Biopatinas on Peperino Stone: Three Eco-Friendly Methods for Their Control and Multi-Technique Approach to Evaluate Their Efficacy
Abstract
1. Introduction
2. Materials and Methods
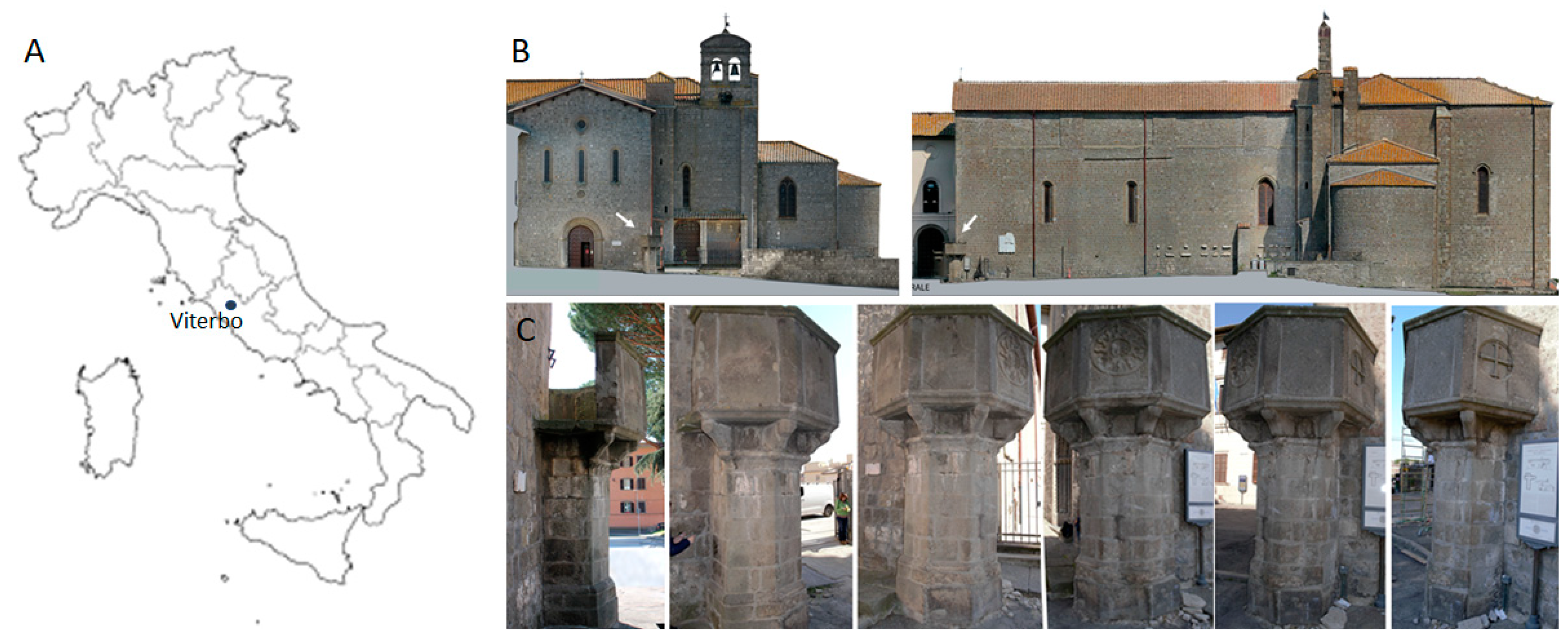
2.1. Lithotype Features and Artifact Under Study
2.2. Innovative Products and Respective Cleaning Protocols
2.2.1. DMSO-Based Gel
2.2.2. BioTersus®—Essential Oil-Based Product
2.2.3. Nasier L01—Enzyme-Based Detergent
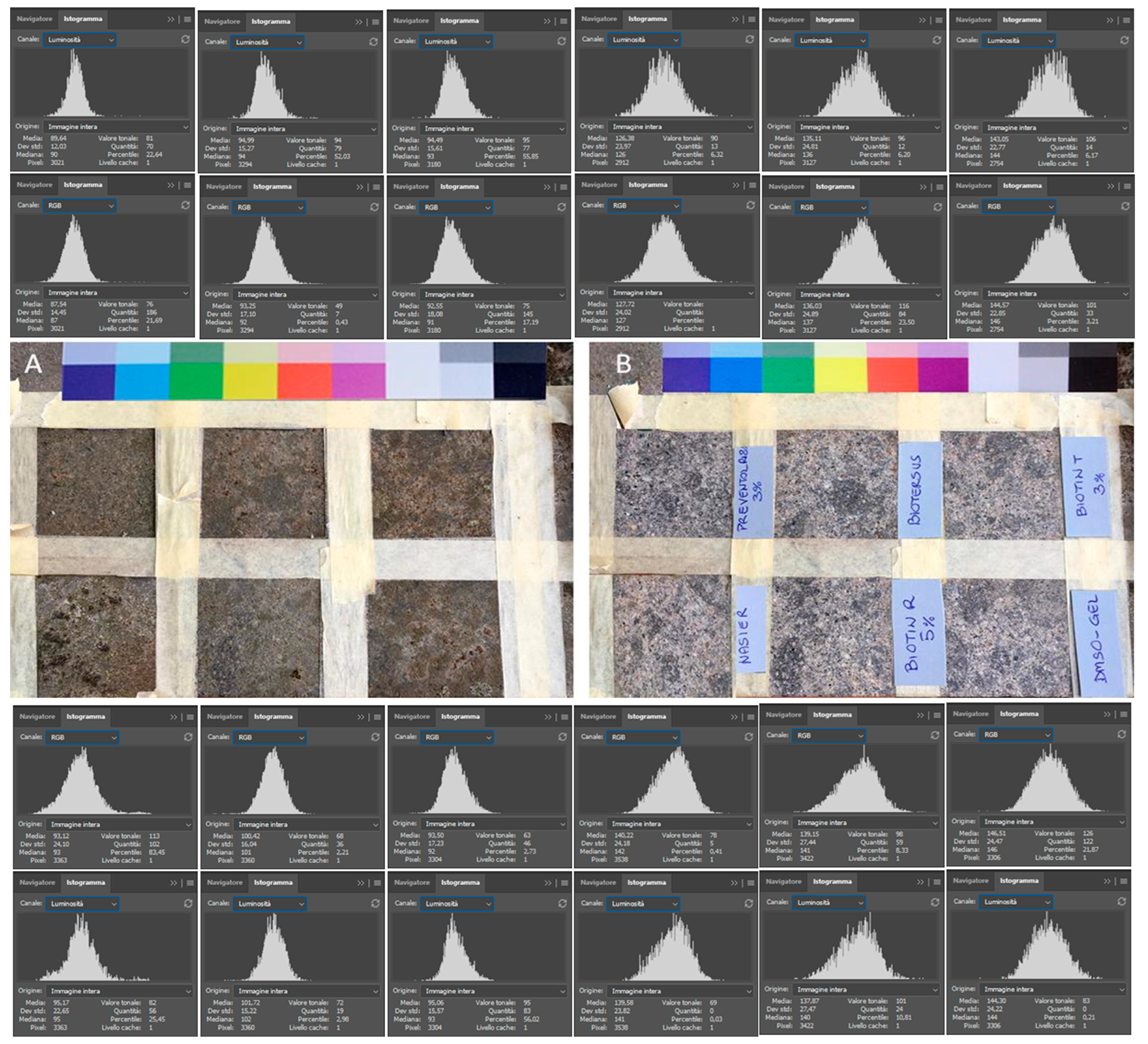
2.3. Ex Situ Comparative Experiment
2.4. In Situ Experiment
2.5. Physical Measurements
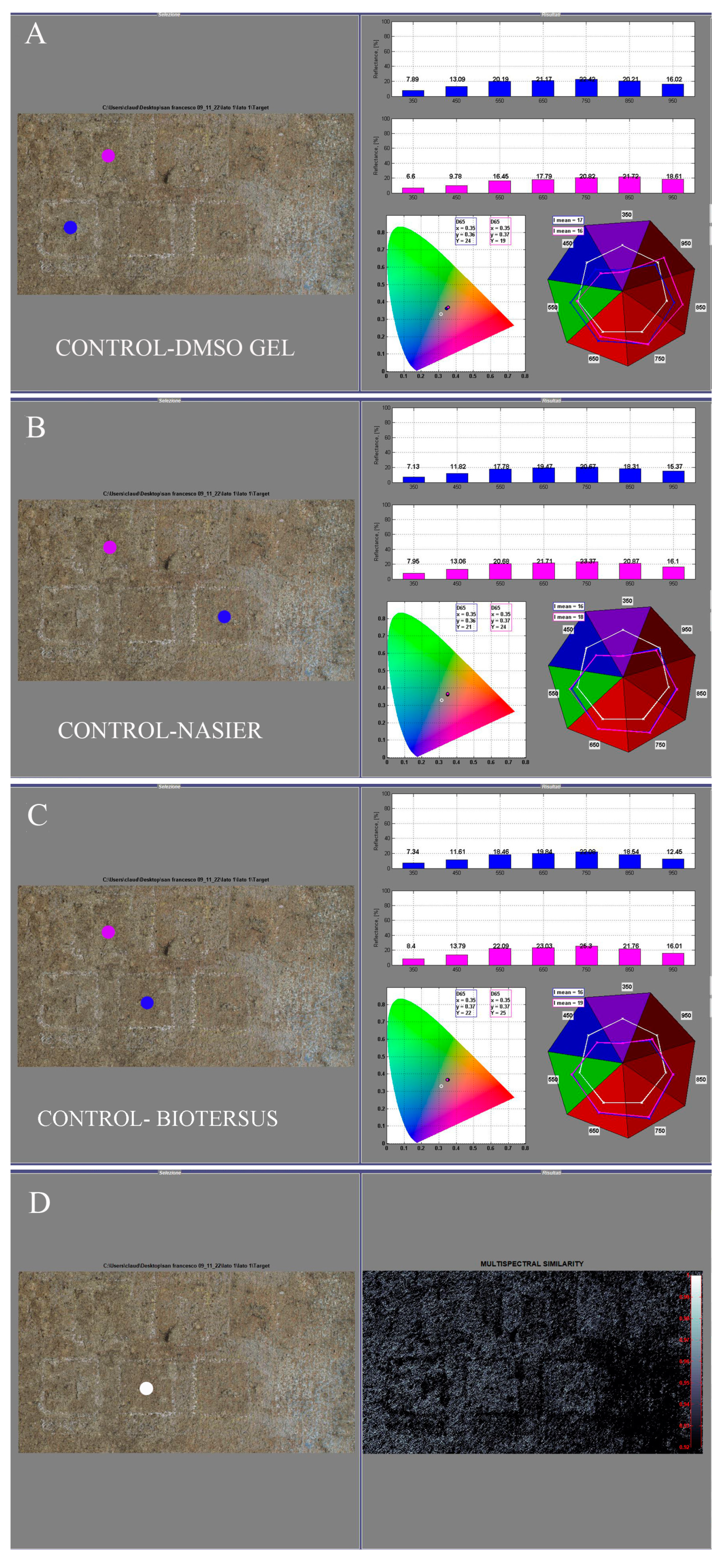
2.5.1. Reflectance Spectroradiometry
Multivariate Data Analysis on Reflectance Data
Preprocessing
Principal Component Analysis (PCA)
PCA-LDA for Classifying and Estimating Biological Patina
2.5.2. Laser-Induced Fluorescence (LIF)
LIF Data Processing
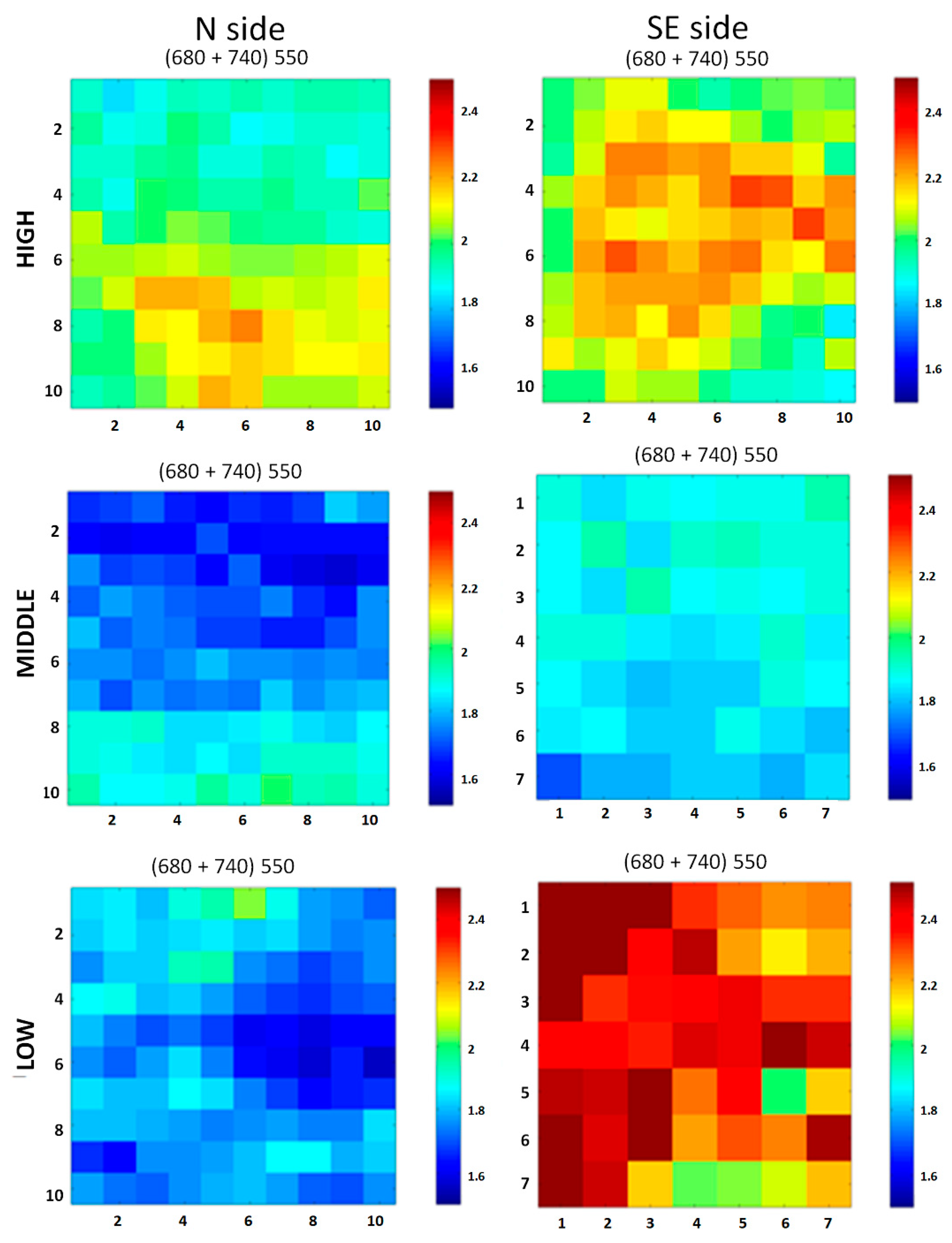
2.5.3. Hypercolorimetric Multispectral Imaging (HMI)
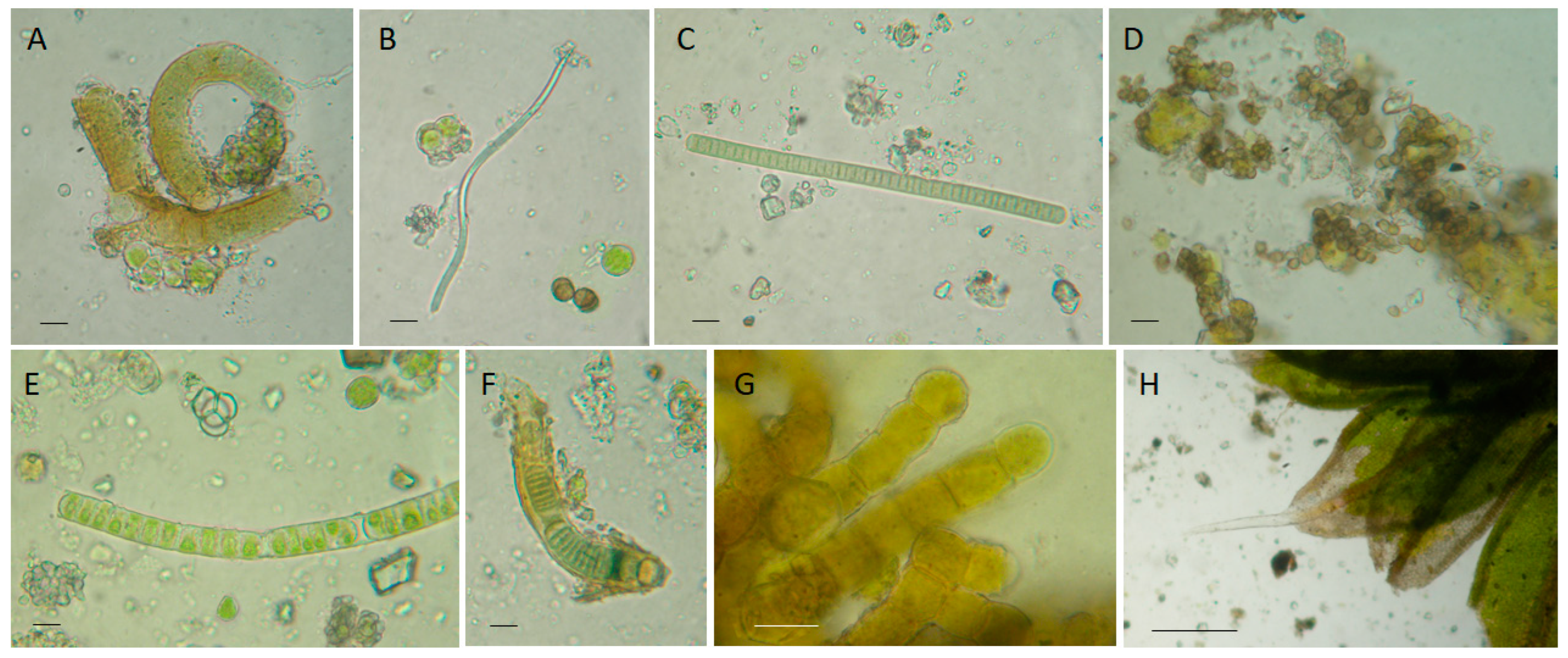
2.6. Sampling and Biopatina Characterization
2.7. Experiment Synopsis
3. Results
3.1. Ex Situ Comparative Experiment
3.2. In Situ Experiment: Biological Patina Composition and Removal Yields
3.2.1. Biological Patina Composition
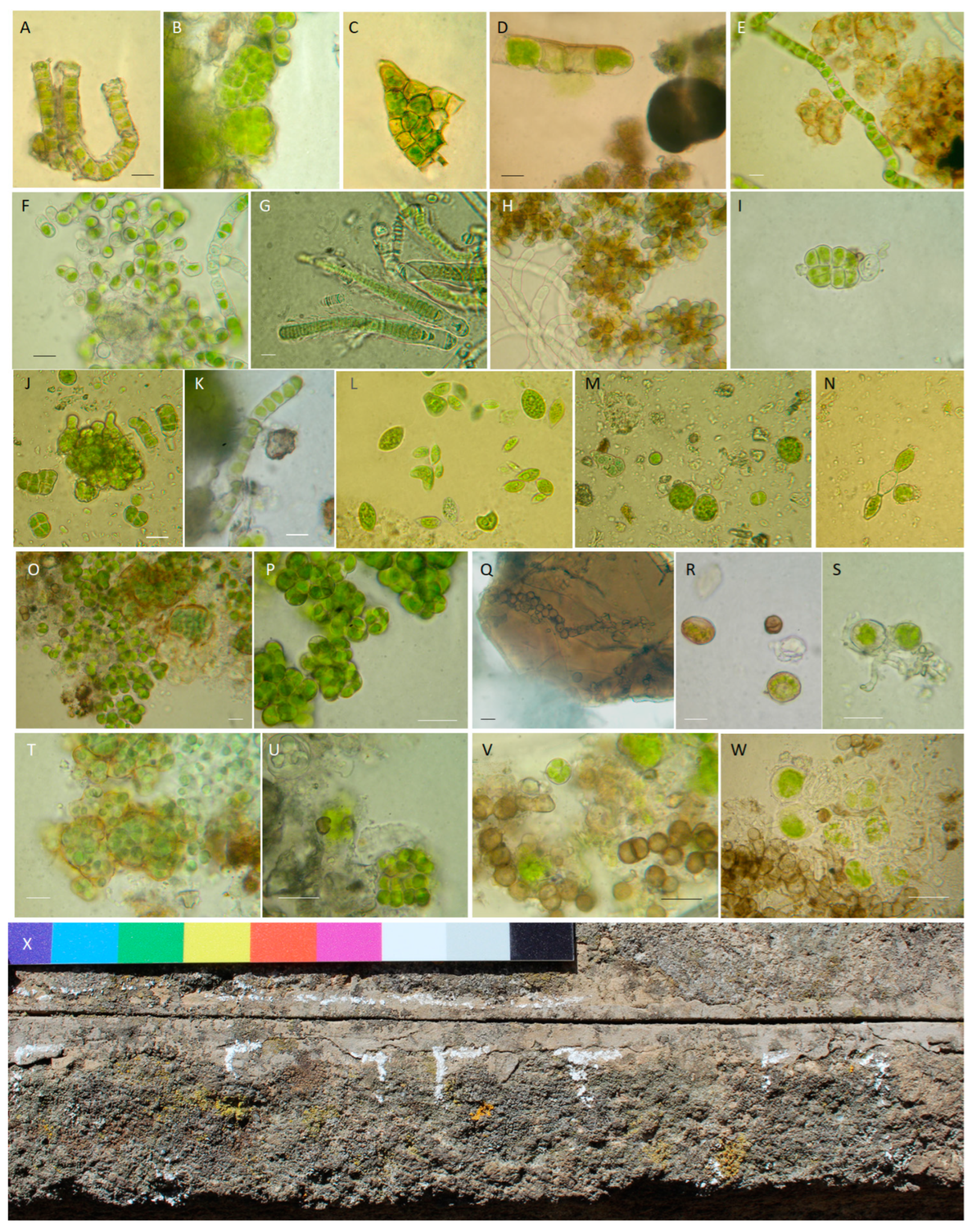
| Ex situ | In situ | |||
|---|---|---|---|---|
| Sampled Area | Peperino Slab | Pulpit, North (N) | Pulpit, Southeast (SE) | Organisms |
| Slab | Tortula muralis | Mosses | ||
| Calothrix parietina Leptolyngbya sp. Lyngbya sp. | Cyanobacteria | |||
| Klebsormidium flaccidum Trentepohlia sp. | Algae | |||
| Coniosporium sp. | Fungi | |||
| High (H) | Calothrix parietina Chroococcus turgidus Pleurocapsa sp. | Chlorogloea sp. Gloeocapsa novacekii | Cyanobacteria | |
| Apatococcus lobatus Klebsormidium flaccidum Klebsormudium sp. | Apatococcus lobatus | Algae | ||
| Setosphaeosphaeria sp | Knufia marmoricola Coniosporium uncinatum Constantinomyces sp. Exophiala sp. Dothideomycetes sp. Herpotrichiellaceae sp. | Fungi | ||
| / | Acarospora fuscata Candelariella vitellina Xanthoria parietina Scoliciosporum umbrinum | Lichens | ||
| Middle (M) | / | None | Cyanobacteria | |
| Klebsormidium sp. | Algae | |||
| Knufia marmoricola Knufia petricola | Fungi | |||
| Low (L) | Chlorogloea sp. Pleurocapsa sp. | None | Cyanobacteria | |
| Euglena sp. * Euglena splendens * Klebsormidium flaccidum Oocystis sp. | Algae | |||
| Scolecobasidium sp. Setosphaeosphaeria sp. | Fungi | |||
3.2.2. Field Practical Evidence on the Application and Removal of Treatments
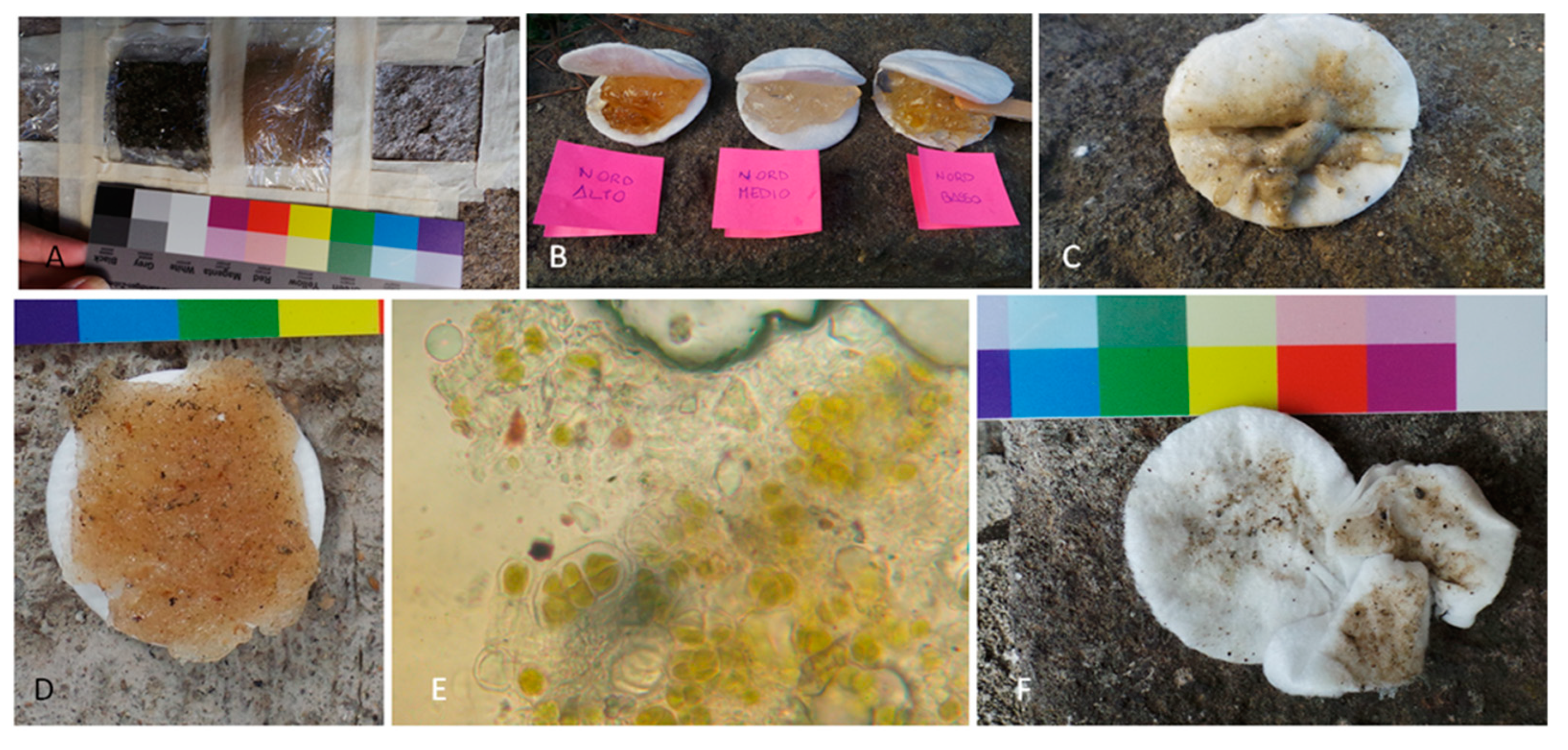
3.2.3. Biopatina Removal Yields

3.3. In Situ Experiment: Instrumental Measurements Before and After Treatments
3.3.1. Reflectance Spectroradiometry

3.3.2. LIF
3.3.3. HMI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onofri, S.; Zucconi, L.; Isola, D.; Selbmann, L. Rock-inhabiting fungi and their role in deterioration of stone monuments in the Mediterranean area. Plant Biosyst. 2014, 148, 384–391. [Google Scholar] [CrossRef]
- Török, Á.; Přikryl, R. Current methods and future trends in testing, durability analyses and provenance studies of natural stones used in historical monuments. Eng. Geol. 2010, 115, 139–142. [Google Scholar] [CrossRef]
- Marra, F.; Palladino, D.M.; Licht, O.B. The peperino rocks: Historical and volcanological overview. Bull. Volcanol. 2022, 84, 69. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and reanalysing the concept of bioreceptivity 25 years on. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Mesquita, N.; Trovão, J.; Soares, F.; Tiago, I.; Coelho, C.; de Carvalho, H.P.; Gil, F.; Catarino, L.; Piñar, G.; et al. Limestone biodeterioration: A review on the Portuguese cultural heritage scenario. J. Cult. Herit. 2019, 36, 275–285. [Google Scholar] [CrossRef]
- Trovão, J.; Portugal, A.; Soares, F.; Paiva, D.S.; Mesquita, N.; Coelho, C.; Pinheiro, A.C.; Catarino, L.; Gil, F.; Tiago, I. Fungal diversity and distribution across distinct biodeterioration phenomena in limestone walls of the old cathedral of Coimbra, UNESCO World Heritage Site. Int. Biodeterior. Biodegrad. 2019, 142, 91–102. [Google Scholar] [CrossRef]
- Isola, D.; Bartoli, F.; Meloni, P.; Caneva, G.; Zucconi, L. Black fungi and stone heritage conservation: Ecological and metabolic assays for evaluating colonization potential and responses to traditional biocides. Appl. Sci. 2022, 12, 2038. [Google Scholar] [CrossRef]
- Selbmann, L.; Isola, D.; Fenice, M.; Zucconi, L.; Sterflinger, K.; Onofri, S. Potential extinction of Antarctic endemic fungal species as a consequence of global warming. Sci. Total Environ. 2012, 438, 127–134. [Google Scholar] [CrossRef]
- Matteucci, E.; Scarcella, A.V.; Croveri, P.; Marengo, A.; Borghi, A.; Benelli, C.; Hamdan, O.; Favero-Longo, S.E. Lichens and other lithobionts on the carbonate rock surfaces of the heritage site of the tomb of Lazarus (Palestinian territories): Diversity, biodeterioration, and control issues in a semi-arid environment. Ann. Microbiol. 2019, 69, 1033–1046. [Google Scholar] [CrossRef]
- Gaylarde, C.; Little, B. Biodeterioration of stone and metal—Fundamental microbial cycling processes with spatial and temporal scale differences. Sci. Total Environ. 2022, 823, 153193. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.; Gu, J.D.; He, D.; Zhang, G.; Liu, X.; Guo, Q.; Cui, H.; Zhao, J.; Feng, H. Community assembly, potential functions and interactions between fungi and microalgae associated with biodeterioration of sandstone at the Beishiku Temple in Northwest China. Sci. Total Environ. 2022, 835, 155372. [Google Scholar] [CrossRef]
- Zucconi, L.; Onofri, S.; Cecchini, C.; Isola, D.; Ripa, C.; Fenice, M.; Madonna, S.; Riboleiro-Rivas, P.; Selbmann, L. Mapping the lithic colonization at the boundaries of life in Northern Victoria Land, Antarctica. Polar Biol. 2016, 39, 91–102. [Google Scholar] [CrossRef]
- Pinna, D. Can we do without biocides to cope with biofilms and lichens on stone heritage? Int. Biodeterior. Biodegrad. 2022, 172, 105437. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F. Novel antibiofilm non-biocidal strategies. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021; p. 117. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Tabasso, M.L.; Brigadeci, F.; Capua, M.C.; Morelli, A.; Pastorello, P.; Sohrabi, M.; Chaverdi, A.A.; Callieri, P. A first assessment of the biocidal efficacy of plant essential oils against lichens on stone cultural heritage, and the importance of evaluating suitable application protocols. J. Cult. Herit. 2022, 55, 68–77. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Caldeira, A.T. Green mitigation strategy for cultural heritage using bacterial biocides. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021; p. 137. [Google Scholar] [CrossRef]
- Isola, D.; Bartoli, F.; Municchia, A.C.; Lee, H.J.; Jeong, S.H.; Chung, Y.J.; Caneva, G. Green biocides for the conservation of hypogeal mural paintings raised from Western and Eastern traditions: Evaluation of interference on pigments and substrata and multifactor parameters affecting their activity. J. Cult. Herit. 2023, 61, 116–126. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The essential oil of Thymbra capitata and its application as a biocide on stone and derived surfaces. Plants 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Schröer, L.; Fiers, G.; Deprez, M.; Boon, N.; Cnudde, V.; Soens, L.; De Kock, T. Examining the potential of enzyme-based detergents to remove biofouling from limestone heritage. Coatings 2022, 12, 375. [Google Scholar] [CrossRef]
- Gabriele, F.; Ranaldi, R.; Bruno, L.; Casieri, C.; Rugnini, L.; Spreti, N. Biodeterioration of stone monuments: Studies on the influence of bioreceptivity on cyanobacterial biofilm growth and on the biocidal efficacy of essential oils in natural hydrogel. Sci. Total Environ. 2023, 870, 161901. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Diamanti, A.; Palleschi, G. New bio-cleaning strategies on porous building materials affected by biodeterioration event. Appl. Surf. Sci. 2010, 256, 6550–6563. [Google Scholar] [CrossRef]
- Bartoli, F.; Ellwood, N.T.W.; Bruno, L.; Ceschin, S.; Rugnini, L.; Caneva, G. Ecological and taxonomic characterisation of Trentepohlia umbrina (Kützing) Bornet growing on stone surfaces in Lazio (Italy). Ann. Microbiol. 2019, 69, 1059–1070. [Google Scholar] [CrossRef]
- Toreno, G.; Isola, D.; Meloni, P.; Carcangiu, G.; Selbmann, L.; Onofri, S.; Caneva, G.; Zucconi, L. Biological colonization on stone monuments: A new low impact cleaning method. J. Cult. Herit. 2018, 30, 100–109. [Google Scholar] [CrossRef]
- Toreno, G.; Zucconi, L.; Caneva, G.; Meloni, P.; Isola, D. Recolonization dynamics of marble monuments after cleaning treatments: A nine-year follow-up study. Sci. Total Environ. 2024, 912, 169350. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.; Marra, F.; Terrenato, R. Geochemical identification criteria for “peperino” stones employed in ancient Roman buildings: A Lapis Gabinus case study. J. Archaeol. Sci. Rep. 2015, 3, 41–51. [Google Scholar] [CrossRef]
- Berry, P.; Sciotti, M. I Peperini del Lazio [Peperini tuffs of the Latium Region]. In Proceedings of the Convegno Internazionale sulla Coltivazione di Pietre e Minerali Litoidi, Torino, Italy, 4–6 October 1974. II Sess C5:1–35. [Google Scholar]
- Lazzarini, L. Genesi e classificazione delle rocce. Cenni di mineralogia. Pietre da costruzione della Lombardia. Cenni di mineralogia e di petrografia delle argille. Morfologia del degrado dei materiali lapidei. In Primo Corso di Aggiornamento Sui Problemi Della Salvaguardia del Patrimonio Artistico Monumentale, Milano, maggio-giugno 1981 (Vol. 3).
- Fratini, F.; Manganelli Del Fa, C.; Pecchioni, E.; Pierattani, P.; Scala, A. Il Peperino laziale. Problemi di alterazione e conservazione nel Parco di Bomarzo. Arkos Sci. Restor. 1990, 11, 21–55. [Google Scholar]
- Puxeddu, M. Studio chimico-petrografico delle vulcaniti del M. Cimino (Viterbo). Atti Soc. Toscana Sci. Nat. 1971, 78, 329–394. [Google Scholar]
- Scriattoli, A. Viterbo Nei Suoi Monumenti; Capaccini: Roma, Italy, 1920; p. 470. [Google Scholar]
- Bartoli, F.; Isola, D.; Casanova Municchia, A.; Kumbaric, A.; Caneva, G. Science for art: Multi-years’ evaluations of biocidal efficacy in support of artwork conservation. Front. Microbiol. 2023, 14, 1178900. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, R. Un Approccio Acquoso Alla Pulitura Dei Dipinti, Quaderni Del Cesmar7, n. 1; Casa Editrice Il Prato: Padova, Italy, 2004. [Google Scholar]
- Reale, R.; Medeghini, L.; Botticelli, M. Stealing from Phytotherapy—Heritage Conservation with Essential Oils: A Review, from Remedy to Sustainable Restoration Product. Sustainability 2024, 16, 5110. [Google Scholar] [CrossRef]
- Devreux, G.; Santamaria, U.; Morresi, F.; Rodolfo, A.; Barbabietola, N.; Fratini, F.; Reale, R. Fitoconservazione, trattamenti alternativi sulle opere in materiale lapideo nei Giardini Vaticani. In Proceedings of the Lo Stato dell’Arte XIII, Atti del XIII Congresso IGIIC, Torino, Italy, 24–26 October 2015; Nardini Editore: Firenze, Italy, 2015; pp. 199–206. [Google Scholar]
- Macchia, A.; Aureli, H.; Biribicchi, C.; Docci, A.; Alisi, C.; Prestileo, F.; Galiano, F.; Figoli, A.; Mancuso, R.; Gabriele, B.; et al. In Situ Application of Anti-Fouling Solutions on a Mosaic of the Archaeological Park of Ostia Antica. Materials 2022, 15, 5671. [Google Scholar] [CrossRef] [PubMed]
- Macchia, A.; Aureli, H.; Prestileo, F.; Ortenzi, F.; Sellathurai, S.; Docci, A.; Cerafogli, E.; Colasanti, I.A.; Ricca, M.; La Russa, M.F. In-Situ Comparative Study of Eucalyptus, Basil, Cloves, Thyme, Pine Tree, and Tea Tree Essential Oil Biocide Efficacy. Methods Protoc. 2022, 5, 37. [Google Scholar] [CrossRef]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 73–83. [Google Scholar] [CrossRef]
- Cremonesi, P.; Casoli, A. Enzymes as tools for conservation of works of art. J. Cult. Herit. 2021, 50, 73–87. [Google Scholar] [CrossRef]
- Alfani, E.; Biadaioli, I.; Lupi, C. Utilizzo di gel a base di enzimi (NASIER) per la pulitura eco-compatibile di superfici lapidee e decorate dell’architettura. Indagini e studi applicativi. In Tutela e Restauro 2021 Notiziario della Soprintendenza Archeologia, Belle Arti e Paesaggio per la Città Metropolitana di Firenze e le Province di Pistoia e Prato; Pessina, A., Tarantini, M., Eds.; All’insegna del Giglio: Sesto Fiorentino, Italy, 2023; Available online: https://www.insegnadelgiglio.it/wp-content/uploads/2023/05/Notizie-Generali.pdf (accessed on 25 September 2023).
- Colantonio, C.; Lanteri, L.; Bocci, R.; Valentini, V.; Pelosi, C. “A Woman Clothed with the Sun”: The Diagnostic Study and Testing of Enzyme-Based Green Products for the Restoration of an Early 17th Century Wall Painting in the Palazzo Gallo in Bagnaia (Italy). Appl. Sci. 2023, 13, 12884. [Google Scholar] [CrossRef]
- Vannini, A.; Contardo, T.; Paoli, L.; Scattoni, M.; Favero-Longo, S.E.; Loppi, S. Application of commercial biocides to lichens: Does a physiological recovery occur over time? Int. Biodeterior. Biodegrad. 2018, 129, 189–194. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Matteucci, E.; Pinna, D.; Ruggiero, M.G.; Riminesi, C. Efficacy of the environmentally sustainable microwave heating compared to biocide applications in the devitalization of phototrophic communities colonizing rock engravings of Valle Camonica, UNESCO world heritage site, Italy. Int. Biodeterior. Biodegrad. 2021, 165, 105327. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Benes, E.; Fodor, M.; Kovács, S.; Gere, A. Application of detrended fluctuation analysis and yield stability index to evaluate near infrared spectra of green and roasted coffee samples. Processes 2020, 8, 913. [Google Scholar] [CrossRef]
- Amigo, J.M.; Cruz, J.; Bautista, M.; Maspoch, S.; Coello, J.; Blanco, M. Study of pharmaceutical samples by NIR chemical-image and multivariate analysis. TrAC Trends Anal. Chem. 2008, 27, 696–713. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Z.; Chen, X.; Fei, S. Preprocessing methods for near-infrared spectrum calibration. J. Chemom. 2020, 34, e3306. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chemom. 2003, 17, 16–33. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Tominaga, Y. Comparative study of class data analysis with PCA-LDA, SIMCA, PLS, ANNs, and k-NN. Chemom. Intell. Lab. Syst. 1999, 49, 105–115. [Google Scholar] [CrossRef]
- Schaffer, C. Selecting a classification method by cross-validation. Mach. Learn. 1993, 13, 135–143. [Google Scholar] [CrossRef]
- Cecchi, G.; Pantani, L.; Raimondi, V.; Tomaselli, L.; Lamenti, G.; Tiano, P.; Chiari, R. Fluorescence lidar technique for the remote sensing of stone monuments. J. Cult. Herit. 2000, 1, 29–36. [Google Scholar] [CrossRef]
- Spizzichino, V.; Angelini, F.; Caneve, L.; Colao, F.; Corrias, R.; Ruggiero, L. In situ study of modern synthetic materials and pigments in contemporary paintings by laser-induced fluorescence scanning. Stud. Conserv. 2015, 60, S178–S184. [Google Scholar] [CrossRef]
- Caneve, L.; Guarneri, M.; Lai, A.; Spizzichino, V.; Ceccarelli, S.; Mazzei, B. Non-destructive laser based techniques for biodegradation analysis in cultural heritage. NDT Int. 2019, 104, 108–113. [Google Scholar] [CrossRef]
- Palucci, A.; Caponero, M.A.; Caneve, L.; Di Frischia, S.; Francucci, M.; Guarneri, M.; Spizzichino, V. ENEA’s Optical Sensors for Local and Remote Sensing of Cultural Heritage. In The Safety and Security of Cultural Heritage in Zones of War or Instability; Romiti, B., Folliero, A., Eds.; IOS Press: Amsterdam, The Netherlands, 2021; pp. 62–79. [Google Scholar] [CrossRef]
- Brown, J.S. Absorption and fluorescence of chlorophyll a in particle fractions from different plants. Biophys. J. 1961, 9, 1542–1552. [Google Scholar] [CrossRef]
- Peterson, R.B.; Oja, V.; Laisk, A. Chlorophyll fluorescence at 680 and 730 nm and leaf photosynthesis. Photosynth. Res. 2001, 70, 185–196. [Google Scholar] [CrossRef]
- Lyons, W.H.; Glascock, M.D.; Mehringer, P.J., Jr. Silica from sources to site: Ultraviolet fluorescence and trace elements identify cherts from Lost Dune, southeastern Oregon, USA. J. Archaeol. Sci. 2003, 30, 1139–1159. [Google Scholar] [CrossRef]
- Melis, M.; Miccoli, M.; Quarta, D. Multispectral hypercolorimetry and automatic guided pigment identification: Some masterpieces case studies. In Proceedings of the SPIE 8790, Optics for Arts, Architecture, and Archaeology IV, Munich, Germany, 30 May 2013; Pezzati, L., Targowski, P., Eds.; SPIE: Bellingham, WA, USA, 2013; Volume 33, pp. 1–14. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Caglio, S.; Galli, A.; Lanteri, L.; Pelosi, C. Materials and Technique: The First Look at Saturnino Gatti. Appl. Sci. 2023, 13, 6842. [Google Scholar] [CrossRef]
- Pogliani, P.; Pelosi, C.; Lanteri, L.; Bordi, G. Imaging Based Techniques Combined with Color Measurements for the Enhancement of Medieval Wall Paintings in the Framework of EHEM Project. J. Imaging 2024, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- NORMAL: 9/88 Microflora Autotrofa ed Eterotrofa: Tecniche di Isolamento in Coltura; CNR-ICR: Roma, Italy, 1990.
- Isola, D.; Bartoli, F.; Morretta, S.; Caneva, G. The Roman Houses of the Caelian Hill (Rome, Italy): Multitemporal Evaluation of Biodeterioration Patterns. Microorganisms 2023, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Ruibal, C.; Selbmann, L.; Avci, S.; Martin-Sanchez, P.M.; Gorbushina, A.A. Roof-inhabiting cousins of rock-inhabiting fungi: Novel melanized microcolonial fungal species from photocatalytically reactive subaerial surfaces. Life 2018, 8, 30. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Cecchini, A.; Caneva, G. Dark-pigmented biodeteriogenic fungi in Etruscan hypogeal tombs: New data on their culture-dependent diversity, favouring conditions, and resistance to biocidal treatments. Fungal Biol. 2021, 125, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase, version 4.2. World-Wide Electronic Publication. National University of Ireland: Maynooth, Ireland, 2007.
- Martellos, S.; Conti, M.; Nimis, P.L. Aggregation of Italian Lichen Data in ITALIC 7.0. J. Fungi 2023, 9, 556. [Google Scholar] [CrossRef]
- Gaylarde, C. Influence of environment on microbial colonization of historic stone buildings with emphasis on cyanobacteria. Heritage 2020, 3, 1469–1482. [Google Scholar] [CrossRef]
- Ortega Calvo, J.J.; Sánchez Castillo, P.M.; Hernández Mariné, M.; Sáiz-Jiménez, C. Isolation and characterization of epilithic chlorophytes and cyanobacteria from two Spanish cathedrals (Salamanca and Toledo). Nova Hedwig. 1993, 57, 239–253. [Google Scholar]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef]
- Gulotta, D.; Villa, F.; Cappitelli, F.; Toniolo, L. Biofilm colonization of metamorphic lithotypes of a renaissance cathedral exposed to urban atmosphere. Sci. Total Environ. 2018, 639, 1480–1490. [Google Scholar] [CrossRef]
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56. [Google Scholar] [CrossRef]
- Tomaselli, L.; Lamenti, G.; Bosco, M.; Tiano, P. Biodiversity of photosynthetic micro-organisms dwelling on stone monuments. Int. Biodeterior. Biodegrad. 2000, 46, 251–258. [Google Scholar] [CrossRef]
- Bolivar-Galiano, F.; Cuzman, O.A.; Abad-Ruiz, C.; Sánchez-Castillo, P. Facing phototrophic microorganisms that colonize artistic fountains and other wet stone surfaces: Identification keys. Appl. Sci. 2021, 11, 8787. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.; Androv, M.; Uzunov, B.; Ivanov, K.; Gärtner, G. Algal Biodiversity of Nine Megaliths in South-East Bulgaria. Life 2024, 14, 948. [Google Scholar] [CrossRef]
- Singh, H. Desiccation and radiation stress tolerance in cyanobacteria. J. Basic Microbiol. 2018, 58, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Aigner, S.; Arc, E.; Schletter, M.; Karsten, U.; Holzinger, A.; Kranner, I. Metabolite profiling in green microalgae with varying degrees of desiccation tolerance. Microorganisms 2022, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Nimis, P.L.; Monte, M.; Tretiach, M. Flora e Vegetazione Lichenica di Aree Archeologiche del Lazio; Dipartimento di Biologia Sezione di Geobotanica ed Ecologia Vegetale, Università degli Studi di Trieste: Trieste, Italy, 1987. [Google Scholar]
- Roccardi, A.; Bianchetti, P. The distribution of lichens on some stoneworks in the surroundings of Rome. Stud. Geobot. 1988, 8, 89–97. [Google Scholar]
- Owczarek-Koscielniak, M.; Sterflinger, K. First records of Knufia marmoricola from limestone outcrops in the Wyżyna Krakowsko-Częstochowska Upland, Poland. Phytotaxa 2018, 357, 94–106. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Two new species of the genus Ochroconis, O. lascauxensis and O. anomala isolated from black stains in Lascaux Cave, France. Fungal Biol. 2012, 116, 574–589. [Google Scholar] [CrossRef]
- Isola, D.; Scano, A.; Orrù, G.; Prenafeta-Boldú, F.X.; Zucconi, L. Hydrocarbon-contaminated sites: Is there something more than Exophiala xenobiotica? New insights into black fungal diversity using the long cold incubation method. J. Fungi 2021, 7, 817. [Google Scholar] [CrossRef]
- Caneva, G.; Isola, D.; Lee, H.J.; Chung, Y.J. Biological risk for hypogea: Shared data from Etruscan tombs in Italy and ancient tombs of the Baekje dynasty in Republic of Korea. Appl. Sci. 2020, 10, 6104. [Google Scholar] [CrossRef]
- Sasso, S.; Miller, A.Z.; Rogerio-Candelera, M.A.; Cubero, B.; Coutinho, M.L.; Scrano, L.; Bufo, S.A. Potential of natural biocides for biocontrolling phototrophic colonization on limestone. Int. Biodeterior. Biodegrad. 2016, 107, 102–110. [Google Scholar] [CrossRef]
- Di Turo, F.; Medeghini, L. How green possibilities can help in a future sustainable conservation of cultural heritage in Europe. Sustainability 2021, 13, 3609. [Google Scholar] [CrossRef]
- De Silva, M.; Henderson, J. Sustainability in conservation practice. J. Inst. Conserv. 2011, 34, 5–15. [Google Scholar] [CrossRef]
- Gueidão, M.; Vieira, E.; Bordalo, R.; Moreira, P. Available green conservation methodologies for the cleaning of cultural heritage: An overview. Estud. Conserv. Restor. 2020, 12, 22–44. [Google Scholar]
- da Silva, J.C.; Lombardi, A.T. Chlorophylls in microalgae: Occurrence, distribution, and biosynthesis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Queiroz Zepka, L., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isola, D.; Capobianco, G.; Tovazzi, V.; Pelosi, C.; Trotta, O.; Serranti, S.; Lanteri, L.; Zucconi, L.; Spizzichino, V. Biopatinas on Peperino Stone: Three Eco-Friendly Methods for Their Control and Multi-Technique Approach to Evaluate Their Efficacy. Microorganisms 2025, 13, 375. https://doi.org/10.3390/microorganisms13020375
Isola D, Capobianco G, Tovazzi V, Pelosi C, Trotta O, Serranti S, Lanteri L, Zucconi L, Spizzichino V. Biopatinas on Peperino Stone: Three Eco-Friendly Methods for Their Control and Multi-Technique Approach to Evaluate Their Efficacy. Microorganisms. 2025; 13(2):375. https://doi.org/10.3390/microorganisms13020375
Chicago/Turabian StyleIsola, Daniela, Giuseppe Capobianco, Valery Tovazzi, Claudia Pelosi, Oriana Trotta, Silvia Serranti, Luca Lanteri, Laura Zucconi, and Valeria Spizzichino. 2025. "Biopatinas on Peperino Stone: Three Eco-Friendly Methods for Their Control and Multi-Technique Approach to Evaluate Their Efficacy" Microorganisms 13, no. 2: 375. https://doi.org/10.3390/microorganisms13020375
APA StyleIsola, D., Capobianco, G., Tovazzi, V., Pelosi, C., Trotta, O., Serranti, S., Lanteri, L., Zucconi, L., & Spizzichino, V. (2025). Biopatinas on Peperino Stone: Three Eco-Friendly Methods for Their Control and Multi-Technique Approach to Evaluate Their Efficacy. Microorganisms, 13(2), 375. https://doi.org/10.3390/microorganisms13020375













