Fate and Removal of Antibiotics and Antibiotic Resistance Genes in a Rural Wastewater Treatment Plant: A Microbial Perspective of Nature-Based Versus Advanced Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Treatment Plant and Experimentation
2.2. Sample Collection and Analysis
2.3. Monitoring of Physicochemical Parameters
2.4. Monitoring of Antibiotics Levels
2.5. Monitoring of Microbial Parameters
2.5.1. Quantification of Antibiotic Resistance, Class 1 Integrase, and 16S rRNA Genes
2.5.2. Culture-Based Enumeration of Indicator Bacteria
2.5.3. Flow Cytometric Enumeration of Total and Intact Bacteria
2.5.4. Bacterial Community Profiling with Metabarcoding
2.5.5. Antibiotic Resistance Gene Profiling with Metagenomics
2.6. Data Treatment
2.6.1. Macropollutants and Antibiotics
2.6.2. Microbial Parameters
3. Results
3.1. Impact of Treatment on Nutrients
3.2. Impact of Conventional Activated Sludge Treatment on the Occurrence and Distribution of Antibiotics and Antibiotic Resistance Genes in Treated Wastewater
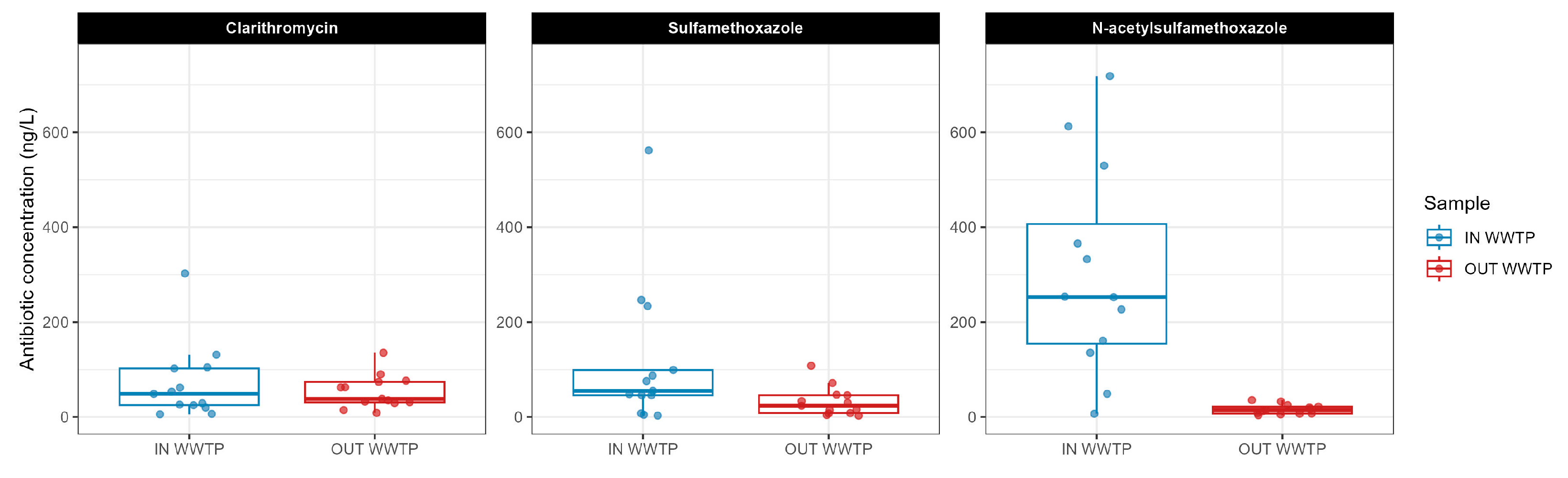
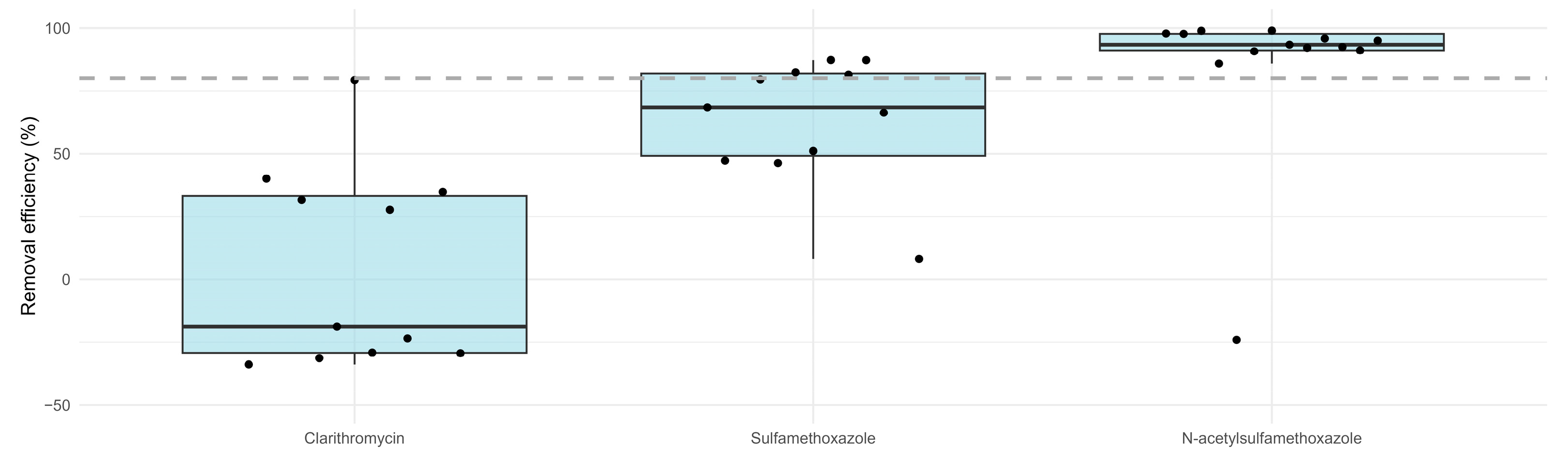
3.2.1. Antibiotics
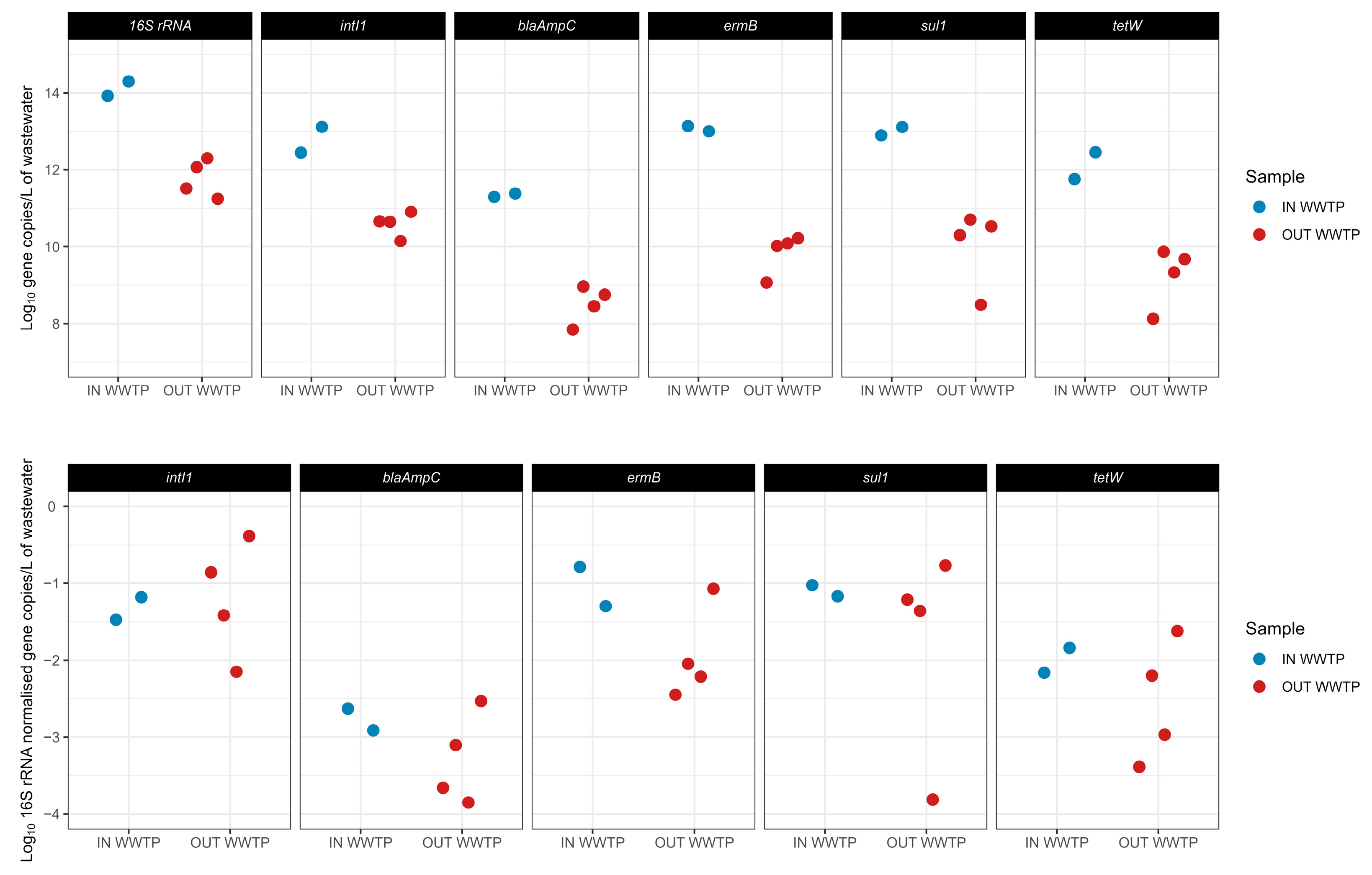
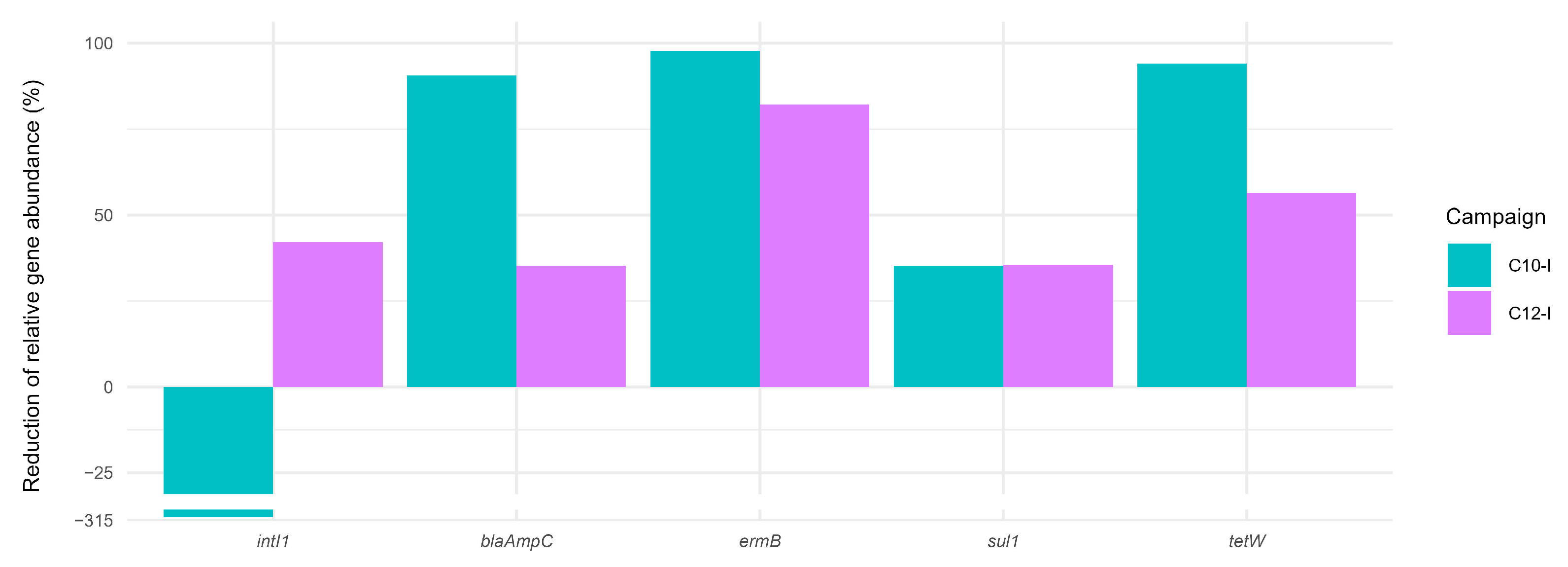
3.2.2. Antibiotic Resistance Genes and Class 1 Integrase Gene
3.3. Impact of Quaternary Treatment on Occurrence and Distribution of Antibiotics, Antibiotic Resistance Genes, and Microbial Indicator Organisms in Treated Wastewater
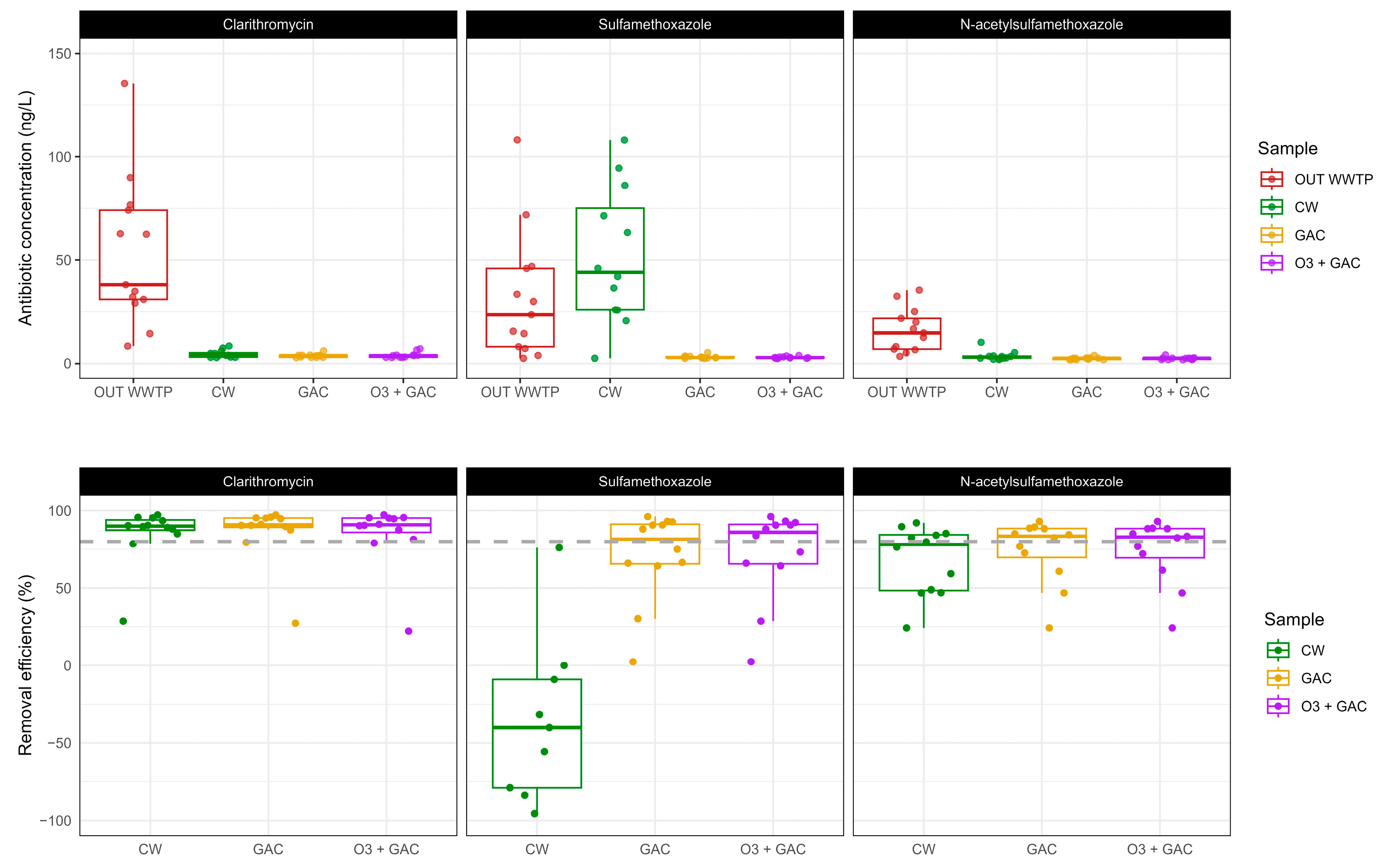
3.3.1. Antibiotics
3.3.2. Antibiotic Resistance Genes and Class 1 Integrase Gene
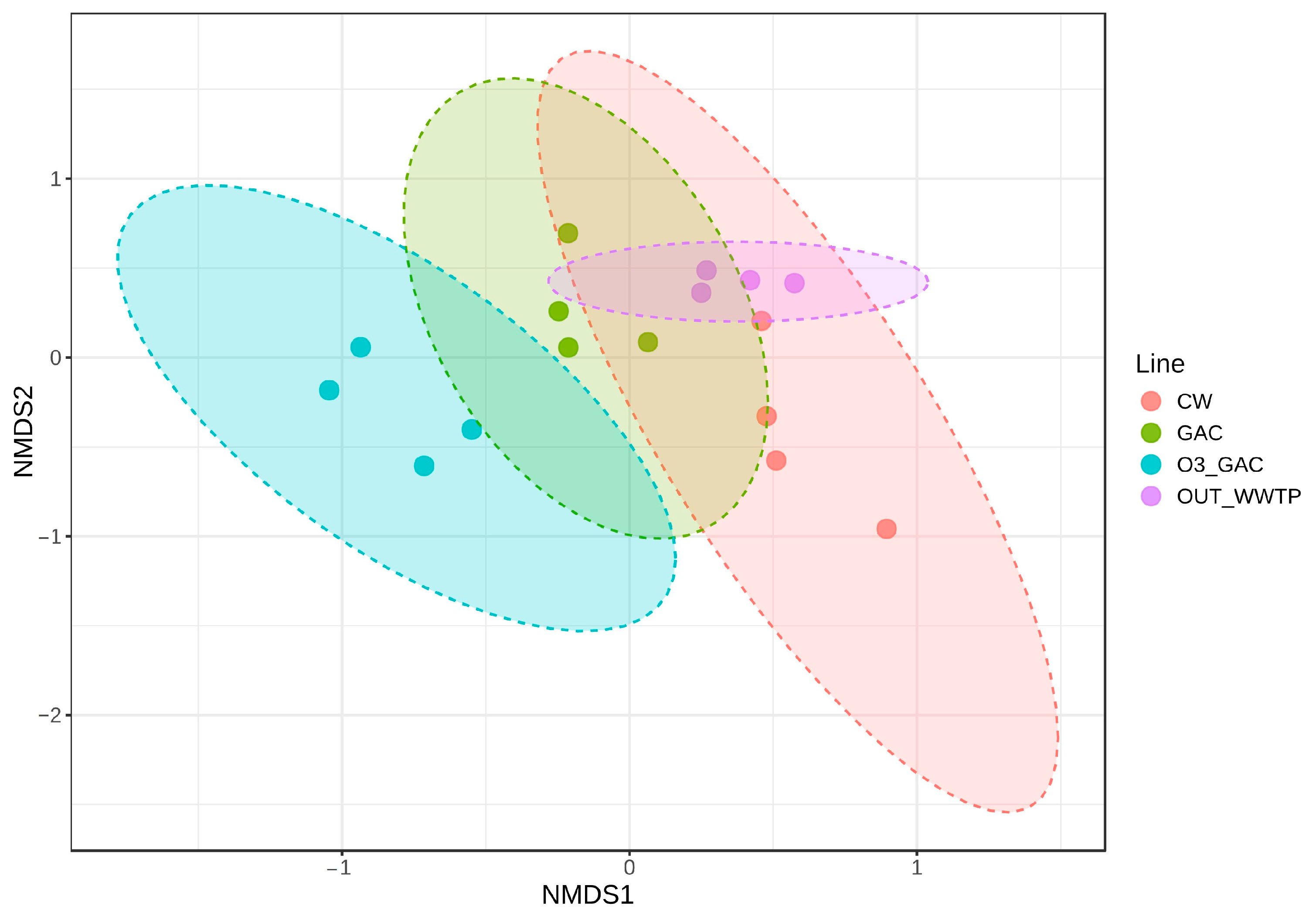
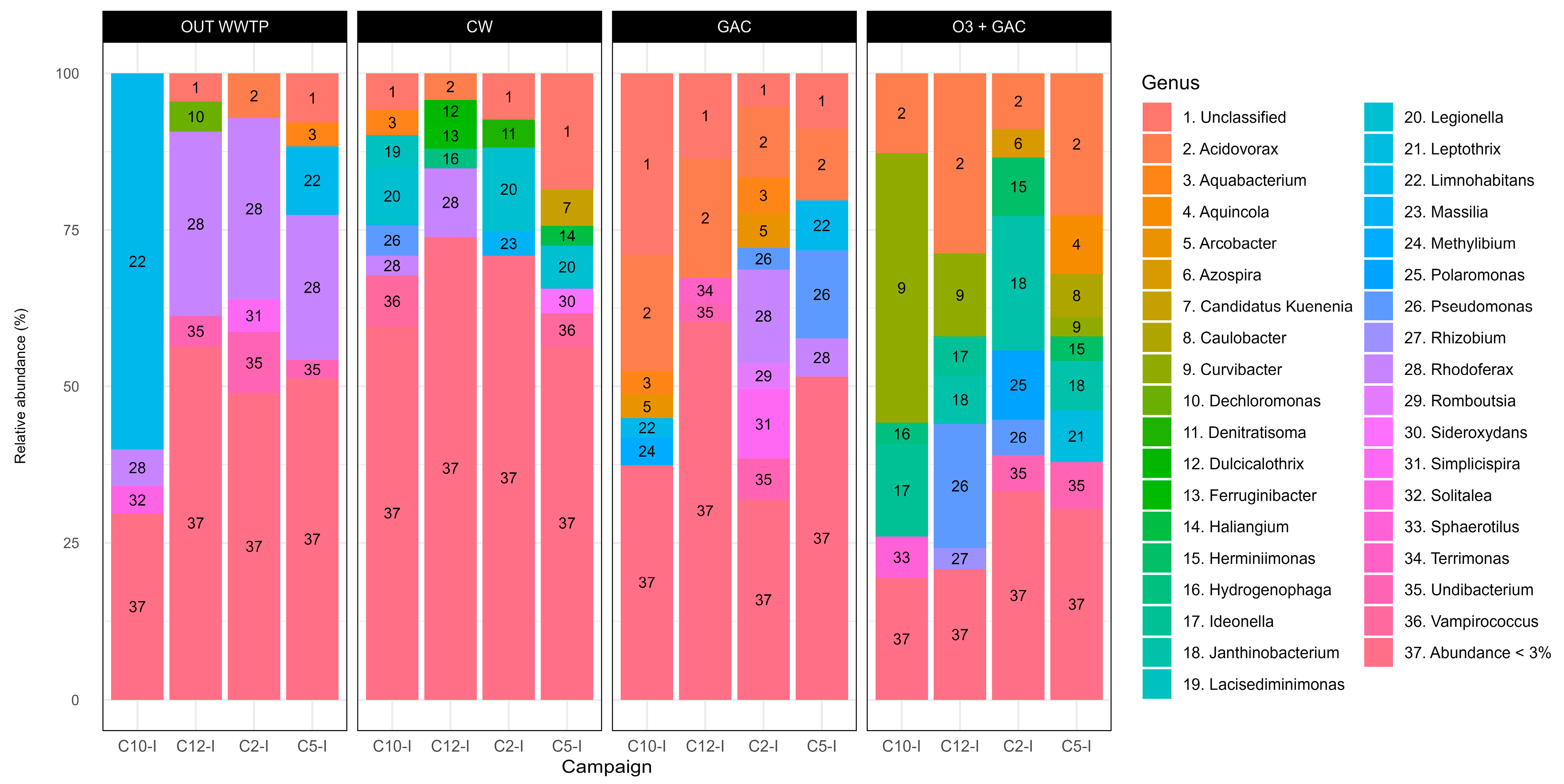
3.3.3. Bacterial Community Composition
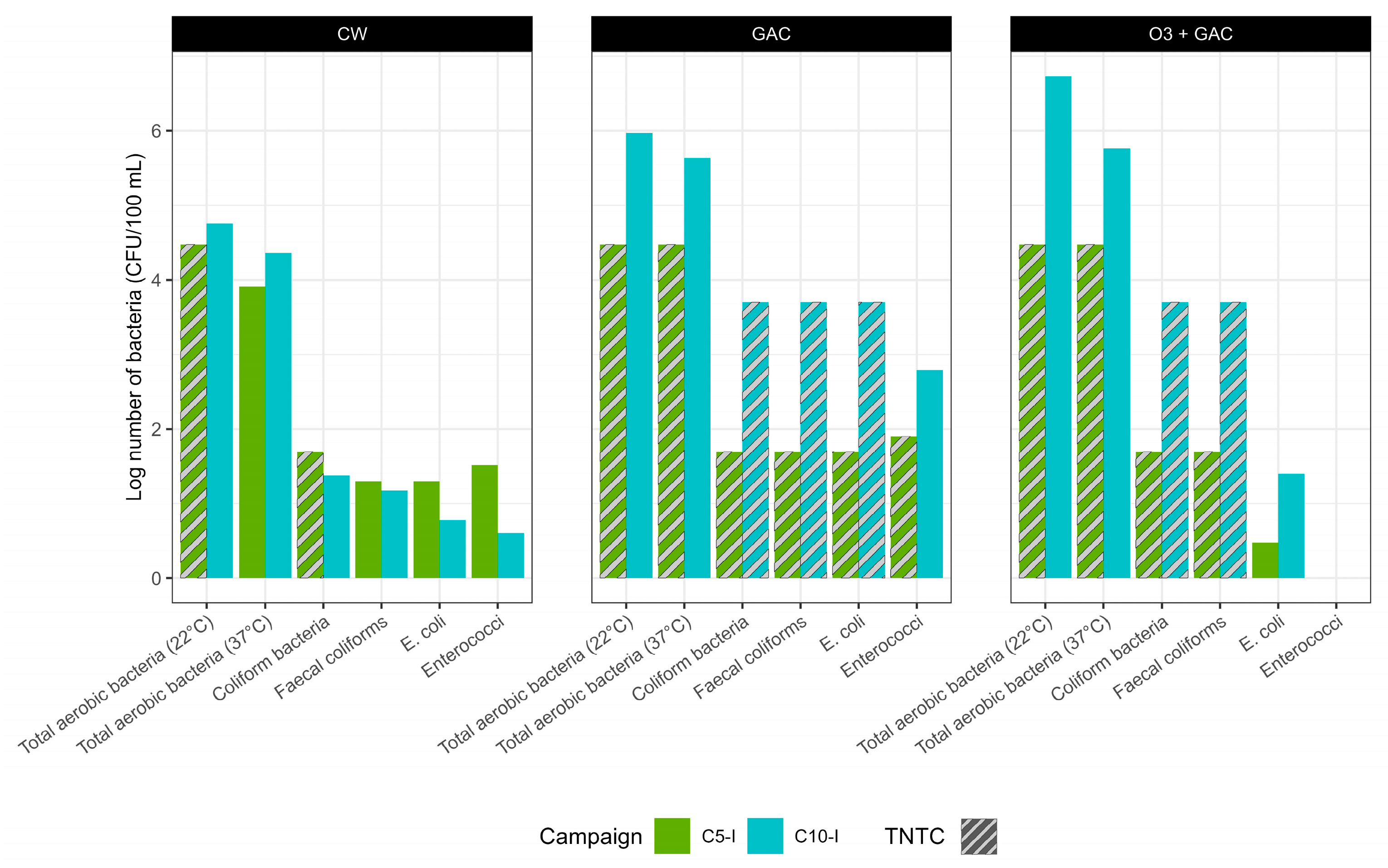
3.3.4. Indicator Bacteria
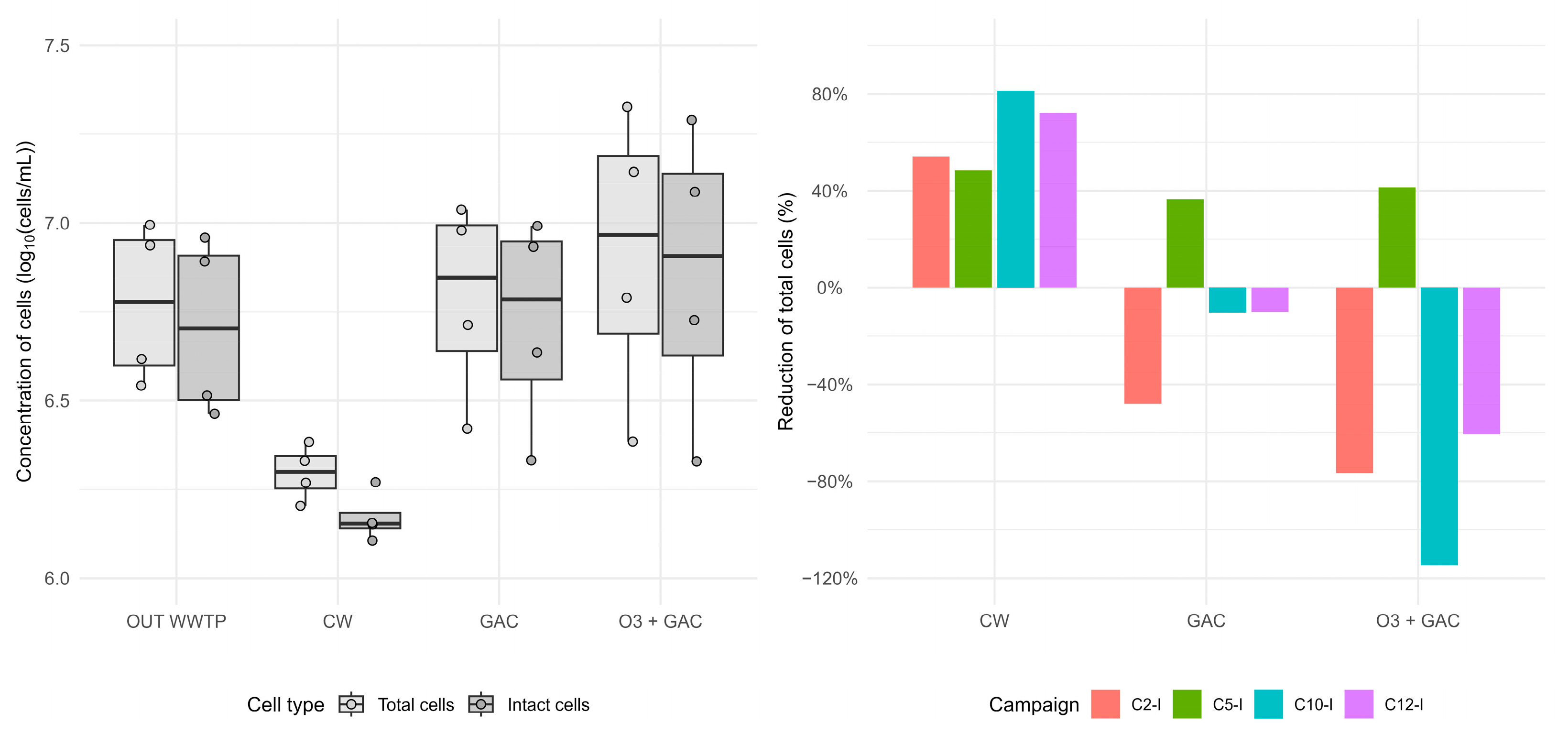
3.3.5. Total and Viable Cell Counts
3.3.6. Metagenomics Campaign C12-I (CW and OUT WWTP)
4. Discussion
4.1. Compliance with Minimum Requirements for Water Reuse
4.2. Heterogenous Fate of Antibiotics During Wastewater Treatment
4.3. Antimicrobial Resistance Propagation
4.4. Considerations for Accurate Monitoring of ARG Removal
4.5. Microbial Perspective Through Bacterial Community Profiling
5. Conclusions: Constructed Wetlands as a Resilient Alternative to Advanced Treatment
- Generating effluent with excellent water quality regarding macropollutants, with particularly stable nitrogen and phosphorus removal. Water quality was compliant with the stringent standards imposed for medium and small WWTPs.
- Showing good performance regarding the removal of CLA by 90%, ensuring compliance with its EQS values and N-SMX (~80%). Negative removals were instead observed for SMX due to the possible reversible transformation of N-SMX in its parent compound.
- Demonstrating adequate and reliable removal of all ARGs and intI1, with low temporal variability compared to O3 + GAC and GAC. Only minor ARG release effects were observed in CW (e.g., sul1 and blaAmpC). Although O3 + GAC achieved an average removal similar to that of CW, its inability to eliminate intI1 underscored the comparative advantage of CW.
- Demonstrating the added value of CW for the removal of antibiotics. It underperformed regarding the removal of sulfamethoxazole, but not regarding its acetylated metabolite, showing the importance of monitoring transformation products.
- Producing effluent that is compliant with Reuse Category B standards—reuse water for applications including food crops consumed raw, processed crops, and non-food crops—as E. coli concentrations remained below 100 CFU/100 mL.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| ATB | Antibiotic |
| ARB | Antimicrobial Resistant Bacteria |
| ARGs | Antibiotic Resistance Genes |
| AWaRe | Access, Watch, and Reserve Classification of Antibiotics |
| BOD | Biological Oxygen Demand |
| CLA | Clarithromycin |
| COD | Chemical Oxygen Demand |
| CW | Constructed Wetland |
| EBCT | Empty Bed Contact Time |
| EQS | Environmental Quality Standard |
| EU | European Union |
| GAC | Granular Activated Carbon |
| HLR | Hydraulic Loading Rate |
| ICC | Intact Cell Count |
| IQR | Inter-Quartile Range |
| LOD | Limit Of Detection |
| LOQ | Limit Of Quantification |
| MAG | Metagenome-Assembled Genome |
| MP | Micropollutant |
| N | Nitrogen |
| NH4 | Ammonium |
| NO2 | Nitrite |
| NO3 | Nitrate |
| N-SMX | N-acetylsulfamethoxazole |
| O3 + GAC | Ozonation Followed by Granular Activated Carbon |
| ONT | Oxford Nanopore Technologies |
| P | Phosphorous |
| PCR (qPCR) | (Quantitative) Polymerase Chain Reaction |
| p.e. | Population Equivalent |
| PES | Polyethersulfone |
| SMX | Sulfamethoxazole |
| TCC | Total Cell Count |
| TSS | Total Suspended Solid |
| TNTC | Too Numerous To Count |
| TOC | Total Organic Carbon |
| UWWTD | Urban Wastewater Treatment Directive |
| UWWTP | Urban Wastewater Treatment Plant |
| VF-CW | Vertical-Flow Constructed Wetland |
| WWTP | Wastewater Treatment Plant |
References
- Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E.; Zorpas, A.A.; Menegaki, A.N. Chapter 3—Aquatic Environment. In Environment and Development Basic Principles, Human Activities, and Environmental Implications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 137–212. ISBN 978-0-444-62733-9. [Google Scholar]
- European Union. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj/eng (accessed on 22 August 2025).
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (WFD). Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj/eng (accessed on 22 August 2025).
- European Union Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2015) 1756). Available online: https://eur-lex.europa.eu/eli/dec_impl/2015/495/oj/eng (accessed on 22 August 2025).
- Oekotoxzentrum Proposals for Quality Criteria for Surface Waters. Available online: https://www.ecotoxcentre.ch/expert-service/quality-criteria/quality-criteria-for-surface-waters (accessed on 16 October 2025).
- European Union. Directive (EU) 2024/3019 Concerning Urban Wastewater Treatment. Available online: https://eur-lex.europa.eu/eli/dir/2024/3019/oj/eng (accessed on 22 August 2025).
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for the Release of Antibiotics in the Environment: A Review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for Antibiotic Resistant Bacteria and Genes Spread into the Environment: A Review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Sabri, N.A.; Van Holst, S.; Schmitt, H.; Van Der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate of Antibiotics and Antibiotic Resistance Genes during Conventional and Additional Treatment Technologies in Wastewater Treatment Plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef]
- Schwermer, C.U.; Krzeminski, P.; Anglès d’Auriac, M.; Gjeitnes, M.; Moe, J.; Bellanger, X.; Bürgmann, H.; Carapeto García, R.; Crespo Iniesta, P.; Dagot, C.; et al. Pilot Study on Antimicrobial Resistance Monitoring in European Surface Waters-Final Report of the Eionet Working Group (2025); Zenodo: Geneva, Switzerland, 2025. [Google Scholar] [CrossRef]
- Hazra, M.; Watts, J.E.M.; Joshi, H. An Evaluation of Conventional and Nature-Based Technologies for Controlling Antibiotic-Resistant Bacteria and Antibiotic-Resistant Genes in Wastewater Treatment Plants. Sci. Total Environ. 2024, 917, 170433. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in Treated Wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-K.; Lin, C.; Bui, X.-T.; Rakib, M.R.J.; Nguyen, H.-L.; Truong, Q.-M.; Hoang, H.-G.; Tran, H.-T.; Malafaia, G.; Idris, A.M. Occurrence and Fate of Pharmaceutical Pollutants in Wastewater: Insights on Ecotoxicity, Health Risk, and State–of–the-Art Removal. Chemosphere 2024, 354, 141678. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Bains, A.; Sridhar, K.; Chawla, P.; Sharma, M. Environmental Impact and Source-Controlled Approaches for Emerging Micropollutants: Current Status and Future Prospects. Food Chem. Toxicol. 2024, 193, 115038. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of Global Health Risk of Antibiotic Resistance Genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)-Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Desiante, W.L.; Minas, N.S.; Fenner, K. Micropollutant Biotransformation and Bioaccumulation in Natural Stream Biofilms. Water Res. 2021, 193, 116846. [Google Scholar] [CrossRef]
- Garner, E.; Organiscak, M.; Dieter, L.; Shingleton, C.; Haddix, M.; Joshi, S.; Pruden, A.; Ashbolt, N.J.; Medema, G.; Hamilton, K.A. Towards Risk Assessment for Antibiotic Resistant Pathogens in Recycled Water: A Systematic Review and Summary of Research Needs. Environ. Microbiol. 2021, 23, 7355–7372. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The Potential Implications of Reclaimed Wastewater Reuse for Irrigation on the Agricultural Environment: The Knowns and Unknowns of the Fate of Antibiotics and Antibiotic Resistant Bacteria and Resistance Genes – A Review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef]
- Meradji, S.; Basher, N.S.; Sassi, A.; Ibrahim, N.A.; Idres, T.; Touati, A. The Role of Water as a Reservoir for Antibiotic-Resistant Bacteria. Antibiotics 2025, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Pistocchi, A.; Andersen, H.R.; Bertanza, G.; Brander, A.; Choubert, J.M.; Cimbritz, M.; Drewes, J.E.; Koehler, C.; Krampe, J.; Launay, M.; et al. Treatment of Micropollutants in Wastewater: Balancing Effectiveness, Costs and Implications. Sci. Total Environ. 2022, 850, 157593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Leung, K.M.Y.; Snyder, S.A.; Alvarez, P.J.J. Which Micropollutants in Water Environments Deserve More Attention Globally? Environ. Sci. Technol. 2022, 56, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gamboa, L.; Martínez-López, S.; Ayuso-García, L.M.; Lahora, A.; Martínez-Alcalá, I. Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive. Environments 2024, 11, 105. [Google Scholar] [CrossRef]
- Muylaert, A.; Mainil, J. Résistance bactérienne aux antibiotiques, les mécanismes et leur “contagiosité”. Ann. Médecine Vét. 2013, 156, 109–123. [Google Scholar]
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. 2023. Available online: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 5 September 2025).
- Coleman, J.P.; Smith, C.J. Microbial Resistance. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–3. ISBN 978-0-08-055232-3. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655, Erratum in Lancet. 2022, 400, 1102. https://doi.org/10.1016/S0140-6736(21)02653-2. [Google Scholar] [CrossRef]
- Elliott, T.S.J.; Lambert, P.A. Antibacterial Resistance in the Intensive Care Unit: Mechanisms and Management. Br. Med. Bull. 1999, 55, 259–276. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, H.; Haraoka, M.; Saika, T.; Kobayashi, I.; Naito, S. Antimicrobial Resistance of Neisseria Gonorrhoeae and High Prevalence of Ciprofloxacin-Resistant Isolates in Japan, 1993 to 1998. J. Clin. Microbiol. 2000, 38, 521–525. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) Increases in Fluoroquinolone-Resistant Neisseria Gonorrhoeae--Hawaii and California, 2001. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1041–1044.
- Wang, S.A.; Lee, M.V.C.; O’Connor, N.; Iverson, C.J.; Ohye, R.G.; Whiticar, P.M.; Hale, J.A.; Trees, D.L.; Knapp, J.S.; Effler, P.V.; et al. Multidrug-Resistant Neisseria Gonorrhoeae with Decreased Susceptibility to Cefixime—Hawaii, 2001. Clin. Infect. Dis. 2003, 37, 849–852. [Google Scholar] [CrossRef]
- Organisation mondiale de la Santé. Système Mondial de Surveillance de la Résistance aux Antimicrobiens: Manual de Mise en Oeuvre Initiale; Organisation Mondiale de la Santé: Geneva, Switzerland, 2016; ISBN 978-92-4-254940-9. [Google Scholar]
- Fleischmann, S.; Robben, C.; Alter, T.; Rossmanith, P.; Mester, P. How to Evaluate Non-Growing Cells—Current Strategies for Determining Antimicrobial Resistance of VBNC Bacteria. Antibiotics 2021, 10, 115. [Google Scholar] [CrossRef]
- Ishii, S. Quantification of Antibiotic Resistance Genes for Environmental Monitoring: Current Methods and Future Directions. Curr. Opin. Environ. Sci. Health 2020, 16, 47–53. [Google Scholar] [CrossRef]
- Aldea, A.C.; Diguṭă, F.C.; Presacan, O.; Voaideṣ, C.; Toma, R.C.; Matei, F. Detecting Antibiotic Resistance: Classical, Molecular, Advanced Bioengineering, and AI-Enhanced Approaches. Front. Microbiol. 2025, 16, 1673343. [Google Scholar] [CrossRef] [PubMed]
- Bolzon, V.; Bulfoni, M.; Nencioni, A.; Nencioni, E. Development of Real-Time PCR Methods for Quality Control Detection of Pathogenic Bacteria in Cosmetic Preparations. Front. Public Health 2025, 13, 1572201. [Google Scholar] [CrossRef]
- Bai, S.; Wang, X.; Zhang, Y.; Liu, F.; Shi, L.; Ding, Y.; Wang, M.; Lyu, T. Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR). Sustainability 2022, 14, 14989. [Google Scholar] [CrossRef]
- Ofiera, L.M.; Wintgens, T.; Kazner, C. Comparative Analysis of Conventional and Modified Constructed Wetlands for the Removal of Trace Organic Compounds from Municipal Wastewater Effluent. Sci. Total Environ. 2025, 987, 179796. [Google Scholar] [CrossRef]
- Wagner, T.V.; Rempe, F.; Hoek, M.; Schuman, E.; Langenhoff, A. Key Constructed Wetland Design Features for Maximized Micropollutant Removal from Treated Municipal Wastewater: A Literature Study Based on 16 Indicator Micropollutants. Water Res. 2023, 244, 120534. [Google Scholar] [CrossRef] [PubMed]
- Brunsch, A.F.; Ter Laak, T.L.; Christoffels, E.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Retention Soil Filter as Post-Treatment Step to Remove Micropollutants from Sewage Treatment Plant Effluent. Sci. Total Environ. 2018, 637–638, 1098–1107. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; Van Der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Performance of Full Scale Constructed Wetlands in Removing Antibiotics and Antibiotic Resistance Genes. Sci. Total Environ. 2021, 786, 147368. [Google Scholar] [CrossRef]
- Salmerón, I.; Venditti, S.; Hansen, J. Upgrading Constructed Wetlands Using Upstream (Advanced) Oxidation Technology to Improve the Removal of Micropollutants and Reduce the Ecotoxicological Risks. Water Sci. Technol. 2025, 92, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I.; Venditti, S.; Hansen, J. Technical Assessment Of Natural-Based And Advanced Technologies For The Removal Of Micropollutants From Municipal Wastewater In A Real Scenario In The Greater Region. In Proceedings of the Proceedings of the 39th IAHR World Congress, Granada, Spain, 19–24 June 2022; International Association for Hydro-Environment Engineering and Research (IAHR): Madrid, Spain, 2022; pp. 2019–2024. [Google Scholar]
- European Union. Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/dec_impl/2020/1161/oj (accessed on 22 August 2025).
- Gomez Cortes, L.; Marinov, D.; Sanseverino, I.; Navarro Cuenca, A.; Niegowska, M.; Porcel Rodriguez, E.; Lettieri, T. Selection of Substances for the 3rd Watch List under the Water Framework Directive; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-19426-2. [Google Scholar]
- Wang, J.; Mao, D.; Mu, Q.; Luo, Y. Fate and Proliferation of Typical Antibiotic Resistance Genes in Five Full-Scale Pharmaceutical Wastewater Treatment Plants. Sci. Total Environ. 2015, 526, 366–373. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Li, J.; Liang, P.; Huang, X. Removal of Antibiotic Resistance Genes in Four Full-Scale Membrane Bioreactors. Sci. Total Environ. 2019, 653, 112–119. [Google Scholar] [CrossRef]
- Suzuki, M.T.; Taylor, L.T.; DeLong, E.F. Quantitative Analysis of Small-Subunit rRNA Genes in Mixed Microbial Populations via 5′-Nuclease Assays. Appl. Environ. Microbiol. 2000, 66, 4605–4614. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Egli, T. Cytometric Methods for Measuring Bacteria in Water: Advantages, Pitfalls and Applications. Anal. Bioanal. Chem. 2010, 397, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- De Coster, W.; Rademakers, R. NanoPack2: Population-Scale Evaluation of Long-Read Sequencing Data. Bioinformatics 2023, 39, btad311. [Google Scholar] [CrossRef]
- Wick, R.; Menzel, P. Filtlong: Quality Filtering Tool for Long Reads (2019). Available Online: Https://Github.Com/Rrwick/Filtlong (accessed on 21 August 2025).
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-Level Microbial Community Profiling of Full-Length 16S rRNA Oxford Nanopore Sequencing Data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; Van Den Brand, T. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; R Package Version 2019, 2, 173. Available Online: Https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 21 August 2025).
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive Statistical, Functional and Integrative Analysis of Microbiome Data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Li, H. New Strategies to Improve Minimap2 Alignment Accuracy. Bioinformatics 2021, 37, 4572–4574. [Google Scholar] [CrossRef]
- Tarasov, A.; Vilella, A.J.; Cuppen, E.; Nijman, I.J.; Prins, P. Sambamba: Fast Processing of NGS Alignment Formats. Bioinformatics 2015, 31, 2032–2034. [Google Scholar] [CrossRef]
- Kang, D.D.; Li, F.; Kirton, E.; Thomas, A.; Egan, R.; An, H.; Wang, Z. MetaBAT 2: An Adaptive Binning Algorithm for Robust and Efficient Genome Reconstruction from Metagenome Assemblies. PeerJ 2019, 7, e7359. [Google Scholar] [CrossRef] [PubMed]
- Cornet, L.; Durieu, B.; Baert, F.; D’hooge, E.; Colignon, D.; Meunier, L.; Lupo, V.; Cleenwerck, I.; Daniel, H.-M.; Rigouts, L.; et al. The GEN-ERA Toolbox: Unified and Reproducible Workflows for Research in Microbial Genomics. GigaScience 2023, 12, giad022. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A Toolkit to Classify Genomes with the Genome Taxonomy Database. Bioinforma. Oxf. Engl. 2019, 36, 1925–1927. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.-H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19, Erratum in Antimicrob. Agents Chemother. 2020, 64, e00361-20. https://doi.org/10.1128/AAC.00361-20. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, Y.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse-Easily Install and Load the “Tidyverse” 2.0.0. J. Open Source Softw. 2016, 4, 1686. [Google Scholar] [CrossRef]
- Camper, A.K.; LeChevallier, M.W.; Broadaway, S.C.; McFeters, G.A. Growth and Persistence of Pathogens on Granular Activated Carbon Filters. Appl. Environ. Microbiol. 1985, 50, 1378–1382. [Google Scholar] [CrossRef]
- Santos, J.; Rodrigues, S.; Magalhães, M.; Rodrigues, K.; Pereira, L.; Marinho, G. A State-of-the-Art Review (2019–2023) on Constructed Wetlands for Greywater Treatment and Reuse. Environ. Chall. 2024, 16, 100973. [Google Scholar] [CrossRef]
- Castellar, J.A.C.; Torrens, A.; Buttiglieri, G.; Monclús, H.; Arias, C.A.; Carvalho, P.N.; Galvao, A.; Comas, J. Nature-Based Solutions Coupled with Advanced Technologies: An Opportunity for Decentralized Water Reuse in Cities. J. Clean. Prod. 2022, 340, 130660. [Google Scholar] [CrossRef]
- Suárez, S.; Reif, R.; Lema, J.M.; Omil, F. Mass Balance of Pharmaceutical and Personal Care Products in a Pilot-Scale Single-Sludge System: Influence of T, SRT and Recirculation Ratio. Chemosphere 2012, 89, 164–171. [Google Scholar] [CrossRef]
- Venditti, S.; Brunhoferova, H.; Hansen, J. Behaviour of 27 Selected Emerging Contaminants in Vertical Flow Constructed Wetlands as Post-Treatment for Municipal Wastewater. Sci. Total Environ. 2022, 819, 153234. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Pallares-Vega, R.; Blaak, H.; Van Der Plaats, R.; De Roda Husman, A.M.; Hernandez Leal, L.; Van Loosdrecht, M.C.M.; Weissbrodt, D.G.; Schmitt, H. Determinants of Presence and Removal of Antibiotic Resistance Genes during WWTP Treatment: A Cross-Sectional Study. Water Res. 2019, 161, 319–328. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, J.H.; Shin, J.; Jang, H.M.; Kim, S.; Song, M.S.; Kim, Y.M. Quantitative and Qualitative Changes in Antibiotic Resistance Genes after Passing through Treatment Processes in Municipal Wastewater Treatment Plants. Sci. Total Environ. 2017, 605–606, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Makowska, N.; Koczura, R.; Mokracka, J. Class 1 Integrase, Sulfonamide and Tetracycline Resistance Genes in Wastewater Treatment Plant and Surface Water. Chemosphere 2016, 144, 1665–1673. [Google Scholar] [CrossRef]
- Rafraf, I.D.; Lekunberri, I.; Sànchez-Melsió, A.; Aouni, M.; Borrego, C.M.; Balcázar, J.L. Abundance of Antibiotic Resistance Genes in Five Municipal Wastewater Treatment Plants in the Monastir Governorate, Tunisia. Environ. Pollut. 2016, 219, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, Y.; Yu, G.; Wang, G.; Zhou, Z.; Li, P.; Zhang, Y.; Yang, K.; Wang, S. A Review on Microorganisms in Constructed Wetlands for Typical Pollutant Removal: Species, Function, and Diversity. Front. Microbiol. 2022, 13, 845725. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lu, Z.; Zhang, Z.; Zhang, Y.; Shi, B.; Wang, H. Ozone and Fenton Oxidation Affected the Bacterial Community and Opportunistic Pathogens in Biofilms and Effluents from GAC. Water Res. 2022, 218, 118495. [Google Scholar] [CrossRef]
- Dueholm, M.K.D.; Andersen, K.S.; Korntved, A.-K.C.; Rudkjøbing, V.; Alves, M.; Bajón-Fernández, Y.; Batstone, D.; Butler, C.; Cruz, M.C.; Davidsson, Å.; et al. MiDAS 5: Global Diversity of Bacteria and Archaea in Anaerobic Digesters. Nat. Commun. 2024, 15, 5361. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Gao, J.; Lei, Y.; Yuan, Y.; Jiang, Y.; Xu, Z.; He, C. Microbial Action and Mechanisms for Cr(VI) Removal Performance by Layered Double Hydroxide Modified Zeolite and Quartz Sand in Constructed Wetlands. J. Environ. Manage. 2019, 246, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Chauhan, M.; Kumar Sharma, P.; Kumari, M.; Mitra, D.; Joshi, S. Microbiological Dimensions and Functions in Constructed Wetlands: A Review. Curr. Res. Microb. Sci. 2024, 7, 100311. [Google Scholar] [CrossRef]
- Man, Y.; Wang, J.; Tam, N.F.; Wan, X.; Huang, W.; Zheng, Y.; Tang, J.; Tao, R.; Yang, Y. Responses of Rhizosphere and Bulk Substrate Microbiome to Wastewater-Borne Sulfonamides in Constructed Wetlands with Different Plant Species. Sci. Total Environ. 2020, 706, 135955. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Microbial Degradation of Sulfamethoxazole in the Environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Zerva, I.; Alexandropoulou, I.; Panopoulou, M.; Melidis, P.; Ntougias, S. Antibiotic Resistance Genes Dynamics at the Different Stages of the Biological Process in a Full-Scale Wastewater Treatment Plant. In Proceedings of the EWaS3 2018, Lefkada Island, Greece, 27–30 June 2018; MDPI: Basel, Switzerland, 2018; p. 650. [Google Scholar]
- Chen, H.; Zhang, M. Occurrence and Removal of Antibiotic Resistance Genes in Municipal Wastewater and Rural Domestic Sewage Treatment Systems in Eastern China. Environ. Int. 2013, 55, 9–14. [Google Scholar] [CrossRef]
- Chen, B.; Liang, X.; Nie, X.; Huang, X.; Zou, S.; Li, X. The Role of Class I Integrons in the Dissemination of Sulfonamide Resistance Genes in the Pearl River and Pearl River Estuary, South China. J. Hazard. Mater. 2015, 282, 61–67. [Google Scholar] [CrossRef]


| Gene | Sequence (5′–3′) | Thermal Cycle | Reference | ||
|---|---|---|---|---|---|
| blaAmpC | Forward | CCTCTTGCTCCACATTTGCT | 94 °C, 10 min | 1× 40× | [46] |
| Reverse | ACAACGTTTGCTGTGTGACG | 94 °C, 30 s; 59 °C, 60 s; 72 °C, 30 s | |||
| ermB | Forward | GATACCGTTTACGAAATTGG | 94 °C, 10 min | 1× 40× | [47] |
| Reverse | GAATCGAGACTTGAGTGTGC | 94 °C, 30 s; 59 °C, 60 s; 72 °C, 30 s | |||
| sul1 | Forward | CGCACCGGAAACATCGCTGCAC | 94 °C, 10 min | 1× 40× | [47] |
| Reverse | TGAAGTTCCGCCGCAAGGCTCG | 94 °C, 30 s; 59 °C, 60 s; 72 °C, 30 s | |||
| tetW | Forward | GAGAGCCTGCTATATGCCAGC | 94 °C, 10 min | 1× 40× | [47] |
| Reverse | GGGCGTATCCACAATGTTAAC | 94 °C, 30 s; 59 °C, 60 s; 72 °C, 30 s | |||
| 16S rRNA | Forward | GGCTTCGTGATGCCTGCTT | 95 °C, 10 min | 1× 40× | [48] |
| Reverse | GGWTACCTTGTTACGACTT | 95 °C, 30 s; 56 °C, 30 s; 72 °C, 30 s | |||
| intI1 | Forward | GGGCGTATCCACAATGTTAAC | 94 °C, 10 min | 1× 40× | [46] |
| Reverse | CATTCCTGGCCGTGGTTCT | 94 °C, 30 s; 54 °C, 60 s; 72 °C, 30 s | |||
| Date | Campaign Duration | Season | Rainy or Dry Weather | Temperature | pH | Conductivity | Redox |
|---|---|---|---|---|---|---|---|
| 20.1.22 | 24 h | Winter | Dry | 10 | 6.8 | 542 | 364 |
| 24.2.22 | 72 h | Winter | Rainy | 9 | 6.5 | 267 | 167 |
| 24.3.22 | 24 h | Spring | - | 11 | 6.8 | 498 | 112 |
| 20.4.22 | 24 h | Spring | Dry | 12 | 6.7 | 471 | 442 |
| 13.5.22 | 72 h | Spring | Rainy | 16 | 6.7 | 526 | 492 |
| 25.5.22 | 24 h | Spring | Dry | 16 | 6.4 | 269 | 522 |
| 16.6.22 | 24 h | Spring | Rainy | 18 | 6.9 | 563 | 464 |
| 29.6.22 | 24 h | Summer | Dry | 19 | 6.7 | 381 | 484 |
| 04.8.22 | 24 h | Summer | Dry | 21 | 7.1 | 687 | 455 |
| 24.8.22 | 72 h | Summer | Dry | 21 | 6.9 | 714 | 440 |
| 14.9.22 | 24 h | Summer | Dry | 18 | 6.8 | 374 | 501 |
| 07.10.22 | 72 h | Autumn | Dry | 15 | 6.8 | 435 | 485 |
| 17.10.22 | 24 h | Autumn | - | 16 | 7.1 | 355 | 432 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouwir, L.; KleinJan, H.; Balent, C.; Quabron, G.; Salmerón, I.; Venditti, S.; Gritten, F. Fate and Removal of Antibiotics and Antibiotic Resistance Genes in a Rural Wastewater Treatment Plant: A Microbial Perspective of Nature-Based Versus Advanced Technologies. Microorganisms 2025, 13, 2663. https://doi.org/10.3390/microorganisms13122663
Brouwir L, KleinJan H, Balent C, Quabron G, Salmerón I, Venditti S, Gritten F. Fate and Removal of Antibiotics and Antibiotic Resistance Genes in a Rural Wastewater Treatment Plant: A Microbial Perspective of Nature-Based Versus Advanced Technologies. Microorganisms. 2025; 13(12):2663. https://doi.org/10.3390/microorganisms13122663
Chicago/Turabian StyleBrouwir, Lena, Hetty KleinJan, Charlotte Balent, Gilles Quabron, Irene Salmerón, Silvia Venditti, and Fanny Gritten. 2025. "Fate and Removal of Antibiotics and Antibiotic Resistance Genes in a Rural Wastewater Treatment Plant: A Microbial Perspective of Nature-Based Versus Advanced Technologies" Microorganisms 13, no. 12: 2663. https://doi.org/10.3390/microorganisms13122663
APA StyleBrouwir, L., KleinJan, H., Balent, C., Quabron, G., Salmerón, I., Venditti, S., & Gritten, F. (2025). Fate and Removal of Antibiotics and Antibiotic Resistance Genes in a Rural Wastewater Treatment Plant: A Microbial Perspective of Nature-Based Versus Advanced Technologies. Microorganisms, 13(12), 2663. https://doi.org/10.3390/microorganisms13122663








