Molecular and Structural Characterization of an Immunopurified Telomerase from Leishmania major and the Effect of Telomerase Inhibitors
Abstract
1. Introduction
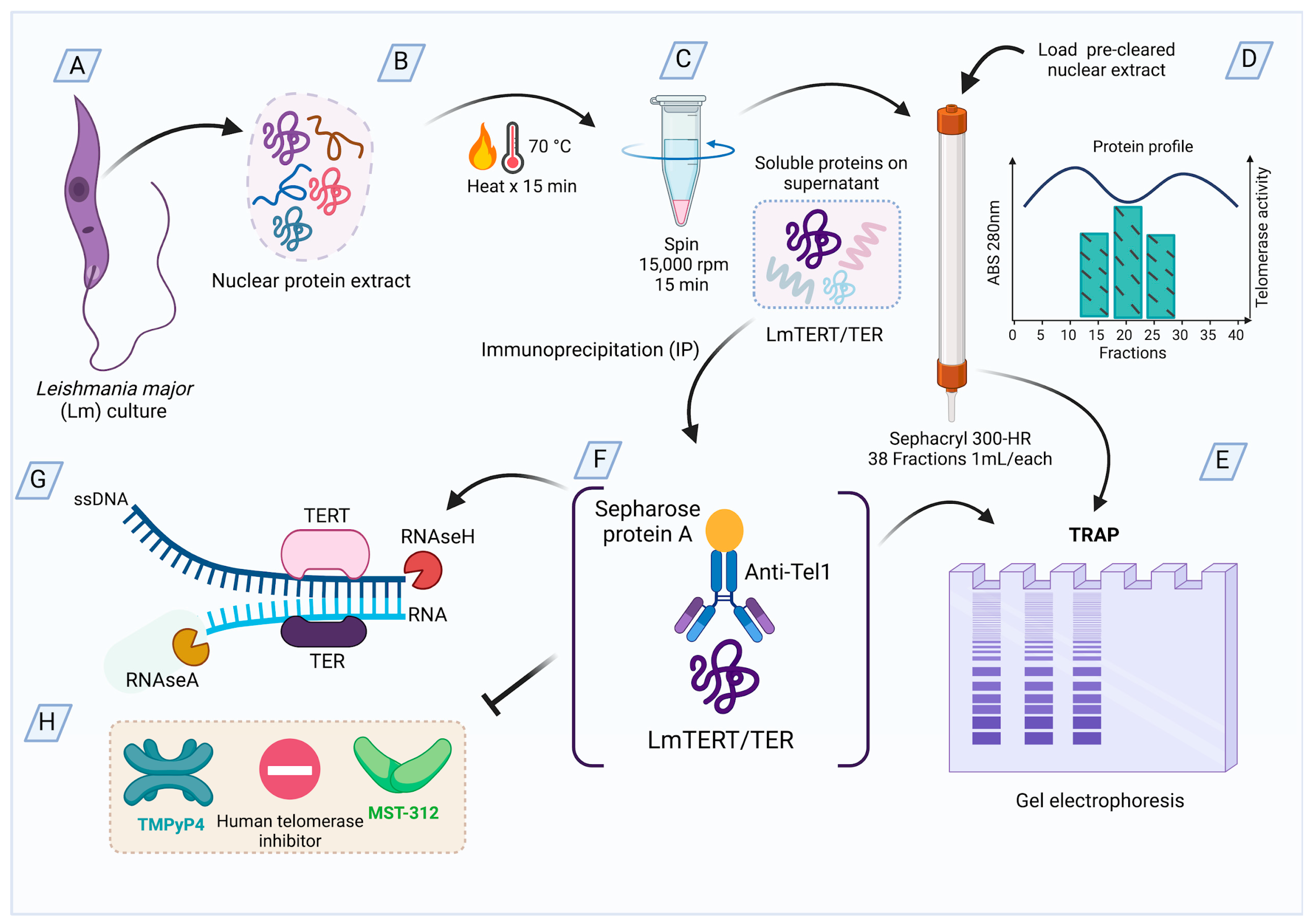
2. Materials and Methods
2.1. Parasitic Cells
2.2. Preparation of Nuclear Extracts from L. major Promastigotes
2.3. Preparation of Fractions Enriched in L. major Telomerase Using Sephacryl-300HR Gel Filtration
2.4. Production of Anti-Telomerase Antibodies
2.5. Immunoprecipitation Assays
2.6. Electrophoresis and Western Blots of L. major Telomerase
2.7. Telomerase Detection Assay
2.8. RNase H Assays
2.9. Structural Simulations
2.10. Assays with Telomerase Inhibitors
2.11. Effect of Telomerase Inhibitors on Promastigotes of L. major
3. Results
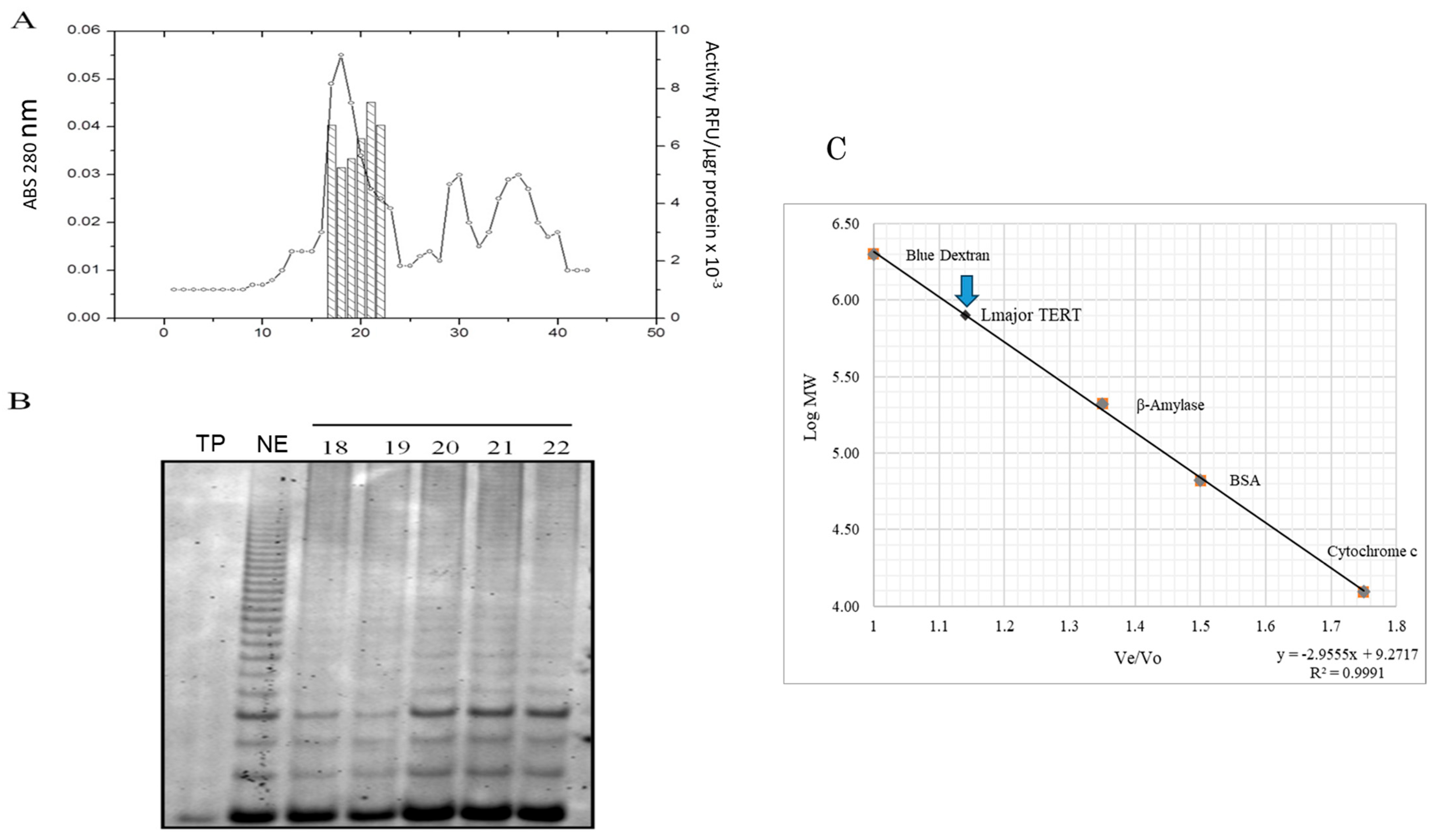
3.1. Sephacryl-300HR Gel Filtration of L. major Nuclear Extracts
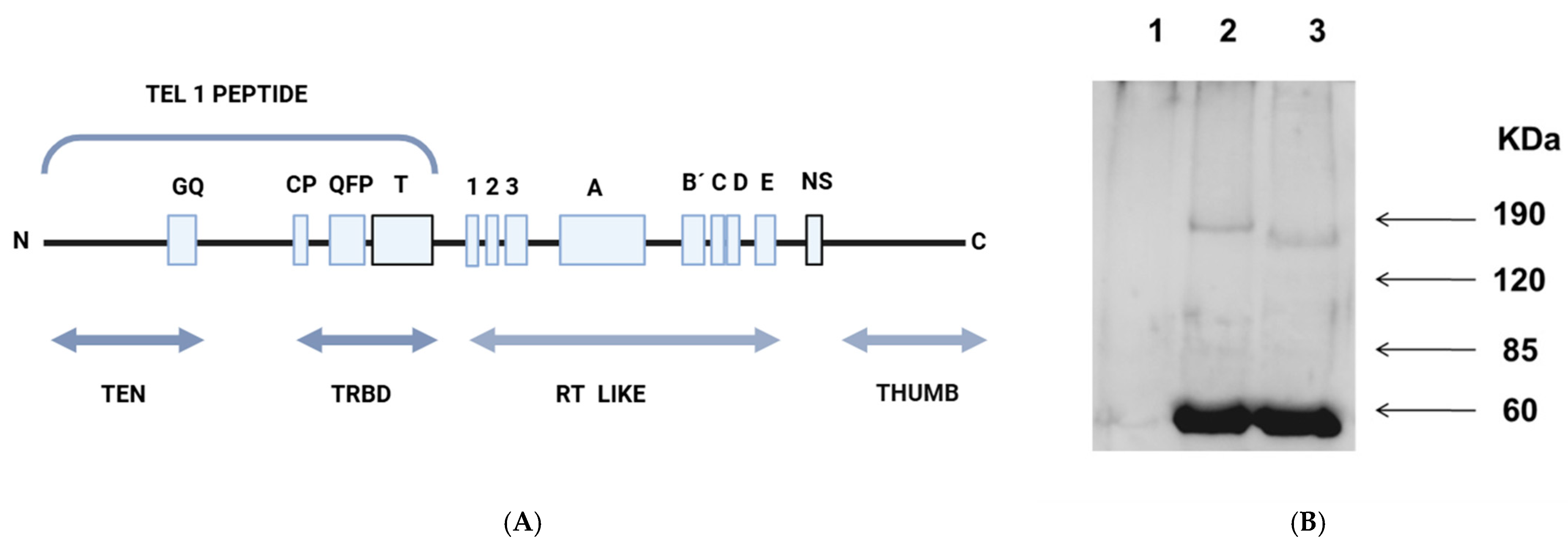
3.2. Immunopurified L. major Telomerase
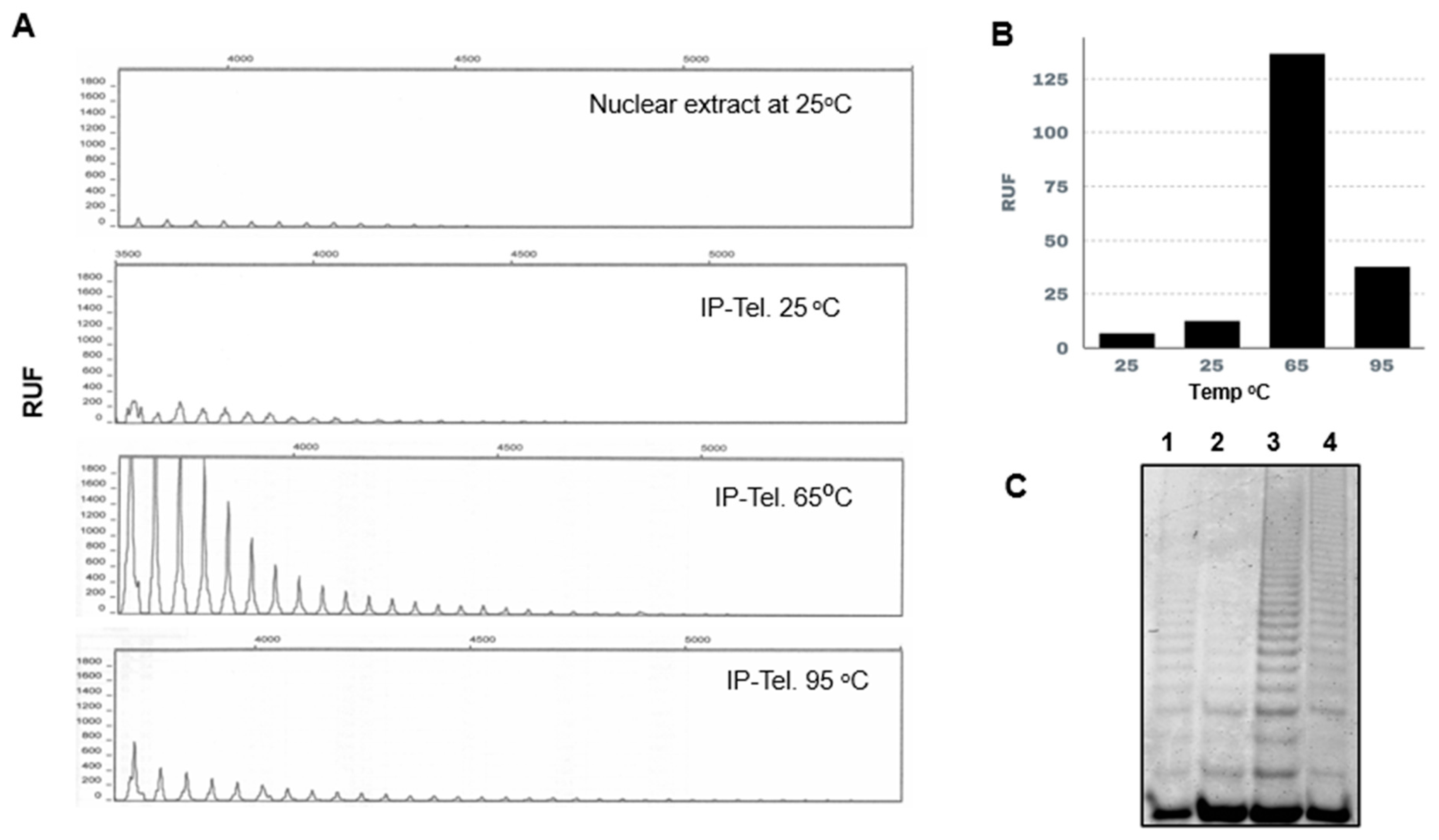
3.3. Measurement of Telomerase Activity in Immunopurified Enzyme and the Effect of the Extension Primer Sequence
3.4. Effect of Temperature on L. major Immunopurified-Telomerase Activity
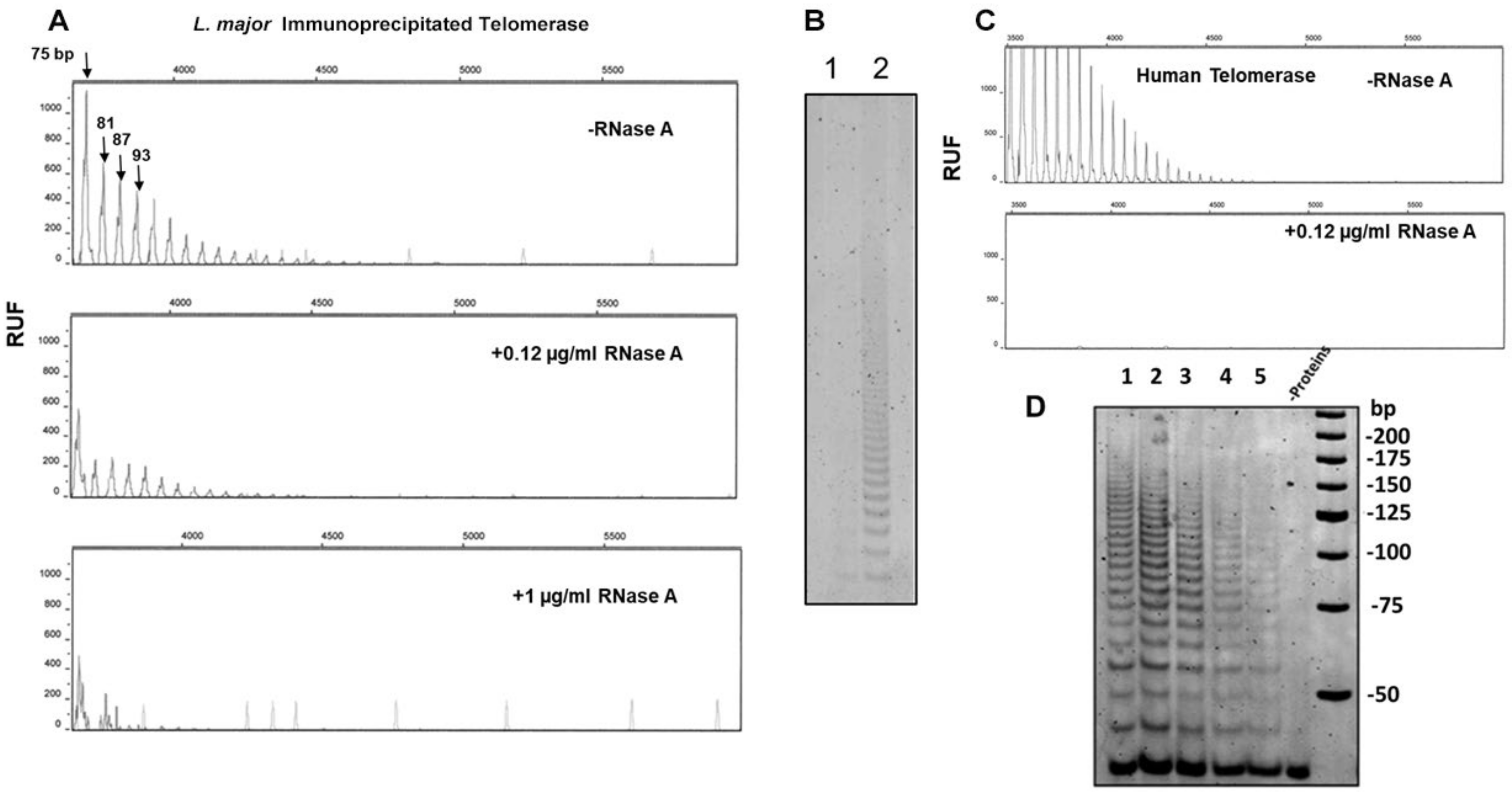
3.5. RNases A and H Treatment
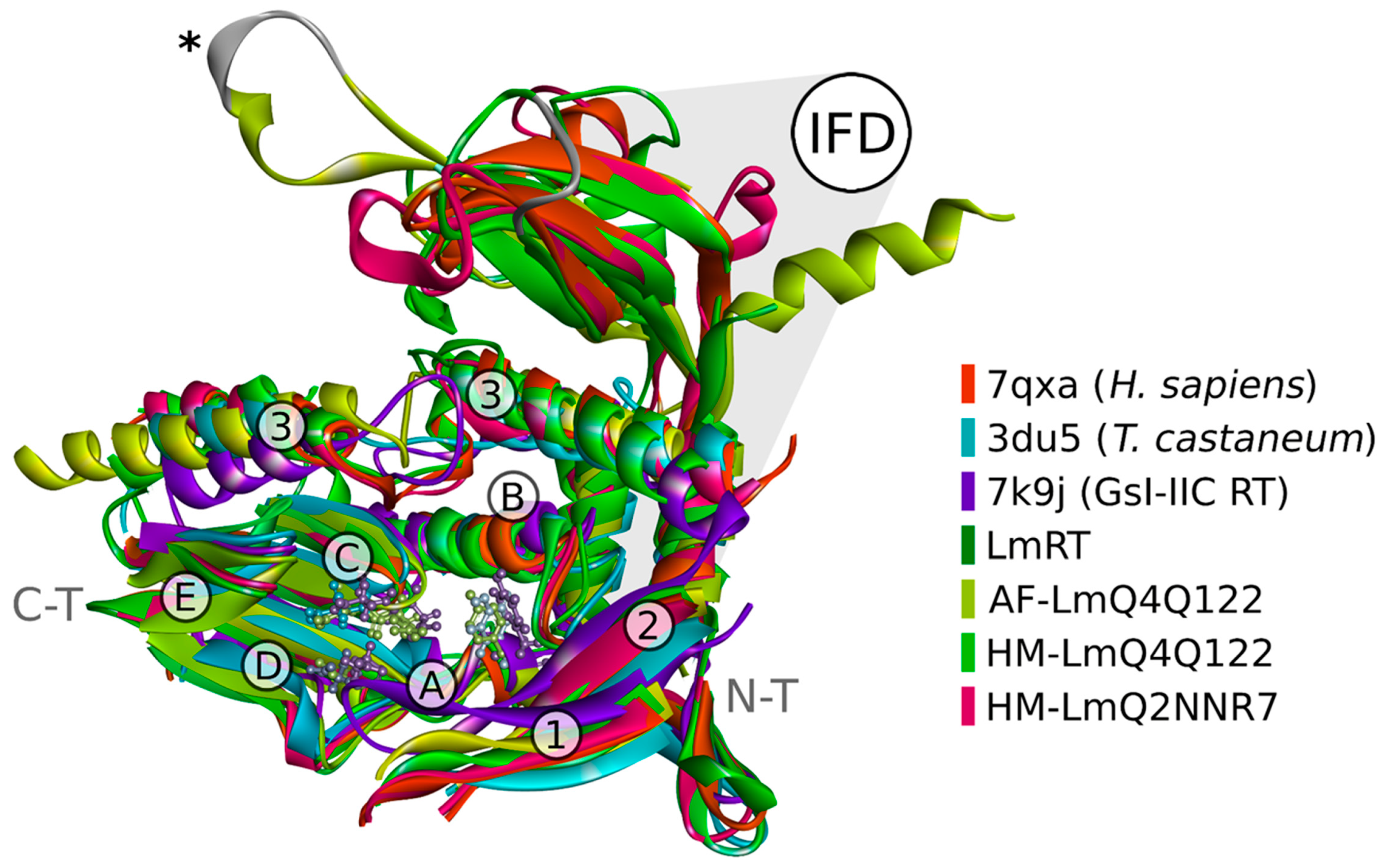
3.6. Structural Simulations to Obtain the Structural Models of L. major
3.7. Telomerase Inhibitors of L. major Telomerase Activity
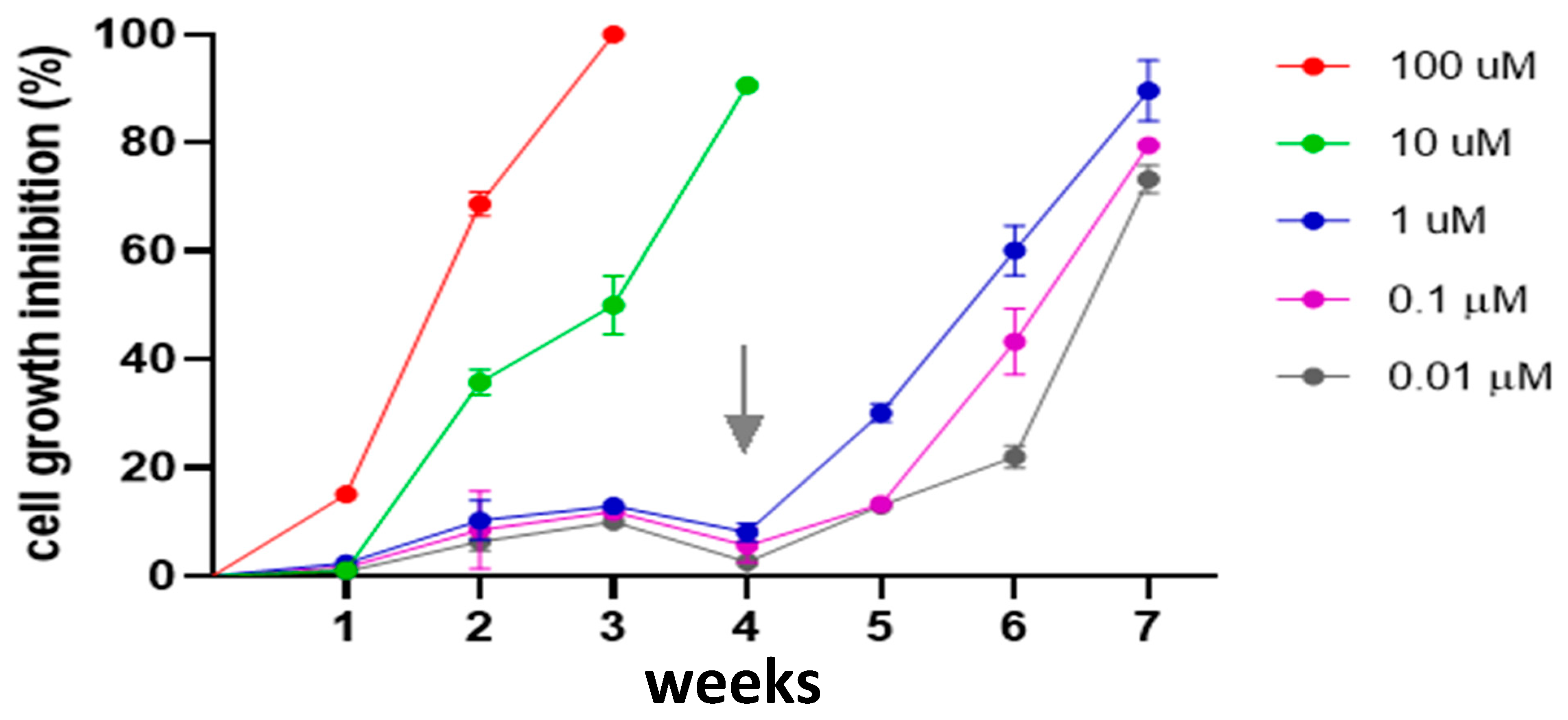
3.8. Effect MST-312 on L. major Promastigote Cell Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Greenberger, S.; Baum, S.; Pavlotsky, F.; Barzilai, A.; Schwartz, E. Unusual forms of cutaneous leishmaniasis due to Leishmania major. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1171–1175. [Google Scholar] [CrossRef]
- Griffin, W.A.; Freudenthal, B.D. Recent advancements in the structural biology of human telomerase and their implications for improved design of cancer therapeutics. Nucleic Acids Res. Cancer 2023, 5, zcad010. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomere states and cell fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef]
- Cortez-Gonzalez, X.; Zanetti, M. Telomerase immunity from bench to bedside: Round one. J. Transl. Med. 2007, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Dunham, M.A.; Neumann, A.A.; Fasching, C.L.; Reddel, R.R. Telomere maintenance by recombination in human cells. Nat. Genet. 2000, 26, 447–450. [Google Scholar] [CrossRef]
- Grobelny, J.V.; Broccoli, D. Effects of reconstitution of telomerase activity on telomere maintenance by the alternative lengthening of telomeres (ALT) pathway. Hum. Mol. Genet. 2001, 10, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Barker, D.C. Characterization of Leishmania telomeres reveals unusual telomeric repeats and conserved telomere-associated sequences. Nucleic Acids Res. 1998, 26, 2161–2167. [Google Scholar] [CrossRef]
- Chiurillo, M.A.; Beck, A.E.; Devos, T.; Myler, P.J.; Stuart, K.; Ramirez, J.L. Cloning and Characterization of Leishmania donovani Telomeres. Exp. Parasitol. 2000, 94, 248–258. [Google Scholar] [CrossRef]
- Cano, M.I.; Dungan, J.M.; Agabian, N.; Blackburn, E.H. Telomerase in kinetoplastid parasitic protozoa. Proc. Natl. Acad. Sci. USA 1999, 96, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Galindo, M.M.; Rodriguez, E.; Rojas, M.G.; Figarella, K.; Campelo, R.; Ramírez, J.L. A heat-activated and thermoresistant telomerase activity in Leishmania major Friedlin. Acta Trop. 2009, 111, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Ray, D.S. Nuclear extracts of Crithidia fasciculata contain a factor(s) that binds to the 5′-untranslated regions of TOP2 and RPA1 mRNAs containing sequences required for their cell cycle regulation. J. Biol. Chem. 1998, 273, 23729–23734. [Google Scholar] [CrossRef][Green Version]
- Campelo, R.; Díaz Lozano, I.; Figarella, K.; Osuna, A.; Ramírez, J.L. Leishmania major telomerase TERT protein has a nuclear/mitochondrial eclipsed distribution that is affected by oxidative stress. Infect. Immun. 2015, 83, 57–66. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [PubMed]
- Forsythe, H.L.; Jarvis, J.L.; Turner, J.W.; Elmore, L.W.; Holt, S.E. Stable association of hsp90 and p23, but not hsp70, with active human telomerase. J. Biol. Chem. 2001, 276, 15571–15574. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Fiser, A. Template-based protein structure modeling. Methods Mol. Biol. 2010, 673, 73–94. [Google Scholar] [CrossRef]
- Haddad, Y.; Adam, V.; Heger, Z. Ten quick tips for homology modeling of high-resolution protein 3D structures. PLoS Comput. Biol. 2020, 16, e1007449. [Google Scholar] [CrossRef]
- Stamos, J.L.; Lentzsch, A.M.; Lambowitz, A.M. Structure of a Thermostable Group II Intron Reverse Transcriptase with Template-Primer and Its Functional and Evolutionary Implications. Mol. Cell 2017, 68, 926–939.e4. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, N.; Romaniuk-Drapała, A.; Lisiak, N.; Totoń, E.; Paszel-Jaworska, A.; Kaczmarek, M.; Rubiś, B. Telomerase Inhibitor TMPyP4 Alters Adhesion and Migration of Breast-Cancer Cells MCF7 and MDA-MB-231. Int. J. Mol. Sci. 2019, 20, 2670. [Google Scholar] [CrossRef]
- Seimiya, H.; Oh-hara, T.; Suzuki, T.; Naasani, I.; Shimazaki, T.; Tsuchiya, K.; Tsuruo, T. Telomere shortening and growth inhibition of human cancer cells by novel synthetic telomerase inhibitors MST-312, MST-295, and MST-1991. Mol. Cancer Therap. 2002, 1, 657–665. [Google Scholar]
- Chiurillo, M.A.; Ramirez, J.L. Characterization of Leishmania major telomeric terminus. Mem. Inst. Oswaldo Cruz. 2002, 97, 343–346. [Google Scholar] [CrossRef]
- Wang, Y.; Gallagher-Jones, M.; Sušac, L.; Song, H.; Feigon, J. A structurally conserved human and Tetrahymena telomerase catalytic core. Proc. Natl. Acad. Sci. USA 2020, 117, 31078–31087. [Google Scholar] [CrossRef] [PubMed]
- Garrido Ruiz, D.; Sandoval-Perez, A.; Rangarajan, A.V.; Gunderson, E.L.; Jacobson, M.P. Cysteine Oxidation in Proteins: Structure, Biophysics, and Simulation. Biochemistry 2022, 61, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Schaich, M.A.; Sanford, S.L.; Welfer, G.A.; Johnson, S.A.; Khoang, T.H.; Opresko, P.L.; Freudenthal, B.D. Mechanisms of nucleotide selection by telomerase. eLife 2020, 9, e55438. [Google Scholar] [CrossRef]
- da Silva, V.L.; de Paiva, S.C.; de Oliveira, H.C.; Fernandes, C.A.H.; Salvador, G.H.M.; Fontes, M.R.M.; Cano, M.I. Biochemical and structural characterization of the RT domain of Leishmania sp. telomerase reverse transcriptase. Biochim. Biophys. Acta 2023, 1867, 130451. [Google Scholar] [CrossRef]
- Xi, L.; Cech, T.R. Inventory of telomerase components in human cells reveals multiple subpopulations of hTR and hTERT. Nucleic Acids Res. 2014, 42, 8565–8577. [Google Scholar] [CrossRef] [PubMed]
- Mozdy, A.D.; Cech, T.R. Low abundance of telomerase in yeast: Implications for telomerase haploinsufficiency. RNA 2006, 12, 1721–1737. [Google Scholar] [CrossRef]
- Ivens, A.C.; Peacock, C.S.; Worthey, E.A.; Murphy, L.; Aggarwal, G.; Berriman, M. The genome of the kinetoplastid parasite, Leishmania major. Science 2005, 309, 436–442. [Google Scholar] [CrossRef]
- Vasconcelos, E.J.R.; Nunes, V.S.; da Silva, M.S.; Segatto, M.; Myler, P.J.; Cano, M.I.N. The Putative Leishmania Telomerase RNA (LeishTER) Undergoes Trans-Splicing and Contains a Conserved Template Sequence. PLoS ONE 2014, 9, e112061. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.P.; Collins, K. Biochemical properties of Trypanosoma cruzi telomerase. Nucleic Acids Res. 2004, 32, 5214–5222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giardini, M.A.; Fernandez, M.F.; Lira, C.B.; Cano, M.I. Leishmania amazonensis: Partial purification and study of the biochemical properties of the telomerase reverse transcriptase activity from promastigote-stage. Exp. Parasitol. 2011, 127, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Campelo, R.; Galindo, M.M.; Ramirez, J.L. Characterization of Trypanosoma cruzi telomerase. Acta Trop. 2011, 120, 173–178. [Google Scholar] [CrossRef]
- de Oliveira, B.C.D.; Shiburah, M.E.; Paiva, S.C.; Vieira, M.R.; Morea, E.G.O.; da Silva, M.S.; Alves, C.S.; Segatto, M.; Gutierrez-Rodrigues, F.; Borges, J.C.; et al. Possible Involvement of Hsp90 in the Regulation of Telomere Length and Telomerase Activity During the Leishmania amazonensis Developmental Cycle and Population Proliferation. Front. Cell Dev. Biol. 2021, 9, 713415. [Google Scholar] [CrossRef]
- Gerard, G.F.; Potter, R.J.; Smith, M.D.; Rosenthal, K.; Dhariwal, G.; Lee, J.; Chatterjee, D.K. The role of template-primer in the protection of reverse transcriptase from thermal inactivation. Nucleic Acids Res. 2002, 30, 3118–3129. [Google Scholar] [CrossRef]
- Yasukawa, K.; Nemoto, D.; Inouye, K. Comparison of the Thermal Stabilities of Reverse Transcriptases from Avian Myeloblastosis Virus and Moloney Murine Leukaemia Virus. J. Biochem. 2008, 143, 261–268. [Google Scholar] [CrossRef]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural biology of telomeres and telomerase. Cell Mol. Life Sci. 2020, 77, 61–79. [Google Scholar] [CrossRef]
- Garavıs, M.; Gonzalez, C.; Villasante, A. On the Origin of the Eukaryotic Chromosome: The Role of Noncanonical DNA Structures in Telomere Evolution. Genome Biol. Evol. 2013, 5, 1142–1150. [Google Scholar] [CrossRef]
- Qu, G.; Kaushal, P.S.; Wang, J.; Shigematsu, H.; Piazza, C.L.; Agrawal, R.K.; Belfort, M.; Wang, H.W. Structure of a group II intron in complex with its reverse transcriptase. Nat. Struct. Mol. Biol. 2016, 23, 549–557. [Google Scholar] [CrossRef]
- Lambowitz, A.M.; Belfort, M. Mobile Bacterial Group II Introns at the Crux of Eukaryotic Evolution. Microbiol. Spectr. 2015, 3, MDNA3-0050-2014. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.E.; Cheng, A.; Yadav, G.P.; Ludlow, A.T.; Shay, J.W.; Wright, W.E.; Jiang, Q.X. Catalysis-dependent inactivation of human telomerase and its reactivation by intracellular telomerase-activating factors (iTAFs). J. Biol. Chem. 2019, 294, 11579–11596. [Google Scholar] [CrossRef]
- Matagne, A.; Joris, B.; Frere, J.-M. Anomalous behavior of a protein during SDS/PAGE corrected by chemical modification of carboxylic groups. Biochem. J. 1991, 280, 553–556. [Google Scholar] [CrossRef]
- Rath, A.; Glibowicka, M.; Nadeau, V.G.; Chen, G.; y Deber, C.M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kaila, P.; Guptasarma, P. Understanding anomalous mobility of proteins on SDS-PAGE with special reference to the highly acidic extracellular domains of human E- and N-cadherins. Electrophoresis 2019, 40, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, G.; Gouzelou, E.; Côrtes Boité, M.; Kherachi, I.; Harrat, Z.; Eddaikra, N.; Mottram, J.C.; Antoniou, M.; Christodoulou, V.; Bali, A.; et al. Leishmania Genome Dynamics during Environmental Adaptation Reveal Strain-Specific Differences in Gene Copy Number Variation, Karyotype Instability, and Telomeric Amplification. mBio 2018, 9, e01399-e18. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, B.C.D.; Shiburah, M.E.; Assis, L.H.C.; Fontes, V.S.; Bisetegn, H.; Passos, A.D.O.; de Oliveira, L.S.; Alves, C.S.; Ernst, E.; Martienssen, R.; et al. Leishmania major telomerase RNA knockout: From altered cell proliferation to decreased parasite infectivity. Int. J. Biol. Macromol. 2024, 279, 135150. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges, and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Hallinan, J.P.; Doyle, L.A.; Shen, B.W.; Gewe, M.M.; Takushi, B.; Kennedy, M.A.; Friend, D.; Roberts, J.M.; Bradley, P.; Stoddard, B.L. Design of functionalised circular tandem repeat proteins with longer repeat topologies and enhanced subunit contact surfaces. Commun Biol. 2021, 4, 1240. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campelo Morillo, R.; Casique, L.; Figarella, K.; Ramírez, J.L. Molecular and Structural Characterization of an Immunopurified Telomerase from Leishmania major and the Effect of Telomerase Inhibitors. Microorganisms 2025, 13, 357. https://doi.org/10.3390/microorganisms13020357
Campelo Morillo R, Casique L, Figarella K, Ramírez JL. Molecular and Structural Characterization of an Immunopurified Telomerase from Leishmania major and the Effect of Telomerase Inhibitors. Microorganisms. 2025; 13(2):357. https://doi.org/10.3390/microorganisms13020357
Chicago/Turabian StyleCampelo Morillo, Riward, Liliana Casique, Katherine Figarella, and José Luis Ramírez. 2025. "Molecular and Structural Characterization of an Immunopurified Telomerase from Leishmania major and the Effect of Telomerase Inhibitors" Microorganisms 13, no. 2: 357. https://doi.org/10.3390/microorganisms13020357
APA StyleCampelo Morillo, R., Casique, L., Figarella, K., & Ramírez, J. L. (2025). Molecular and Structural Characterization of an Immunopurified Telomerase from Leishmania major and the Effect of Telomerase Inhibitors. Microorganisms, 13(2), 357. https://doi.org/10.3390/microorganisms13020357






