Exploring the Interplay Between Fatigue and the Oral Microbiome: A Longitudinal Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants and Sample Collection
2.3. Lifestyle Survey and Standardized Assessments
2.4. DNA Extraction, 16S rRNA Library Preparation, and Sequencing
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The Oral–Gut Microbiome Axis in Health and Disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Truyens, M.; Lernout, H.; De Vos, M.; Laukens, D.; Lobaton, T. Unraveling the Fatigue Puzzle: Insights into the Pathogenesis and Management of IBD-Related Fatigue Including the Role of the Gut-Brain Axis. Front. Med. 2024, 11, 1424926. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Crespi, C.M.; Petersen, L.; Asher, A.; Hurvitz, S.A.; Cole, S.W. Type I Interferons, Inflammation, and Fatigue in a Longitudinal RNA Study of Women with Breast Cancer. Brain Behav. Immun. 2024, 118, 312–317. [Google Scholar] [CrossRef]
- Norton, S.A.; Blaydon, L.M.; Niehaus, M.; Miller, A.P.; Hill, P.L.; Oltmanns, T.F.; Bogdan, R. Inflammation Is Associated with Pain and Fatigue in Older Adults. Brain Behav. Immun. Health 2024, 42, 100874. [Google Scholar] [CrossRef] [PubMed]
- Latimer, K.M.; Gunther, A.; Kopec, M. Fatigue in Adults: Evaluation and Management. Am. Fam. Physician 2023, 108, 58–69. [Google Scholar] [PubMed]
- Park, N.-H.; Kang, Y.-E.; Yoon, J.-H.; Ahn, Y.-C.; Lee, E.-J.; Park, B.-J.; Son, C.-G. Comparative Study for Fatigue Prevalence in Subjects with Diseases: A Systematic Review and Meta-Analysis. Sci. Rep. 2024, 14, 23348. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Fujiwara, N.; Murakami, Y.; Ishida, S.; Kinguchi, S.; Haze, T.; Azushima, K.; Fujiwara, A.; Wakui, H.; Sakakura, M.; et al. Visualizing Fatigue Mechanisms in Non-Communicable Diseases: An Integrative Approach with Multi-Omics and Machine Learning. BMC Med. Inf. Inform. Decis. Mak. 2025, 25, 204. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F.; Weinstock, G.M.; et al. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Kapila, Y.L. Oral Health’s Inextricable Connection to Systemic Health: Special Populations Bring to Bear Multimodal Relationships and Factors Connecting Periodontal Disease to Systemic Diseases and Conditions. Periodontology 2000 2021, 87, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.F.D.; Rocchetti, G.; Lucini, L.; Lorusso, L.; Manara, E.; Bertelli, M.; Puglisi, E.; Capelli, E. Potential Role of Microbiome in Chronic Fatigue Syndrome/Myalgic Encephalomyelits (CFS/ME). Sci. Rep. 2021, 11, 7043. [Google Scholar] [CrossRef]
- Moon, J.-H.; Lee, J.-H. Probing the Diversity of Healthy Oral Microbiome with Bioinformatics Approaches. BMB Rep. 2016, 49, 662–670. [Google Scholar] [CrossRef]
- Blasche, G.; Khanaqa, T.A.K.; Wagner-Menghin, M. Mentally Demanding Work and Strain: Effects of Study Duration on Fatigue, Vigor, and Distress in Undergraduate Medical Students. Healthcare 2023, 11, 1674. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Tsiligianni, I.; Stathakis, G.; Fanaridis, M.; Koloi, A.; Bakiri, E.; Moudatsaki, M.; Pouladaki, E.; Schiza, S. Sleep Quality and Fatigue during Exam Periods in University Students: Prevalence and Associated Factors. Healthcare 2023, 11, 2389. [Google Scholar] [CrossRef]
- Underdahl, L.; Ditri, M.; Duthely, L.M. Physician Burnout: Evidence-Based Roadmaps to Prioritizing and Supporting Personal Wellbeing. J. Healthc. Leadersh. 2024, 16, 15–27. [Google Scholar] [CrossRef]
- Walters, W.A.; Caporaso, J.G.; Lauber, C.L.; Berg-Lyons, D.; Fierer, N.; Knight, R. PrimerProspector: De Novo Design and Taxonomic Analysis of Barcoded Polymerase Chain Reaction Primers. Bioinformatics 2011, 27, 1159–1161. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glockner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive Statistical, Functional and Integrative Analysis of Microbiome Data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Dyrbye, L.N.; West, C.P.; Satele, D.; Boone, S.; Tan, L.; Sloan, J.; Shanafelt, T.D. Burnout Among U.S. Medical Students, Residents, and Early Career Physicians Relative to the General U.S. Population. Acad. Med. 2014, 89, 443–451. [Google Scholar] [CrossRef]
- Dyrbye, L.; Shanafelt, T. A Narrative Review on Burnout Experienced by Medical Students and Residents. Med. Educ. 2016, 50, 132–149. [Google Scholar] [CrossRef]
- McKinley, B.; Daines, B.; Allen, M.; Pulsipher, K.; Zapata, I.; Wilde, B. Mental Health and Sleep Habits during Preclinical Years of Medical School. Sleep Med. 2022, 100, 291–297. [Google Scholar] [CrossRef]
- Matud, M.P.; Díaz, A.; Bethencourt, J.M.; Ibáñez, I. Stress and Psychological Distress in Emerging Adulthood: A Gender Analysis. J. Clin. Med. 2020, 9, 2859. [Google Scholar] [CrossRef]
- Maslach, C. Understanding Job Burnout. In Stress and Quality of Working Life: Current Perspectives; Emerald Publishing: Bingley, UK, 2006. [Google Scholar]
- Lin, Y.K.; Lin, C.-D.; Lin, B.Y.-J.; Chen, D.-Y. Medical Students’ Resilience: A Protective Role on Stress and Quality of Life in Clerkship. BMC Med. Educ. 2019, 19, 473. [Google Scholar] [CrossRef]
- West, C.P.; Dyrbye, L.N.; Sinsky, C.; Trockel, M.; Tutty, M.; Nedelec, L.; Carlasare, L.E.; Shanafelt, T.D. Resilience and Burnout Among Physicians and the General US Working Population. JAMA Netw. Open 2020, 3, e209385. [Google Scholar] [CrossRef]
- Nel Van Zyl, K.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of Antibiotics on the Human Microbiome: A Systematic Review. Int. J. Antimicrob. Agents 2022, 59, 106502. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in Healthy and Diseased Individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Menting, J.; Tack, C.J.; Bleijenberg, G.; Donders, R.; Droogleever Fortuyn, H.A.; Fransen, J.; Goedendorp, M.M.; Kalkman, J.S.; Strik-Albers, R.; van Alfen, N.; et al. Is Fatigue a Disease-Specific or Generic Symptom in Chronic Medical Conditions? Health Psychol. 2018, 37, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J.; Braamse, A.M.J.; Spruit, M.A.; Janssen, D.J.A.; Ebadi, Z.; Van Herck, M.; Burtin, C.; Peters, J.B.; Sprangers, M.A.G.; Lamers, F.; et al. Fatigue in Patients with Chronic Disease: Results from the Population-Based Lifelines Cohort Study. Sci. Rep. 2021, 11, 20977. [Google Scholar] [CrossRef] [PubMed]
- Shoeleh, C.; Sandstrom, R.; Pierce, D.P.; Patel, T. Evolution and Effects of Caffeine Utilization Throughout Medical and Surgical Training. Cureus 2025, 17, e77600. [Google Scholar] [CrossRef]
- Oreskovich, M.R.; Shanafelt, T.; Dyrbye, L.N.; Tan, L.; Sotile, W.; Satele, D.; West, C.P.; Sloan, J.; Boone, S. The Prevalence of Substance Use Disorders in American Physicians. Am. J. Addict. 2015, 24, 30–38. [Google Scholar] [CrossRef]
- Zhou, S.; Van Devanter, N.; Fenstermaker, M.; Cawkwell, P.; Sherman, S.; Weitzman, M. A Study of the Use, Knowledge, and Beliefs About Cigarettes and Alternative Tobacco Products Among Students at One U.S. Medical School. Acad. Med. 2015, 90, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Czeisler, C.A.; Shea, S.A. Resident Physician Extended Work Hours and Burnout. Sleep 2018, 41, zsy112. [Google Scholar] [CrossRef]
- Claponea, R.M.; Pop, L.M.; Iorga, M.; Iurcov, R. Symptoms of Burnout Syndrome among Physicians during the Outbreak of COVID-19 Pandemic-A Systematic Literature Review. Healthcare 2022, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, E.G.; Mériaux, S.B.; Guebels, P.; Muller, C.P.; Leenen, F.A.D.; Elwenspoek, M.M.C.; Thiele, I.; Hertel, J.; Turner, J.D. The Oral Microbiome Is Associated with HPA Axis Response to a Psychosocial Stressor. Sci. Rep. 2024, 14, 15841. [Google Scholar] [CrossRef] [PubMed]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Merlo, G.; Rippe, J. Physician Burnout: A Lifestyle Medicine Perspective. Am. J. Lifestyle Med. 2021, 15, 148–157. [Google Scholar] [CrossRef]
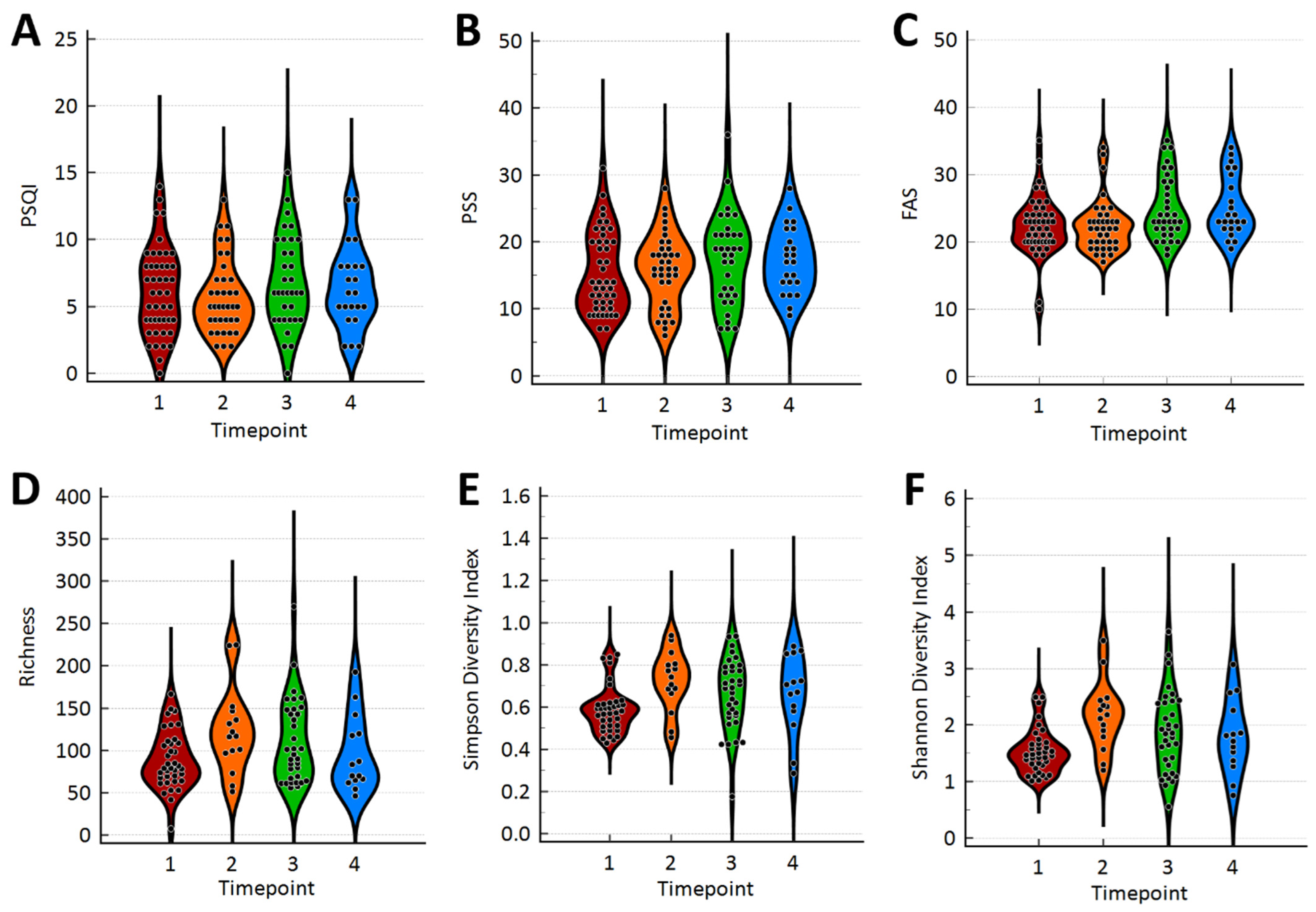
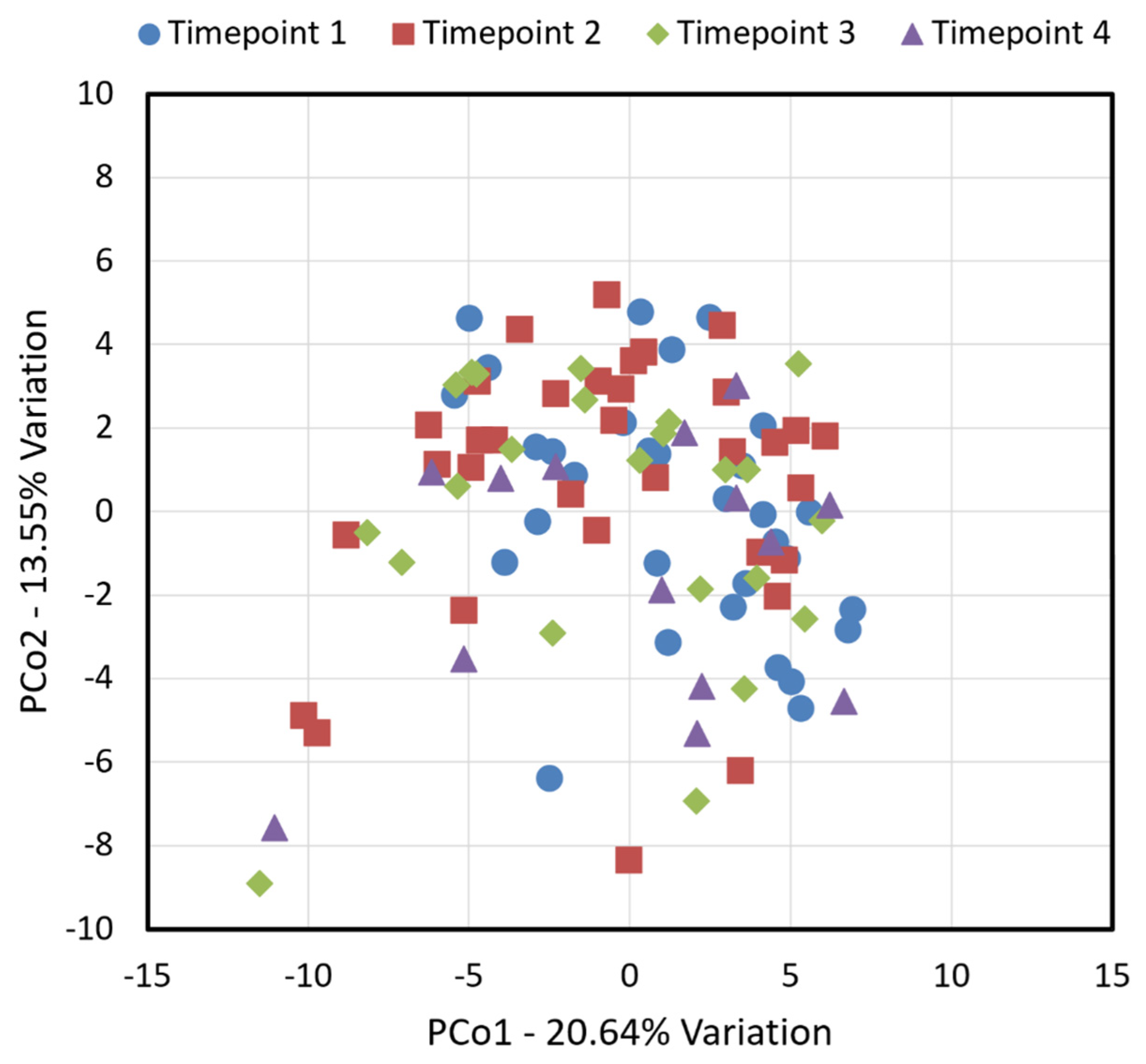
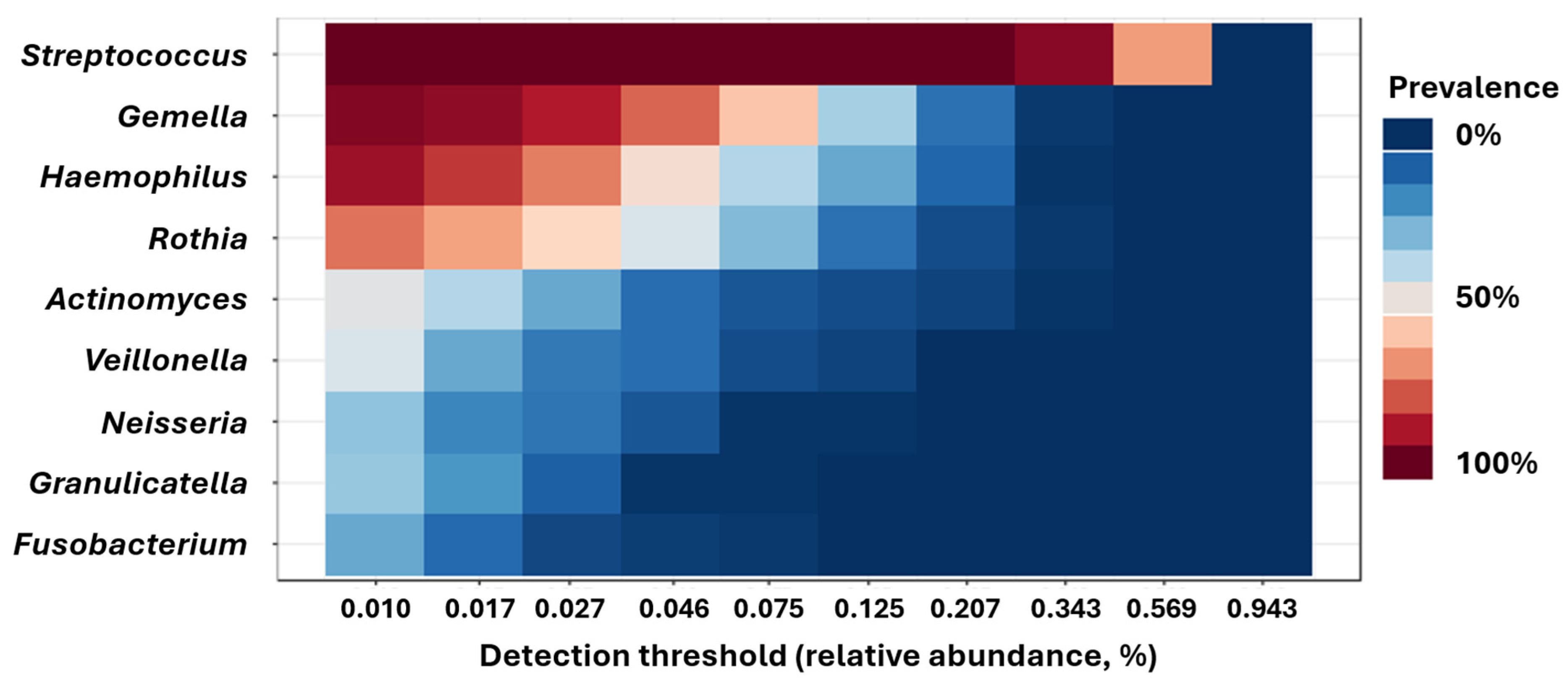
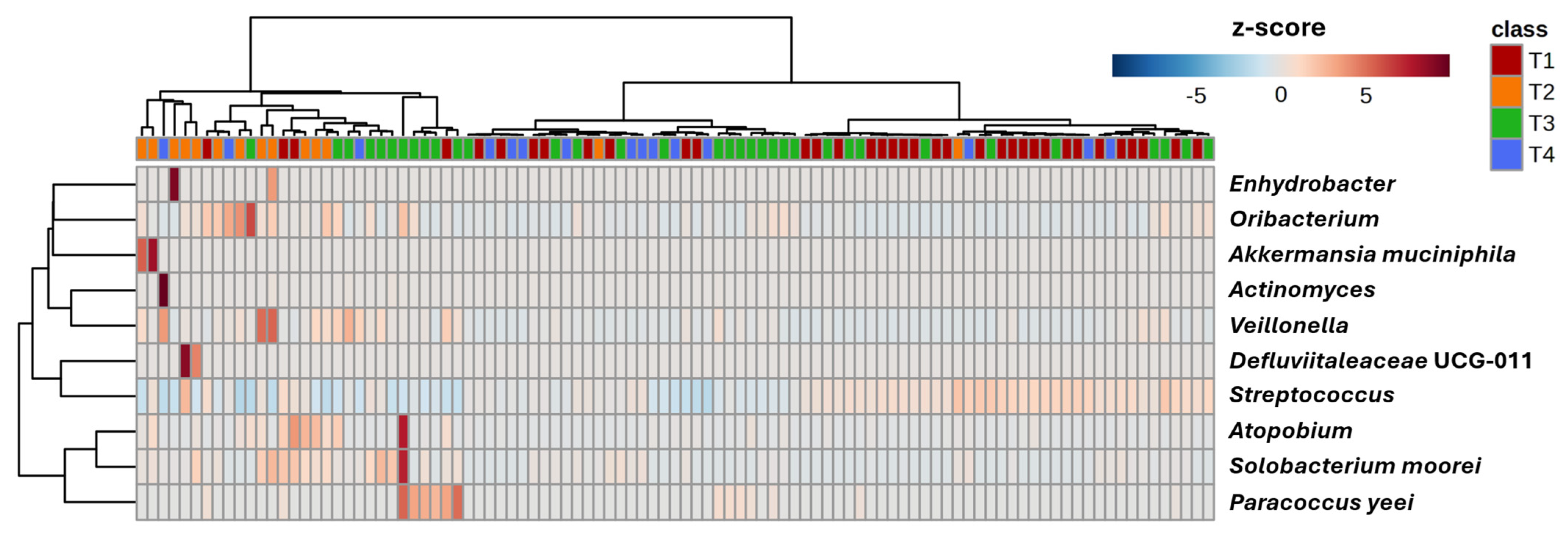
| Timepoint 1 (Baseline) N = 45 | Timepoint 2 N = 37 | Timepoint 3 N = 34 | Timepoint 4 N = 23 | |||||
|---|---|---|---|---|---|---|---|---|
| Freq | % | Freq | % | Freq | % | Freq | % | |
| Consume Coffee | ||||||||
| No | 9 | 20 | 12 | 32.43 | 9 | 26.47 | 9 | 39.13 |
| Yes | 36 | 80 | 25 | 67.57 | 25 | 73.53 | 14 | 60.87 |
| Consume Energy Drinks | ||||||||
| No | 28 | 62.22 | 21 | 56.76 | 16 | 47.06 | 13 | 56.52 |
| Yes | 17 | 37.78 | 16 | 43.24 | 18 | 52.94 | 10 | 43.48 |
| Consume Tea | ||||||||
| No | 24 | 53.33 | 24 | 64.86 | 19 | 55.88 | 11 | 47.83 |
| Yes | 21 | 46.67 | 13 | 35.14 | 15 | 44.12 | 12 | 52.17 |
| Consume Other Caffeine | ||||||||
| No | 36 | 80 | 33 | 89.19 | 31 | 91.18 | 22 | 95.65 |
| Yes | 9 | 20 | 4 | 10.81 | 3 | 8.82 | 1 | 4.35 |
| Consume Beer | ||||||||
| No | 28 | 62.22 | 24 | 66.67 | 25 | 73.53 | 17 | 73.91 |
| Yes | 17 | 37.78 | 12 | 33.33 | 9 | 26.47 | 6 | 26.09 |
| Consume Wine | ||||||||
| No | 34 | 75.56 | 34 | 91.89 | 30 | 88.24 | 23 | 100 |
| Yes | 11 | 24.44 | 3 | 8.11 | 4 | 11.76 | 0 | 0 |
| Consume Hard Liquor | ||||||||
| No | 32 | 71.11 | 34 | 91.89 | 27 | 79.41 | 21 | 91.3 |
| Yes | 13 | 28.89 | 3 | 8.11 | 7 | 20.59 | 2 | 8.7 |
| Consume Other Alcoholic drinks | ||||||||
| No | 42 | 93.33 | 36 | 97.3 | 34 | 100 | 23 | 100 |
| Yes | 3 | 6.67 | 1 | 2.7 | 0 | 0 | 0 | 0 |
| Consume Nicotine products | ||||||||
| No | 44 | 97.78 | 36 | 97.3 | 34 | 100 | 23 | 100 |
| Yes | 1 | 2.22 | 1 | 2.7 | 0 | 0 | 0 | 0 |
| Timepoint 2 N = 37 | Timepoint 3 N = 34 | Timepoint 4 N = 23 | ||||
|---|---|---|---|---|---|---|
| Freq | % | Freq | % | Freq | % | |
| Environmental changes from previous | ||||||
| No | 29 | 78.38 | 31 | 91.18 | 16 | 69.57 |
| Yes | 8 | 21.62 | 3 | 8.82 | 7 | 30.43 |
| Suffered any illness | ||||||
| No | 33 | 89.19 | 24 | 70.59 | 14 | 60.87 |
| Yes | 4 | 10.81 | 10 | 29.41 | 9 | 39.13 |
| Took time off because of illness | ||||||
| No | 3 | 75 | 7 | 70 | 8 | 88.89 |
| Yes | 1 | 25 | 3 | 30 | 1 | 11.11 |
| Used Antibiotics | ||||||
| No | 4 | 100 | 8 | 80 | 7 | 77.78 |
| Yes | 0 | 0 | 2 | 20 | 2 | 22.22 |
| Added a new medication | ||||||
| No | 33 | 89.19 | 29 | 85.29 | 15 | 65.22 |
| Yes | 4 | 10.81 | 5 | 14.71 | 8 | 34.78 |
| Raw | Adjusted by Demographics (Age, BMI, Gender, Previous Experience, Relocation, Living Arrangement Setting, and Diet) | Adjusted by Consumption Patterns (Caffeine, Alcohol, and Nicotine Product Consumption) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Error | p-Value | Estimate | Standard Error | p-Value | Estimate | Standard Error | p-Value | ||
| Richness | Timepoint | |||||||||
| Timepoint 1 | 86.641 | 7.357 | 0.0340 | 73.416 | 16.190 | 0.0382 | 90.297 | 10.296 | 0.1138 | |
| Timepoint 2 | 121.820 | 11.435 | 102.320 | 18.511 | 120.720 | 15.039 | ||||
| Timepoint 3 | 112.590 | 7.657 | 99.279 | 16.330 | 111.460 | 11.167 | ||||
| Timepoint 4 | 100.450 | 12.344 | 81.985 | 20.113 | 97.411 | 15.238 | ||||
| Standardized Assessment Tools | ||||||||||
| PSQI | 0.597 | 1.624 | 0.7144 | 0.674 | 1.668 | 0.6875 | 1.697 | 1.707 | 0.3251 | |
| FAS | −1.807 | 1.103 | 0.1069 | −2.147 | 1.066 | 0.0488 | −1.280 | 1.204 | 0.2931 | |
| PSS | 0.040 | 0.888 | 0.9644 | −0.278 | 0.884 | 0.7542 | 0.206 | 0.922 | 0.8238 | |
| Simpson Diversity Index | Timepoint | |||||||||
| Timepoint 1 | 0.585 | 0.025 | 0.0206 | 0.490 | 0.054 | 0.0087 | 0.570 | 0.035 | 0.0694 | |
| Timepoint 2 | 0.720 | 0.039 | 0.630 | 0.062 | 0.680 | 0.052 | ||||
| Timepoint 3 | 0.668 | 0.026 | 0.574 | 0.055 | 0.655 | 0.038 | ||||
| Timepoint 4 | 0.665 | 0.042 | 0.587 | 0.068 | 0.655 | 0.052 | ||||
| Standardized Assessment Tools | ||||||||||
| PSQI | 0.000 | 0.006 | 0.9689 | −0.005 | 0.006 | 0.3324 | 0.001 | 0.006 | 0.8392 | |
| FAS | −0.001 | 0.004 | 0.7856 | −0.003 | 0.004 | 0.4802 | −0.002 | 0.004 | 0.5639 | |
| PSS | −0.003 | 0.003 | 0.2592 | −0.002 | 0.003 | 0.5666 | −0.003 | 0.003 | 0.3360 | |
| Shannon Diversity Index | Timepoint | |||||||||
| Timepoint 1 | 1.516 | 0.099 | 0.0049 | 1.166 | 0.222 | 0.0046 | 1.507 | 0.140 | 0.0297 | |
| Timepoint 2 | 2.152 | 0.154 | 1.774 | 0.254 | 2.045 | 0.205 | ||||
| Timepoint 3 | 1.892 | 0.103 | 1.548 | 0.224 | 1.867 | 0.152 | ||||
| Timepoint 4 | 1.838 | 0.166 | 1.509 | 0.276 | 1.807 | 0.208 | ||||
| Standardized Assessment Tools | ||||||||||
| PSQI | 0.006 | 0.022 | 0.7723 | −0.011 | 0.023 | 0.6212 | 0.017 | 0.023 | 0.4557 | |
| FAS | −0.006 | 0.015 | 0.6724 | −0.012 | 0.015 | 0.3991 | −0.009 | 0.016 | 0.5712 | |
| PSS | −0.012 | 0.012 | 0.3014 | −0.006 | 0.012 | 0.6012 | −0.010 | 0.013 | 0.4191 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presutti, L.; Gueningsman, M.C.; Fredericksen, B.; Smith, A.; Taylor, R.; Tuckett, A.; Folsom, C.; Wainwright, R.; Klena, C.; Ericsson, A.C.; et al. Exploring the Interplay Between Fatigue and the Oral Microbiome: A Longitudinal Approach. Microorganisms 2025, 13, 2721. https://doi.org/10.3390/microorganisms13122721
Presutti L, Gueningsman MC, Fredericksen B, Smith A, Taylor R, Tuckett A, Folsom C, Wainwright R, Klena C, Ericsson AC, et al. Exploring the Interplay Between Fatigue and the Oral Microbiome: A Longitudinal Approach. Microorganisms. 2025; 13(12):2721. https://doi.org/10.3390/microorganisms13122721
Chicago/Turabian StylePresutti, Laura, Madison C. Gueningsman, Blake Fredericksen, Andrew Smith, Ryan Taylor, Austin Tuckett, Christina Folsom, Rachel Wainwright, Christian Klena, Aaron C. Ericsson, and et al. 2025. "Exploring the Interplay Between Fatigue and the Oral Microbiome: A Longitudinal Approach" Microorganisms 13, no. 12: 2721. https://doi.org/10.3390/microorganisms13122721
APA StylePresutti, L., Gueningsman, M. C., Fredericksen, B., Smith, A., Taylor, R., Tuckett, A., Folsom, C., Wainwright, R., Klena, C., Ericsson, A. C., Zapata, I., & Brooks, A. E. (2025). Exploring the Interplay Between Fatigue and the Oral Microbiome: A Longitudinal Approach. Microorganisms, 13(12), 2721. https://doi.org/10.3390/microorganisms13122721







