Abstract
Proteus mirabilis is a well-known opportunistic pathogen predominantly associated with urinary tract infections. It exhibits natural resistance to multiple antibiotics, including last-resort options like colistin. The emergence and spread of multidrug-resistant P. mirabilis isolates, including those producing ESBLs, AmpC cephalosporinases, and carbapenemases, are now more frequently reported. The most common carbapenemase types found in P. mirabilis are KPC-2, IMP, VIM, NDM, and OXA-48. We sequenced the genomes of three carbapenem-resistant P. mirabilis isolates harboring both blaVIM-4 and blaVIM-75 from Germany using both short-read and long-read sequencing techniques. We found that the isolates were only distantly related genetically. Both blaVIM-4 and blaVIM-75 genes were located on a class I integron, which in two cases was located on the chromosome and in one case on a plasmid. This is the first report on the complete genomes of P. mirabilis strains harboring a rare genetic element encoding both blaVIM-4 and blaVIM-75. Our results emphasize a key role for class 1 integrons in the transmission of VIM carbapenemases in P. mirabilis.
1. Introduction
Proteus mirabilis is a commensal of the human digestive tract, also present in various environmental sources, particularly in sewage water and soil. In the medical context, the species is mostly known for causing urinary tract infections and infections originating from the intestines, such as peritonitis. When these infections breach the immune system or overcome medical interventions, P. mirabilis can cause bloodstream infections (BSIs) and subsequently septicemias. Typical pathogenicity factors include fimbriae, adhesion molecules, hemolysis (not visible on Columbia sheep blood agar), and flagella, which enable strains to swarm over agar plates in a typical specific pattern [1,2,3,4]. Common treatment options include aminopenicillins, second- and third-generation cephalosporines, trimethoprime–sulfamethoxazole, and fluoroquinolones. Like other Enterobacterales, P. mirabilis can develop or acquire resistance to any or all of these substances, demanding physicians to draw and reserve antibiotics, most typically on carbapenems. Risk factors are linked with the acquisition of multidrug-resistant (to three or more classes of antimicrobials) strains.
Proteus are members of the family Morganellaceae and have a certain degree of intrinsic resistance to imipenem. However, this intrinsic resistance can usually be overcome at high doses or in infections at sites with high concentrations of imipenem, especially in the urinary tract. Nevertheless, Proteus can also acquire carbapenemases, which render them virtually immune to treatment with carbapenems. In Germany, P. mirabilis isolates harboring carbapenemases are rare, but pose a significant threat to human health when encountered in potentially life-threatening infections. Proteus are also naturally resistant to polymyxins like colistin, which is generally only rarely used because of severe neurological and nephrological side effects but remains as one of the important last-resort antibiotics for infection with carbapenem-resistant Gram-negative bacteria [5].
Recently, the emergence and spread of carbapenemase-producing P. mirabilis encoding types blaKPC-2, blaNDM, blaOXA-23, and blaOXA-48 have been reported in many countries around the world [6]. The acquisition of blaVIM genes has occurred in Greece, Italy, and Bulgaria [7,8]. In Germany, carbapenemase-producing Enterobacterales are increasing in prevalence [9]. Nationwide surveillance reports note that E. coli and Klebsiella species are the most common carbapenemase-producers, while P. mirabilis harboring carbapenemases have only rarely been detected.
We carried out a surveillance study of carbapenem-resistant Gram-negative bacteria in the state of Hesse in Germany, in which carbapenem-resistant Enterobacterales collected within a three-year period were subjected to whole-genome sequencing. In the present study, we included three P. mirabilis isolates exhibiting phenotypic carbapenem resistance and containing both blaVIM-75 and blaVIM-4 genes encoding VIM carbapenemase. Herein, we present a detailed description of the nature of the resistance elements in the context of their genomes and their plasmids.
2. Materials and Methods
2.1. Study Design and Bacterial Isolates
An epidemiologic surveillance study on carbapenem-resistant Gram-negative bacteria was conducted from 2017 to 2019 across 61 hospitals in the State of Hesse in Germany. This molecular epidemiologic investigation yielded 520 Enterobacteriaceae isolates that exhibited non-susceptibility to at least one carbapenem, including five P. mirabilis from various hospitals in 2018 and 2019. Three isolates with duplicated VIM alleles were identified from the genome analysis with the short-read sequence data and no known carbapenemase genes were found in two other isolates. Long-read sequencing was then performed for the three blaVIM-carrying isolates for future genomic analyses. They were isolated from groin swabs, blood cultures, and rectal swabs and derived from three epidemiologically unrelated patients aged 82, 80, and 92 years in three different hospitals, respectively, in Hesse, Germany, in 2019.
2.2. Antibiotic Susceptibility Testing
The antibiotic susceptibility test was performed using the VITEK®2 system (bioMérieux, Nürtingen, Germany) and interpreted in accordance with EUCAST guidelines. The quantitative MICs of imipenem and meropenem were determined using a Liofilchem MIC Test Strip (bestbion dx GmbH, Köln, Germany). Taxonomy was validated by utilizing MALDI-TOF-MS (Vitek MS, bioMérieux, Nürtingen, Germany).
According to the criteria for multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) bacteria by the ECDC and/or CDC [10], our three isolates analyzed belonged to XDR (extensively drug-resistant) because they were non-susceptible to at least 1 agent in all but 2 or fewer antimicrobial categories (see Table 1).

Table 1.
Sources and antibiotic susceptibility testing results of the P. mirabilis isolates. VITEK®2 MIC (mg/L) (interpretation).
2.3. Whole-Genome Sequencing and Genome Analyses
Short-read whole-genome sequencing, post-sequencing quality control, and assembly were performed as described previously [11]. For detailed genomic characterization, these isolates were re-sequenced using PacBio long-read sequencing as described previously [12]. Genome assembly following quality control was performed using ASA3P [13]. Plasmid incompatibility (Inc) groups, antimicrobial resistance genes (ARGs), and insertion sequences (ISs) were identified using the Center for Genomic Epidemiology website (https://cge.food.dtu.dk/ accessed on 13 December 2023) [14,15] and ISFinder [16]. To identify the VIM alleles, blastN in the NCBI database screening was performed and validated with the bldb database (www.bldb.eu accessed on on 5 December 2024) [17]. oriTfinder (https://bioinfo-mml.sjtu.edu.cn/oriTfinder/ accessed on 5 December 2024) was used to determine virulent factor genes and predict OriT sites [18]. Mobile genetic elements (MGEs) associated with the ARCs were detected with the web tool MobileElementFinder [19]. The genetic structure surrounding the VIM genes was annotated and visualized by using Galileo AMR of ARC Bio [20]. To detect CRISPR-Cas sequences, CRISPRCasFinder was used [21].
2.4. Ethical Approval
Ethical approval was sought from the Ethics Committee of the State Medical Association of Hesse in Frankfurt/Main. The Committee decided on 24 January 2018 that ethical approval for the project was not necessary as this study’s patient data were rendered anonymous [11].
3. Results
3.1. Antibiotic Susceptibility
The isolates were phenotypically resistant to β-lactams (ampicillin, ampicillin–sulbactam, piperacillin, piperacillin–tazobactam, cefepime, cefpodoxime, cefotaxime, ceftazidime, cefuroxime, imipenem, and aztreonam), fluoroquinolones (ciprofloxacin, moxifloacine, and ofloxacin), aminoglycosides (gentamicin, tigecycline, and trimethroprime–sulfamethaoxal) and displayed reduced sensitivity towards to meropenem and ertapenem (Table 1). The colistin test indicated an MIC of >64 µg/mL for isolates Survcare401 and Survcare357.
3.2. Whole-Genome Sequencing and Genomic Features
The draft genomes of the isolates from the short-read sequencing exhibit a total length between 4.118 and 4.250 Mb. In combination with long-read-sequencing, the chromosome and one to three plasmids of each isolate were circular-closed (Table 2). The completed chromosomal sequence of Survcare401 is 4,218,249 bps in size and encodes 3852 CDSs, 22 rRNAs, and 86 tRNAs. The annotated CDSs are shown in Figure S1 in detail. The genomic comparison of the three isolates revealed significant sequence orthologue similarity among them through BRIG (Figure S1).

Table 2.
Genomic characteristics, genome sizes, and antimicrobial resistance genes (ARGs).
3.3. Antimicrobial Resistance Genes and the Co-Occurrences of Both blaVIM-75 and blaVIM-4
The genomes each contained a large number of antimicrobial resistance genes (ARGs), which localized mostly on chromosomes (Table 2). Twenty-six different ARGs were identified on Survcare401, which belonged to seven antibiotic classes, including aminoglycoside (armA, aac(6′)-IIc, strA, strB, aac(3)-IId, and aph(3′)-Ic), beta-lactam (blaVIM-4, blaVIM-75, blaCTX-M-15, blaTEM-1, and blaTEM-2), marcrolide/lincosamide/streptogramin B (lnu(F)), (mph(E) and msr(E)), phenicols (cat and catA1), sulfonamides (sul1 and sul2), tetracycline (tet(J)), and trimethoprim (dfrA1 and dfrA17). A resistance gene against quaternary ammonium compounds (ΔqacE) was also identified.
The ARG repertoires of the isolates Survcare357 and Survcare372 were like Survcare401, differing only in the genetic location for some ARGs of Survcare372 which were located on a plasmid instead of the chromosome (Table 2).
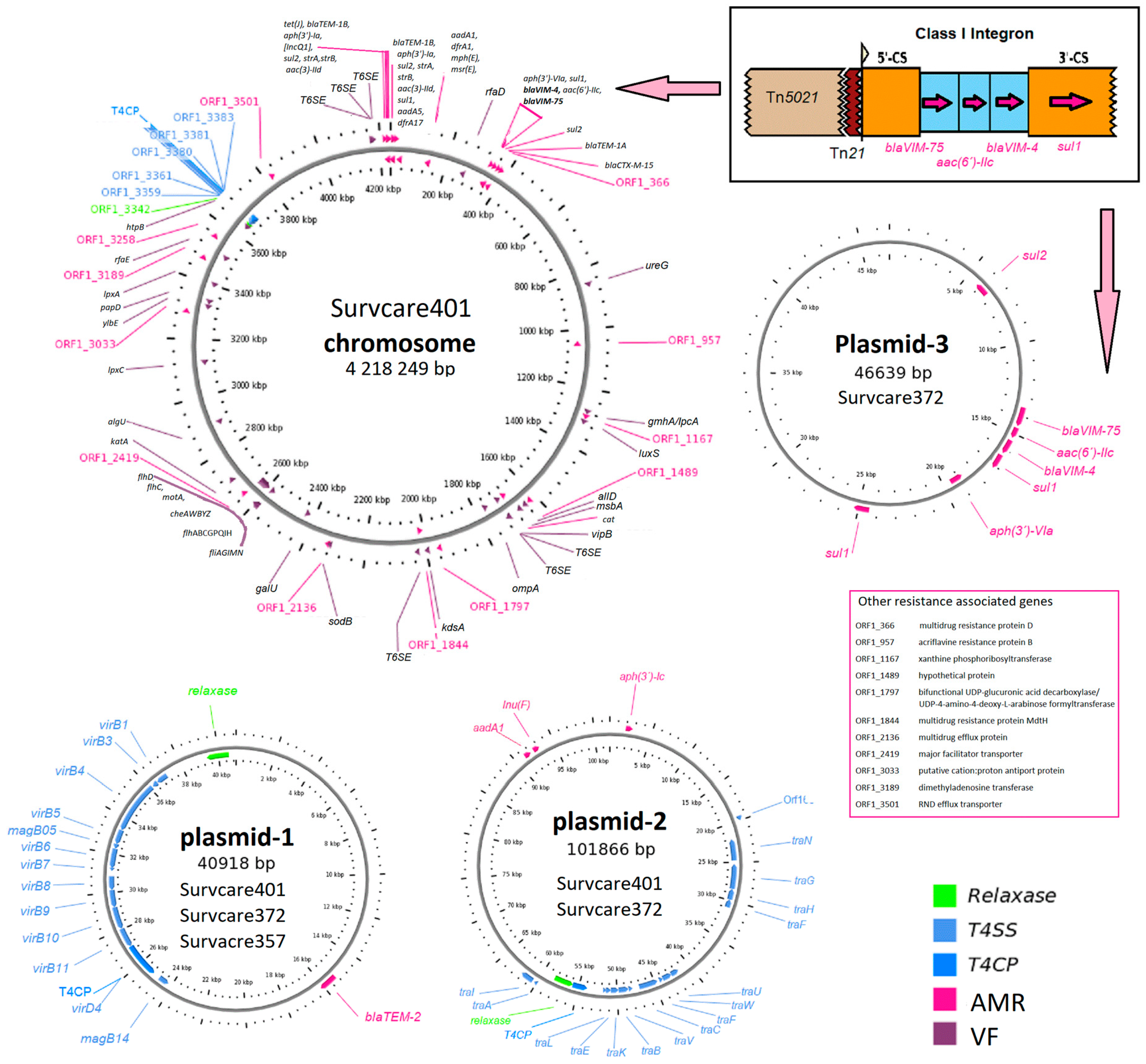
Of note, both the blaVIM-75 and blaVIM-4 genes were embedded in a single class 1 integron whose genetic structure comprises the gene cassette [blaVIM-75, aac(6)-IIc, blaVIM-4, ∆qacE, and sul1] as identified on the chromosomes of Survcare401 and Survcare357 and on plasmid p372-3 of Survcare372, as shown in detail in Figure 1. The blaVIM-4 and blaVIM-75 genes are variants of blaVIM-1, each with a single amino acid substitution of S206R in blaVIM-4 and Q60R in blaVIM-75.
Figure 1.
Genetic maps of the closed chromosome of Survcare401 and the plasmids of the isolates Survcare401, Survcare357, and Survcare372 show the antimicrobial resistance genes (ARGs) and virulence factor predicted as well as the Type 4 secretion system (T4SS), with the genetic structure of the class 1 integron encoding both blaVIM-75 and blaVIM-4 (top right). The arrows indicate the position of the class 1 integron on the chromosome (to the left) and the plasmid (downwards). Plasmid-1 represents plasmids p410-1, p357-1, and p372-1 presented in three strains, plasmid-2 represents p401-2 and p372-2 in strains Survcare402 and Survcare372, and plasmid-3 indicates p372-3, which was exclusively detected in Survcare372.
3.4. Plasmids
Three different plasmids were identified. One plasmid was present in all three isolates, another was present in two, and a unique plasmid was present only in one isolate (Figure 1).
Two closed plasmids, p401-1 (40,917 bps) and p401-2 (101,867 bps), were identified in the genome of Survcare401. Like Survcare401, Survcare357 harbored the identical 41 kb plasmid 1, p357-1, while Survcare372 harbored an additional third plasmid, p372-3 (46,639 bps), compared to Survcare401 (Figure 1). All plasmids of the isolates were non-typeable according to the plasmid Inc-group scheme, but a disrupted IncQ1 replicon CDS was detected on their chromosomes.
The 41 kb plasmids, e.g., p401-1, p357-1, and p372-1, encode the beta-lactamase blaTEM-2 gene located within a 5 kb Tn801 transposon. A type IV secretion system, colicin immunity protein Cui, and type II toxin–antitoxin system (RelE/ParE) were identified (Figure 1). Plasmids with high sequence identity (>99.9%) and 100% coverage in Proteus and Providence strains have been frequently reported from different countries, such as p52260_1, p52808_2, P1-FZP3115, and p16Pre36_1 from Australia, China, and the Czech Republic (GenBank accession numbers: CP070570, CP070574, CP098451, and KX832926). Notably, they were only isolated after 2019. As p-1 is present in all three isolates studied, it appears to be commonly conserved in clinical P. mirabilis isolates.
The plasmids p401-2 and p372-2 both encoded lnu(F), aadA1, and aph(3′)-Ia, conferring antibiotic resistance to lincomycin and aminoglycosides like streptomycin, kanamycin as well neomycin, respectively. In comparison, aph(3′)-Ia was embedded within a putative composite transposon, cn_2938_IS26. A type IV secretion system was also found in these plasmids. We found several plasmids with high identities (>99%) to the backbone of our p_2 plasmids, such as pIB-NDM-1, pPp47, pPv1-49741, and p06-1619-1 and p-dmpro_5749a_NDM1 from Proteus strains in Italy, Australia, the USA, and Bangladesh, respectively (GenBank accession numbers: CP045540, MG516912, CP104122, KX832929, and CP095677).
The third plasmid of Survcare372, p372-3, that harbored the class 1 integron encoding both blaVIM-75 and blaVIM-4 and other ARGs exhibited a backbone structure similar to plasmid sequences from different Enterobacterales species like E. coli, Enterobacter spp., Klebsiella spp., and Providencia spp. Interestingly, none of them carried the blaVIM-75 and blaVIM-4 encoding class 1 integron.
3.5. Virulence Factor Genes
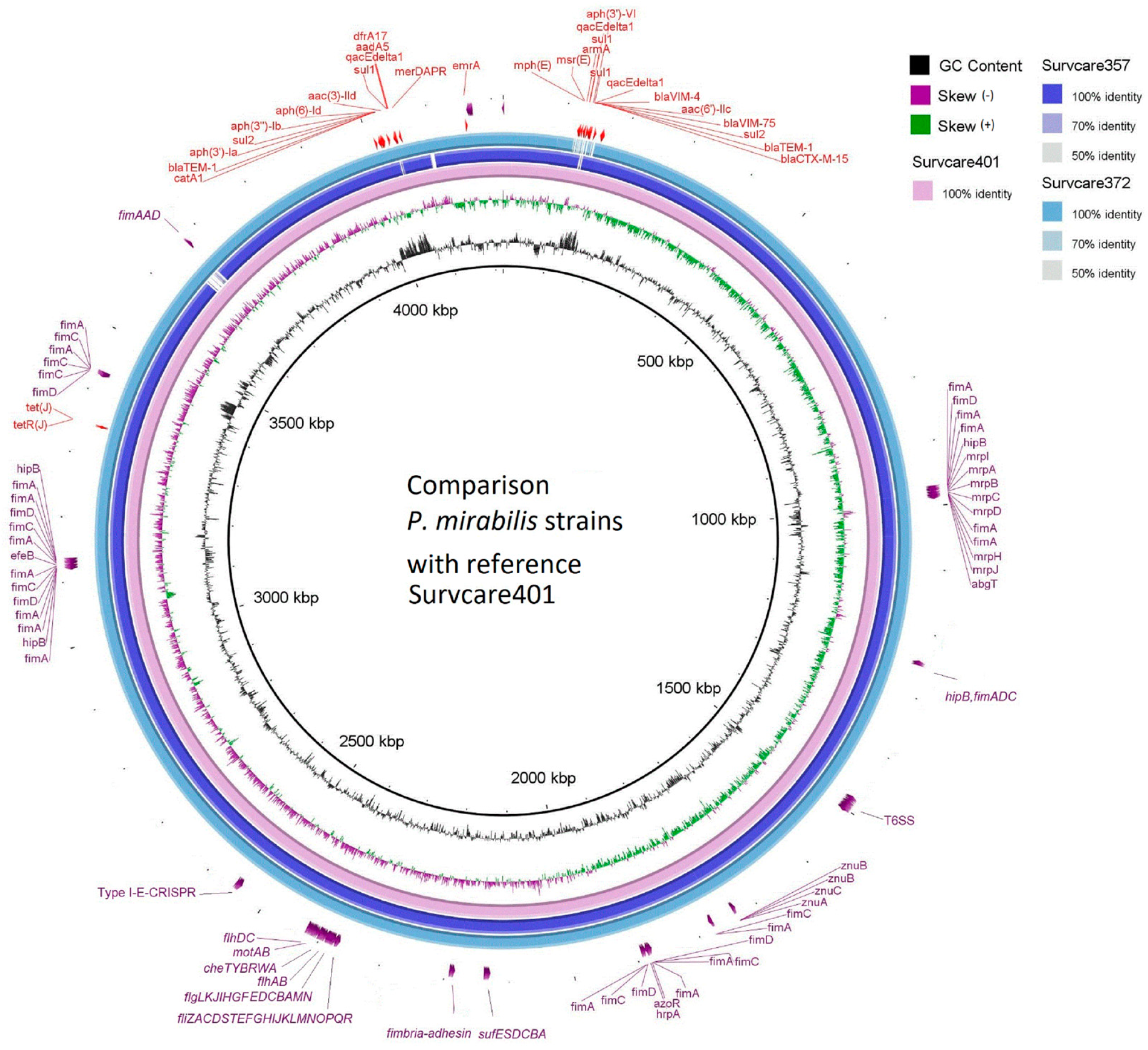
Forty-six virulence genes were predicted in the genome of Survcare401 and forty-four genes each were predicted for Survcare372 and Survcare357 on their chromosomes (Table S1). They comprise a type VI secretion system, flagella biosynthesis, fimbria syntheses, fimbria adhesin, metalloproteases, iron transporter, and Fe-S cluster assembly. The BRIG genome comparison (Figure 2) shows the different virulence groups on the chromosomes of the isolates. In the chromosome of Survcare357, the region coding a prophage was not detected but was present in the genome of Survcare372 and Survcare401.
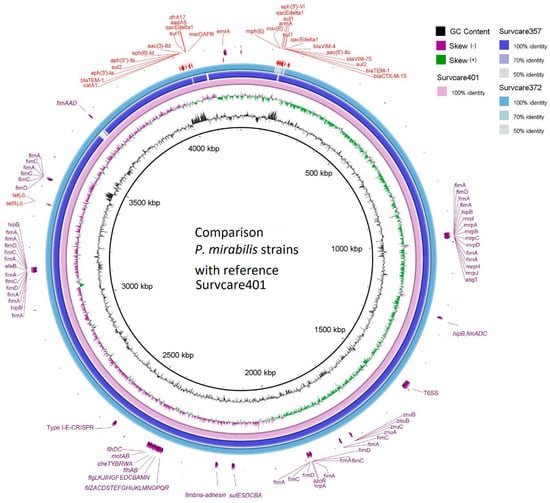
Figure 2.
Comparison of the chromosomes of three isolates with Survcare401 as the reference sequence, indicating the presence of different virulence groups. The blaVIM-75- and blaVIM-4-carrying class 1 integron was present in the chromosomes of Survcare401 and Survcare357, and it was also present in Survcare372 on plasmid p372-3.
Notably, a type I-E CRISPR-Cas system was present in all isolates, including Cas3, CasABCDE, Cas1e, Cas2e, and a CRISPR array consisting of 14 repeats, each 29 nucleotides in length, with a spacer length of 32 nucleotides, which typically provides P. mirabilis with immunity against bacteriophages.
3.6. Phylogeny
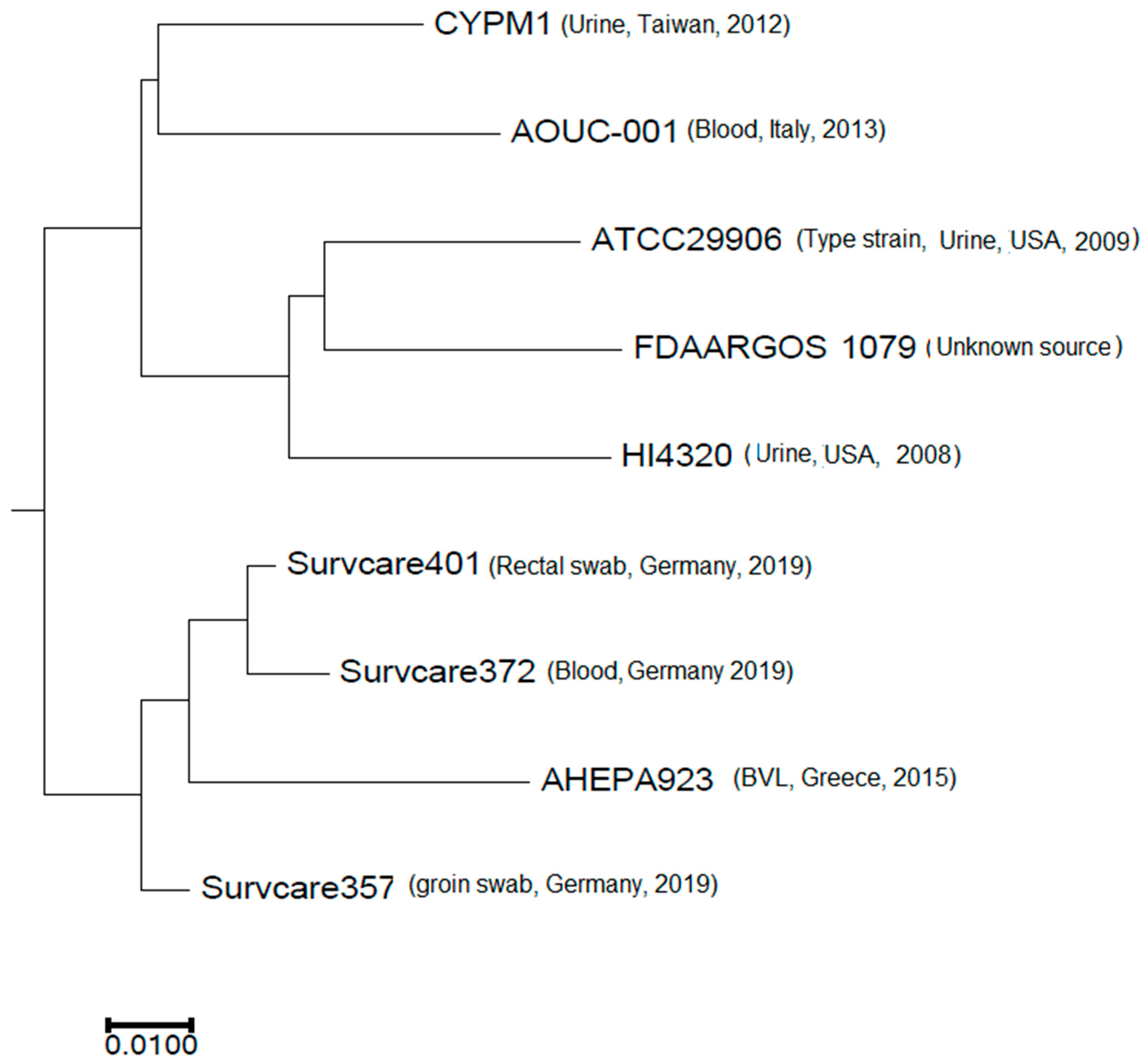
The phylogenetic analysis revealed that the isolates are phylogenetically not related to each other, indicating different origins (Figure 3). Nevertheless, the best-matching type-strain was the genome of P. mirabilis strain ATCC 29906 with an Average Nucleotide Identity (ANI) of 99.31% to 99.33% and a coverage of 0.86, which was identical for all three isolates.
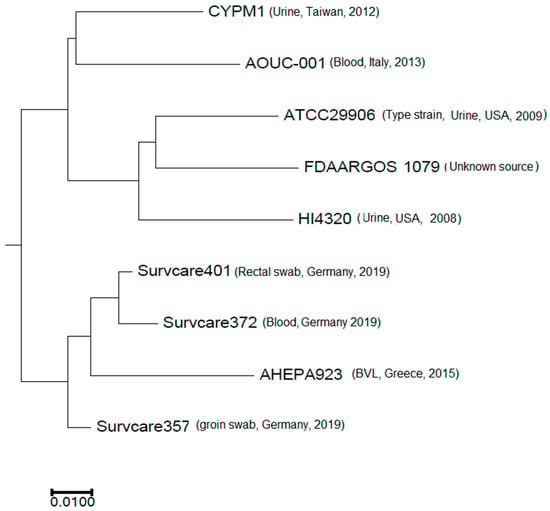
Figure 3.
Phylogenetic comparison of the three isolates studied with selected publicly available complete Proteus mirabilis genomes.
4. Discussion
The co-occurrence of carbapenemase genes blaVIM-4 and blaVIM-75 in P. mirabilis has never been reported before. Herein, we have for the first time genomically characterized three P. mirabilis isolates co-harboring these two genes. Whole-genome analysis revealed that these genes were located on a class I integron in all three cases, which itself was located chromosomally in two isolates and plasmids in the third isolate. The three isolates originated from independent sources and are not genetically clonally related. We therefore suggest that the class I integron with the carbapenemase genes was acquired independently from different origins by the P. mirabilis strains.
Verona integron-encoded metallo-β-lactamases (VIM) in general are probably better known for Pseudomonas aeruginosa but have also been found in Enterobacterales, including Proteus, as well [6,22,23,24,25]. Currently, about 80 different VIM types have been identified, and the most reported genes are blaVIM-1 and blaVIM-4. P. mirabilis has been described as carrying blaVIM-1 in Greece, Bulgaria, and the Netherlands, for example [8,26,27]. The presence of the blaVIM-4 gene (a single amino acid mutation of the blaVIM-1 gene (S206R)) has also been described in P. mirabilis, for example, in Greece [28]. The blaVIM-75 gene is also a single amino acid mutation of blaVIM-1 (Q60R) but, to the best of our knowledge, has not been described in this species before.
We identified potential sources or potentially similar, parallel, acquisitions of blaVIM-75 by P. mirabilis in data in public databases: blaVIM-75 has apparently been detected in a P. mirabilis strain 07C16CRGN002 from a surveillance project in Canada (access no. NG_076844.1). Additionally, entries with an identical nucleotide sequence were found in an isolate from urine in Poland (designated as In2239, access no. OQ116828.1) and an animal isolate (Gallus) from Iraq (access no. LC848470.1).
The three isolates we studied herein harbored both blaVIM-4 and blaVIM-75, so the question arises as to how this double-carbapenemase status came to pass or how it was transmitted. We found a similar genetic construct in the literature, within a Vibrio cholerae strain described by Aberkane et al. [29]: a strain isolated from Yellow-legged gulls (Larus michaellis) co-carried blaVIM-1 and blaVIM-4. They were located on a class I integron with aac(6′)-IIc in between and a sul1-gene downstream, carried by its IncA/C plasmid. However, apart from this similarity, we have no further data to conclude that our isolates have an environmental epidemiological link.
Even though the specific combination of genes detected herein has never been published before, the genetic vicinity has been described in P. mirabilis; the genes are located on a class I integron, which is common in P. mirabilis carrying blaVIM-1. These integrons can carry other resistance genes as well, like aacA7, dhfr, and aadA [7]. Our isolates each carry an almost identical class I integron with the gene cassettes [blaVIM-75—aac(6′)-IIc—blaVIM-4—∆qacE—sul1], downstream to the integrase (Figure 1). We speculate that the three isolates may independently have acquired the same integron from an unknown source and that this integron is a development from previously known types of P. mirabilis integrons, through gene replacement, and possibly duplication with a mutation, as in the case of blaVIM-4 and blaVIM-75.
IS26 was not found in the integron structure, so high-level carbapenem resistance from the increased expression of the carbapenemase gene VIM through an increase in its copy number via the association of IS26 with the integron was not to be expected, as demonstrated in a previous study on blaVIM-1-carrying P. mirabilis [30].
Our isolates also harbored a large number of acquired ARGs which conferred resistance to several antibiotic classes. Acquired fluoroquinolone resistance genes were not found (Table 2), but amino acid substitutions in DNA gyrase subunit A GyrA (S83I) and topoisomerase IV ParC (S84I), compared to P. mirabilis HI4320 (NC_010554), were identified. Such alterations result in decreased susceptibility to fluoroquinolones in Enterobacterales [31,32], including P. mirabilis [33,34].
As far as the pathogenicity of the strains themselves is concerned, we have only limited clinical data. One was isolated from a rectal swab and another from a groin swab, which leaves little room for speculation. However, one isolate originates from a blood culture, which suggests the pathogenic potential of Survcare372. This is in accordance with the results of our virulence gene analysis, which revealed (for all three isolates) a plethora of common genes associated with P. mirabilis pathogenicity such as fimbriae, flagella, and gene clusters of type III, IV, and VI secretion systems. An interesting feature of our isolates is a type I-E CAS system in their genomes. CRISPRCas systems have been found only in about one-third of sequenced P. mirabilis genomes [1].
Plasmid type 1 (41 kb) in all three of our isolates seems to be a conserved common genome component of P. mirabilis, as we found many matching entries from P. mirabilis in the NCBI genome database. Plasmid type 2 (101 kb), which is present in two isolates, is also similar to numerous plasmids in the species, for example, pPp47 from silver gulls [35]. Notably, the segment containing the tra-operon is frequently found in strains of both human and environmental origin.
The overall increase in carbapenemases found in Enterobacterales constitutes a potential threat to modern healthcare facilities. National surveillance programs seek to quantify and assess the extent of this spread. Studies have shown that the spread of multidrug resistance, including carbapenem resistance, is associated with multiple species with different genetic backgrounds. Surveillance programs serve to identify early emerging resistance patterns and the emergence and spread of carbapenemase genes. Herein, we report rather unusual findings from a surveillance program based on WGS in Hesse, Germany: we identified three P. mirabilis isolates, each possessing a unique genetic element that harbors both blaVIM-75 and blaVIM-4 genes.
From a clinical perspective, our findings demonstrate a worrisome development: carbapenemase-producing P. mirabilis was previously rare in Germany. However, the detection of three strains with a combination of two carbapenemase genes could be a potential entry point for the spread of carbapenem resistance in P. mirabilis in clinical care in larger numbers.
Notably, the integron with these genes integrated into the chromosome in two of the three isolates and into a plasmid in one isolate. This indicated that the VIM-metallo-β-lactamase could easily spread by the integron both vertically and horizontally as they all carried the transferable conjugative plasmids (plasmid type 1 and type 2).
The present study has limitations. The first of which is that we only collected clinical strains from human medicine samples voluntarily submitted by participating laboratories. Thus, we can be wary of a potential threat in clinical care, but we have limited data about the full extent of the problem. It would be interesting to know if strains like ours can be found in the general population as well. Likewise, it is possible that additional data from animal or environmental sources could elucidate the origin of these strains and/or of the integron.
In summary, our results reveal dual carbapenemase-carrying P. mirabilis strains (blaVIM-4 and blaVIM-75) in Hesse, Germany, which may disseminate and complicate patient treatment by conferring carbapenem resistance. A mobile integron facilitates this resistance and may allow further dissemination to various Enterobacterales, particularly those of greater clinical relevance, such as Escherichia coli and Klebsiella pneumoniae. Furthermore, our data could support global efforts to trace the acquisition of two carbapenemase genes, blaVIM-75 and blaVIM-4 in the simultaneous presence of the 16S-rRNA-methylase gene armA by this species.
5. Conclusions
Herein, we report for the first time the complete genomes and the chromosomal and plasmidic co-occurrence of blaVIM-4 and blaVIM-75 of three multidrug-resistant and highly virulent P. mirabilis isolates from patients in Germany. The comparable genetic organization of the VIM gene-carrying class I integron structure with a previously documented variant (with blaVIM-1—and blaVIM-4) in a V. cholerae strain from wild avians suggests the potential for zoonotic transmission of VIM-carrying organisms, which may contribute to the spread of carbapenem resistance in humans. The high incidence of VIM-carrying Proteus in the environment underscores the significance of a One Health perspective in controlling multidrug resistance in Enterobacterales.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13020266/s1. Table S1: Predicted virulence factors on the genome of Survcare401 as well as their presence in the genomes of Survcare357 and Survcare372. Figure S1: Comparison of the genomes and the locations of different virulence factor groups and ARGs.
Author Contributions
Conceptualization, Y.Y.; methodology, J.F.; validation, M.F. and C.I.; formal analysis, Y.Y. and J.F.; investigation, Y.Y.; resources, T.C., T.H. and C.I.; data curation, Y.Y., M.F. and J.F.; writing—original draft preparation, Y.Y. and M.F.; writing—review and editing, T.C., T.H., C.I., M.F. and Y.Y.; visualization, Y.Y.; supervision, Y.Y.; funding acquisition, T.C., T.H., C.I. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded as part of the DZIF-R-Net 2.0 project, TTU 08.824, funding code 8032808824, awarded to T.C. and T.H. (2021–2025), and the SurvCARE, Hessian Ministry for Social Affairs and Integration, funding code V3B, awarded to T.C. und C.I. (2017–2019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The complete genome sequences of the P. mirabilis isolates have been deposited at the National Center for Biotechnology Information (NCBI) under accession no. JAFHGA01 for Survcare357, JAFHGO01 for Survcare372, and CP177077-CP177079 for Survcare401, within the Bioproject accession no. PRJNA692829.
Acknowledgments
We would like to thank the hospitals in Frankfurt/M., Heppenheim, and Lampertheim for providing the isolates, Anja M. Hauri and Petra Heimüller for their support in collecting the isolates, Christina Gerstmann and Laura Hildebrand for their excellent technical support, and Linda Falgenhauer for valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8, 1. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Grimont, P.A.D.; Grimont, F.; Lefevre, M.; Giammanco, G.; Pignato, S. Phylogenetic analysis of the genera Proteus, Morganella and Providencia by comparison of rpoB gene sequences of type and clinical strains suggests the reclassification of Proteus myxofaciens in a new genus, Cosenzaea gen. nov., as Cosenzaea myxofaciens comb. nov. Int. J. Syst. Evol. Microbiol. 2011, 61, 1638–1644. [Google Scholar]
- Pearson, M.M.; Sebaihia, M.; Churcher, C.; Quail, M.A.; Seshasayee, A.S.; Luscombe, N.M.; Abdellah, Z.; Arrosmith, C.; Atkin, B.; Chillingworth, T.; et al. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J. Bacteriol. 2008, 190, 4027–4037. [Google Scholar] [CrossRef]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and Urinary Tract Infections. In Urinary Tract Infections: Molecular Pathogenesis and Clinical Management; Wiley: Hoboken, NJ, USA, 2015; Volume 3. [Google Scholar]
- Stock, I. Natural antibiotic susceptibility of Proteus spp., with special reference to P. mirabilis and P. penneri strains. J. Chemother. 2003, 15, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Bonnin, R.A.; Dortet, L.; Naas, T. Genetics of Acquired Antibiotic Resistance Genes in Proteus spp. Front. Microbiol. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Vourli, S.; Tsorlini, H.; Katsifa, H.; Polemis, M.; Tzouvelekis, L.S.; Kontodimou, A.; Vatopoulos, A.C. Emergence of Proteus mirabilis carrying the blaVIM-1 metallo-β-lactamase gene. Clin. Microbiol. Infect. 2006, 12, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Markovska, R.; Schneider, I.; Keuleyan, E.; Ivanova, D.; Lesseva, M.; Stoeva, T.; Sredkova, M.; Bauernfeind, A.; Mitov, I. Dissemination of a multidrug-resistant VIM-1- and CMY-99-producing Proteus mirabilis clone in Bulgaria. Microb. Drug Resist. 2017, 23, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. Bericht des Nationalen Referenzzentrums für Gramnegative Krankenhauserreger; Robert Koch-Institut: Berlin, Germany, 2023. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Yao, Y.; Imirzalioglu, C.; Falgenhauer, L.; Falgenhauer, J.; Heinmüller, P.; Domann, E.; Chakraborty, T. Plasmid-Mediated Spread of Carbapenem Resistance in Enterobacterales: A Three-Year Genome-Based Survey. Antibiotics 2024, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Doijad, S.; Falgenhauer, J.; Schmiedel, J.; Imirzalioglu, C.; Chakraborty, T. Co-occurrence of dual carbapenemases KPC-2 and OXA-48 with the mobile colistin resistance gene mcr-9.1 in Enterobacter xiangfangensis. Front. Cell. Infect. Microbiol. 2022, 12, 960892. [Google Scholar] [CrossRef] [PubMed]
- Schwengers, O.; Hoek, A.; Fritzenwanker, M.; Falgenhauer, L.; Hain, T.; Chakraborty, T.; Goesmann, A. ASA3P: An automatic and scalable pipeline for the assembly, annotation and higher level analysis of closely related bacterial isolates. PLoS Comput. Biol. 2020, 16, e1007134. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)–structure and function. J. Enzyme Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Liu, M.; Tai, C.; Sun, J.; Deng, Z.; Ou, H.Y. OriTfinder: A web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 2018, 46, W229–W234. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Tsafnat, G. Automated annotation of mobile antibiotic resistance in Gram-negative bacteria: The Multiple Antibiotic Resistance Annotator (MARA) and database. J. Antimicrob. Chemother. 2018, 73, 883–890. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-Metallo-β-Lactamases: Where Do We Stand? Curr. Drug Targets 2015, 17, 1029–1050. [Google Scholar] [CrossRef]
- Fuchs, F.; Ahmadzada, A.; Plambeck, L.; Wille, T.; Hamprecht, A. Susceptibility of Clinical Enterobacterales Isolates With Common and Rare Carbapenemases to Mecillinam. Front. Microbiol. 2021, 11, 627267. [Google Scholar] [CrossRef] [PubMed]
- Heiden, S.E.; Sydow, K.; Schaefer, S.; Klempien, I.; Balau, V.; Bauer, P.; Hübner, N.O.; Schaufler, K. Nearly identical plasmids encoding vim-1 and mercury resistance in enterobacteriaceae from north-eastern germany. Microorganisms 2021, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Campos-Madueno, E.I.; Sigrist, T.; Flückiger, U.M.; Risch, L.; Bodmer, T.; Endimiani, A. First report of a blaVIM-1 metallo-β-lactamase-possessing Klebsiella michiganensis. J. Glob. Antimicrob. Resist. 2021, 25, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Papagiannitsis, C.C.; Miriagou, V.; Kotsakis, S.D.; Tzelepi, E.; Vatopoulos, A.C.; Petinaki, E.; Tzouvelekis, L.S. Characterization of a transmissible plasmid encoding VEB-1 and VIM-1 in Proteus mirabilis. Antimicrob. Agents Chemother. 2012, 56, 4024–4025. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.; Voets, G.M.; Scharringa, J.; Voskuil, S.; Fluit, A.C.; Rottier, W.C.; Leverstein-Van Hall, M.A.; Cohen Stuart, J.W.T. A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin. Microbiol. Infect. 2014, 20, 345–349. [Google Scholar] [CrossRef]
- Protonotariou, E.; Poulou, A.; Politi, L.; Meletis, G.; Chatzopoulou, F.; Malousi, A.; Metallidis, S.; Tsakris, A.; Skoura, L. Clonal outbreak caused by VIM-4-producing Proteus mirabilis in a Greek tertiary-care hospital. Int. J. Antimicrob. Agents 2020, 56, 106060. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, S.; Compain, F.; Barraud, O.; Ouédraogo, A.S.; Bouzinbi, N.; Vittecoq, M.; Jean-Pierre, H.; Decré, D.; Godreuil, S. Non-O1/non-O139 Vibrio cholerae avian isolate from France cocarrying the blaVIM-1 and blaVIM-4 genes. Antimicrob. Agents Chemother. 2015, 59, 6594–6596. [Google Scholar] [CrossRef] [PubMed]
- Bontron, S.; Poirel, L.; Kieffer, N.; Savov, E.; Trifonova, A.; Todorova, I.; Kueffer, G.; Nordmann, P. Increased resistance to carbapenems in Proteus mirabilis mediated by amplification of the blaVIM-1-carrying and IS26-associated class 1 integron. Microb. Drug Resist. 2019, 25, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, Y.S.; Park, Y.K.; Kim, B.S. Alterations in the GyrA and GyrB subunits of topoisomerase II and the ParC and ParE subunits of topoisomerase IV in ciprofloxacin-resistant clinical isolates of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2005, 25, 290–295. [Google Scholar] [CrossRef]
- Varughese, L.R.; Rajpoot, M.; Goyal, S.; Mehra, R.; Chhokar, V.; Beniwal, V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PLoS ONE 2018, 13, e0190729. [Google Scholar] [CrossRef] [PubMed]
- Abdelkreem, R.H.; Yousuf, A.M.; Elmekki, M.A.; Elhassan, M.M. Dna gyrase and topoisomerase iv mutations and their effect on quinolones resistant Proteus mirabilis among utis patients. Pak. J. Med. Sci. 2020, 36, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Sato, K.; Kumita, W.; Inami, N.; Nishiyama, H.; Okamura, N.; Moriya, K.; Koike, K. Mutations of DNA gyrase and topoisomerase IV in clinical isolates of fluoroquinolone-resistant Proteus mirabilis. Jpn. J. Antibiot. 2006, 59, 41–43. [Google Scholar] [PubMed]
- Bitar, I.; Marchetti, V.M.; Mercato, A.; Nucleo, E.; Anesi, A.; Bracco, S.; Rognoni, V.; Hrabak, J.; Migliavacca, R. Complete genome and plasmids sequences of a clinical Proteus mirabilis isolate producing plasmid mediated ndm-1 from Italy. Microorganisms 2020, 8, 339. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).