Efficacy of Antimicrobials Against Enveloped and Non-Enveloped Viruses on Porous Materials: A Review
Abstract
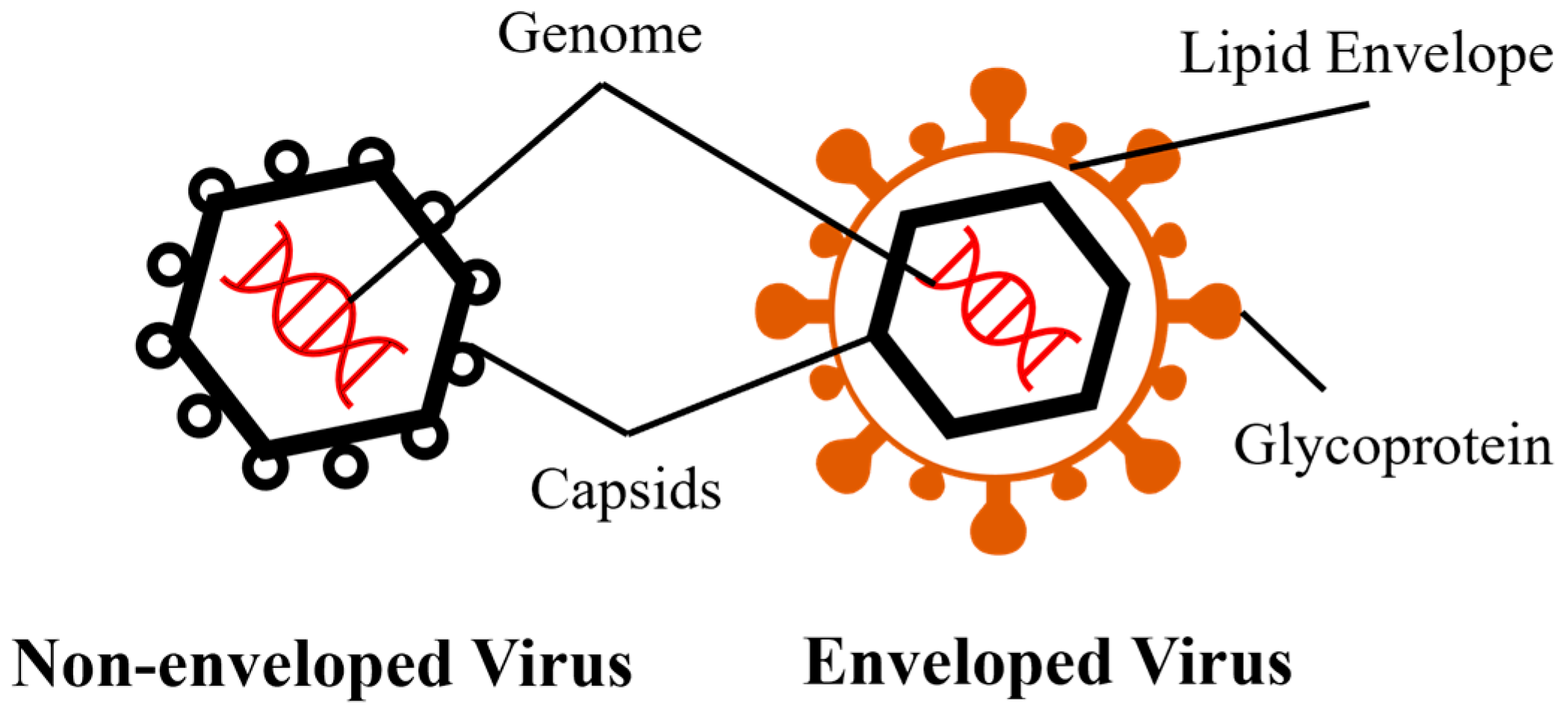
1. Introduction
2. Persistence of Viruses on Porous Materials
2.1. The Effect of Material Characteristics
2.2. The Effect of Temperature and Relative Humidity
2.3. The Effect of Transmission Medium, Deposition Method, Strain Subtype and pH
3. Disinfection of Viruses on Porous Materials
3.1. Launderable Materials
3.2. Non-Launderable Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPA | United States Environmental Protection Agency |
| T | Temperature |
| RH | Relative humidity |
| MC | Material characteristics |
| TM | Transmission media |
| FCV | Feline calicivirus |
| MNV | Murine norovirus |
| PV | Poliovirus |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| NaClO | Sodium hypochlorite |
| QAC | Quaternary ammonium compound |
| UV | Ultraviolet |
References
- Li, X.C.; Zhang, Y.Y.; Zhang, Q.Y.; Liu, J.S.; Ran, J.J.; Han, L.F.; Zhang, X.X. Global burden of viral infectious diseases of poverty based on Global Burden of Diseases Study 2021. Infect. Dis. Poverty 2024, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.T.; Ren, J.J.; Li, R.H.; Gao, Y.; Zhang, H.Y.; Li, J.M.; Zhang, J.J.; Wang, X.C.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. eClinicalMedicine 2021, 37, 100986. [Google Scholar] [CrossRef]
- Cheesbrough, J.S.; Green, J.; Gallimore, C.I.; Wright, P.A.; Brown, D.W. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol. Infect. 2000, 125, 93–98. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef]
- Morter, S.; Bennet, G.; Fish, J.; Richards, J.; Allen, D.J.; Nawaz, S.; Iturriza-Gomara, M.; Brolly, S.; Gray, J. Norovirus in the hospital setting: Virus introduction and spread within the hospital environment. J. Hosp. Infect. 2011, 77, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Fornek, M.; Schwab, K.J.; Chapin, A.R.; Gibson, K.; Schwab, E.; Spencer, C.; Henning, K. A norovirus outbreak at a long-term-care facility: The role of environmental surface contamination. Infect. Control Hosp. Epidemiol. 2005, 26, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Yeargin, T.; Buckley, D.; Fraser, A.; Jiang, X.P. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am. J. Infect. Control 2016, 44, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.M.; Minter, A.; Edmunds, W.J.; Lau, C.L.; Kucharski, A.J.; Lowe, R. Transmission modelling of environmentally persistent zoonotic diseases: A systematic review. Lancet Planet. Health 2021, 5, E466–E478. [Google Scholar] [CrossRef]
- Hernon-Kenny, L.A.; Behringer, D.L.; Crenshaw, M.D. Comparison of latex body paint with wetted gauze wipes for sampling the chemical warfare agents VX and sulfur mustard from common indoor surfaces. Forensic Sci. Int. 2016, 262, 143–149. [Google Scholar] [CrossRef]
- Kruszewska, E.; Czupryna, P.; Pancewicz, S.; Martonik, D.; Buklaha, A.; Moniuszko-Malinowska, A. Is peracetic acid fumigation effective in public transportation? Int. J. Environ. Res. Public Health 2022, 19, 2526. [Google Scholar] [CrossRef]
- Sexton, J.D.; Wilson, A.M.; Sassi, H.P.; Reynolds, K.A. Tracking and controlling soft surface contamination in health care settings. Am. J. Infect. Control 2018, 46, 39–43. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Rogues, C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Huang, J.; Fraser, A.; Jiang, X. Efficacy of three EPA-registered antimicrobials and steam against two human norovirus surrogates on nylon carpets with two backing types. Appl. Environ. Microbiol. 2024, 90, e0038424. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fraser, A.; Jiang, X. Efficacy of three chemical disinfectants and steam against Clostridioides difficile endospores on nylon carpet with two different backing systems. Appl. Environ. Microbiol. 2025, 91, e0086125. [Google Scholar] [CrossRef]
- Pittet, D. Improving adherence to hand hygiene practice: A multidisciplinary approach. Emerg. Infect. Dis. 2001, 7, 234–240. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Verhougstraete, M.P.; Mena, K.D.; Sattar, S.A.; Scott, E.A.; Gerba, C.P. Quantifying pathogen infection risks from household laundry practices. J. Appl. Microbiol. 2022, 132, 1435–1448. [Google Scholar] [CrossRef]
- Dissanayake, M.R.; Johnson, B.E.E.; Leong, B.M.E.; Fraser, A.M. Review of state regulations related to environmental sanitation in long-term care facilities. Am. J. Infect. Control 2025, 53, 843–848. [Google Scholar] [CrossRef]
- Environmental Protection Agency. OCSPP 810.2200 Disinfectants for Use on Environmental Surfaces, Guidance for Efficacy Testing; Environmental Protection Agency: Washington, DC, USA, 2018. [Google Scholar]
- Environmental Protection Agency. OCSPP 810.2400—Disinfectants and Sanitizers for Use on Fabrics and Textiles; Environmental Protection Agency: Washington, DC, USA, 2013. [Google Scholar]
- AOAC 955.14 Testing disinfectants against Salmonella enterica. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; AOAC Publications: New York, NY, USA, 2023.
- AOAC 955.15 Testing disinfectants against Staphylococcus aureus. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; AOAC Publications: New York, NY, USA, 2023.
- AOAC 964.02 Testing disinfectants against Pseudomonas aeruginosa. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; AOAC Publications: New York, NY, USA, 2023.
- ASTM E1153-22; Standard Test Method for Efficacy of Sanitizers Recommended for Inanimate, Hard, Nonporous Non-Food Contact Surfaces. ASTM International: West Conshohocken, PA, USA, 2025.
- ASTM E2274-24; Standard Test Method for Evaluation of Laundry Sanitizers and Disinfectants. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM E2406-24; Standard Test Method for Evaluation of Laundry Sanitizers and Disinfectants for Use in High Efficiency Washing Operations. ASTM International: West Conshohocken, PA, USA.
- AATCC TM100-2004; Antibacterial Finishes on Textile Materials. AATCC: Research Triangle Park, NC, USA, 2004.
- ASTM E2149-25; Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2025.
- Ivanova, P.T.; Myers, D.S.; Milne, S.B.; McClaren, J.L.; Thomas, P.G.; Brown, H.A. Lipid composition of the viral envelope of three strains of influenza virus-Not all viruses are created equal. Acs Infect. Dis. 2015, 1, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A.; Ibrahim, I.M. Zika virus envelope—Heat shock protein A5 (GRP78) binding site prediction. J. Biomol. Struct. Dyn. 2021, 39, 5248–5260. [Google Scholar] [CrossRef]
- Cox, C.S. Roles of water-molecules in bacteria and viruses. Orig. Life Evol. Biosph. 1993, 23, 29–36. [Google Scholar] [CrossRef]
- Simon, M.; Veit, M.; Osterrieder, K.; Gradzielski, M. Surfactants—Compounds for inactivation of SARS-CoV-2 and other enveloped viruses. Curr. Opin. Colloid Interface Sci. 2021, 55, 101479. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, A.C.; Murugkar, H.V.; Kumar, M.; Nagarajan, S.; Tosh, C.; Pathak, A.; Rajendrakumar, A.M.; Agarwal, R.K. Survivability of highly pathogenic avian influenza virus (H5N1) in naturally preened duck feathers at different temperatures. Transbound. Emerg. Dis. 2019, 66, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Abad, F.X.; Pinto, R.M.; Bosch, A. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 1994, 60, 3704–3710. [Google Scholar] [CrossRef]
- Savage, C.E.; Jones, R.C. The survival of avian reoviruses on materials associated with the poultry house environment. Avian Pathol. 2003, 32, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Beiza, A.A.; Mohammad, Z.H.; Sirsat, S.A. Persistence of foodborne pathogens on farmers’ market fomites. J. Food Prot. 2021, 84, 1169–1175. [Google Scholar] [CrossRef]
- Nastasi, N.; Renninger, N.; Bope, A.; Cochran, S.J.; Greaves, J.; Haines, S.R.; Balasubrahmaniam, N.; Stuart, K.; Panescu, J.; Bibby, K.; et al. Persistence of viable MS2 and Phi6 bacteriophages on carpet and dust. Indoor Air 2022, 32, e12969. [Google Scholar] [CrossRef]
- Moce-Llivina, L.; Papageorgiou, G.T.; Jofre, J. A membrane-based quantitative carrier test to assess the virucidal activity of disinfectants and persistence of viruses on porous fomites. J. Virol. Methods 2006, 135, 49–55. [Google Scholar] [CrossRef]
- Makison Booth, C.; Frost, G. Survival of a norovirus surrogate on surfaces in synthetic gastric fluid. J. Hosp. Infect. 2020, 86, 468–473. [Google Scholar] [CrossRef]
- Buckley, D.; Fraser, A.; Huang, G.; Jiang, X. Recovery optimization and survival of the human norovirus surrogates feline calicivirus and murine norovirus on carpet. Appl. Environ. Microbiol. 2017, 83, e01336-17. [Google Scholar] [CrossRef]
- Kim, A.N.; Park, S.Y.; Bae, S.C.; Oh, M.H.; Ha, S.D. Survival of norovirus surrogate on various food-contact surfaces. Food and Environ. Virol. 2014, 6, 182–188. [Google Scholar] [CrossRef]
- Tamrakar, S.B.; Henley, J.; Gurian, P.L.; Gerba, C.P.; Mitchell, J.; Enger, K.; Rose, J.B. Persistence analysis of poliovirus on three different types of fomites. J. Appl. Microbiol. 2017, 122, 522–530. [Google Scholar] [CrossRef]
- Dixon, G.J.; Sidwell, R.W.; Mcneil, E. Quantitative studies on fabrics as disseminators of viruses. II. Persistence of poliomyelitis virus on cotton and wool fabrics. Appl. Microbiol. 1966, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.A.; Lloyd-Evans, N.; Springthorpe, V.S.; Nair, R.C. Institutional outbreaks of rotavirus diarrhoea: Potential role of fomites and environmental surfaces as vehicles for virus transmission. Epidemiol. Infect. 1986, 96, 277–289. [Google Scholar] [CrossRef]
- Fijan, S.; Steyer, A.; Poljsak-Prijatelj, M.; Cencic, A.; Ostar-Turk, S.; Koren, S. Rotaviral RNA found on various surfaces in a hospital laundry. J. Virol. Methods 2008, 148, 66–73. [Google Scholar] [CrossRef]
- Tiwari, A.; Patnayak, D.P.; Chander, Y.; Parsad, M.; Goyal, S.M. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006, 50, 284–287. [Google Scholar] [CrossRef]
- Baker, C.A.; Gutierrez, A.; Gibson, K.E. Factors impacting persistence of Phi6 bacteriophage, an enveloped virus surrogate, on fomite surfaces. Appl. Environ. Microbiol. 2022, 88, e02552-21. [Google Scholar] [CrossRef]
- Whitworth, C.; Mu, Y.; Houston, H.; Martinez-Smith, M.; Noble-Wang, J.; Coulliette-Salmond, A.; Rosea, L. Persistence of bacteriophage Phi 6 on porous and nonporous surfaces and the potential for its use as an Ebola virus or coronavirus surrogate. Appl. Environ. Microbiol. 2020, 86, e01482-20. [Google Scholar] [CrossRef]
- Stowell, J.D.; Forlin-Passoni, D.; Din, E.; Radford, K.; Brown, D.; White, A.; Bate, S.L.; Dollard, S.C.; Bialek, S.R.; Cannon, M.J.; et al. Cytomegalovirus survival on common environmental surfaces: Opportunities for viral transmission. J. Infect. Dis. 2012, 205, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Faix, R. Survival of cytomegalovirus (CMV) on environmental surfaces. Pediatr. Res. 1984, 18, 321. [Google Scholar] [CrossRef]
- Cook, B.W.; Cutts, T.A.; Nikiforuk, A.M.; Poliquin, P.G.; Court, D.A.; Strong, J.E.; Theriault, S.S. Evaluating environmental persistence and disinfection of the Ebola virus Makona variant. Viruses 2015, 7, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Saklou, N.T.; Burgess, B.A.; Ashton, L.V.; Morley, P.S.; Goehring, L.S. Environmental persistence of equid herpesvirus type-1. Equine Vet. J. 2021, 53, 349–355. [Google Scholar] [CrossRef]
- Tracy, S.; Derby, C.; Virjee, N.; Hardwick, M. Virus inactivation on common indoor contact fabrics. Indoor Built Environ. 2022, 31, 1381–1392. [Google Scholar] [CrossRef]
- Oxford, J.; Berezin, E.N.; Courvalin, P.; Dwyer, D.E.; Exner, M.; Jana, L.A.; Kaku, M.; Lee, C.; Letlape, K.; Low, D.E.; et al. The survival of influenza A(H1N1)pdm09 virus on 4 household surfaces. Am. J. Infect. Control 2014, 42, 423–425. [Google Scholar] [CrossRef]
- Zuo, Z.L.; de Abin, M.; Chander, Y.; Kuehn, T.H.; Goyal, S.M.; Pui, D.Y.H. Comparison of spike and aerosol challenge tests for the recovery of viable influenza virus from non-woven fabrics. Influenza Other Respir. Viruses 2013, 7, 637–644. [Google Scholar] [CrossRef]
- Guan, J.; Chan, M.; VanderZaag, A. Inactivation of avian influenza viruses on porous and non-porous surfaces is enhanced by elevating absolute humidity. Transbound. Emerg. Dis. 2017, 64, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Greatorex, J.S.; Digard, P.; Curran, M.D.; Moynihan, R.; Wensley, H.; Wreghitt, T.; Varsani, H.; Garcia, F.; Enstone, J.; Nguyen-Van-Tam, J.S. Survival of influenza A(H1N1) on materials found in households: Implications for infection control. PLoS ONE 2011, 6, e27932. [Google Scholar] [CrossRef]
- Mukherjee, D.V.; Cohen, B.; Bovino, M.E.; Desai, S.; Whittier, S.; Larson, E.L. Survival of influenza virus on hands and fomites in community and laboratory settings. Am. J. Infect. Control 2012, 40, 590–594. [Google Scholar] [CrossRef]
- Thompson, K.A.; Bennett, A.M. Persistence of influenza on surfaces. J. Hosp. Infect. 2017, 95, 194–199. [Google Scholar] [CrossRef]
- Kim, Y.; Krishna, V.D.; Torremorell, M.; Goyal, S.M.; Cheeran, M.C.J. Stability of porcine epidemic diarrhea virus on fomite materials at different temperatures. Vet. Sci. 2018, 5, 21. [Google Scholar] [CrossRef]
- Hall, C.B.; Douglas, R.G.; Geiman, J.M. Possible transmission by fomites of respiratory syncytial virus. J. Infect. Dis. 1980, 141, 98–102. [Google Scholar] [CrossRef]
- Duan, S.M.; Zhao, X.S.; Wen, R.F.; Huang, J.J.; Pi, G.H.; Zhang, S.X.; Han, J.; Bi, S.L.; Ruan, L.; Dong, X.P.; et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003, 16, 246–255. [Google Scholar]
- Lai, M.Y.Y.; Cheng, P.K.C.; Lim, W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005, 41, e67–e71. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.; Kay, G.A.; Aljayyoussi, G.; Owen, S.I.; Harland, A.R.; Pierce, N.S.; Calder, J.D.F.; Fletcher, T.E.; Adams, E.R. SARS-CoV-2 viability on sports equipment is limited, and dependent on material composition. Sci. Rep. 2022, 12, 1416. [Google Scholar] [CrossRef]
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Sturdivant, R.X.; Barr, K.L.; Harris, D. SARS-CoV-2 viability on 16 common indoor surface finish materials. HERD 2021, 14, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Dixon, G.J.; Mcneil, E. Quantitative studies on fabrics as disseminators of viruses: III. Persistence of vaccinia virus on fabrics impregnated with a virucidal agent. Appl. Microbiol. 1967, 15, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Dixon, G.J.; Mcneil, E. Quantitative studies on fabrics as disseminators: I. Persistence of vaccinia virus on cotton and wool fabrics. Appl. Microbiol. 1966, 14, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.P.; Choi, Y.W.; Wendling, M.Q.; Rogers, J.V.; Chappie, D.J. Environmental persistence of vaccinia virus on materials. Lett. Appl. Microbiol. 2013, 57, 399–404. [Google Scholar] [CrossRef]
- Haines, S.R.; Adams, R.I.; Boor, B.E.; Bruton, T.A.; Downey, J.; Ferro, A.R.; Gall, E.; Green, B.J.; Hegarty, B.; Horner, E.; et al. Ten questions concerning the implications of carpet on indoor chemistry and microbiology. Build. Environ. 2019, 170, 106589. [Google Scholar] [CrossRef]
- Paton, S.; Spencer, A.; Garratt, I.; Thompson, K.A.; Dinesh, I.; Aranega-Bou, P.; Stevenson, D.; Clark, S.; Dunning, J.; Bennett, A.; et al. Persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and viral RNA in relation to surface type and contamination concentration. Appl. Environ. Microbiol. 2021, 87, e0052621. [Google Scholar] [CrossRef]
- Gibson, K.E.; Crandall, P.G.; Ricke, S.C. Removal and Transfer of Viruses on Food Contact Surfaces by Cleaning Cloths. Appl. Environ. Microbiol. 2012, 78, 3037–3044, Erratum in Appl. Environ. Microbiol. 2017, 83, e03373-16. [Google Scholar] [CrossRef]
- Shields, P.A.; Farrah, S.R. Characterization of virus adsorption by using DEAE-sepharose and octyl-sepharose. Appl. Environ. Microbiol. 2002, 68, 3965–3968. [Google Scholar] [CrossRef]
- Mannan, M.; Al-Ghamdi, S.G. Indoor air quality in buildings: A comprehensive review on the factors influencing air pollution in residential and commercial structure. Int. J. Environ. Res. Public Health 2021, 18, 3276. [Google Scholar] [CrossRef] [PubMed]
- Lowen, A.C.; Steel, J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Goggins, W.B.; Chan, E.Y.Y. A time-series study of the association of rainfall, relative humidity and ambient temperature with hospitalizations for rotavirus and norovirus infection among children in Hong Kong. Sci. Total Environ. 2018, 643, 414–422. [Google Scholar] [CrossRef]
- Rimmer, D.L.; Smith, A.M. Antioxidants in soil organic matter and in associated plant materials. Eur. J. Soil Sci. 2009, 60, 170–175. [Google Scholar] [CrossRef]
- Zidar, M.; Rozman, P.; Belko-Parkel, K.; Ravnik, M. Control of viscosity in biopharmaceutical protein formulations. J. Colloid. Interf. Sci. 2020, 580, 308–317. [Google Scholar] [CrossRef]
- Owen, L.; Shivkumar, M.; Cross, R.B.M.; Laird, K. Porous surfaces: Stability and recovery of coronaviruses. Interface Focus 2021, 12, 20210039. [Google Scholar] [CrossRef]
- Yang, W.; Marr, L.C. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl. Environ. Microbiol. 2012, 78, 6781–6788. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef]
- Gerba, C.P.; Kennedy, D. Enteric virus survival during household laundering and impact of disinfection with sodium hypochlorite. Appl. Environ. Microbiol. 2007, 73, 4425–4428. [Google Scholar] [CrossRef]
- String, G.M.; White, M.R.; Gute, D.M.; Muhlberger, E.; Lantagne, D.S. Selection of a SARS-CoV-2 surrogate for use in surface disinfection efficacy studies with chlorine and antimicrobial surfaces. Environ. Sci. Technol. Lett. 2021, 8, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Tanner, B.D. Reduction in infection risk through treatment of microbially contaminated surfaces with a novel, portable, saturated steam vapor disinfection system. Am. J. Infect. Control 2009, 37, 20–27. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, S.A.; Mullin, T.J.; Munoz, S.; Ontiveros, C.C.; Gagnon, G.A. Immersive ultraviolet disinfection of E. coli and MS2 phage on woven cotton textiles. Sci. Rep. 2022, 12, 13260. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.S.; Allwood, P.B.; Hedberg, C.W.; Goyal, S.M. Disinfection of fabrics and carpets artificially contaminated with calicivirus: Relevance in institutional and healthcare centres. J. Hosp. Infect. 2006, 63, 205–210. [Google Scholar] [CrossRef]
- Buckley, D.; Dharmasena, M.; Fraser, A.; Pettigrew, C.; Anderson, J.; Jiang, X. Efficacy of silver dihydrogen citrate and steam vapor against a human norovirus surrogate, feline calicivirus, in suspension, on glass, and on carpet. Appl. Environ. Microbiol. 2018, 84, e00233-18. [Google Scholar] [CrossRef]
- Yeargin, T.; Fraser, A.; Huang, G.; Jiang, X. Recovery and disinfection of two human norovirus surrogates, feline calicivirus and murine norovirus, from hard nonporous and soft porous surfaces. J. Food Prot. 2015, 78, 1842–1850. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Petric, M. Inactivation of norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect. 2007, 66, 40–45. [Google Scholar] [CrossRef]
- Gabbert, L.R.; Neilan, J.G.; Rasmussen, M. Recovery and chemical disinfection of foot-and-mouth disease and African swine fever viruses from porous concrete surfaces. J. Appl. Microbiol. 2020, 129, 1092–1101. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Dixon, G.J.; Westbrook, L.; Forziati, F.H. Quantitative studies on fabrics as disseminators of viruses. V. Effect of laundering on poliovirus-contaminated fabrics. Appl. Microbiol. 1971, 21, 227–234. [Google Scholar] [CrossRef]
- Heinzel, M.; Kyas, A.; Weide, M.; Breves, R.; Bockmuhl, D.P. Evaluation of the virucidal performance of domestic laundry procedures. Int. J. Hyg. Environ. Health 2010, 213, 334–337. [Google Scholar] [CrossRef] [PubMed]
- String, G.M.; Kamal, Y.; Gute, D.M.; Lantagne, D.S. Chlorine efficacy against bacteriophage Phi6, a surrogate for enveloped human viruses, on porous and non-porous surfaces at varying temperatures and humidity. J. Environ. Health 2022, 57, 685–693. [Google Scholar] [CrossRef]
- Smither, S.; Phelps, A.; Eastaugh, L.; Ngugi, S.; O’Brien, L.; Dutch, A.; Lever, M.S. Effectiveness of Four Disinfectants against Ebola Virus on Different Materials. Viruses 2016, 8, 185, Erratum in Viruses 2016, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Hardison, R.L.; Nelson, S.W.; Barriga, D.; Ghere, J.M.; Fenton, G.A.; James, R.R.; Stewart, M.J.; Lee, S.D.; Calfee, M.W.; Ryan, S.P.; et al. Efficacy of detergent-based cleaning methods against coronavirus MHV-A59 on porous and non-porous surfaces. J. Occup. Environ. Hyg. 2022, 19, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hardison, R.L.; Nelson, S.W.; Barriga, D.; Ruiz, N.F.; Ghere, J.M.; Fenton, G.A.; Lindstrom, D.J.; James, R.R.; Stewart, M.J.; Lee, S.D.; et al. Evaluation of surface disinfection methods to inactivate the beta coronavirus murine hepatitis virus. J. Occup. Environ. Hyg. 2022, 19, 455–468. [Google Scholar] [CrossRef]
- Mello Mares-Guia, M.A.M.; Pereira Paiva, A.A.; Mello, V.M.; Eller, C.M.; Salvio, A.L.; Nascimento, F.F.; Silva, E.S.R.F.; Martins Guerra Campos, V.T.; Mendes, Y.d.S.; Sampaio Lemos, E.R.; et al. Effectiveness of household disinfection techniques to remove SARS-CoV-2 from cloth masks. Pathogens 2022, 11, 916. [Google Scholar] [CrossRef]
- Tomas, A.L.; Reichel, A.; Silva, P.M.; Silva, P.G.; Pinto, J.; Calado, I.; Campos, J.; Silva, I.; Machado, V.; Laranjeira, R.; et al. UV-C irradiation-based inactivation of SARS-CoV-2 in contaminated porous and non-porous surfaces. J. Photochem. Photobiol. B 2022, 234, 112531. [Google Scholar] [CrossRef]
- Wolfgruber, S.; Loibner, M.; Puff, M.; Melischnig, A.; Zatloukal, K. SARS-CoV2 neutralizing activity of ozone on porous and non-porous materials. New Biotechnol. 2022, 66, 36–45. [Google Scholar] [CrossRef]
- Metolina, P.; de Oliveira, L.G.; Ramos, B.; Angelo, Y.d.S.; Minoprio, P.; Silva Costa Teixeira, A.C. Evaluation of the effectiveness of UV-C dose for photoinactivation of SARS-CoV-2 in contaminated N95 respirator, surgical and cotton fabric masks. Photochem. Photobiol. Sci. 2022, 21, 1915–1929. [Google Scholar] [CrossRef]
- Gerhardts, A.; Bockmühl, D.; Kyas, A.; Hofmann, A.; Weide, M.; Rapp, I.; Höfer, D. Testing of the adhesion of herpes simplex virus on textile substrates and its inactivation by household laundry processes. J. Biosci. Med. 2016, 4, 111–125. [Google Scholar] [CrossRef]
- Ijaz, M.K.; Nims, R.W.; McKinney, J.; Gerba, C.P. Virucidal efficacy of laundry sanitizers against SARS-CoV-2 and other coronaviruses and influenza viruses. Sci. Rep. 2022, 12, 5247. [Google Scholar] [CrossRef]
- Luijkx, G.; Hild, R.; Krijnen, E.; Lodewick, R.; Rechenbach, T.; Reinhardt, G. Testing of the fabric damage properties of bleach containing detergents. Tenside Surfactants Deterg. 2004, 41, 164–168. [Google Scholar] [CrossRef]
- Tyan, K.; Jin, K.; Kang, J. Novel colour additive for bleach disinfectant wipes reduces corrosive damage on stainless steel. J. Hosp. Infect. 2019, 103, 227–230. [Google Scholar] [CrossRef]
- Rideout, K.; Teschke, K.; Dimich-Ward, H.; Kennedy, S.M. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 2005, 59, 4–11. [Google Scholar] [CrossRef]
- Schmitz, B.W.; Wang, H.W.; Schwab, K.; Jacangelo, J. Selected mechanistic aspects of viral inactivation by peracetic acid. Environ. Sci. Technol. 2021, 55, 16120–16129. [Google Scholar] [CrossRef]
- Jo, H.; West, A.M.; Teska, P.J.; Oliver, H.F.; Howarter, J.A. Assessment of early onset surface damage from accelerated disinfection protocol. Antimicrob. Resist. Infect. Control 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- MacCannell, T.; Umscheid, C.A.; Agarwal, R.K.; Lee, I.; Kuntz, G.; Stevenson, K.B.; Healthcare Infection Control Practices Advisory Committee, H. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect. Control Hosp. Epidemiol. 2011, 32, 939–969. [Google Scholar] [CrossRef]
- Lutz, E.A.; Sharma, S.; Casto, B.; Needham, G.; Buckley, T.J. Effectiveness of UV-C equipped vacuum at reducing culturable surface-bound microorganisms on carpets. Environ. Sci. Technol. 2010, 44, 9451–9455. [Google Scholar] [CrossRef]
| Treatment | Antimicrobials | Testing Methods | Evaluation of the Successful Efficacy 1 |
|---|---|---|---|
| Launderable material: | |||
| Pre-soak treatments | limited and broad-spectrum disinfectant | AOAC Use-Dilution Methods (AOAC 955.14; 955.15; 964.02) [20,21,22] | 6 log reduction in S. enterica 2 and S. aureus ≤ 10 min |
| healthcare disinfectants | AOAC Use-Dilution Methods (AOAC 955.14; 955.15; 964.02) [20,21,22] | 6 log reduction in Pseudomonas aeruginosa and S. aureus in ≤10 min | |
| Sanitizers | ASTM E1153 [23] | 3 log reduction in S. aureus and K. pneumoniae in ≤5 min | |
| Laundry additives | Disinfectants | ASTM E2274 or E2406 [24,25] | 4 log reduction in S. aureus and K. pneumoniae in a wash cycle, in addition to P. aeruginosa for healthcare facilities |
| Sanitizers | ASTM E2274 or E2406 [24,25] | 3 log reduction in S. aureus and K. pneumoniae in a wash cycle | |
| Self-sanitizing additives | AATCC Test Method 100 or ASTM E2149 [26,27] | 3 log reduction in S. aureus and K. pneumoniae in no more than 24 h intervals on a case-by-case basis | |
| Non-launderable material: | |||
| Carpet sanitizers | Not available 3 | 3 log reduction in S. aureus and E. aerogenes, in addition to P. aeruginosa for healthcare facilities | |
| Mattress, pillows and upholstered furniture treatments | Not available | Efficacy is on a case-by-case basis. Tested microorganisms are B. subtilis and C. sporogenes for claims of sterilization, S. aureus and S. enterica for claims of broad-spectrum disinfection, in addition to P. aeruginosa for healthcare facilities, S. aureus and K. pneumoniae (or E. aerogenes) for claims of sanitization | |
| Material sanitizers for fabric and textiles | ASTM E1153 [23] | 3 log reduction in S. aureus and K. pneumoniae (or E. aerogenes) | |
| Virus | Material | Key Findings on Virus Persistence 1 | Refs. |
|---|---|---|---|
| Non-enveloped virus: | |||
| Adenovirus | Cotton cloth |
| [33] |
| Avian reovirus | Cotton, wood |
| [34] |
| Bacteriophage MS2 | Polyester tablecloth, polyester carpet |
| [35,36] |
| Echovirus | Cellulose membrane |
| [37] |
| Feline calicivirus | Cotton fabric, unknown carpet, wool/nylon carpet, formica |
| [38,39] |
| Hepatitis A virus | Cotton cloth |
| [33] |
| Murine norovirus | Wool/nylon carpet, wood |
| [39,40] |
| Poliovirus | Cotton cloth, wool, cellulose membrane |
| [33,37,41,42] |
| Rotavirus | Cotton/polyester cloth |
| [33,43,44] |
| Enveloped Virus: | |||
| Avian metapneumovirus | Wood, cotton and polyester fabric, feather |
| [45] |
| Bacteriophage Phi6 | Polyester fabric with and without zinc pyrithione, wood, polyester carpet |
| [36,46,47] |
| Cytomegalovirus | Cotton blanket, sanded pine plywood and cotton cloth |
| [48,49] |
| Ebola virus | Cotton gown |
| [50] |
| Equine herpesvirus type-1 | Pinewood shavings, polyester-cotton fabric |
| [51] |
| Human coronavirus OC43 | Polyester, wool and cotton |
| [52] |
| Influenza A virus | Silver containing fabric, soft toy, wood, cotton cloth, microfiber, pinewood, duck feather (preen oil removed), polypropylene, polyester, polyamide, and polyester fabric |
| [32,45,53,54,55,56,57,58] |
| Porcine epidemic diarrhea virus | Unknown cloth |
| [59] |
| Respiratory syncytial virus | Cloth gown (cotton/polyester) |
| [60] |
| SARS-CoV | Wood board, cloth, polypropylene gown and cotton gown |
| [61,62] |
| SARS-CoV-2 | Cotton cloth, treated wood, gym pit foam, nylon and PET carpet |
| [63,64,65,66,67] |
| Vaccinia virus | Cotton, wool, industrial carpet |
| [68,69,70] |
| Virus | Materials | Disinfectants | Key Findings on Disinfection Efficacy | Refs. |
|---|---|---|---|---|
| Non-enveloped virus: | ||||
| Adenovirus | Cotton clothes | Laundry: Drying cycle and NaClO |
| [83] |
| Bacteriophage MS2 | Porous unglazed red clay coupon, cotton T-shirt, polyester carpet, wood | Non-laundry: Steam, UV-C, chlorine-based disinfectant |
| [36,84,85,86] |
| Coxsackievirus | Cellulose membrane | Non-laundry: Glutaraldehyde, chlorine |
| [37] |
| Echoviruses | Cellulose membrane | Non-laundry: Glutaraldehyde, chlorine |
| [37] |
| Feline calicivirus | Nylon, wool, and olefin polyester carpets, cotton fabric, cotton, polyester and cotton blended cloth | Non-laundry: Silver dihydrogen citrate, steam vapor, hydrogen peroxide-, chlorine-based, glutaraldehyde-based, chlorophenol/phenylphenol-based, QAC-based, and alcohol-based disinfectants, ozone |
| [87,88,89,90] |
| Foot-and-mouth disease virus | Concrete | Non-laundry: Virkon (potassium peroxymonosulfate) |
| [91] |
| Hepatitis A | Cotton clothes | Laundry: Drying cycle and NaClO |
| [83] |
| Murine norovirus | Cotton fabric | Non-laundry: Hydrogen peroxide- and chlorine-based disinfectants |
| [89] |
| Poliovirus | Cotton, wool, and nylon clothes in laundry studies; Cellulose membrane | Laundry: Detergent, washing time, and water temperature Non-laundry: Glutaraldehyde, NaClO |
| [37,92,93] |
| Rotavirus | Cotton clothes | Laundry: Detergent, washing time, and water temperature |
| [83] |
| Enveloped virus: | ||||
| African swine fever virus | Concrete | Non-laundry: Virkon (Potassium peroxymonosulfate) |
| [91] |
| Bacteriophage Phi6 | wood, concrete, polyester carpet | Non-laundry: NaClO (0.5%), steam, PurTabs® (sodium troclosene and hypochlorous acid, 1076 ppm free chlorine) |
| [36,84,94] |
| Ebola virus | Pilot seat-belt strapping | Non-laundry: QAC-based disinfectants and sodium hypochlorite |
| [95] |
| Murine hepatitis virus | Bus seat fabric | Non-laundry: Detergent, QAC-, oxide-, chlorine-based disinfectants |
| [96,97] |
| SARS-CoV-2 | Tricoline fabric, wood, fabrics from bus seat, car, hospital bed linen, hospital clothing, cotton fabric and strap flap | Laundry: Detergent, formaldehyde-based disinfectant, sodium hypochlorite, 70% alcohol Non-Laundry: UV-C, ozone, heat (40 °C) |
| [84,98,99,100,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Kimbrell, B.; Yan, R.; Fraser, A.M.; Jiang, X. Efficacy of Antimicrobials Against Enveloped and Non-Enveloped Viruses on Porous Materials: A Review. Microorganisms 2025, 13, 2827. https://doi.org/10.3390/microorganisms13122827
Huang J, Kimbrell B, Yan R, Fraser AM, Jiang X. Efficacy of Antimicrobials Against Enveloped and Non-Enveloped Viruses on Porous Materials: A Review. Microorganisms. 2025; 13(12):2827. https://doi.org/10.3390/microorganisms13122827
Chicago/Turabian StyleHuang, Jinge, Breanna Kimbrell, Runan Yan, Angela M. Fraser, and Xiuping Jiang. 2025. "Efficacy of Antimicrobials Against Enveloped and Non-Enveloped Viruses on Porous Materials: A Review" Microorganisms 13, no. 12: 2827. https://doi.org/10.3390/microorganisms13122827
APA StyleHuang, J., Kimbrell, B., Yan, R., Fraser, A. M., & Jiang, X. (2025). Efficacy of Antimicrobials Against Enveloped and Non-Enveloped Viruses on Porous Materials: A Review. Microorganisms, 13(12), 2827. https://doi.org/10.3390/microorganisms13122827






