Role of Açaí (Euterpe oleracea) in Modulating the Immune Response During Experimental Oral Infection with Trypanosoma cruzi
Abstract
1. Introduction
2. Materials and Methods
2.1. Açaí
2.2. Animals and Infection with T. cruzi
2.3. Parasitemia Curve Assessment
2.4. Survival RateEvaluation
2.5. Euthanasia and Tissue Sampling
2.6. Parasite Burden
2.7. Morphometric Analyses
2.8. Cytokine Production Quantification
2.9. Mass Spectrometry for Proteomics Analysis
2.10. In-Solution Digestion
2.11. Liquid Chromatography-Mass Spectrometry
2.12. Analysis of Proteomic Data
2.13. Statistical Analysis
3. Results
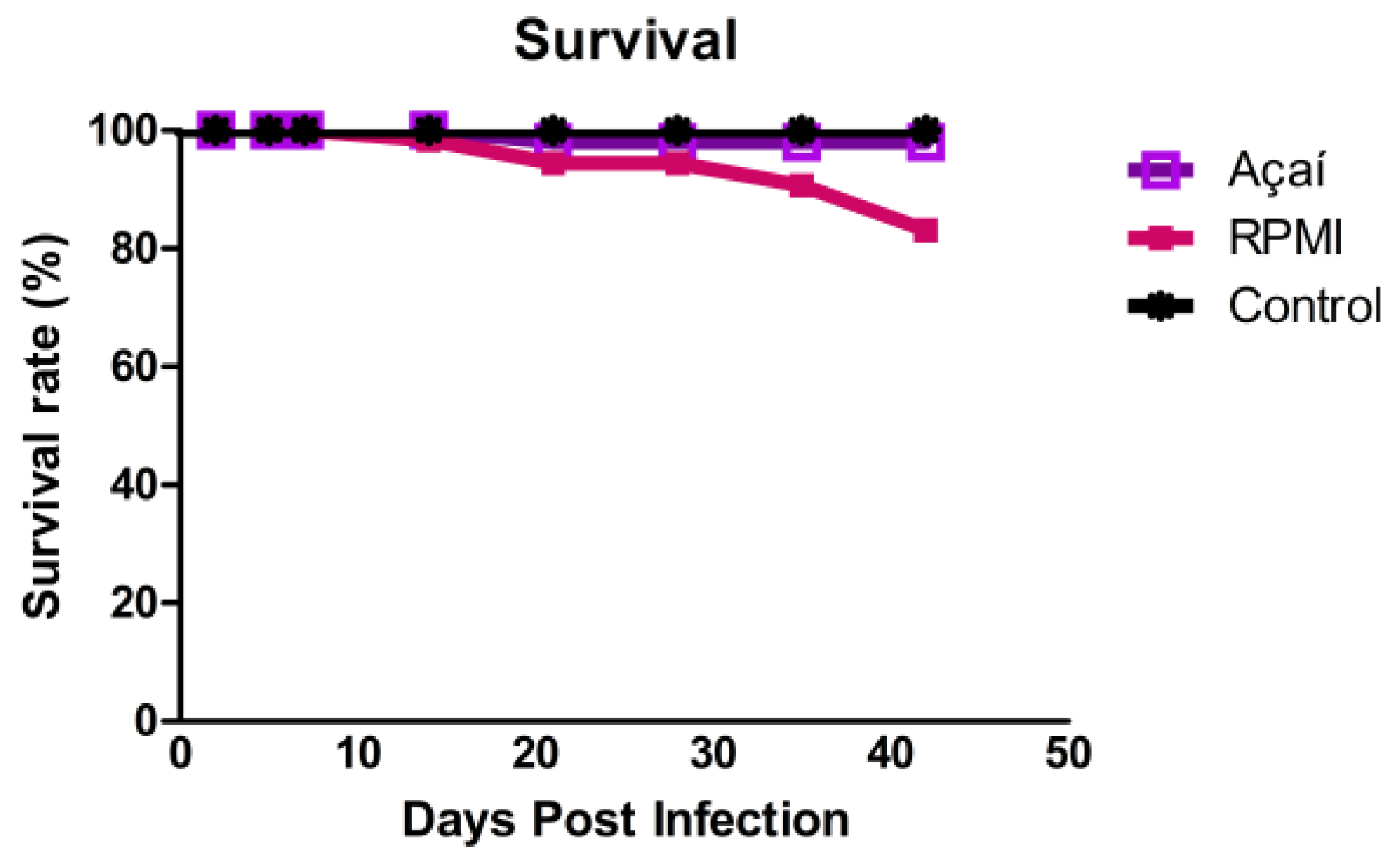
3.1. Survival Rate Outcomes
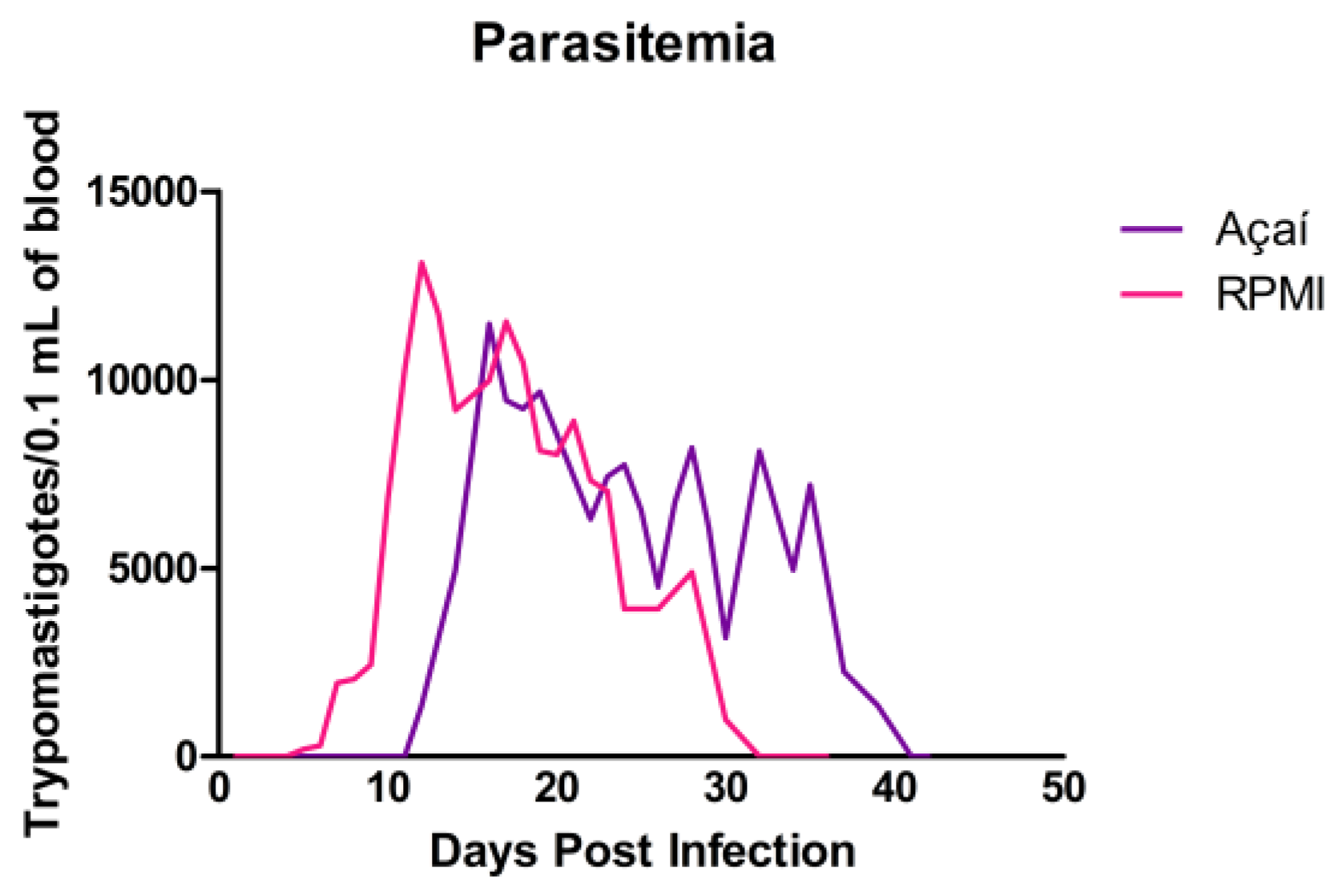
3.2. Parasitemia Curve Results
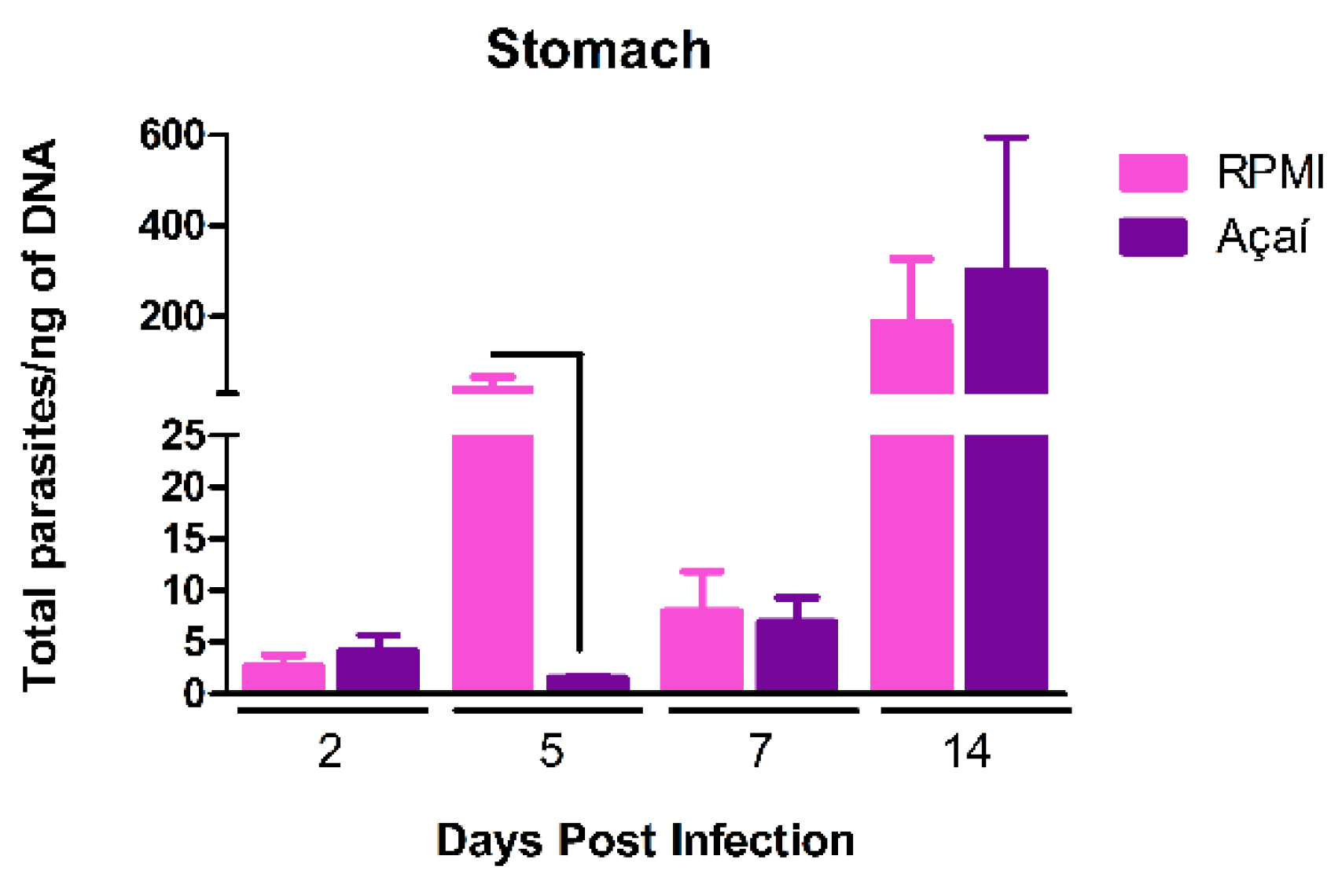
3.3. Parasitic Load
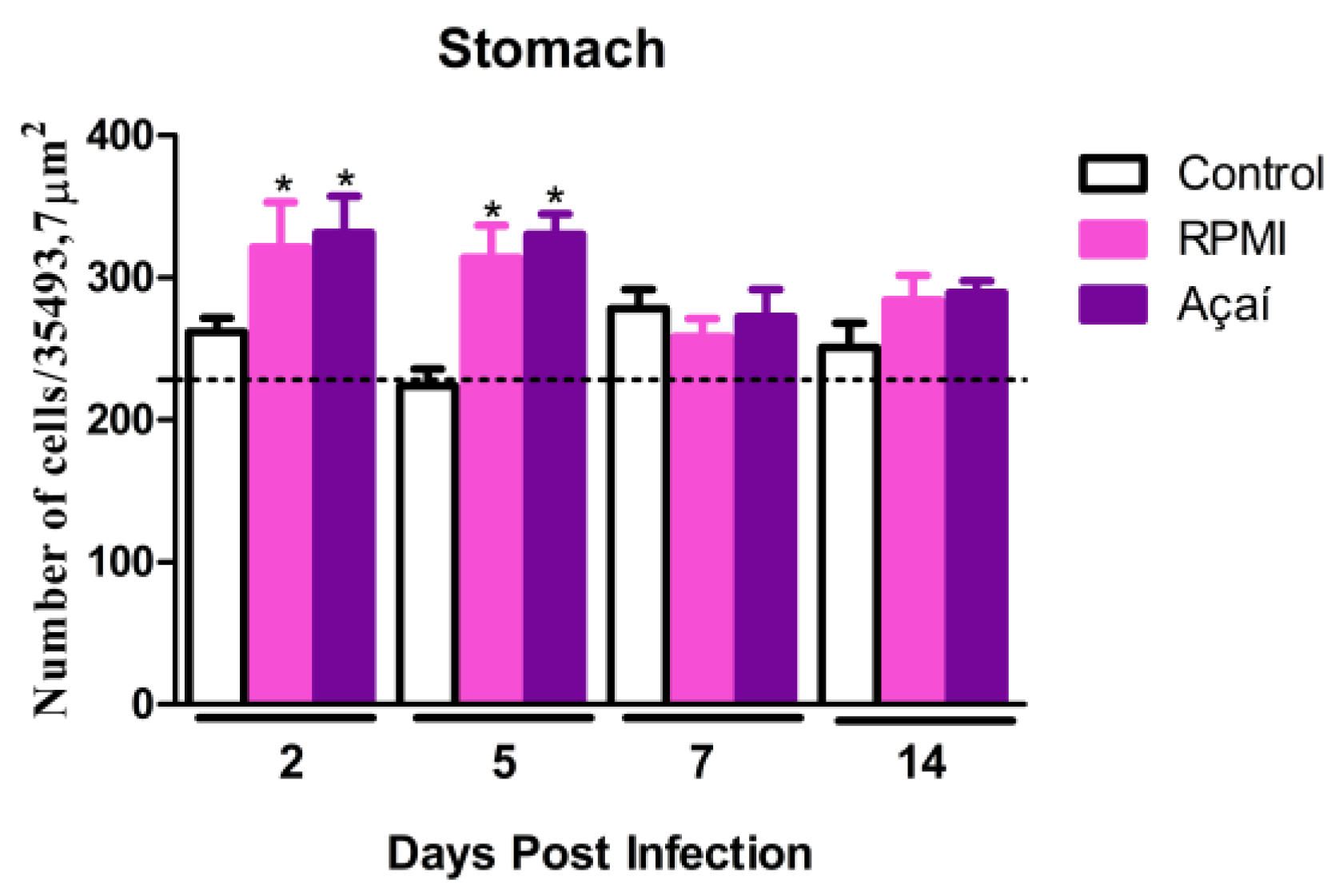
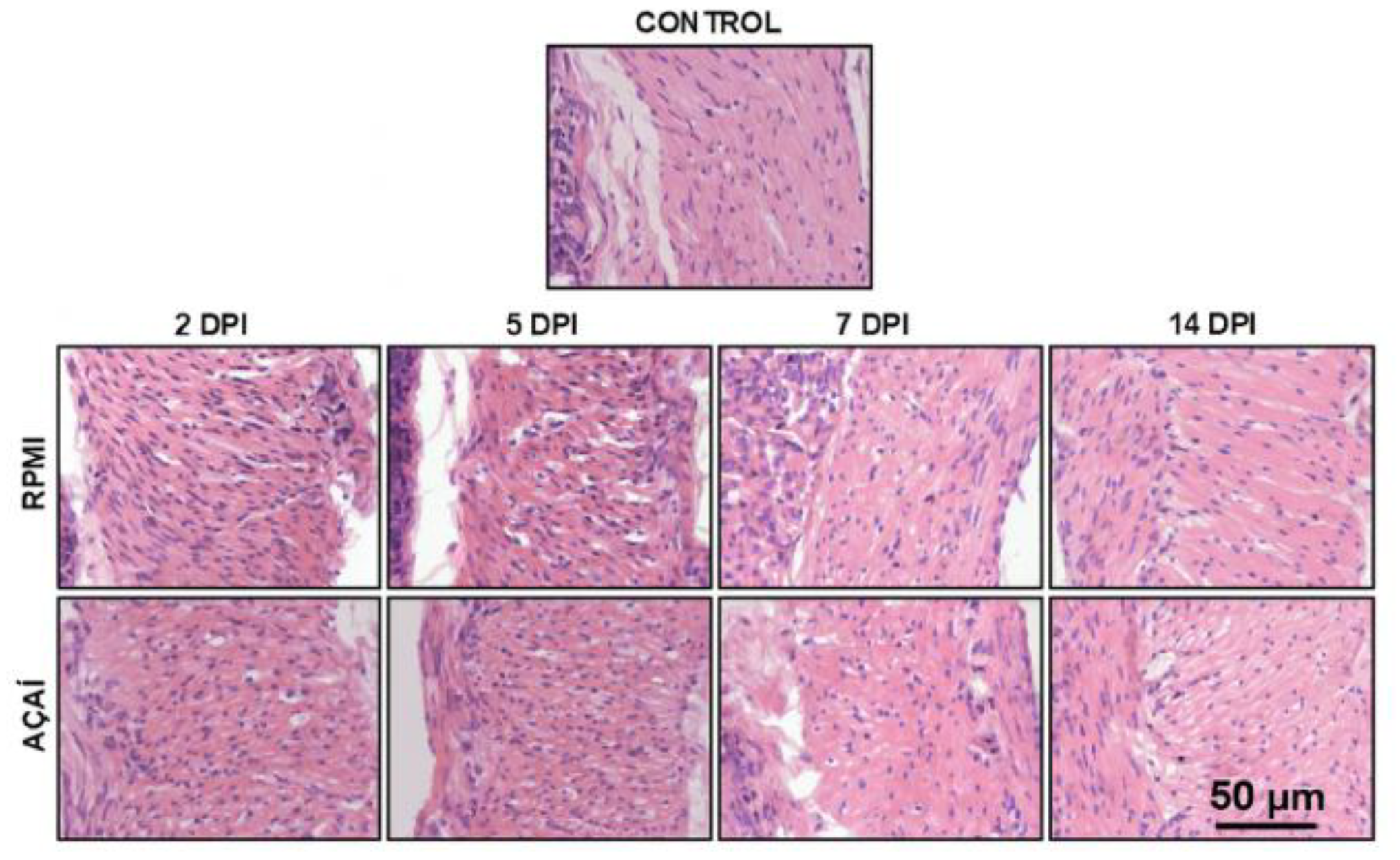
3.4. Inflammatory Process
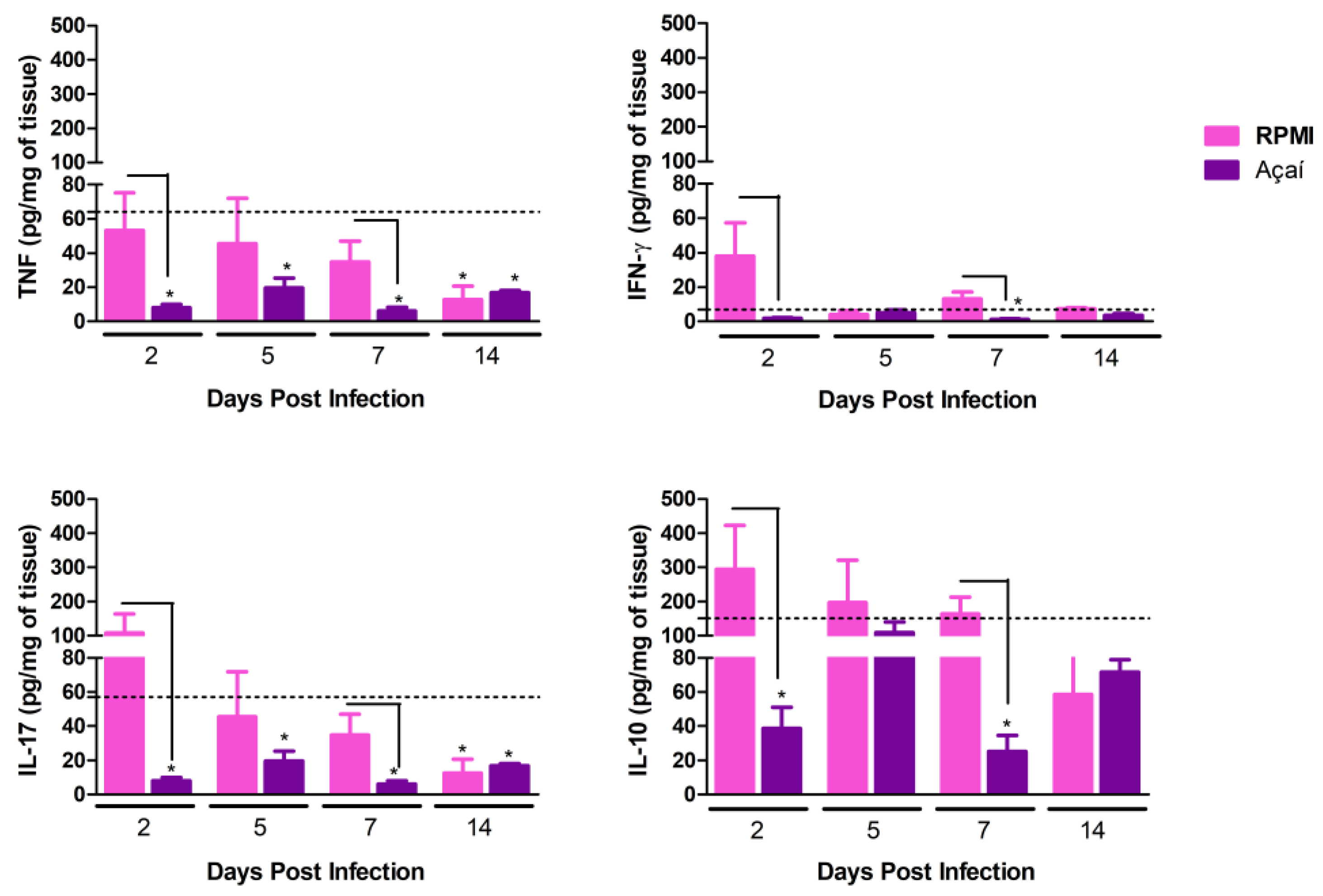
3.5. Cytokine Production
3.6. Stomach Proteomic Analysis Based on Mass Spectrometry
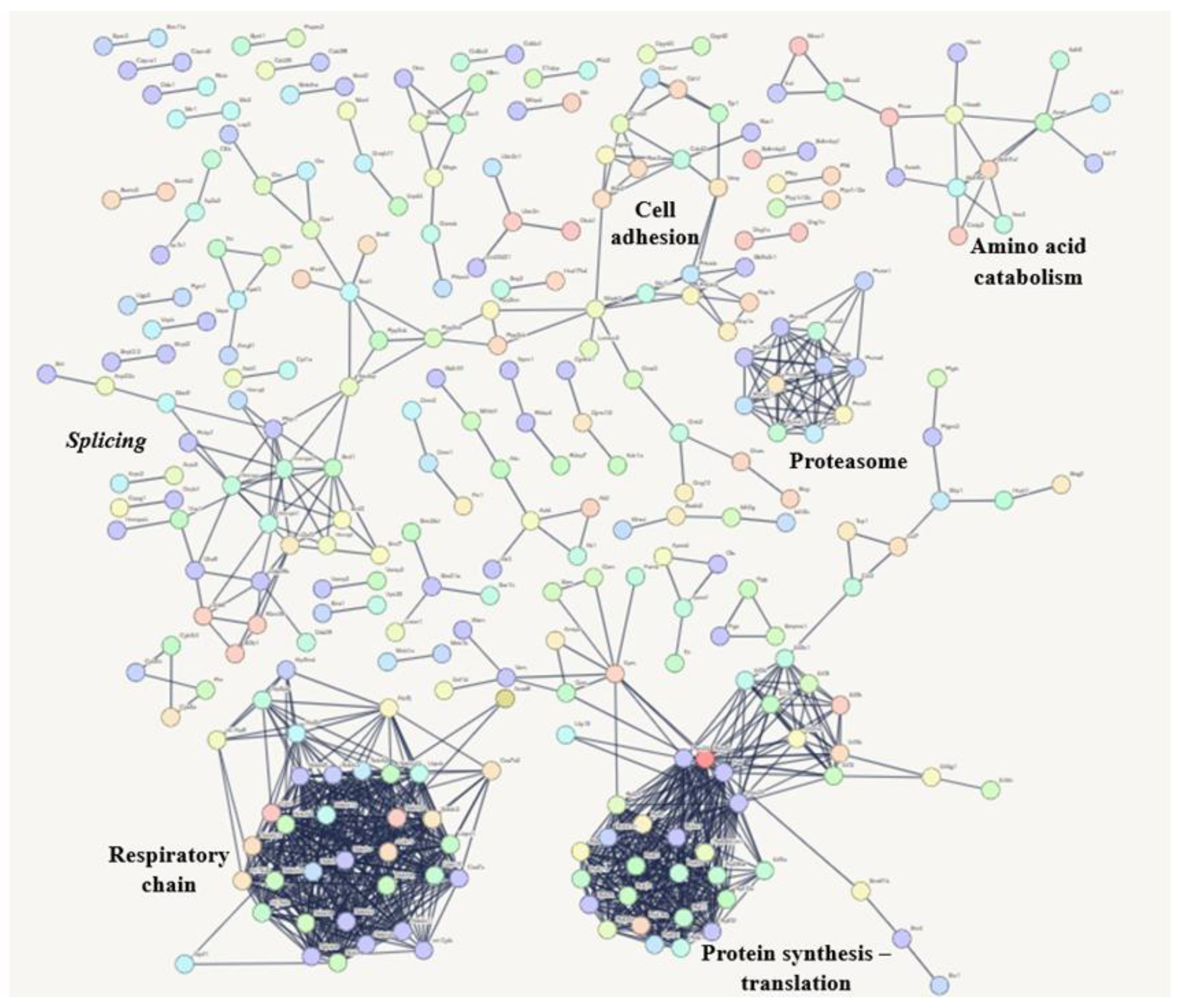
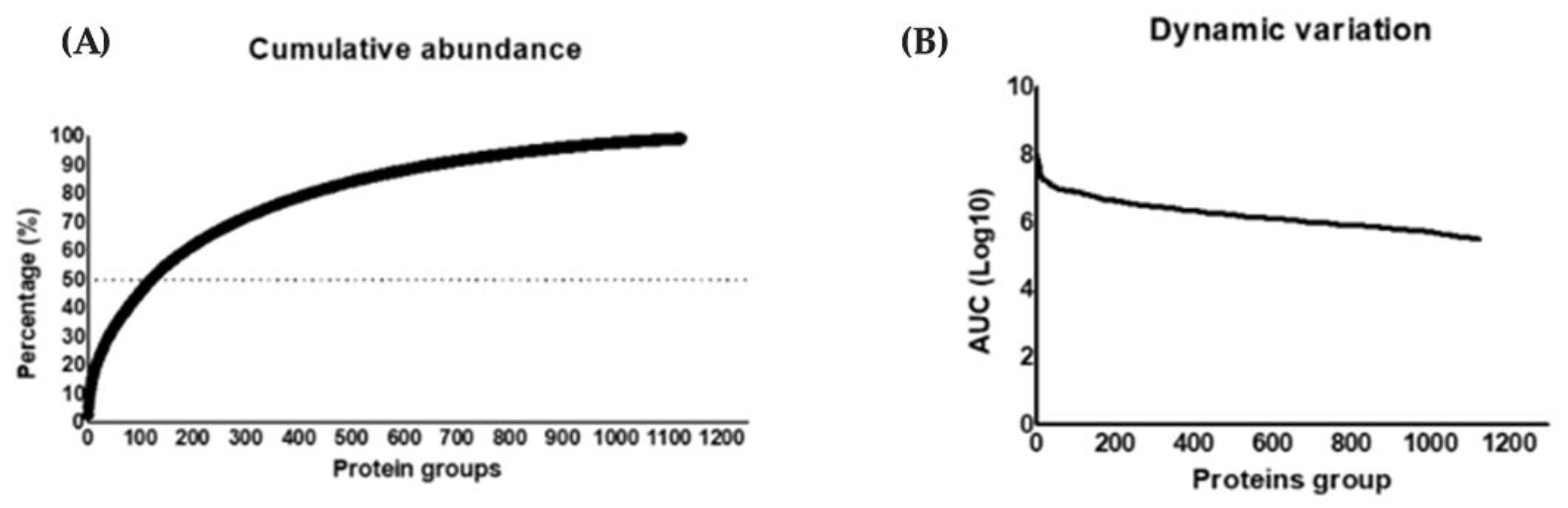
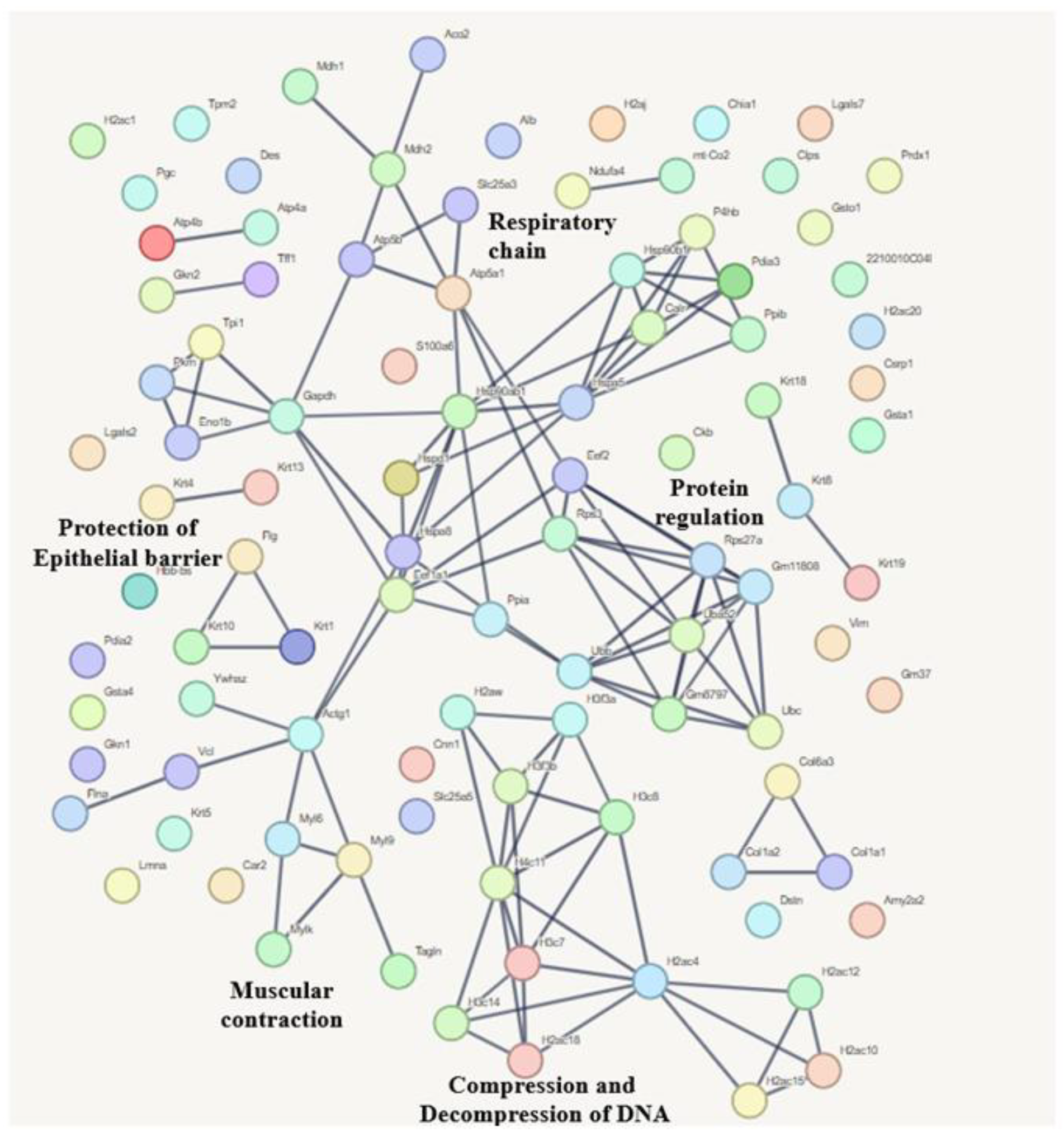
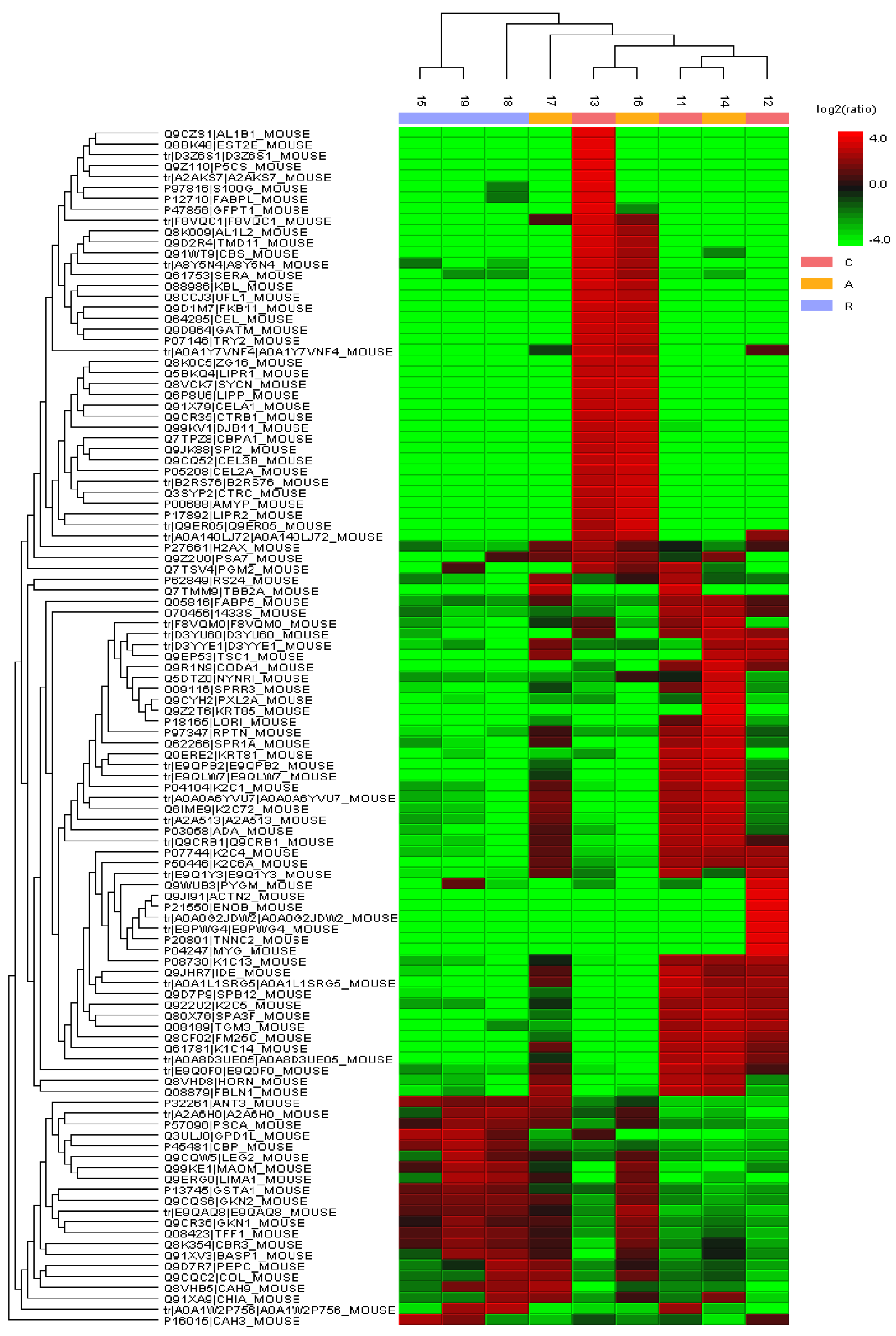
3.6.1. Compositional Analysis
3.6.2. Quantitative Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | Cytometric Bead Array |
| CCA | Ciência Animal (Universidade Federal de Ouro Preto) |
| CONCEA | Conselho Nacional de Controle de Experimentação Animal |
| DDA | Data-Dependent Acquisition |
| DPI | Days Post Infection |
| FDR | False Discovery Rate |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| NCE | Normalized Collision Energy |
| NUPEB | Núcleo de Pesquisas em Ciências Biológicas |
| TFA | Trifluoroacetic Acid (solution)~ |
| T. cruzi | Trypanosoma cruzi |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| WHO | World Health Organization |
References
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., agente etiológico de nova entidade mórbida do homem. Mem. Inst. Oswaldo Cruz. 1909, 1, 159–218. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease: Key Facts; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease (accessed on 10 February 2024).
- Brener, Z. Biology of Trypanosoma cruzi. Ann. Rev. in Microbiol. 1973, 27, 347–382. [Google Scholar] [CrossRef]
- Coura, J.R.; Dias, J.C.P. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem. Inst. Oswaldo Cruz. 2009, 104, 31–40. [Google Scholar] [CrossRef]
- Coura, J.R.; Viñas, P.A. Chagas disease: A new worldwide challenge. Nature 2010, 465, S6–S7. [Google Scholar] [CrossRef]
- Schmunis, G.A. Epidemiology of Chagas disease in non endemic countries: The role of international migration. Mem. Inst. Oswaldo Cruz. 2007, 102, 75–86. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Brisola, M.C.; Guedes, L.; Siqueira, G.; Barone, A.; Dias, J.C.P.; Neto, V.A.; Tolezano, J.; Peres, B.A.; Júnior, E.R.A.; et al. Possible oral transmission of acute Chagas disease in Brazil. Rev. Inst. Med. Trop. São Paulo. 1991, 33, 351–357. [Google Scholar] [CrossRef]
- Steindel, M.; Pacheco, L.K.; Scholl, D.; Soares, M.; de Moraes, M.H.; Eger, I.; Kosmann, C.; Sincero, T.C.M.; Stoco, P.H.; Murta, S.M.F.; et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn. Microbiol. Infect. Dis. 2008, 60, 25–32. [Google Scholar] [CrossRef]
- Nóbrega, A.A.; Garcia, M.H.; Tatto, E.; Obara, M.T.; Costa, E.; Sobel, J.; Araujo, W.N. Oral transmission of Chagas disease by consumption of açaí palm fruit, Brazil. Emerging Infect. Dis. 2009, 15, 653–655. [Google Scholar] [CrossRef]
- Valente, S.A.d.S.; da Costa, V.V.; das Neves Pinto, A.Y.; de Jesus Barbosa César, M.; dos Santos, M.P.; Miranda, C.O.S.; Cuervo, P.; Fernandes, O. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: Human cases, triatomines, reservoir mammals and parasites. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Alarcón de Noya, B.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Zavala-Jaspe, R.; Suarez, J.A.; Abate, T.; Naranjo, L.; Paiva, M.; et al. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J. Infect. Dis. 2010, 201, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions–A comprehensive review. Mem. Inst. Oswaldo Cruz. 2014, 110, 277–282. [Google Scholar]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi, A., Jr.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America–Public health and travel medicine importance. Travel Med. Infect. Dis. 2020, 36, 101565. [Google Scholar] [CrossRef]
- Bastos, C.J.C.; Aras, R.; Mota, G.; Reis, F.; Dias, J.P.; de Jesus, R.S.; Freire, M.S.; De Araújo, E.G.; Prazeres, J.; Grassi, M.F.R. Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl. Trop. Dis. 2010, 4, e651. [Google Scholar] [CrossRef]
- Oliveira, M.; de Farias Neto, J.; Nogueira, O.; Rogez, H. Açaí: Manejo, Produção e Processamento; Embrapa: Belém, Brazil, 2006. [Google Scholar]
- Carvalho, L.M.; de Carvalho, T.V.; Ferraz, A.T.; Marques, F.d.S.; Roatt, B.M.; Fonseca, K.d.S.; Reis, L.E.S.; Carneiro, C.M.; Vieira, P.M.d.A. Histopathological changes in the gastrointestinal tract and systemic alterations triggered by experimental oral infection with Trypanosoma cruzi. Exp. Parasitol. 2020, 218, 108012. [Google Scholar] [CrossRef]
- Barreto-De-Albuquerque, J.; Silva-Dos-Santos, D.; Pérez, A.R.; Berbert, L.R.; De Santana-Van-Vliet, E.; Farias-De-Oliveira, D.A.; Moreira, O.C.; Roggero, E.; De Carvalho-Pinto, C.E.; Jurberg, J.; et al. Trypanosoma cruzi infection through the oral route promotes a severe infection in mice: New disease form from an old infection? PLoS Negl. Trop. Dis. 2015, 9, e0003849. [Google Scholar] [CrossRef]
- Santana, R.A.G.; Guerra, M.G.V.B.; Sousa, D.R.; Couceiro, K.; Ortiz, J.V.; Oliveira, M.; Ferreira, L.S.; Souza, K.R.; Tavares, I.C.; Morais, R.F. Oral transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg. Infect. Dis. 2019, 25, 132–135. [Google Scholar]
- De Lima Yamaguchi, K.K.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon açaí: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 1962, 4, 389–396. [Google Scholar]
- Coscia, F.; Doll, S.; Bech, J.M.; Schweizer, L.; Mund, A.; Lengyel, E.; Lindebjerg, J.; Madsen, G.I.; Moreira, J.M.A.; Mann, M. A streamlined mass spectrometry–based proteomics workflow for large-scale FFPE tissue analysis. J. Pathol. 2020, 1, 100–112. [Google Scholar]
- Brasil, Ministério da Saúde. Sistema de Informação de Agravos de Notificação (SINAN)–Doença de Chagas: Dados Epidemiológicos 2021; Ministério da Saúde: Brasília, Brazil, 2021. Available online: https://www.gov.br/saude/pt-br (accessed on 10 February 2024).
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, L.V. American trypanosomiasis (Chagas disease)—A tropical disease now in the United States. N. Engl. J. Med. 1993, 329, 639–644. [Google Scholar]
- Hoft, D.F.; Farrar, P.L.; Kratz-Owens, K.; Shaffer, D. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect. Immun. 1996, 64, 3800–3810. [Google Scholar] [CrossRef]
- Camandaroba, E.L.P.; Pinheiro Lima, C.M.; Andrade, S.G. Oral transmission of Chagas disease: Importance of Trypanosoma cruzi biodeme in the intragastric experimental infection. Rev. Inst. Med. Trop. São Paulo 2002, 44, 97–103. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Fonseca, K.d.S.; Perin, L.; de Paiva, N.C.N.; da Silva, B.C.; Duarte, T.H.C.; Marques, F.d.S.; Costa, G.d.P.; Molina, I.; Correa-Oliveira, R.; Vieira, P.M.d.A.; et al. Benznidazole treatment: Time- and dose-dependence varies with the Trypanosoma cruzi strain. Pathogens 2021, 10, 729. [Google Scholar] [CrossRef]
- Marsden, P. Trypanosoma cruzi infections in CFI mice: II—Infections induced by different routes. Ann. Trop. Med. Parasitol. 1967, 61, 62–67. [Google Scholar] [CrossRef]
- Caradonna, K.; PereiraPerrin, M. Preferential brain homing following intranasal administration of Trypanosoma cruzi. Infect. Immun. 2009, 77, 1349–1356. [Google Scholar] [CrossRef]
- De Meis, J.; de Albuquerque, J.B.; dos Santos, D.S.; Farias-De-Oliveira, D.A.; Berbert, L.R.; Cotta-De-Almeida, V.; Savino, W. Trypanosoma cruzi entrance through systemic or mucosal infection sites differentially modulates regional immune response following acute infection in mice. Front. Immunol. 2013, 4, 216. [Google Scholar] [CrossRef]
- Dias, G.B.M.; Gruendling, A.P.; Araújo, S.M.; Gomes, M.L.; de Ornelas Toledo, M.J. Evolution of infection in mice inoculated by the oral route with different developmental forms of Trypanosoma cruzi I and II. Exp. Parasitol. 2013, 135, 511–517. [Google Scholar] [CrossRef]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef]
- Tosello Boari, J.; Amezcua Vesely, M.C.; Bermejo, D.A.; Ramello, M.C.; Montes, C.L.; Cejas, H.; Gruppi, A.; Rodríguez, E.V.A. IL-17RA signaling reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL-10-producing neutrophils. PLoS Pathog. 2012, 8, e1002658. [Google Scholar] [CrossRef]
- Blaschitz, C.; Raffatellu, M. Th17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 2010, 30, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Lane, E. Keratin mutations and intestinal pathology. J. Pathol. 2004, 204, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.; VandeVord, P.; Van Dyke, M. Keratin biomaterials augment anti-inflammatory macrophage phenotype in vitro. Acta Biomater. 2018, 66, 213–223. [Google Scholar] [CrossRef]
- Fearing, B.V.; Van Dyke, M.E. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater 2014, 7, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Maeda, F.Y.; Cortez, C.; Yoshida, N. Cell signaling during Trypanosoma cruzi invasion. Front. Immunol. 2012, 3, 361. [Google Scholar] [CrossRef]
- Barrias, E.S.; Carvalho, T.M.U.; De Souza, W. Trypanosoma cruzi: Entry into mammalian host cells and parasitophorous vacuole formation. Front. Immunol. 2013, 4, 186. [Google Scholar] [CrossRef]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the immune response by Trypanosoma cruzi during acute infection. Front. Immunol. 2016, 6, 659. [Google Scholar] [CrossRef]
- Rodríguez-Bejarano, O.H.; Avendaño, C.; Patarroyo, M.A. Mechanisms associated with Trypanosoma cruzi host target cell adhesion, recognition and internalization. Life 2021, 6, 534. [Google Scholar] [CrossRef]
- Rodriguez, A.; Samoff, E.; Rioult, M.G.; Chung, A.; Andrews, N.W. Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin-mediated transport. Int. J. Biochem. Cell Biol. 1996, 2, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.F.; Cazes, A.; Butt, E.; Grunewald, T.G. An update on the LIM and SH3 domain protein 1 (LASP1): A versatile structural, signaling, and biomarker protein. Onco. Targets Ther. 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Negedu, M.N.; Duckworth, C.A.; Yu, L.-G. Galectin-2 in health and diseases. Int. J. Mol. Sci. 2022, 1, 341. [Google Scholar] [CrossRef]
- Paclik, D.; Werner, L.; Guckelberger, O.; Wiedenmann, B.; Sturm, A. Galectins distinctively regulate central monocyte and macrophage function. Cell. Immunol. 2011, 1, 97–103. [Google Scholar] [CrossRef]
- Vannella, K.M.; Ramalingam, T.R.; Hart, K.M.; de Queiroz Prado, R.; Sciurba, J.; Barron, L.; Borthwick, L.A.; Smith, A.D.; Mentink-Kane, M.; White, S.; et al. Acidic chitinase primes the protective immune response to gastrointestinal nematodes. Nat. Immunol. 2016, 5, 538–544. [Google Scholar] [CrossRef]
- Esposito, R.; Montefusco, S.; Ferro, P.; Monti, M.C.; Baldantoni, D.; Tosco, A.; Marzullo, L. Trefoil Factor 1 is involved in gastric cell copper homeostasis. Int. J. Biochem. Cell Biol. 2015, 59, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Shimada, T.; Fujii, Y.; Chen, G.; Tabei, K.; Namatame, T.; Yamagata, M.; Tajima, A.; Yoneda, M.; Terano, A. Up-regulation of TFF1 (pS2) expression by TNF-α in gastric epithelial cells. J. Gastroenterol. Hepatol. 2007, 6, 936–942. [Google Scholar] [CrossRef]
- Toback, F.G.; Walsh-Reitz, M.M.; Musch, M.W.; Chang, E.B.; Del Valle, J.; Ren, H.; Huang, E.; Martin, T.E. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 2, G344–G353. [Google Scholar] [CrossRef]
- Walsh-Reitz, M.M.; Huang, E.F.; Musch, M.W.; Chang, E.B.; Martin, T.E.; Kartha, S.; Toback, F.G. AMP-18 protects barrier function of colonic epithelial cells: Role of tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 1, G163–G171. [Google Scholar] [CrossRef]

| Groups | PPP | PP | MPP | DMPP |
|---|---|---|---|---|
| RPMI | 4 | 26 | 13,108 | 12 |
| Açaí | 11 | 29 | 11,475 | 16 |
| Indicators | Results |
|---|---|
| MS | 242.783 |
| MS/MS | 178.876 |
| Peptide-Spectrum Matches (PSM) | 52.802 |
| Peptide sequences | 7.760 |
| Protein groups | 1251 |
| Proteins (unique peptides) | 1012 (>2); 369 (=2); 506 (=1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, F.d.S.; Duarte, T.H.C.; Xavier, V.F.; Mercês, A.C.d.; Silva, T.V.d.C.; Medeiros, L.d.F.; Vital, C.E.; Carneiro, C.M.; Borges, W.d.C.; Vieira, P.M.d.A. Role of Açaí (Euterpe oleracea) in Modulating the Immune Response During Experimental Oral Infection with Trypanosoma cruzi. Microorganisms 2025, 13, 2711. https://doi.org/10.3390/microorganisms13122711
Marques FdS, Duarte THC, Xavier VF, Mercês ACd, Silva TVdC, Medeiros LdF, Vital CE, Carneiro CM, Borges WdC, Vieira PMdA. Role of Açaí (Euterpe oleracea) in Modulating the Immune Response During Experimental Oral Infection with Trypanosoma cruzi. Microorganisms. 2025; 13(12):2711. https://doi.org/10.3390/microorganisms13122711
Chicago/Turabian StyleMarques, Flávia de Souza, Thays Helena Chaves Duarte, Viviane Flores Xavier, Aline Coelho das Mercês, Thaís Vieira de Carvalho Silva, Luciana da Fonseca Medeiros, Camilo Elber Vital, Cláudia Martins Carneiro, William de Castro Borges, and Paula Melo de Abreu Vieira. 2025. "Role of Açaí (Euterpe oleracea) in Modulating the Immune Response During Experimental Oral Infection with Trypanosoma cruzi" Microorganisms 13, no. 12: 2711. https://doi.org/10.3390/microorganisms13122711
APA StyleMarques, F. d. S., Duarte, T. H. C., Xavier, V. F., Mercês, A. C. d., Silva, T. V. d. C., Medeiros, L. d. F., Vital, C. E., Carneiro, C. M., Borges, W. d. C., & Vieira, P. M. d. A. (2025). Role of Açaí (Euterpe oleracea) in Modulating the Immune Response During Experimental Oral Infection with Trypanosoma cruzi. Microorganisms, 13(12), 2711. https://doi.org/10.3390/microorganisms13122711






