Smykovskaya, R.S.; Samoilova, N.A.; Novikov, V.A.; Shakhov, A.M.; Aybush, A.V.; Kuznetsova, O.P.; Lomakin, S.M.; Ryzhmanova, Y.V. Development of Poly(Lactic Acid)-Based Biocomposites with Silver Nanoparticles and Investigation of Their Characteristics. Polymers 2024, 16, 2758. [Google Scholar] [CrossRef]
- Stanley, J.; Xanthopoulou, E.; Finšgar, M.; Zemljič, L.F.; Klonos, P.A.; Kyritsis, A.; Koltsakidis, S.; Tzetzis, D.; Lambropoulou, D.A.; Baciu, D.; et al. Synthesis of Poly(Ethylene Furanoate) Based Nanocomposites by In Situ Polymerization with Enhanced Antibacterial Properties for Food Packaging Applications. Polymers 2023, 15, 4502. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial Activity of Alginate/Clay Nanocomposite Films Enriched with Essential Oils against Three Common Foodborne Pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Malagurski, I.; Levic, S.; Nesic, A.; Mitric, M.; Pavlovic, V.; Dimitrijevic-Brankovic, S. Mineralized Agar-Based Nanocomposite Films: Potential Food Packaging Materials with Antimicrobial Properties. Carbohydr. Polym. 2017, 175, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, B.S.; Buazar, F.; Hosseini, S.M.; Mousavi, S.M. Enhanced Antibacterial Activity, Mechanical and Physical Properties of Alginate/Hydroxyapatite Bionanocomposite Film. Int. J. Biol. Macromol. 2018, 116, 786–792. [Google Scholar] [CrossRef]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of Novel Nanohybrids by Impregnation of CuO Nanoparticles into Bacterial Cellulose and Chitosan Nanofibers: Characterization, Antimicrobial and Release Properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium Dioxide (TiO2) for the Manufacture of Multifunctional Active Food Packaging Films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Jin, T.; Yan, L.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Preparation and Physicochemical/Antimicrobial Characteristics of Asparagus Cellulose Films Containing Quercetin. Food Sci. Human. Wellness 2021, 10, 251–257. [Google Scholar] [CrossRef]
- Sharma, S.; Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Natural Antimicrobials from Fruits and Plant Extract for Food Packaging and Preservation. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2024; pp. 133–152. [Google Scholar]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.-W. Alginate-Coated Functional Wrapping Paper Incorporated with Sulfur Quantum Dots and Grapefruit Seed Extract for Preservation of Potato Hash Browns. Sustain. Mater. Technol. 2024, 40, e00942. [Google Scholar] [CrossRef]
- Ahsan, S.; Tariq, A.; Liaqat, A.; Khaliq, A.; Farooq, M.A.; Hussain, A.; Kauser, S.; Ali, S.; Firdous, N.; Ahmed, A.; et al. Development of Active Packaging Film, Based on Phyllanthus Wightianus and Their Application to Meat Product Preservation. J. Food Meas. Charact. 2024, 18, 4905–4919. [Google Scholar] [CrossRef]
- Sreekanth, K.; Sharath, K.P.; CD, M.D.; Mathew, D.; Radhakrishnan, E.K. Microbial Load Reduction in Stored Raw Beef Meat Using Chitosan/Starch-Based Active Packaging Films Incorporated with Cellulose Nanofibers and Cinnamon Essential Oil. Meat Sci. 2024, 216, 109552. [Google Scholar] [CrossRef]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of Antimicrobial/Ultraviolet Protective Bacterial Nanocellulose Film with Carbon Dots Synthesized from Lactic Acid Bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Chitosan/Gelatin-Based Multifunctional Films Integrated with Sulfur-Functionalized Chitin for Active Packaging Applications. Food Hydrocoll. 2024, 149, 109537. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional Biocompatible Nanocomposite Films Consisting of Selenium and Zinc Oxide Nanoparticles Embedded in Gelatin/Cellulose Nanofiber Matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, K.Y. Native Marine Bacteriophages. FEMS Microbiol. Lett. 1993, 102, 141–159. [Google Scholar] [CrossRef]
- Ackermann, H.-W.; Prangishvili, D. Prokaryote Viruses Studied by Electron Microscopy. Arch. Virol. 2012, 157, 1843–1849. [Google Scholar] [CrossRef]
- Batinovic, S.; Wassef, F.; Knowler, S.A.; Rice, D.T.F.; Stanton, C.R.; Rose, J.; Tucci, J.; Nittami, T.; Vinh, A.; Drummond, G.R.; et al. Bacteriophages in Natural and Artificial Environments. Pathogens 2019, 8, 100. [Google Scholar] [CrossRef]
- Zrelovs, N.; Dislers, A.; Kazaks, A. Motley Crew: Overview of the Currently Available Phage Diversity. Front. Microbiol. 2020, 11, 579452. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W. Frequency of Morphological Phage Descriptions in 1995. Arch. Virol. 1996, 141, 209–218. [Google Scholar] [CrossRef]
- Jurczak-Kurek, A.; Gąsior, T.; Nejman-Faleńczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of Bacteriophages: Morphological and Biological Properties of a Large Group of Phages Isolated from Urban Sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Sen, G.C. Viruses and Interferons. Annu. Rev. Microbiol. 2001, 55, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W. Phage Classification and Characterization. Methods Mol Biol. 2009, 501, 127–140. [Google Scholar] [PubMed]
- Ackermann, H.-W. Tailed Bacteriophages: The Order Caudovirales. Adv Virus Res. 1998, 51, 135–201. [Google Scholar] [PubMed]
- King, A.M.Q.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011; Volume 9, ISBN 0123846854. [Google Scholar]
- Ackermann, H.W. Frequency of Morphological Phage Descriptions in the Year 2000. Arch. Virol. 2001, 146, 843–857. [Google Scholar] [CrossRef]
- Ackermann, H.W. 5500 Phages Examined in the Electron Microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Lithgow, T. Filamentous Phages: Masters of a Microbial Sharing Economy. EMBO Rep. 2019, 20, e47427. [Google Scholar] [CrossRef]
- Chipman, P.R.; Agbandje-McKenna, M.; Renaudin, J.; Baker, T.S.; McKenna, R. Structural Analysis of the Spiroplasma Virus, SpV4: Implications for Evolutionary Variation to Obtain Host Diversity among the Microviridae. Structure 1998, 6, 135–145. [Google Scholar] [CrossRef]
- Brentlinger, K.L.; Hafenstein, S.; Novak, C.R.; Fane, B.A.; Borgon, R.; McKenna, R.; Agbandje-McKenna, M. Microviridae, a Family Divided: Isolation, Characterization, and Genome Sequence of ΦMH2K, a Bacteriophage of the Obligate Intracellular Parasitic Bacterium Bdellovibrio bacteriovorus. J. Bacteriol. 2002, 184, 1089–1094. [Google Scholar] [CrossRef]
- Oksanen, H.M. ICTV Virus Taxonomy Profile: Corticoviridae. J. General. Virol. 2017, 98, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Abrescia, N.G.A.; Cockburn, J.J.B.; Grimes, J.M.; Sutton, G.C.; Diprose, J.M.; Butcher, S.J.; Fuller, S.D.; San Martín, C.; Burnett, R.M.; Stuart, D.I.; et al. Insights into Assembly from Structural Analysis of Bacteriophage PRD1. Nature 2004, 432, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.M.; deCarvalho, T.N.; Huynh, A.; Morcos, G.; Kuo, N.; Parsa, S.; Erill, I. A Novel Genus of Actinobacterial Tectiviridae. Viruses 2019, 11, 1134. [Google Scholar] [CrossRef]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for Unifying the Terminology in Biological Control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Bollback, J.P.; Huelsenbeck, J.P. Phylogeny, Genome Evolution, and Host Specificity of Single-Stranded RNA Bacteriophage (Family Leviviridae). J. Mol. Evol. 2001, 52, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Poranen, M.M.; Mäntynen, S. ICTV Virus Taxonomy Profile: Cystoviridae. J. General. Virol. 2017, 98, 2423–2424. [Google Scholar] [CrossRef]
- Bath, C.; Cukalac, T.; Porter, K.; Dyall-Smith, M.L. His1 and His2 Are Distantly Related, Spindle-Shaped Haloviruses Belonging to the Novel Virus Group, Salterprovirus. Virology 2006, 350, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Kukkaro, P.; Bamford, J.K.H.; Bath, C.; Kivelä, H.M.; Dyall-Smith, M.L.; Bamford, D.H. SH1: A Novel, Spherical Halovirus Isolated from an Australian Hypersaline Lake. Virology 2005, 335, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.; Tang, L.; Stedman, K.; Roberto, F.; Spuhler, J.; Gillitzer, E.; Johnson, J.E.; Douglas, T.; Young, M. The Structure of a Thermophilic Archaeal Virus Shows a Double-Stranded DNA Viral Capsid Type That Spans All Domains of Life. Proc. Natl. Acad. Sci. USA 2004, 101, 7716–7720. [Google Scholar] [CrossRef]
- Prangishvili, D.; Krupovic, M. ICTV Virus Taxonomy Profile: Bicaudaviridae. J. General. Virol. 2018, 99, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D.; Stedman, K.; Zillig, W. Viruses of the Extremely Thermophilic Archaeon Sulfolobus. Trends Microbiol. 2001, 9, 39–43. [Google Scholar] [CrossRef]
- Veesler, D.; Cambillau, C. A Common Evolutionary Origin for Tailed-Bacteriophage Functional Modules and Bacterial Machineries. Microbiol. Mol. Biol. Rev. 2011, 75, 423–433. [Google Scholar] [CrossRef]
- Krupovic, M. ICTV Virus Taxonomy Profile: Plasmaviridae. J. General. Virol. 2018, 99, 617–618. [Google Scholar] [CrossRef]
- Prangishvili, D.; Mochizuki, T.; Krupovic, M. ICTV Virus Taxonomy Profile: Guttaviridae. J. General. Virol. 2018, 99, 290–291. [Google Scholar] [CrossRef]
- Ahn, D.-G.; Kim, S.-I.; Rhee, J.-K.; Pyo Kim, K.; Pan, J.-G.; Oh, J.-W. TTSV1, a New Virus-like Particle Isolated from the Hyperthermophilic Crenarchaeote Thermoproteus Tenax. Virology 2006, 351, 280–290. [Google Scholar] [CrossRef][Green Version]
- Bertani, G. Lysogenic versus Lytic Cycle of Phage Multiplication. Cold Spring Harb. Symp. Quant. Biol. 1953, 18, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Vonasek, E.L.; Choi, A.H.; Sanchez, J.; Nitin, N. Incorporating Phage Therapy into WPI Dip Coatings for Applications on Fresh Whole and Cut Fruit and Vegetable Surfaces. J. Food Sci. 2018, 83, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.W. Bacteriophage Genomics. Curr. Opin. Microbiol. 2003, 6, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, L.; Dong, B.; Kou, E.; Wang, L.; Zhu, Y. Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections. Int. J. Mol. Sci. 2024, 25, 5465. [Google Scholar] [CrossRef]
- Rose, T.; Verbeken, G.; De Vos, D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.-P. Experimental Phage Therapy of Burn Wound Infection: Difficult First Steps. Int. J. Burn. Trauma. 2014, 4, 66–73. [Google Scholar]
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.-K. Phage Therapy for Respiratory Infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86. [Google Scholar] [CrossRef]
- Iszatt, J.J.; Larcombe, A.N.; Chan, H.-K.; Stick, S.M.; Garratt, L.W.; Kicic, A. Phage Therapy for Multi-Drug Resistant Respiratory Tract Infections. Viruses 2021, 13, 1809. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Hooey, P.B.; Cook, J.D.; Burrows, L.L.; Martyniuk, J.; Hynes, A.P.; German, G.J. Phage Therapy in the Management of Urinary Tract Infections: A Comprehensive Systematic Review. PHAGE 2023, 4, 112–127. [Google Scholar] [CrossRef]
- Malik, S.; Sidhu, P.K.; Rana, J.S.; Nehra, K. Managing Urinary Tract Infections through Phage Therapy: A Novel Approach. Folia Microbiol. 2020, 65, 217–231. [Google Scholar] [CrossRef]
- Kutateladze, M. Experience of the Eliava Institute in Bacteriophage Therapy. Virol. Sin. 2015, 30, 80–81. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Zhan, W.; Li, Z.; Zou, L.; Zhao, Q. Challenges for the Application of Bacteriophages as Effective Antibacterial Agents in the Food Industry. J. Sci. Food Agric. 2022, 102, 461–471. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Li, C.; Ye, Y.; Chen, X.; Lin, L. Enhancing Anti-E. Coli O157:H7 Activity of Composite Phage Nanofiber Film by D-Phenylalanine for Food Packaging. Int. J. Food Microbiol. 2022, 376, 109762. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chang, Y. Anti-Salmonella Polyvinyl Alcohol Coating Containing a Virulent Phage PBSE191 and Its Application on Chicken Eggshell. Food Res. Int. 2022, 162, 111971. [Google Scholar] [CrossRef]
- Song, J.; Ruan, H.; Chen, L.; Jin, Y.; Zheng, J.; Wu, R.; Sun, D. Potential of Bacteriophages as Disinfectants to Control of Staphylococcus Aureus Biofilms. BMC Microbiol. 2021, 21, 57. [Google Scholar] [CrossRef]
- Issabekov, S.S.; Syrym, N.S.; Sambetbayev, A.A.; Alikhanov, K.D.; Amanbaevich Yespembetov, B. Prospects of Bacteriophage Collections in Disinfectant Applications. Vet. World 2022, 15, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.E.D.; Dodd, C.E.R. Phage for Rapid Detection and Control of Bacterial Pathogens in Food. Adv. Appl. Microbiol. 2006, 59, 159–186. [Google Scholar]
- Richter, Ł.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Paczesny, J.; Hołyst, R. Recent Advances in Bacteriophage-Based Methods for Bacteria Detection. Drug Discov. Today 2018, 23, 448–455. [Google Scholar] [CrossRef]
- Prangishvili, D.; Arnold, H.P.; Götz, D.; Ziese, U.; Holz, I.; Kristjansson, J.K.; Zillig, W. A Novel Virus Family, the Rudiviridae: Structure, Virus-Host Interactions and Genome Variability of the Sulfolobus Viruses SIRV1 and SIRV2. Genetics 1999, 152, 1387–1396. [Google Scholar] [CrossRef]
- Singh, A.; Poshtiban, S.; Evoy, S. Recent Advances in Bacteriophage Based Biosensors for Food-Borne Pathogen Detection. Sensors 2013, 13, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Endersen, L.; O’Mahony, J.; Hill, C.; Ross, R.P.; McAuliffe, O.; Coffey, A. Phage Therapy in the Food Industry. Annu. Rev. Food Sci. Technol. 2014, 5, 327–349. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, D.S.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone Film Functionalized with Bacteriophage T4 Promotes Antibacterial Activity of Food Packaging toward Escherichia coli. Food Chem. 2021, 346, 128883. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; López, A.; Sáez-Orviz, S.; Marcet, I.; García, P.; Rendueles, M.; Díaz, M. Effectiveness of Bacteriophages Incorporated in Gelatine Films against Staphylococcus Aureus. Food Control 2021, 121, 107666. [Google Scholar] [CrossRef]
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The Antibacterial Effect of Chitosan-based Edible Coating Incorporated with a Lytic Bacteriophage against Escherichia coli O157:H7 on the Surface of Tomatoes. J. Food Saf. 2018, 38, e12571. [Google Scholar] [CrossRef]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ΦIBB-PF7A Loaded on Sodium Alginate-Based Films to Prevent Microbial Meat Spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; López Carballo, G. Development of Biodegradable Films Loaded with Phages with Antilisterial Properties. Polymers 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Characterization of PHBV Films Loaded with FO1 Bacteriophage Using Polyvinyl Alcohol-Based Nanofibers and Coatings: A Comparative Study. Innov. Food Sci. Emerg. Technol. 2021, 69, 102646. [Google Scholar] [CrossRef]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Development of Whey Protein Concentrate/Pullulan Composite Films Containing Bacteriophage A511: Functional Properties and Anti-Listerial Effects during Storage. Food Packag. Shelf Life 2022, 33, 100902. [Google Scholar] [CrossRef]
- Tomat, D.; Soazo, M.; Verdini, R.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of an WPC Edible Film Added with a Cocktail of Six Lytic Phages against Foodborne Pathogens Such as Enteropathogenic and Shigatoxigenic Escherichia coli. LWT 2019, 113, 108316. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Zhang, H.; Zhao, Y.; Shu, M.; Wu, G. Effectiveness of Bacteriophage JN01 Incorporated in Gelatin Film with Protocatechuic Acid on Biocontrol of Escherichia coli O157:H7 in Beef. Int. J. Food Sci. Technol. 2022, 57, 3503–3514. [Google Scholar] [CrossRef]
- Radford, D.; Guild, B.; Strange, P.; Ahmed, R.; Lim, L.-T.; Balamurugan, S. Characterization of Antimicrobial Properties of Salmonella Phage Felix O1 and Listeria Phage A511 Embedded in Xanthan Coatings on Poly(Lactic Acid) Films. Food Microbiol. 2017, 66, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Lin, L. Novel Chitosan Film Embedded with Liposome-Encapsulated Phage for Biocontrol of Escherichia coli O157:H7 in Beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kimmelshue, C.; Goggi, A.S.; Cademartiri, R. The Use of Biological Seed Coatings Based on Bacteriophages and Polymers against Clavibacter michiganensis subsp. nebraskensis in Maize Seeds. Sci. Rep. 2019, 9, 17950. [Google Scholar] [CrossRef]
- Huang, K.; Nitin, N. Edible Bacteriophage Based Antimicrobial Coating on Fish Feed for Enhanced Treatment of Bacterial Infections in Aquaculture Industry. Aquaculture 2019, 502, 18–25. [Google Scholar] [CrossRef]
- Sezer, B.; Tayyarcan, E.K.; Boyaci, I.H. The Use of Bacteriophage-Based Edible Coatings for the Biocontrol of Salmonella in Strawberries. Food Control 2022, 135, 108812. [Google Scholar] [CrossRef]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Poly (Lactic Acid) and Whey Protein/Pullulan Composite Bilayer Film Containing Phage A511 as an Anti-Listerial Packaging for Chicken Breast at Refrigerated Temperatures. LWT 2022, 170, 114085. [Google Scholar] [CrossRef]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a Phage Cocktail and Cinnamaldehyde on Sodium Alginate Emulsion-Based Films to Fight Food Contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, M.; Paczesny, J.; Raza, S. Enhancing the Stability of Bacteriophages Using Physical, Chemical, and Nano-Based Approaches: A Review. Pharmaceutics 2022, 14, 1936. [Google Scholar] [CrossRef]
- Hegedüs, M.; Kovács, G.; Módos, K.; Rontó, G.; Lammer, H.; Panitz, C.; Fekete, A. Exposure of Phage T7 to Simulated Space Environment: The Effect of Vacuum and UV-C Radiation. J. Photochem. Photobiol. B 2006, 82, 94–104. [Google Scholar] [CrossRef]
- Yang, Y.; Du, H.; Zou, G.; Song, Z.; Zhou, Y.; Li, H.; Tan, C.; Chen, H.; Fischetti, V.A.; Li, J. Encapsulation and Delivery of Phage as a Novel Method for Gut Flora Manipulation in Situ: A Review. J. Control. Release 2023, 353, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Bacteriophages: Natural Antimicrobial Bioadditives for Food Preservation in Active Packaging. Int. J. Biol. Macromol. 2024, 276, 133945. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quek, S.-Y.; Huang, K. Advanced Strategies to Overcome the Challenges of Bacteriophage-Based Antimicrobial Treatments in Food and Agricultural Systems. Crit. Rev. Food Sci. Nutr. 2024, 64, 12574–12598. [Google Scholar] [CrossRef]
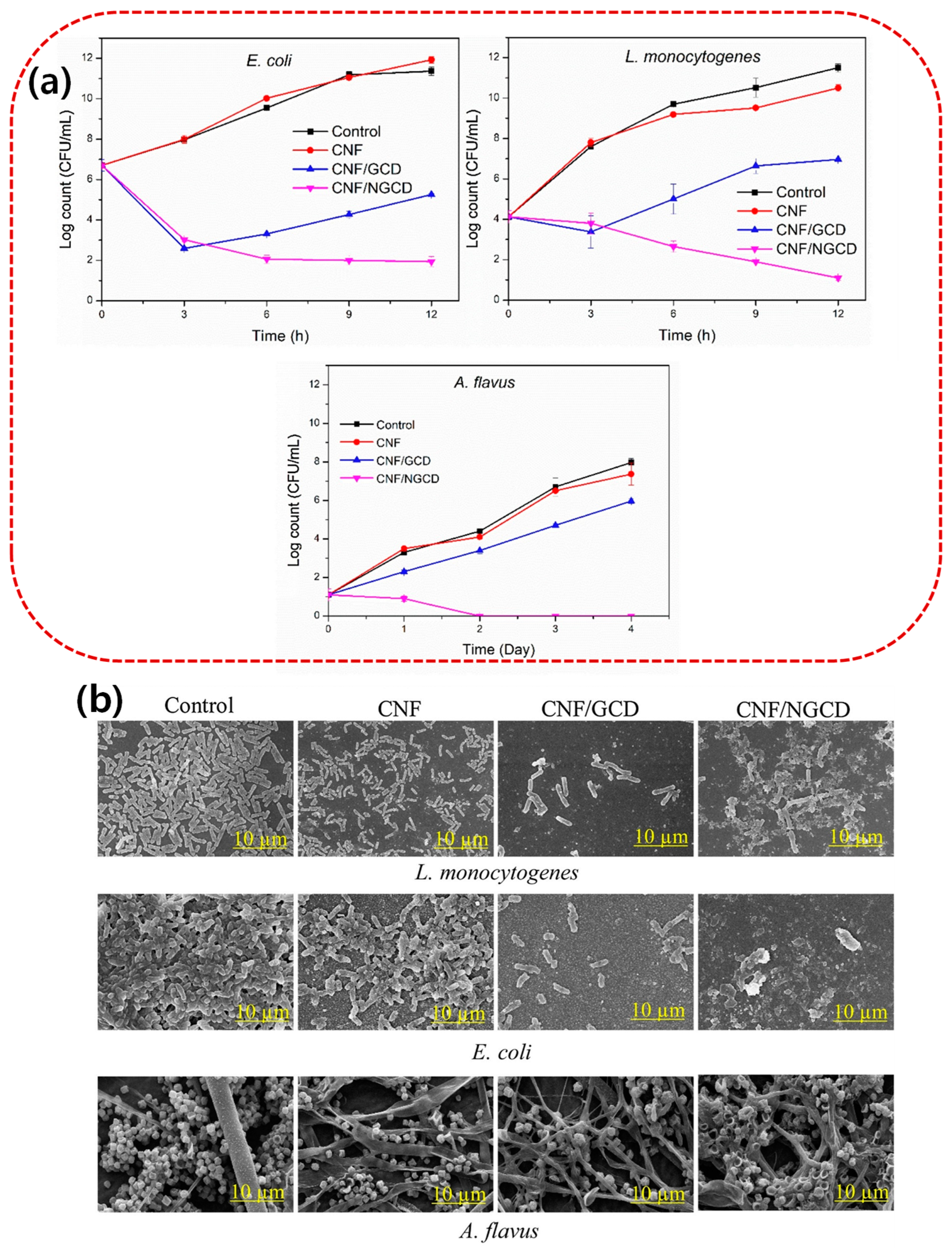
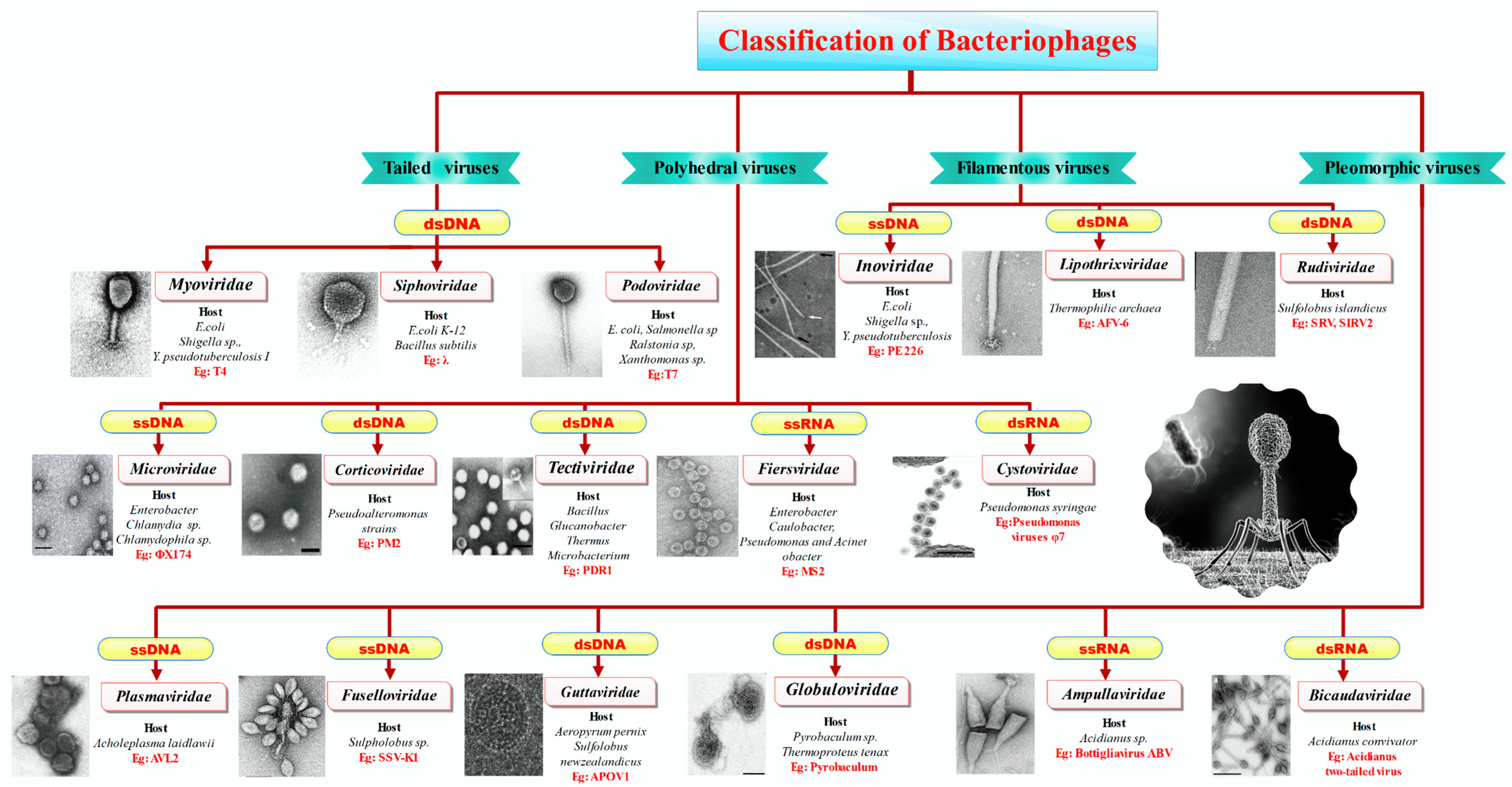

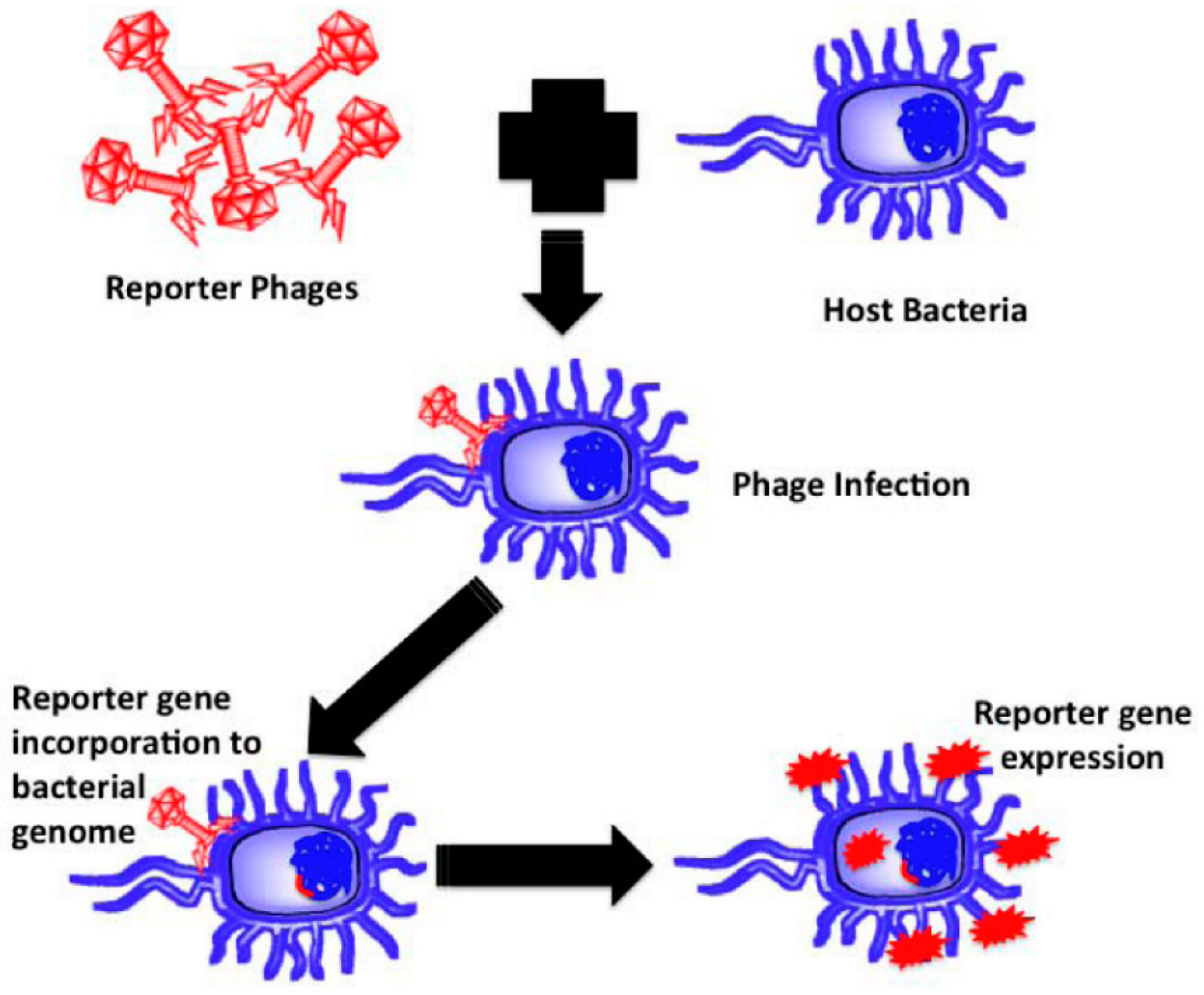
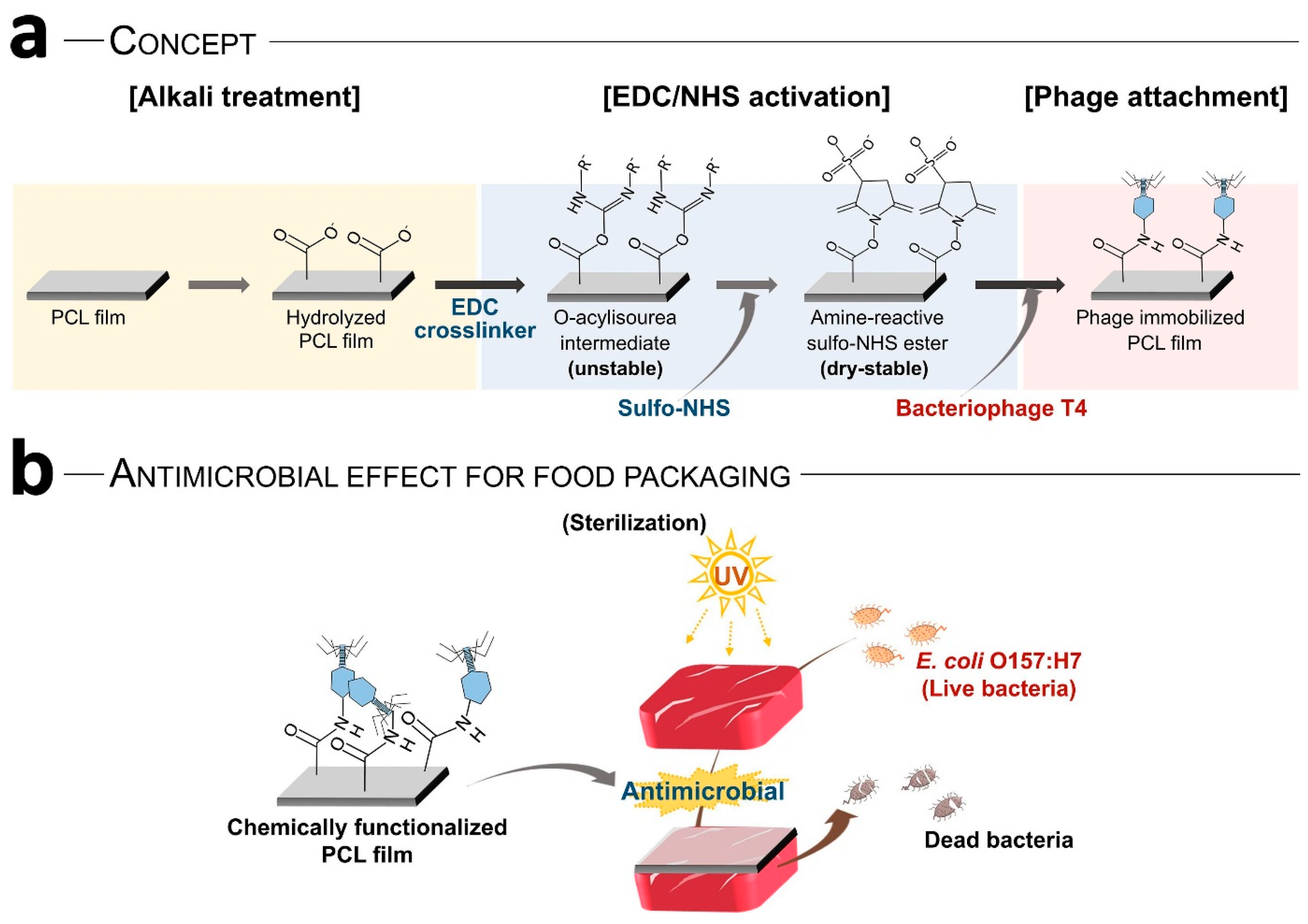
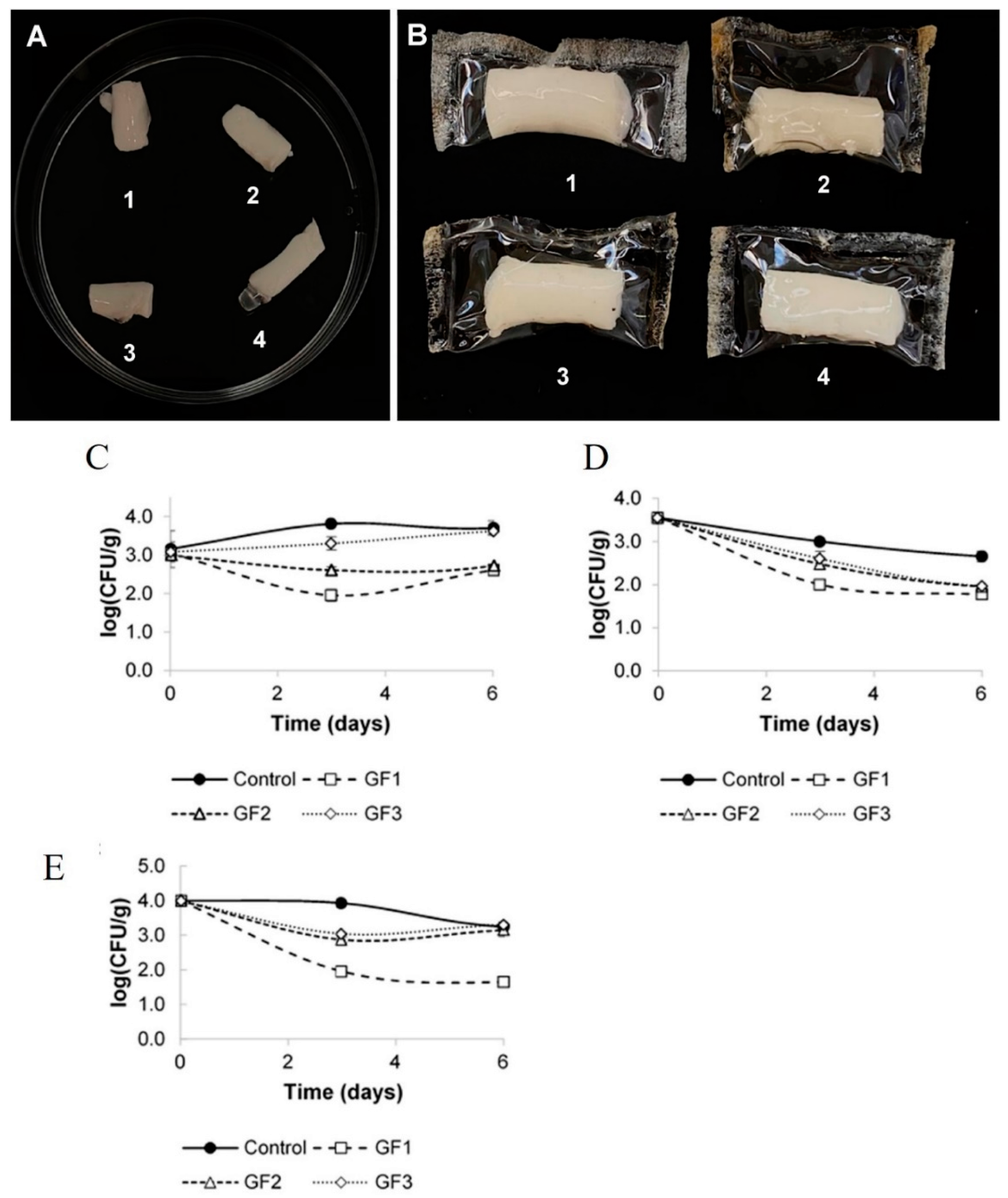
| Biopolymer Film | Functional Fillers | Target Microorganism | Application | Ref |
|---|---|---|---|---|
| PLA | Silver nanoparticles | Bacillus subtilis, E. coli, Micrococcus luteus, Clostridium sporogenes, and Groenewaldozyma auringiensis | Active packaging application | [42] |
| PEF | Ce–bioglass, ZnO, and ZrO2 nanoparticles | E. coli and Staphylococcus aureus | Active packaging application | [43] |
| GEL | Vaccinium corymbosum and derived carbon dots | E. coli and L.monocytogenes | Active and intelligent food packaging | [11] |
| CAR | Rose petals-derived carbon dots | E. coli and L. monocytogenes | Active and intelligent food packaging | [14] |
| CNF and WPI | Rosemary essential oil and titania nanoparticles | Enterobacteriaceae, Pseudomonas spp., Lactobacillus, S. aureus, L. monocytogenes, and E. coli O157: H7 | Preserving lamb meat | [36] |
| Cellulose acetate | Montmorillonite-modified Cu2+ | E. coli | Active food packaging | [37] |
| Cellulose nanofiber | Brassica oleracea-derived CDs | E. coli and L. monocytogenes | Active and intelligent food packaging | [38] |
| Alginate | Clay, essential oils (clove, cinnamon, and marjoram) | E. coli, S. aureus, and L. monocytogenes | Controlling pathogens in food packaging | [44] |
| CMC | Grape seed extract and ZnO | E. coli and L. monocytogenes | Storage life extension studies of meat (high-fat) products | [40] |
| Pectin | AgNPs | E. coli and L. monocytogenes | Active food packaging | [41] |
| Mineralized agar | Zn-minerals (Zn-phosphate, Zn-carbonate) | E. coli, C. albicans, and S. aureus | Food packaging | [45] |
| Alginate | Hydroxyapatite nanoparticles | L. monocytogenes | Fish and seafood packaging | [46] |
| Chitosan | Copper oxide | E. coli, P. aeruginosa, and L. monocytogenes | Water purification and food packaging | [47] |
| Chitosan | Titanium dioxide | S. aureus, E. coli, Candida albicans, and Aspergillus niger | Active food packaging | [48] |
| Cellulose | Quercetin | E. coli and S. aureus | Food packaging, environmental protection, and pharmaceutical industry application | [49] |
| Poly(lactide)/Poly(butylene adipate-co-terephthalate) | Clove and thyme essential oil | S. aureus and E. coli | Active food packaging | [50] |
| GEL/Agar | Sulfur quantum dots | E. coli and L. monocytogenes | Active food packaging | [51] |
| Phyllanthus wightianus | P. wightianus extract, flaxseed gel | S. aureus and E. coli | Meat product packaging (beef patties) | [52] |
| Chitosan/starch | Cellulose nanofibers and cinnamon essential oil | S. aureus and E. coli | Meat product packaging (raw beef meat) | [53] |
| Bacterial nanocellulose | Carbon dots | E. coli and L. monocytogenes | UV screening and forgery-proof packaging | [54] |
| Chitosan/GEL | Sulfur-functionalized chitin | S. aureus, L. monocytogeneses, E. coli, and S. enterica | Chicken meat preservation | [55] |
| GEL, cellulose | ZnO | L. monocytogenes, E. coli, and S. aureus | Active food packaging | [56] |
| Bacteriophage | Polymer Matrix | Packaging Type | Target Pathogen (s) | Food Application | References |
|---|---|---|---|---|---|
| T4 | Polycaprolactone | Film | E. coli O157: H7 | Raw beef | [107] |
| PhiIPLA-RODI | Gelatin | Film Coating | S. aureus | - | [108] |
| vB_EcoM34X vB_EcoSH2Q vB_EcoMH2W | Chitosan | Coating | E. coli | Tomatoes | [109] |
| ϕIBB-PF7A | Sodium alginate | Film | P. fluorescens | Chicken breast fillets | [110] |
| LISTEX™ P100 | Cellulose membranes | Coating | L. monocytogenes | Ready-to-eat turkey breast | [111] |
| Felix O1 | PHBV/PVOH PHBV/nanofiber | Coatings Film | S. Enteritidis | - | [112] |
| A511 | WPC/pullulan | Film | L. monocytogenes | - | [113] |
| Cocktail (DT1 to DT6) | WPC | Film | E. coli | Meat | [114] |
| JN01 | Gelatin | Film | E. coli | Beef | [115] |
| Felix O1 A511 | Xanthan-coated polylactic acid | Film | Salmonella L. monocytogenes | Sliced turkey | [116] |
| E. coli O157: H7 | Chitosan | Film | E. coli | Beef | [117] |
| CN8 | Polyvinyl alcohol–whey protein isolate | Coating | Clavibacter michiganensis | Maize seeds | [118] |
| T7 | Edible WPI | Coatings | E. coli Vibrio spp. | Fish feed pallets | [119] |
| E. coli O157: H7 | Sodium alginate/polyethylene oxide nanofiber | Film | E. coli | Beef | [98] |
| Cocktail (S. Enteritidis F5-4, S. Typhimurium L2-1, and S. Typhimurium ICB1–1) | WPC, carboxymethyl cellulose, chitosan, sodium alginate | Coatings | Salmonella | Strawberries | [120] |
| A511 | Poly (lactic acid) and whey protein/pullulan bilayer | Film | L. monocytogenes | Chicken breast | [121] |
| Cocktail (EC4 and φ135) | Sodium alginate | Film | E. coli and Salmonella | - | [122] |
| LISTEX™ P100 | Sodium caseinate, sodium alginate mixed with gelatin, and polyvinyl alcohol | Film | L. monocytogenes | - | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagh, R.V.; Priyadarshi, R.; Khan, A.; Riahi, Z.; Packialakshmi, J.S.; Kumar, P.; Rindhe, S.N.; Rhim, J.-W. The Role of Active Packaging in the Defense Against Foodborne Pathogens with Particular Attention to Bacteriophages. Microorganisms 2025, 13, 401. https://doi.org/10.3390/microorganisms13020401
Wagh RV, Priyadarshi R, Khan A, Riahi Z, Packialakshmi JS, Kumar P, Rindhe SN, Rhim J-W. The Role of Active Packaging in the Defense Against Foodborne Pathogens with Particular Attention to Bacteriophages. Microorganisms. 2025; 13(2):401. https://doi.org/10.3390/microorganisms13020401
Chicago/Turabian StyleWagh, Rajesh V., Ruchir Priyadarshi, Ajahar Khan, Zohreh Riahi, Jeyakumar Saranya Packialakshmi, Pavan Kumar, Sandeep N. Rindhe, and Jong-Whan Rhim. 2025. "The Role of Active Packaging in the Defense Against Foodborne Pathogens with Particular Attention to Bacteriophages" Microorganisms 13, no. 2: 401. https://doi.org/10.3390/microorganisms13020401
APA StyleWagh, R. V., Priyadarshi, R., Khan, A., Riahi, Z., Packialakshmi, J. S., Kumar, P., Rindhe, S. N., & Rhim, J.-W. (2025). The Role of Active Packaging in the Defense Against Foodborne Pathogens with Particular Attention to Bacteriophages. Microorganisms, 13(2), 401. https://doi.org/10.3390/microorganisms13020401










