The Antimicrobial Effects of Coffee and By-Products and Their Potential Applications in Healthcare and Agricultural Sectors: A State-of-Art Review
Abstract
1. Introduction
2. Classification of Active Compounds in Coffee and Their Biological Effect
2.1. Phenolic Acids
2.2. Alkaloids
2.3. Terpens
3. Chemical Composition of Coffee Extracts and By-Products
- Coffee By-Products
4. Antimicrobial Activities of Coffee and Its By-Products
4.1. Antibacterial Activity of Coffee and Its By-Products
4.2. Antifungal Activity of Coffee and Its By-Products
4.3. Antiviral Activity of Coffee and Its By-Products
5. Regarding the Future of Coffee Residues as a Sustainable Alternative for Antimicrobials Used in Agronomic Practices
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freitas, V.V.; Borges, L.L.R.; Vidigal, M.C.T.R.; dos Santos, M.H.; Stringheta, P.C. Coffee: A comprehensive overview of origin, market, and the quality process. Trends Food Sci. Technol. 2024, 146, 104411. [Google Scholar] [CrossRef]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nunez, O.; Fiorini, D.; Giardinieri, A.; Frega, N.G.; Pacetti, D. Spent espresso coffee grounds as a source of anti-proliferative and antioxidant compounds. Innov. Food Sci. Emerg. Technol. 2020, 59, 102254. [Google Scholar] [CrossRef]
- Viencz, T.; Acre, L.B.; Rocha, R.B.; Alves, E.A.; Ramalho, A.R.; Benassi, M.d.T. Caffeine, trigonelline, chlorogenic acids, melanoidins, and diterpenes contents of Coffea canephora coffees produced in the Amazon. J. Food Compos. Anal. 2023, 117. [Google Scholar] [CrossRef]
- Bondam, A.F.; Diolinda da Silveira, D.; Pozzada dos Santos, J.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Gallardo-Ignacio, J.; Santibáñez, A.; Oropeza-Mariano, O.; Salazar, R.; Montiel-Ruiz, R.M.; Cabrera-Hilerio, S.; Gonzáles-Cortazar, M.; Cruz-Sosa, F.; Nicasio-Torres, P. Chemical and Biological Characterization of Green and Processed Coffee Beans from Coffea arabica Varieties. Molecules 2023, 28, 4685. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Chaves-Ulate, C.; Rodríguez-Sánchez, C.; Arias-Echandi, M.L.; Esquivel, P. Antimicrobial activities of phenolic extracts of coffee mucilage. NFS J. 2023, 31, 50–56. [Google Scholar] [CrossRef]
- Yosboonruang, A.; Ontawong, A.; Thapmamang, J.; Duangjai, A. Antibacterial Activity of Coffea robusta Leaf Extract against Foodborne Pathogens. J. Microbiol. Biotechnol. 2022, 32, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Tasew, T.; Mekonnen, Y.; Gelana, T.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E.; Mohammed, A.M.; Mamo, H. In Vitro Antibacterial and Antioxidant Activities of Roasted and Green Coffee Beans Originating from Different Regions of Ethiopia. Int. J. Food Sci. 2020, 2020, 8490492. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Li, Y.-C.; Peng, S.-L.; Chen, C.-Y.; Chen, H.-F.; Hsueh, P.-R.; Wang, W.-J.; Liu, Y.-Y.; Jiang, C.-L.; Chang, W.-C.; et al. Coffee as a dietary strategy to prevent SARS-CoV-2 infection. Cell Biosci. 2023, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, S.; Suliman, R.; Idriss, M.T.; Aloufi, A.S.; Alolayan, E.; Awadalla, M.; Aodah, A.; Asab, O.A.; Al-Qahtani, J.; Mohmmed, N.; et al. In vivo Evaluation of the Antiviral Effects of Arabian coffee (Coffea arabica) and Green Tea (Camellia sinensis) Extracts on Influenza A Virus. Int. J. Biomed. 2023, 13, 154–161. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Lestari, D.; Khairul Ikram, N.K.; Gazzali, A.M.; Hariono, M.; Wahab, H.A. Decaffeination and Neuraminidase Inhibitory Activity of Arabica Green Coffee (Coffea arabica) Beans: Chlorogenic Acid as a Potential Bioactive Compound. Molecules 2021, 26, 3402. [Google Scholar] [CrossRef] [PubMed]
- Simon-Gruita, A.; Pojoga, M.D.; Constantin, N.; Duta-Cornescu, G.; Grumezescu, A.M.; Holban, A.M. 14—Genetic Engineering in Coffee. In Caffeinated and Cocoa Based Beverages; Woodhead Publishing: Cambridge, UK, 2019; pp. 447–488. [Google Scholar]
- Freitas, V.V.; Rodrigues Borges, L.L.; Dias Castro, G.A.; Henrique dos Santos, M.; Teixeira Ribeiro Vidigal, M.C.; Fernandes, S.A.; Stringheta, P.C. Impact of different roasting conditions on the chemical composition, antioxidant activities, and color of Coffea canephora and Coffea arabica L. samples. Heliyon 2023, 9, e19580. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.A.M. Phenolic compounds: Classification, chemistry, and updated techniques of analysis and synthesis. In Phenolic Compounds; IntechOpen: Rijeka, Croatia, 2021; p. 4. [Google Scholar]
- Chacón-Figueroa, I.H.; Medrano-Ruiz, L.G.; Moreno-Vásquez, M.D.; Ovando-Martínez, M.; Gámez-Meza, N.; Del-Toro-Sánchez, C.L.; Castro-Enríquez, D.D.; López-Ahumada, G.A.; Dórame-Miranda, R.F. Use of Coffee Bean Bagasse Extracts in the Brewing of Craft Beers: Optimization and Antioxidant Capacity. Molecules 2022, 27, 7755. [Google Scholar] [CrossRef]
- Munyendo, L.M.; Njoroge, D.M.; Owaga, E.E.; Mugendi, B. Coffee phytochemicals and post-harvest handling—A complex and delicate balance. J. Food Compos. Anal. 2021, 102, 103995. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Badmos, S.; Lee, S.-H.; Kuhnert, N. Comparison and quantification of chlorogenic acids for differentiation of green Robusta and Arabica coffee beans. Food Res. Int. 2019, 126, 108544. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, S.-J.; Zhang, M.-T.; Jia, J.; Chen, F.-X.; Chen, C.-L.; Yang, P.-F.; Mao, J.-L. Synthesis, configurational analysis and antiviral activities of novel diphenylacrylic acids with caffeic acid as the lead compound. J. Mol. Struct. 2023, 1291. [Google Scholar] [CrossRef]
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef]
- Dos Santos, É.M.; de Macedo, L.M.; Ataide, J.A.; Delafiori, J.; de Oliveira Guarnieri, J.P.; Rosa, P.C.P.; Ruiz, A.L.T.G.; Lancellotti, M.; Jozala, A.F.; Catharino, R.R.; et al. Antioxidant, antimicrobial and healing properties of an extract from coffee pulp for the development of a phytocosmetic. Sci. Rep. 2024, 14, 54797. [Google Scholar] [CrossRef]
- Zhang, G.; Tan, Y.; Yu, T.; Wang, S.; Liu, L.; Li, C. Synergistic antibacterial effects of reuterin and catechin against Streptococcus mutans. LWT 2021, 139, 110527. [Google Scholar] [CrossRef]
- Majumdar, G.; Mandal, S. Evaluation of broad-spectrum antibacterial efficacy of quercetin by molecular docking, molecular dynamics simulation and in vitro studies. Chem. Phys. Impact 2024, 8, 110527. [Google Scholar] [CrossRef]
- Rezaul Islam, M.; Akash, S.; Murshedul Islam, M.; Sarkar, N.; Kumer, A.; Chakraborty, S.; Dhama, K.; Ahmed Al-Shaeri, M.; Anwar, Y.; Wilairatana, P.; et al. Alkaloids as drug leads in Alzheimer’s treatment: Mechanistic and therapeutic insights. Brain Res. 2024, 1834. [Google Scholar] [CrossRef]
- Kar, A.; Mukherjee, S.K.; Barik, S.; Hossain, S.T. Antimicrobial Activity of Trigonelline Hydrochloride Against Pseudomonas aeruginosa and Its Quorum-Sensing Regulated Molecular Mechanisms on Biofilm Formation and Virulence. ACS Infect. Dis. 2024, 10, 746–762. [Google Scholar] [CrossRef]
- Woziwodzka, A.; Krychowiak-Maśnicka, M.; Gołuński, G.; Łosiewska, A.; Borowik, A.; Wyrzykowski, D.; Piosik, J. New Life of an Old Drug: Caffeine as a Modulator of Antibacterial Activity of Commonly Used Antibiotics. Pharmaceuticals 2022, 15, 872. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Berton-Carabin, C.C.; Guyot, S.; Gacel, A.; Fogliano, V.; Schroën, K. Coffee melanoidins as emulsion stabilizers. Food Hydrocoll. 2023, 139, 108522. [Google Scholar] [CrossRef]
- Huang, H.; Gao, Y.; Wang, L.; Yu, X.; Chen, S.; Xu, Y. Maillard reaction intermediates in Chinese Baijiu and their effects on Maillard reaction related flavor compounds during aging. Food Chem. 2024, 22, 101356. [Google Scholar] [CrossRef]
- Gigl, M.; Frank, O.; Gabler, A.; Koch, T.; Briesen, H.; Hofmann, T. Key odorant melanoidin interactions in aroma staling of coffee beverages. Food Chem. 2022, 392, 133291. [Google Scholar] [CrossRef] [PubMed]
- Maesaka, E.; Kukuminato, S.; Aonishi, K.; Koyama, K.; Koseki, S. Antibacterial Effect of Melanoidins Derived From Xylose and Phenylalanine Against Bacillus cereus and Clostridium perfringens. J. Food Prot. 2023, 86, 100140. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, A.; Majie, A.; Karmakar, V.; Das, S.; Dinda, S.C.; Bose, A.; Gorain, B. Terpenoids as potential phytoconstituent in the treatment of diabetes: From preclinical to clinical advancement. Phytomedicine 2024, 129, 155638. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 174238. [Google Scholar] [CrossRef] [PubMed]
- Antoine, G.; Vaissayre, V.; Meile, J.-C.; Payet, J.; Conéjéro, G.; Costet, L.; Fock-Bastide, I.; Joët, T.; Dussert, S. Diterpenes of Coffea seeds show antifungal and anti-insect activities and are transferred from the endosperm to the seedling after germination. Plant Physiol. Biochem. 2023, 194, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.; Astatkie, T.; Boeckx, P. Quality and biochemical composition of Ethiopian coffee varied with growing region and locality. J. Food Compos. Anal. 2023, 115, 105015. [Google Scholar] [CrossRef]
- Sualeh, A.; Tolessa, K.; Mohammed, A. Biochemical composition of green and roasted coffee beans and their association with coffee quality from different districts of southwest Ethiopia. Heliyon 2020, 6, e05812. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.V.; Borges, L.L.R.; Castro, G.A.D.; Almeida, L.F.; Crepalde, L.T.; Kobi, H.d.B.; Vidigal, M.C.T.R.; dos Santos, M.H.; Fernandes, S.A.; Maitan-Alfenas, G.P.; et al. Influence of roasting levels on chemical composition and sensory quality of Arabica and Robusta coffee: A comparative study. Food Biosci. 2024, 59, 104171. [Google Scholar] [CrossRef]
- Alamri, E.; Rozan, M.; Bayomy, H. A study of chemical Composition, Antioxidants, and volatile compounds in roasted Arabic coffee. Saudi J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef] [PubMed]
- Biratu, G.; Woldemariam, H.W.; Gonfa, G. Optimization of pectin yield extracted from coffee Arabica pulp using response surface methodology. Heliyon 2024, 10, e29636. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon 2023, 9, e13917. [Google Scholar] [CrossRef]
- Santos da Silveira, J.; Durand, N.; Lacour, S.; Belleville, M.-P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- Sierra-López, L.D.; Hernandez-Tenorio, F.; Marín-Palacio, L.D.; Giraldo-Estrada, C. Coffee mucilage clarification: A promising raw material for the food industry. Food Humanit. 2023, 1, 689–695. [Google Scholar] [CrossRef]
- Pardo, L.M.F.; Castillo, N.V.; Durán, Y.M.V.; Rosero, J.A.J.; Lozano Moreno, J.A. Comprehensive analysis of ethanol production from coffee mucilage under sustainability indicators. Chem. Eng. Process. Process Intensif. 2022, 182, 109183. [Google Scholar] [CrossRef]
- Reis, R.S.; Tienne, L.G.P.; Souza, D.d.H.S.; Marques, M.d.F.V.; Monteiro, S.N. Characterization of coffee parchment and innovative steam explosion treatment to obtain microfibrillated cellulose as potential composite reinforcement. J. Mater. Res. Technol. 2020, 9, 9412–9421. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, B.; Wang, K.; Li, H.; Peng, L. Carboxymethyl cellulose-based multifunctional film integrated with polyphenol-rich extract and carbon dots from coffee husk waste for active food packaging applications. Food Chem. 2024, 448. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.d.; Moreira, T.F.M.; Paes Silva, B.; Oliveira, G.; Teixeira, V.M.C.; Watanabe, L.S.; Lucy Nixdorf, S.; Eloísa Leal, L.; Pessoa, L.G.A.; Seixas, F.A.V.; et al. Characterization and bioactivities of coffee husks extract encapsulated with polyvinylpyrrolidone. Food Res. Int. 2024, 178, 113878. [Google Scholar] [CrossRef] [PubMed]
- Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Amelia Rachmawati Putri, F.; Puspita, F.; Restu Prihadi, A. Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste. Arab. J. Chem. 2023, 16, 104634. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Benítez, V.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds. Foods 2021, 10, 30653. [Google Scholar] [CrossRef]
- Hoseini, M.; Cocco, S.; Casucci, C.; Cardelli, V.; Corti, G. Coffee by-products derived resources. A review. Biomass Bioenergy 2021, 148, 106009. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Grassino, A.N.E.; Jerković, I.; Pedisić, S.; Dent, M. Hydrodistillation fractions of coffee (green and roasted) and coffee by-product (silver skin and spent grounds) as a source of bioactive compounds. Sustain. Chem. Pharm. 2024, 39, 101592. [Google Scholar] [CrossRef]
- Biondić Fučkar, V.; Božić, A.; Jukić, A.; Krivohlavek, A.; Jurak, G.; Tot, A.; Serdar, S.; Žuntar, I.; Režek Jambrak, A. Coffee Silver Skin—Health Safety, Nutritional Value, and Microwave Extraction of Proteins. Foods 2023, 12, 30518. [Google Scholar] [CrossRef] [PubMed]
- Wale, K.; Tolessa, K.; Atlabachew, M.; Mehari, B.; Alemayehu, M.; Mengistu, D.A.; Kerisew, B. Level of caffeine, trigonelline and chlorogenic acids in green coffee (Coffea arabica L.) beans from Amhara region, Ethiopia. J. Agric. Food Res. 2024, 16, 101082. [Google Scholar] [CrossRef]
- Bomfim, A.S.; Oliveira, D.M.; Voorwald, H.J.; Benini, K.C.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 30437. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 30613. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients 2023, 15, 40994. [Google Scholar] [CrossRef] [PubMed]
- Bouhzam, I.; Cantero, R.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana-i-Palmer, P.; Puig, R. Extraction of Bioactive Compounds from Spent Coffee Grounds Using Ethanol and Acetone Aqueous Solutions. Foods 2023, 12, 24400. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 142064. [Google Scholar] [CrossRef] [PubMed]
- Canci, L.A.; de Toledo Benassi, M.; Canan, C.; Kalschne, D.L.; Colla, E. Antimicrobial potential of aqueous coffee extracts against pathogens and Lactobacillus species: A food matrix application. Food Biosci. 2022, 47, 101756. [Google Scholar] [CrossRef]
- Zubair, M. Antimicrobial and Anti-Biofilm Activities of Coffea arabica L. Against the Clinical Strains Isolated From Diabetic Foot Ulcers. Cureus 2024, 16, 52539. [Google Scholar] [CrossRef] [PubMed]
- Jamalifar, H.; Samadi, N.; Nowroozi, J.; Dezfulian, M.; Fazeli, M.R. Down-regulatory effects of green coffee extract on las I and las R virulence-associated genes in Pseudomonas aeruginosa. DARU J. Pharm. Sci. 2019, 27, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rawangkan, A.; Yosboonruang, A.; Kiddee, A.; Siriphap, A.; Pook-In, G.; Praphasawat, R.; Saokaew, S.; Duangjai, A. Restoring Ampicillin Sensitivity in Multidrug-Resistant Escherichia coli Following Treatment in Combination with Coffee Pulp Extracts. J. Microbiol. Biotechnol. 2023, 33, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; El-Attar, M.M.; Ali, H.S.; Elkhadragy, M.F.; Yehia, H.M.; Farouk, A. Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins 2022, 14, 20109. [Google Scholar] [CrossRef]
- Barbero-López, A.; Monzó-Beltrán, J.; Virjamo, V.; Akkanen, J.; Haapala, A. Revalorization of coffee silverskin as a potential feedstock for antifungal chemicals in wood preservation. Int. Biodeterior. Biodegrad. 2020, 152, 105011. [Google Scholar] [CrossRef]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R.; Fernandes, C.; Gonçalves, T. Antifungal Activity of Spent Coffee Ground Extracts. Microorganisms 2023, 11, 20242. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of Polyphenolic Fraction from Arabica Coffee Pulp and Its Antifungal Applications. Plants 2021, 10, 71422. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Ichinose, M.; Uozaki, M.; Tsujimoto, K.; Yamasaki, H.; Koyama, A.H. Antiviral activities of coffee extracts in vitro. Food Chem. Toxicol. 2008, 46, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.N.; Carneiro, B.M.; Braga, A.C.S.; Rahal, P. Caffeine inhibits hepatitis C virus replication in vitro. Arch. Virol. 2015, 160, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Pariente, A.; Anty, R. Anti-Hepatitis C Virus Treatment and Coffee Drinking. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 343–346. [Google Scholar]
- Zuo, J.; Tang, W.; Xu, Y. Anti-hepatitis B virus activity of chlorogenic acid and its related compounds. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–613. [Google Scholar]
- Azam, M.S.; Islam, M.N.; Wahiduzzaman, M.; Alam, M.; Dhrubo, A.A.K. Antiviral foods in the battle against viral infections: Understanding the molecular mechanism. Food Sci. Nutr. 2023, 11, 4444–4459. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, M.P.; Protopopescu, C.; Marcellin, F.; Rosellini, S.; Wittkop, L.; Esterle, L. Protective effect of coffee consumption on all-cause mortality of French HIV-HCV co-infected patients. J. Hepatol. 2017, 67, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Curto, T.M.; Lindsay, K.L.; Wright, E.C.; Sinha, R.; Everhart, J.E. Coffee consumption is associated with response to peginterferon and ribavirin therapy in patients with chronic hepatitis C. Gastroenterology 2011, 140, 1961–1969. [Google Scholar] [CrossRef]
- Shen, J.; Wang, G.; Zuo, J. Caffeic acid inhibits HCV replication via induction of IFNα antiviral response through p62-mediated Keap1/Nrf2 signaling pathway. Antivir. Res. 2018, 154, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Shirasago, Y.; Suzuki, R.; Abe, R.; Wakita, T.; Hanada, K. Inhibitory effects of caffeic acid, a coffee-related organic acid, on the propagation of hepatitis C virus. Jpn. J. Infect. Dis. 2015, 68, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Namba, T.; Matsuse, T. A historical study of coffee in Japanese and Asian countries: Focusing the medicinal uses in Asian traditional medicines. Yakushigaku Zasshi 2002, 37, 65–75. [Google Scholar] [PubMed]
- Teramoto, M.; Yamagishi, K.; Muraki, I.; Tamakoshi, A.; Iso, H. Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension. J. Am. Heart Assoc. 2022, 12, 12. [Google Scholar] [CrossRef]
- Behne, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk assessment of chlorogenic and isochlorogenic acids in coffee by-products. Molecules 2023, 28, 14. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chiang, H.-M.; Chen, Y.; Chen, C.-Y.; Chen, H.-F.; Su, W.-C.; Wang, W.-J.; Chou, Y.-C.; Chang, W.-C.; Wang, S.-C.; et al. Prospects of coffee leaf against SARS-CoV-2 infection. Int. J. Biol. Sci. 2022, 18, 4677. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.-P.; Mahillon, J.; Bragard, C. On the use of antibiotics to control plant pathogenic bacteria: A genetic and genomic perspective. Front. Microbiol. 2023, 14, 14. [Google Scholar] [CrossRef]
- Taylor, P.; Reeder, R. Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- Pimentão, A.R.; Cuco, A.P.; Pascoal, C.; Cássio, F.; Castro, B.B. Current trends and mismatches on fungicide use and assessment of the ecological effects in freshwater ecosystems. Environ. Pollut. 2024, 347. [Google Scholar] [CrossRef] [PubMed]
- Sett, S.; Prasad, A.; Prasad, M. Resistance genes on the verge of plant–virus interaction. Trends Plant Sci. 2022, 27, 1242–1252. [Google Scholar] [CrossRef]
- García-Estrada, R.S.; Diaz-Lara, A.; Aguilar-Molina, V.H.; Tovar-Pedraza, J.M. Viruses of Economic Impact on Tomato Crops in Mexico: From Diagnosis to Management—A Review. Viruses 2022, 14, 61251. [Google Scholar] [CrossRef]
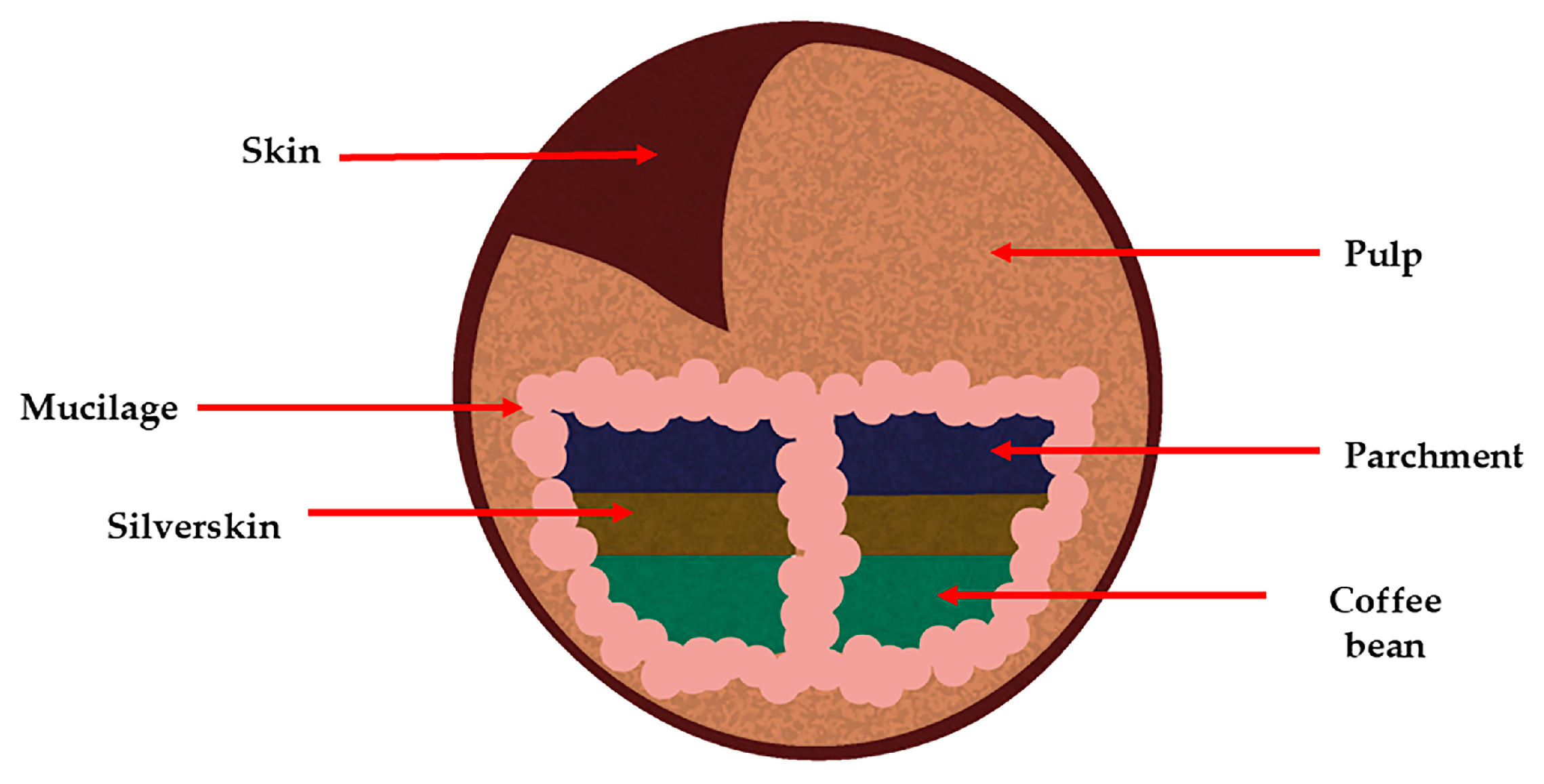

| By-Products | Chemical Composition | Bioactive Compounds | Percentage of the Coffee Cherry | Image |
|---|---|---|---|---|
| Pulp | Carbohydrates Soluble fibers Proteins Minerals | Chlorogenic acid Caffeine Epicatechin Catechin | 40–50% |  |
| Mucilage | Water Carbohydrates Proteins Pectin’s | Chlorogenic acid Caffeine | 14% |  |
| Parchment | (α) cellulose Hemicellulose Lignin Ash | Gallic acid Chlorogenic acid P-cumaric acid Sinapic acid Caffeine | 6.1% |  |
| Husk | Carbohydrates Fibers Proteins | Gallic acid Tannic acid Chlorogenic acid Epicatechin Caffeine | 50% |  |
| Silver skin | Dietary fiber Polysaccharides Proteins Fats Ash | Caffeine Trigonelline 3-feruloylquinic acid 5-caffeoylquinic acid 3-caffeoylquinic acid Chlorogenic acid P-cumaric acid Melanoidins | 4.2% |  |
| Spent coffee grounds | Polysaccharides Proteins Minerals Fats Dietary fiber Vitamin E Lignin | Chlorogenic acid Caffeic acid Gallic acid Ferulic acid Ellagic acid P-coumaric acid Protocatechuic Tannic acid Catechin Epicatechin Quercetin Rutin Trigonelline Caffeine Melanoidins | 90% of the initial coffee beans |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Díaz, R.; Silva-Beltrán, N.P.; Gámez-Meza, N.; Calderón, K. The Antimicrobial Effects of Coffee and By-Products and Their Potential Applications in Healthcare and Agricultural Sectors: A State-of-Art Review. Microorganisms 2025, 13, 215. https://doi.org/10.3390/microorganisms13020215
Castro-Díaz R, Silva-Beltrán NP, Gámez-Meza N, Calderón K. The Antimicrobial Effects of Coffee and By-Products and Their Potential Applications in Healthcare and Agricultural Sectors: A State-of-Art Review. Microorganisms. 2025; 13(2):215. https://doi.org/10.3390/microorganisms13020215
Chicago/Turabian StyleCastro-Díaz, Rosa, Norma Patricia Silva-Beltrán, Nohemi Gámez-Meza, and Kadiya Calderón. 2025. "The Antimicrobial Effects of Coffee and By-Products and Their Potential Applications in Healthcare and Agricultural Sectors: A State-of-Art Review" Microorganisms 13, no. 2: 215. https://doi.org/10.3390/microorganisms13020215
APA StyleCastro-Díaz, R., Silva-Beltrán, N. P., Gámez-Meza, N., & Calderón, K. (2025). The Antimicrobial Effects of Coffee and By-Products and Their Potential Applications in Healthcare and Agricultural Sectors: A State-of-Art Review. Microorganisms, 13(2), 215. https://doi.org/10.3390/microorganisms13020215






