From Dysbiosis to Hepatic Inflammation: A Narrative Review on the Diet-Microbiota-Liver Axis in Steatotic Liver Disease
Abstract
1. Introduction
2. Methodology
3. The Gut-Liver Axis
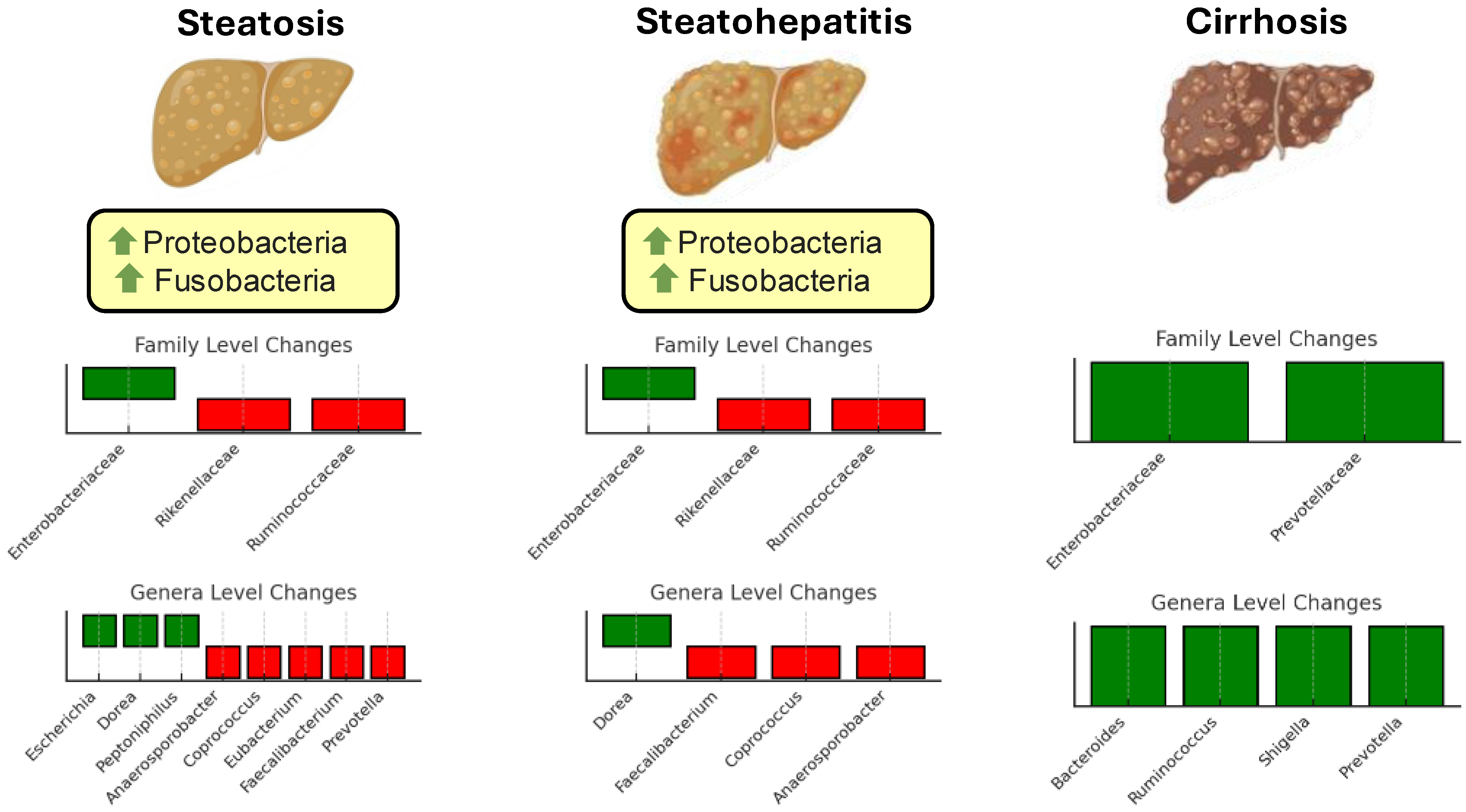
4. Gut Microbiota Signature in Health and Steatotic Liver Disease
5. Mechanistic Links Between Microbiota and MASLD
6. Dietary Interventions and Microbiota
6.1. Western Diet
6.2. Mediterranean Diet
6.3. Other Dietary Patterns and Their Potential Impacts on Gut Microbiota and Liver Steatosis
7. Microbiota-Based Therapeutics
8. Challenges and Knowledge Gaps
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacke, F.; Horn, P.; Wai-Sun Wong, V.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.Y. Steatotic Liver Disease: Know Your Enemies. Clin. Mol. Hepatol. 2024, 30, 171. [Google Scholar] [CrossRef]
- Staufer, K.; Stauber, R.E. Steatotic Liver Disease: Metabolic Dysfunction, Alcohol, or Both? Biomedicines 2023, 11, 2108. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.; Zhang, X. Non-Alcoholic Fatty Liver Disease: An Expanded Review. World J. Hepatol. 2017, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023, 78, 1966. [Google Scholar] [CrossRef]
- Pasta, A.; Borro, P.; Cremonini, A.L.; Formisano, E.; Tozzi, G.; Cecchi, S.; Fresa, R.; Labanca, S.; Djahandideh, A.; Sukkar, S.G.; et al. Effect of a Common Missense Variant in LIPA Gene on Fatty Liver Disease and Lipid Phenotype: New Perspectives from a Single-center Observational Study. Pharmacol. Res. Perspect. 2021, 9, e00820. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Rotman, Y.; Valenti, L. Genetics of Metabolic Dysfunction-Associated Steatotic Liver Disease: The State of the Art Update. Clin. Gastroenterol. Hepatol. 2024, 22, 2177–2187.e3. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, J.M.; Lu, X.L.; Lin, X.Y.; Hong, M.Z.; Weng, S.; Pan, J.S. Global Burden of Adult Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) Has Been Steadily Increasing over the Past Decades and Is Expected to Persist in the Future. Transl. Gastroenterol. Hepatol. 2024, 9, 33. [Google Scholar] [CrossRef]
- Israelsen, M.; Francque, S.; Tsochatzis, E.A.; Krag, A. Steatotic Liver Disease. Lancet 2024, 404, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Zoncapè, M.; Liguori, A.; Tsochatzis, E.A. Multi-Disciplinary Clinic Models for the Management of Non-Alcoholic Fatty Liver Disease. Hepatobiliary Surg. Nutr. 2022, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.M.; Zhang, L.L.; Li, J.M.; Lv, W.L. The Role of Gut Microbiota in Some Liver Diseases: From an Immunological Perspective. Front. Immunol. 2022, 13, 923599. [Google Scholar] [CrossRef]
- Paolella, G.; Mandato, C.; Pierri, L.; Poeta, M.; Di Stasi, M.; Vajro, P. Gut-Liver Axis and Probiotics: Their Role in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 15518. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. Int. J. Biol. Sci. 2021, 17, 818. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2023, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Tan, D.E.L.; Sikka, V.; Ng, C.H.; Xian, Y.; Li, D.; Muthiah, M.; Chew, N.W.S.; Storm, G.; et al. Gut-liver Axis: Potential Mechanisms of Action of Food-derived Extracellular Vesicles. J. Extracell. Vesicles 2024, 13, e12466. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal Mucus Components and Secretion Mechanisms: What We Do and Do Not Know. Exp. Mol. Med. 2023, 55, 681. [Google Scholar] [CrossRef]
- Grüner, N.; Mattner, J. Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver–Gut Axis. Int. J. Mol. Sci. 2021, 22, 1397. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, Z.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Effect of Different Bile Acids on the Intestine through Enterohepatic Circulation Based on FXR. Gut Microbes 2021, 13, 1949095. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Pitcher, M.J.; Dionisi, C.; Spencer, J. Gut-Associated Lymphoid Tissue: A Microbiota-Driven Hub of B Cell Immunity. Trends Immunol. 2024, 45, 211. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The Gut–Liver Axis and Gut Microbiota in Health and Liver Disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; O’Toole, P.W. Disease-Associated Microbiome Signature Species in the Gut. Proc. Natl. Acad. Sci. USA Nexus 2024, 3, pgae352. [Google Scholar] [CrossRef] [PubMed]
- Knights, D.; Parfrey, L.W.; Zaneveld, J.; Lozupone, C.; Knight, R. Human-Associated Microbial Signatures: Examining Their Predictive Value. Cell Host Microbe 2011, 10, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D.; Petifils, C.; De Vos, W.M.; Tilg, H.; El Omar, E.M. What Defines a Healthy Gut Microbiome? Gut 2024, 73, e333378. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic Gut Microbiota Dysbiosis as an Inflammaging and Immunosenescence Condition That Fosters Progression of Retinopathy and Nephropathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Long, Q.; Luo, F.; Li, B.; Li, Z.; Guo, Z.; Chen, Z.; Wu, W.; Hu, M. Gut Microbiota and Metabolic Biomarkers in Metabolic Dysfunction–Associated Steatotic Liver Disease. Hepatol. Commun. 2024, 8, e0310. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yan, Z.; Zhong, H.; Luo, R.; Liu, W.; Xiong, S.; Liu, Q.; Liu, M. Gut Microbial Metabolites in MASLD: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Hepatol. Commun. 2024, 8, e0484. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Pietzner, M.; Bang, C.; Franke, A.; Nauck, M.; Völker, U.; Völzke, H.; Dörr, M.; et al. Long-Term Instability of the Intestinal Microbiome Is Associated with Metabolic Liver Disease, Low Microbiota Diversity, Diabetes Mellitus and Impaired Exocrine Pancreatic Function. Gut 2020, 70, 522. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, J.; Xu, X.; Xu, W.; Tong, B.; Wang, S.; Ji, R.; Tan, Y.; Zhu, Y. Characteristics of Gut Microbiota in Patients with Metabolic Associated Fatty Liver Disease. Sci. Rep. 2023, 13, 9988. [Google Scholar] [CrossRef]
- Lelouvier, B.; Servant, F.; Païssé, S.; Brunet, A.C.; Benyahya, S.; Serino, M.; Valle, C.; Ortiz, M.R.; Puig, J.; Courtney, M.; et al. Changes in Blood Microbiota Profiles Associated with Liver Fibrosis in Obese Patients: A Pilot Analysis. Hepatology 2016, 64, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Sáenz de Miera, L.E.; Ballesteros Pomar, M.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Álvarez-Cuenllas, B.; Linares, P.; Olcoz, J.L.; Arias-Loste, M.T.; García-Lobo, J.M.; et al. An Altered Fecal Microbiota Profile in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) Associated with Obesity. Rev. Esp. Enfermedades Dig. 2019, 111, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Chuaypen, N.; Asumpinawong, A.; Sawangsri, P.; Khamjerm, J.; Iadsee, N.; Jinato, T.; Sutheeworapong, S.; Udomsawaengsup, S.; Tangkijvanich, P. Gut Microbiota in Patients with Non-Alcoholic Fatty Liver Disease without Type 2 Diabetes: Stratified by Body Mass Index. Int. J. Mol. Sci. 2024, 25, 1807. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Yang, X.; Xie, Y.; Zhou, J.; Wang, Q.; Wei, Y.; Yang, Y.; Lu, D.; Ye, L.; Cui, P.; et al. The Role of Gut Microbiota in the Occurrence and Progression of Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2023, 14, 1257903. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional Alterations of Gut Microbiota in Nonalcoholic Fatty Liver Disease Patients: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Schirmer, M.; Franzosa, E.A.; Lloyd-Price, J.; McIver, L.J.; Schwager, R.; Poon, T.W.; Ananthakrishnan, A.N.; Andrews, E.; Barron, G.; Lake, K.; et al. Dynamics of Metatranscription in the Inflammatory Bowel Disease Gut Microbiome. Nat. Microbiol. 2018, 3, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Cocconi, D.; van Sinderen, D.; Ventura, M. Identification of Universal Gut Microbial Biomarkers of Common Human Intestinal Diseases by Meta-Analysis. FEMS Microbiol. Ecol. 2017, 93, 153. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.H.; Peddu, D.; Amin, S.; Elsaid, M.I.; Minacapelli, C.D.; Chandler, T.M.; Catalano, C.; Rustgi, V.K. Nonalcoholic Fatty Liver Disease in Lean/Nonobese and Obese Individuals: A Comprehensive Review on Prevalence, Pathogenesis, Clinical Outcomes, and Treatment. J. Clin. Transl. Hepatol. 2022, 11, 502. [Google Scholar] [CrossRef]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus Spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic Review of the Effects of the Intestinal Microbiota on Selected Nutrients and Non-Nutrients. Eur. J. Nutr. 2017, 57, 25. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; He, Z.; Li, H. Bacteroides and NAFLD: Pathophysiology and Therapy. Front. Microbiol. 2024, 15, 1288856. [Google Scholar] [CrossRef]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus Gnavus: Friend or Foe for Human Health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Yan, S.; Chen, J.; Zhang, Y.; Wang, Y.; Qin, Q.; Li, W.; Li, T.; Huang, M.; Ding, S.; et al. Gut Microbiome Is Associated with Metabolic Syndrome Accompanied by Elevated Gamma-Glutamyl Transpeptidase in Men. Front. Cell. Infect. Microbiol. 2022, 12, 946757. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.X.; et al. Regional Variation Limits Applications of Healthy Gut Microbiome Reference Ranges and Disease Models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Qi, X.; Yang, M.; Stenberg, J.; Dey, R.; Fogwe, L.; Alam, M.S.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Gut Microbiota Mediated Molecular Events and Therapy in Liver Diseases. World J. Gastroenterol. 2020, 26, 7603. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms Underlying the Resistance to Diet-Induced Obesity in Germ-Free Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Wu, C.Y. The Gut Microbiome in Obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025. [Google Scholar] [CrossRef]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015, 4, 250. [Google Scholar] [CrossRef] [PubMed]
- Pasta, A.; Formisano, E.; Calabrese, F.; Plaz Torres, M.C.; Bodini, G.; Marabotto, E.; Pisciotta, L.; Giannini, E.G.; Furnari, M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients 2024, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Davids, M.; Herrema, H.; Aydin, O.; Tremaroli, V.; Rios-Morales, M.; Levels, H.; Bruin, S.; de Brauw, M.; Verheij, J.; et al. Microbiome-Derived Ethanol in Nonalcoholic Fatty Liver Disease. Nat. Med. 2022, 28, 2100–2106. [Google Scholar] [CrossRef]
- Radun, R.; Trauner, M. Role of FXR in Bile Acid and Metabolic Homeostasis in NASH: Pathogenetic Concepts and Therapeutic Opportunities. Semin. Liver Dis. 2021, 41, 461. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of Farnesoid X Receptor and Its Role in Bile Acid Metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef] [PubMed]
- Maliha, S.; Guo, G.L. Farnesoid X Receptor and Fibroblast Growth Factor 15/19 as Pharmacological Targets. Liver Res. 2021, 5, 142–150. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Dore, M.P.; Simonelli, C.; Newton, E.E.; Cammarota, G. Gut Microbiota as a Driver of Inflammation in Nonalcoholic Fatty Liver Disease. Mediat. Inflamm. 2018, 2018, 9321643. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2021, 26, 671. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Chu, H.; Duan, Y.; Schnabl, B. Gut Microbiota in Liver Disease: Too Much Is Harmful, Nothing at All Is Not Helpful Either. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G563–G573. [Google Scholar] [CrossRef] [PubMed]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Forlano, R.; Sivakumar, M.; Mullish, B.H.; Manousou, P. Gut Microbiota-A Future Therapeutic Target for People with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Kang, G.G.; Trevaskis, N.L.; Murphy, A.J.; Febbraio, M.A. Diet-Induced Gut Dysbiosis and Inflammation: Key Drivers of Obesity-Driven NASH. iScience 2022, 26, 105905. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving Healthy Aging through Gut Microbiota-Directed Dietary Intervention: Focusing on Microbial Biomarkers and Host Mechanisms. J. Adv. Res. 2024, 68, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Inci, M.K.; Park, S.H.; Helsley, R.N.; Attia, S.L.; Softic, S. Fructose Impairs Fat Oxidation: Implications for the Mechanism of Western Diet-Induced NAFLD. J. Nutr. Biochem. 2023, 114, 109224. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Scott Rector, R.; Staveley-O’Carroll, K.F.; et al. Western Diet Contributes to the Pathogenesis of Non-Alcoholic Steatohepatitis in Male Mice via Remodeling Gut Microbiota and Increasing Production of 2-Oleoylglycerol. Nat. Commun. 2023, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and Disease Consequences of Nonalcoholic Fatty Liver Disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.d.S.M.d.; Mota, A.C.C.C.; Carvalho, M.C.d.C.; Andrade, E.D.d.O.; Oliveira, É.P.S.F.d.; Galvão, L.L.P.; Maciel, B.L.L. Can Diet Alter the Intestinal Barrier Permeability in Healthy People? A Systematic Review. Nutrients 2024, 16, 1871. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The Microbiome-Driven Impact of Western Diet in the Development of Noncommunicable Chronic Disorders. Best Pract. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef]
- Simas, A.M.; Kramer, C.D.; Genco, C.A. Diet-Induced Non-Alcoholic Fatty Liver Disease and Associated Gut Dysbiosis Are Exacerbated by Oral Infection. Front. Oral Health 2022, 2, 784448. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Liu, Y.; Chopyk, D.M.; Rai, R.P.; Desai, C.; Kumar, P.; Farris, A.B.; Nusrat, A.; Parkos, C.A.; Anania, F.A.; et al. Western Diet-Induced Increase in Colonic Bile Acids Compromises Epithelial Barrier in Nonalcoholic Steatohepatitis. FASEB J. 2020, 34, 7089–7102. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Olayinka, O.T.; Fr, J.; Nisar, M.R.; Kotha, R.; Saad-Omer, S.I.; Nath, T.S. Food Additives’ Impact on Gut Microbiota and Metabolic Syndrome: A Systematic Review. Cureus 2024, 16, e66822. [Google Scholar] [CrossRef]
- Bahitham, W.; Alghamdi, S.; Omer, I.; Alsudais, A.; Hakeem, I.; Alghamdi, A.; Abualnaja, R.; Sanai, F.M.; Rosado, A.S.; Sergi, C.M. Double Trouble: How Microbiome Dysbiosis and Mitochondrial Dysfunction Drive Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Biomedicines 2024, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Olivia Maurine Jasirwan, C.; Muradi, A.; Hasan, I.; Simadibrata, M.; Rinaldi, I. Correlation of Gut Firmicutes/Bacteroidetes Ratio with Fibrosis and Steatosis Stratified by Body Mass Index in Patients with Non-Alcoholic Fatty Liver Disease. Biosci. Microbiota Food Health 2020, 40, 50. [Google Scholar] [CrossRef]
- Cogorno, L.; Formisano, E.; Vignati, A.; Prigione, A.; Tramacere, A.; Borgarelli, C.; Sukkar, S.G.; Pisciotta, L. Non-Alcoholic Fatty Liver Disease: Dietary and Nutraceutical Approaches. Liver Res. 2023, 7, 216–227. [Google Scholar] [CrossRef]
- Anania, C.; Massimo Perla, F.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean Diet and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2018, 24, 2083. [Google Scholar] [CrossRef] [PubMed]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The Effectiveness and Acceptability of Mediterranean Diet and Calorie Restriction in Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zou, J.; Ran, W.; Qi, X.; Chen, Y.; Cui, H.; Guo, J. Advancements in the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD). Front. Endocrinol. 2023, 13, 1087260. [Google Scholar] [CrossRef]
- Pasta, A.; Formisano, E.; Cremonini, A.L.; Maganza, E.; Parodi, E.; Piras, S.; Pisciotta, L. Diet and Nutraceutical Supplementation in Dyslipidemic Patients: First Results of an Italian Single Center Real-World Retrospective Analysis. Nutrients 2020, 12, 2056. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The Effect of Mediterranean Diet on Metabolic Syndrome and Its Components: A Meta-Analysis of 50 Studies and 534,906 Individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Formisano, E.; Pasta, A.; Cremonini, A.L.; Di Lorenzo, I.; Sukkar, S.G.; Pisciotta, L. Effects of a Mediterranean Diet, Dairy, and Meat Products on Different Phenotypes of Dyslipidemia: A Preliminary Retrospective Analysis. Nutrients 2021, 13, 1161. [Google Scholar] [CrossRef]
- Georgoulis, M.; Damigou, E.; Derdelakou, E.; Kosti, R.I.; Chrysohoou, C.; Barkas, F.; Kravvariti, E.; Tsioufis, C.; Pitsavos, C.; Liberopoulos, E.; et al. Adherence to the Mediterranean Diet and 20-Year Incidence of Hypertension: The ATTICA Prospective Epidemiological Study (2002–2022). Eur. J. Clin. Nutr. 2024, 78, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Moorthy, M.V.; Demler, O.V.; Hu, F.B.; Ridker, P.M.; Chasman, D.I.; Mora, S. Assessment of Risk Factors and Biomarkers Associated with Risk of Cardiovascular Disease Among Women Consuming a Mediterranean Diet. JAMA Netw. Open 2018, 1, e185708. [Google Scholar] [CrossRef]
- Maestri, M.; Santopaolo, F.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota Modulation in Patients with Non-Alcoholic Fatty Liver Disease: Effects of Current Treatments and Future Strategies. Front. Nutr. 2023, 10, 1110536. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Victoria Moreno-Arribas, M. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Li, X.Y.; Shen, L. Modulation Effect of Tea Consumption on Gut Microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.M.; Celano, G.; Bonfiglio, C.; Campanella, A.; Franco, I.; Annunziato, A.; Giannelli, G.; Osella, A.R.; De Angelis, M. Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation. Nutrients 2023, 15, 2457. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, A.M.; Ruiz-Limón, P.; Salas-Salvadó, J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Atzeni, A.; Torres-Collado, L.; Álvarez-Sala, A.; et al. Gut Microbiota in Nonalcoholic Fatty Liver Disease: A PREDIMED-Plus Trial Sub Analysis. Gut Microbes 2023, 15, 2223339. [Google Scholar] [CrossRef] [PubMed]
- Khavandegar, A.; Heidarzadeh, A.; Angoorani, P.; Hasani-Ranjbar, S.; Ejtahed, H.S.; Larijani, B.; Qorbani, M. Adherence to the Mediterranean Diet Can Beneficially Affect the Gut Microbiota Composition: A Systematic Review. BMC Med. Genom. 2024, 17, 91. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Gerber, P.A. Fructose Drives de Novo Lipogenesis Affecting Metabolic Health. J. Endocrinol. 2023, 257, e220270. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.J. Effect of Low Carbohydrate Diets on Insulin Resistance and the Metabolic Syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 463. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.A.; Mousavi, S.N.; Chiti, H. A Low-Carb Diet Increases Fecal Short-Chain Fatty Acids in Feces of Obese Women Following a Weight-Loss Program: Randomized Feeding Trial. Sci. Rep. 2023, 13, 18146. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial Effects of the Ketogenic Diet on Nonalcoholic Fatty Liver Disease: A Comprehensive Review of the Literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a Ketogenic Diet on Hepatic Steatosis and Hepatic Mitochondrial Metabolism in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [PubMed]
- Sowah, S.A.; Riedl, L.; Damms-Machado, A.; Johnson, T.S.; Schübel, R.; Graf, M.; Kartal, E.; Zeller, G.; Schwingshackl, L.; Stangl, G.I.; et al. Effects of Weight-Loss Interventions on Short-Chain Fatty Acid Concentrations in Blood and Feces of Adults: A Systematic Review. Adv. Nutr. 2019, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of Caloric Restriction on Gut Permeability, Inflammation Markers, and Fecal Microbiota in Obese Women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Bird, A.R. Comparative Effects of Very Low-Carbohydrate, High-Fat and High-Carbohydrate, Low-Fat Weight-Loss Diets on Bowel Habit and Faecal Short-Chain Fatty Acids and Bacterial Populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajuox, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar] [CrossRef]
- Li, H.Y.; Gan, R.Y.; Shang, A.; Mao, Q.Q.; Sun, Q.C.; Wu, D.T.; Geng, F.; He, X.Q.; Li, H. Bin Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxid. Med. Cell. Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Xue, X.; Al Naggar, Y.; Wu, L.; Wang, K. Understanding the Gastrointestinal Protective Effects of Polyphenols Using Foodomics-Based Approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A Six-Day, Lifestyle-Based Immersion Program Mitigates Cardiovascular Risk Factors and Induces Shifts in Gut Microbiota, Specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium Prausnitzii: A Pilot Study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects with Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef] [PubMed]
- Castelnuovo, G.; Perez-Diaz-del-Campo, N.; Rosso, C.; Armandi, A.; Caviglia, G.P.; Bugianesi, E. A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2024, 16, 2027. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Hanhineva, K. Biomarkers of a Healthy Nordic Diet—From Dietary Exposure Biomarkers to Microbiota Signatures in the Metabolome. Nutrients 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Tertsunen, H.M.; Hantunen, S.; Tuomainen, T.P.; Virtanen, J.K. Adherence to a Healthy Nordic Diet and Risk of Type 2 Diabetes among Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Nutr. 2021, 60, 3927. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Lv, R.; Zou, H.; Zou, T. Beneficial Effects of Intermittent Fasting on Nonalcoholic Fatty Liver Disease: A Narrative Review. Egypt. Liver J. 2024, 14, 63. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Sun, Y.; Zhang, X. Intermittent Fasting and Physical Exercise for Preventing Metabolic Disorders through Interaction with Gut Microbiota: A Review. Nutrients 2023, 15, 2277. [Google Scholar] [CrossRef]
- Paukkonen, I.; Törrönen, E.N.; Lok, J.; Schwab, U.; El-Nezami, H. The Impact of Intermittent Fasting on Gut Microbiota: A Systematic Review of Human Studies. Front. Nutr. 2024, 11, 1342787. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, C.M.; Bruno, A.; Ma, C.; Raman, M. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: A Narrative Review. Nutrients 2022, 14, 4655. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, Prebiotics, and Postbiotics in Health and Disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Schnabl, B.; Knight, R.; Loomba, R. Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease. Cell Metab. 2021, 33, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium Difficile Infection with Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [Google Scholar] [CrossRef]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P.; et al. Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals with Steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients with Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Gan, L.; Feng, Y.; Du, B.; Fu, H.; Tian, Z.; Xue, G.; Yan, C.; Cui, X.; Zhang, R.; Cui, J.; et al. Bacteriophage Targeting Microbiota Alleviates Non-Alcoholic Fatty Liver Disease Induced by High Alcohol-Producing Klebsiella Pneumoniae. Nat. Commun. 2023, 14, 3215. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Govender, P.; Ghai, M. Population-Specific Differences in the Human Microbiome: Factors Defining the Diversity. Gene 2025, 933, 148923. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Elie, C.; Perret, M.; Hage, H.; Sentausa, E.; Hesketh, A.; Louis, K.; Fritah-Lafont, A.; Leissner, P.; Vachon, C.; Rostaing, H.; et al. Comparison of DNA Extraction Methods for 16S RRNA Gene Sequencing in the Analysis of the Human Gut Microbiome. Sci. Rep. 2023, 13, 10279. [Google Scholar] [CrossRef]
- Sarangi, A.N.; Goel, A.; Aggarwal, R. Methods for Studying Gut Microbiota: A Primer for Physicians. J. Clin. Exp. Hepatol. 2019, 9, 62–73. [Google Scholar] [CrossRef]
- McDermaid, A.; Monier, B.; Zhao, J.; Liu, B.; Ma, Q. Interpretation of Differential Gene Expression Results of RNA-Seq Data: Review and Integration. Brief. Bioinform. 2019, 20, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D. Comparison Study of Differential Abundance Testing Methods Using Two Large Parkinson Disease Gut Microbiome Datasets Derived from 16S Amplicon Sequencing. BMC Bioinform. 2021, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Lappa, D.; Herrema, H.; Aydin, O.; Krautkramer, K.A.; Tremaroli, V.; Olofsson, L.E.; Lundqvist, A.; Bruin, S.; Acherman, Y.; et al. A Systems Biology Approach to Study Non-Alcoholic Fatty Liver (NAFL) in Women with Obesity. iScience 2022, 25, 104828. [Google Scholar] [CrossRef]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the Gut-Liver Axis in Liver Disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasta, A.; Formisano, E.; Calabrese, F.; Marabotto, E.; Furnari, M.; Bodini, G.; Torres, M.C.P.; Pisciotta, L.; Giannini, E.G.; Zentilin, P. From Dysbiosis to Hepatic Inflammation: A Narrative Review on the Diet-Microbiota-Liver Axis in Steatotic Liver Disease. Microorganisms 2025, 13, 241. https://doi.org/10.3390/microorganisms13020241
Pasta A, Formisano E, Calabrese F, Marabotto E, Furnari M, Bodini G, Torres MCP, Pisciotta L, Giannini EG, Zentilin P. From Dysbiosis to Hepatic Inflammation: A Narrative Review on the Diet-Microbiota-Liver Axis in Steatotic Liver Disease. Microorganisms. 2025; 13(2):241. https://doi.org/10.3390/microorganisms13020241
Chicago/Turabian StylePasta, Andrea, Elena Formisano, Francesco Calabrese, Elisa Marabotto, Manuele Furnari, Giorgia Bodini, Maria Corina Plaz Torres, Livia Pisciotta, Edoardo Giovanni Giannini, and Patrizia Zentilin. 2025. "From Dysbiosis to Hepatic Inflammation: A Narrative Review on the Diet-Microbiota-Liver Axis in Steatotic Liver Disease" Microorganisms 13, no. 2: 241. https://doi.org/10.3390/microorganisms13020241
APA StylePasta, A., Formisano, E., Calabrese, F., Marabotto, E., Furnari, M., Bodini, G., Torres, M. C. P., Pisciotta, L., Giannini, E. G., & Zentilin, P. (2025). From Dysbiosis to Hepatic Inflammation: A Narrative Review on the Diet-Microbiota-Liver Axis in Steatotic Liver Disease. Microorganisms, 13(2), 241. https://doi.org/10.3390/microorganisms13020241








