Linking N2O Emission with AOB and nirK-Denitrifier in Paddy Fields of Karst and Non-Karst Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Site Description
2.2. Sample Collection and Preparation
2.3. Analysis of Physicochemical Properties
2.4. Measurement of N2O Emissions
- F represents the gas emission fluxes (mg·m−2·h−1);
- H denotes the height of the sampling chamber (m);
- M is the molar mass of the gas (g·mol−1);
- P indicates the atmospheric pressure at the sampling site (Pa);
- R is the universal gas constant (8.314 Pa·m3·mol−1·K−1);
- T represents the average temperature inside the chamber during sampling (°C);
- dc/dt refers to the gas emission fluxes (μL·L−1·min−1);
2.5. Calculation of Global Warming Potential
2.6. Quantification of AOB and nirK-Denitrifierl Abundance
2.7. Data Analysis and Visualization
2.8. Correlation and Importance Prediction
3. Results and Analysis
3.1. Soil Factors and Physicochemical Properties
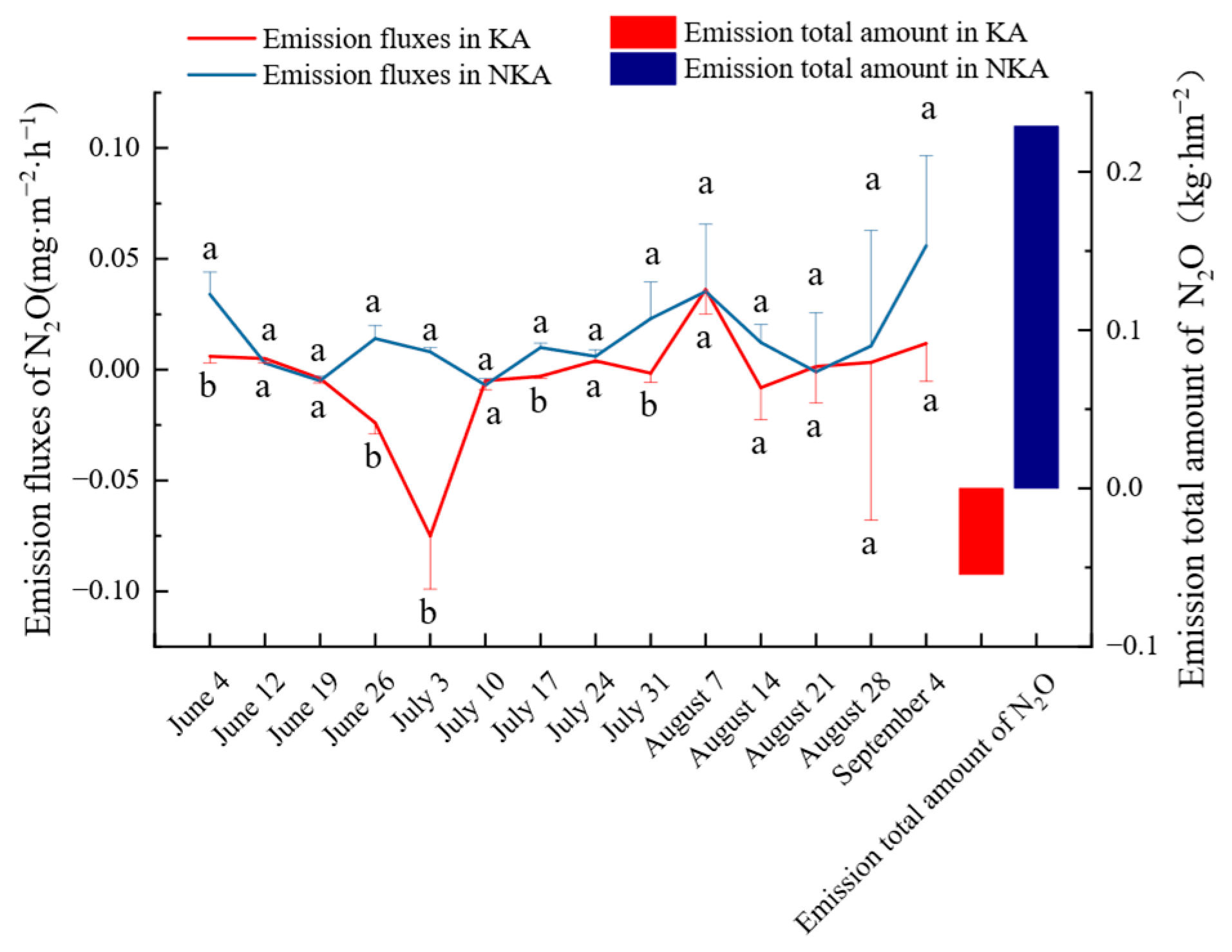
3.2. N2O Emission Fluxes and Cumulative Emissions in KA and NKA
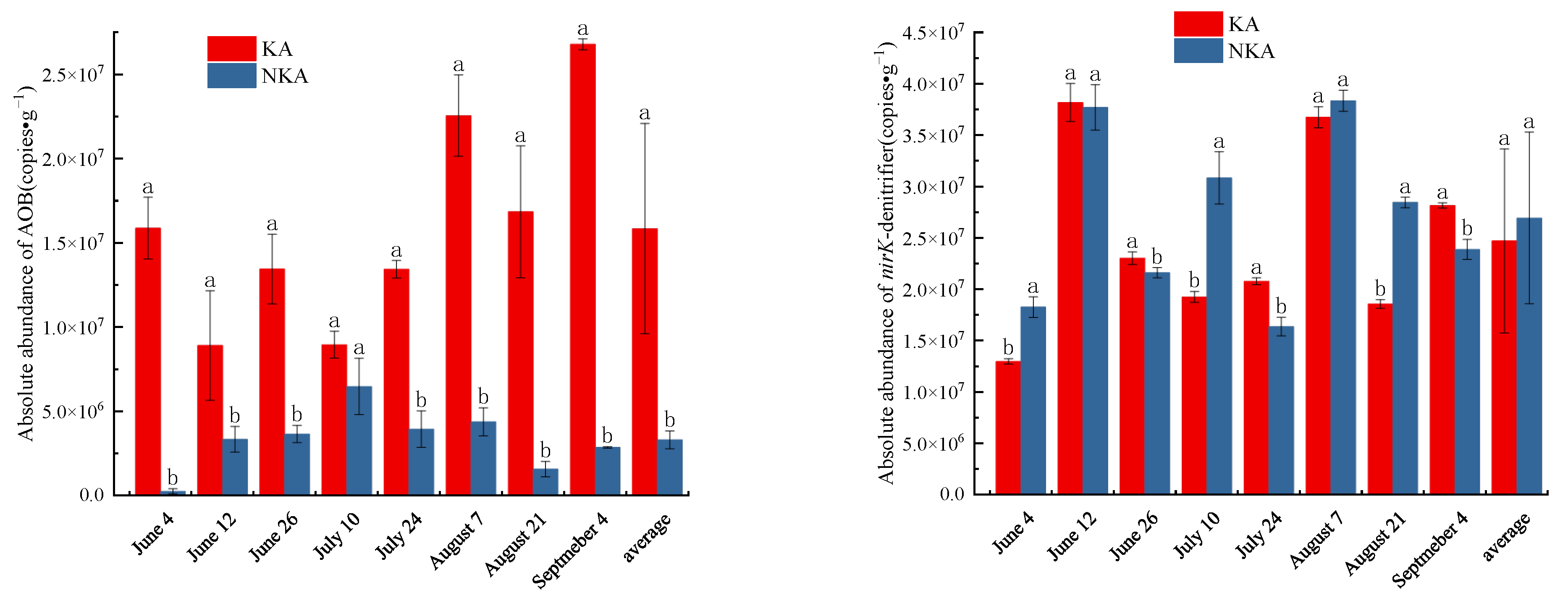
3.3. Absolute Abundance of AOB/nirK-Denitrifier in KA and NKA
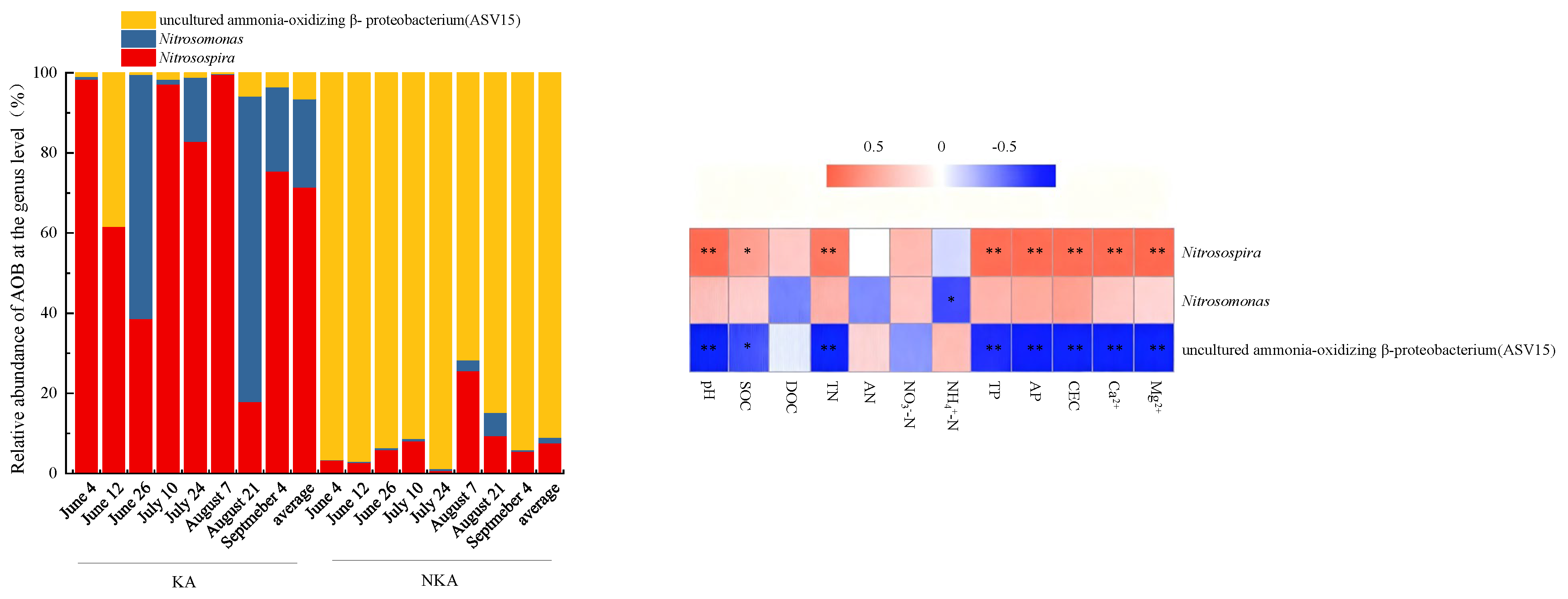
3.4. Community Structure of AOB in KA and NKA
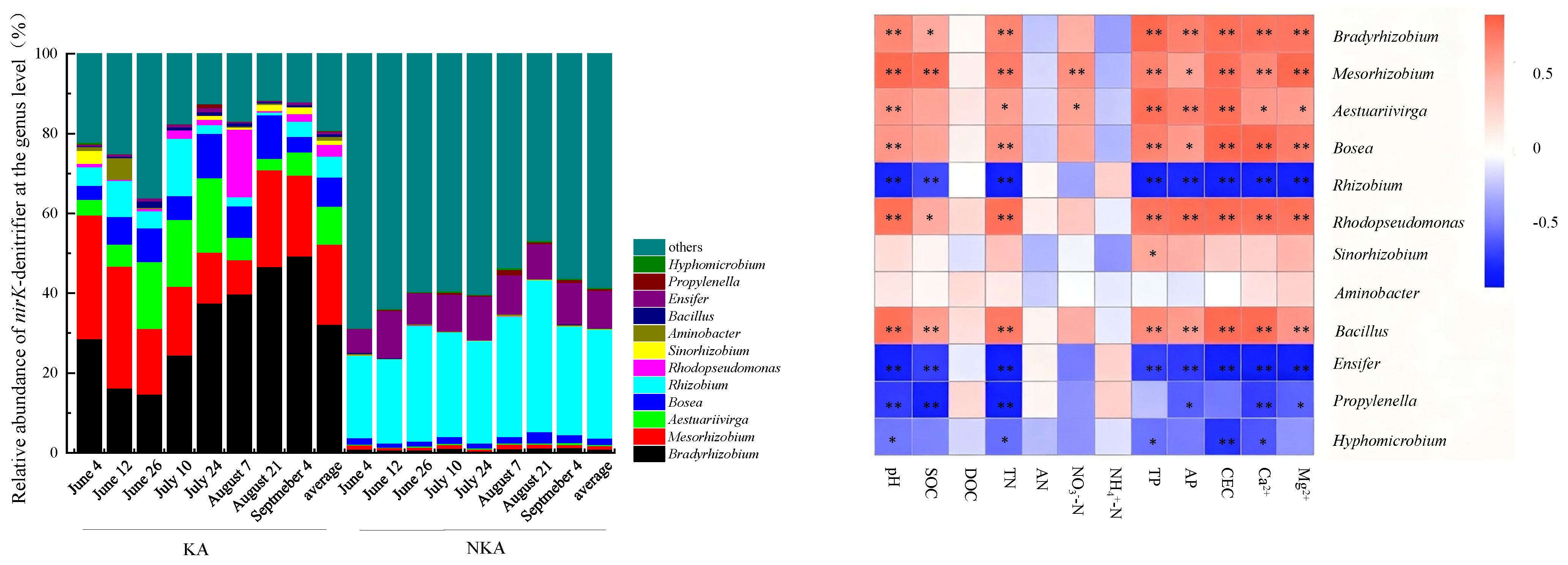
3.5. Community Structure of nirK-Denitrifier in KA and NKA
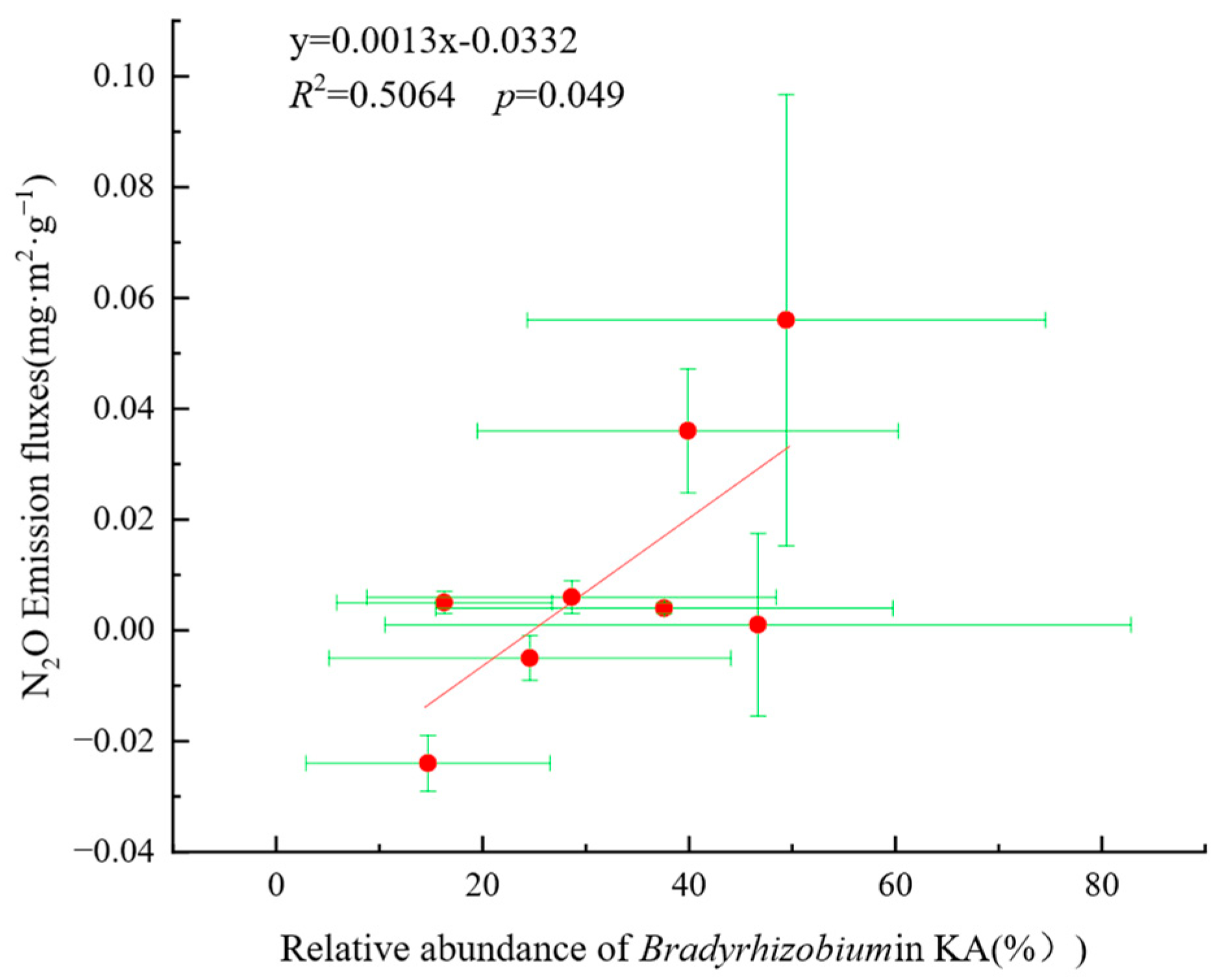
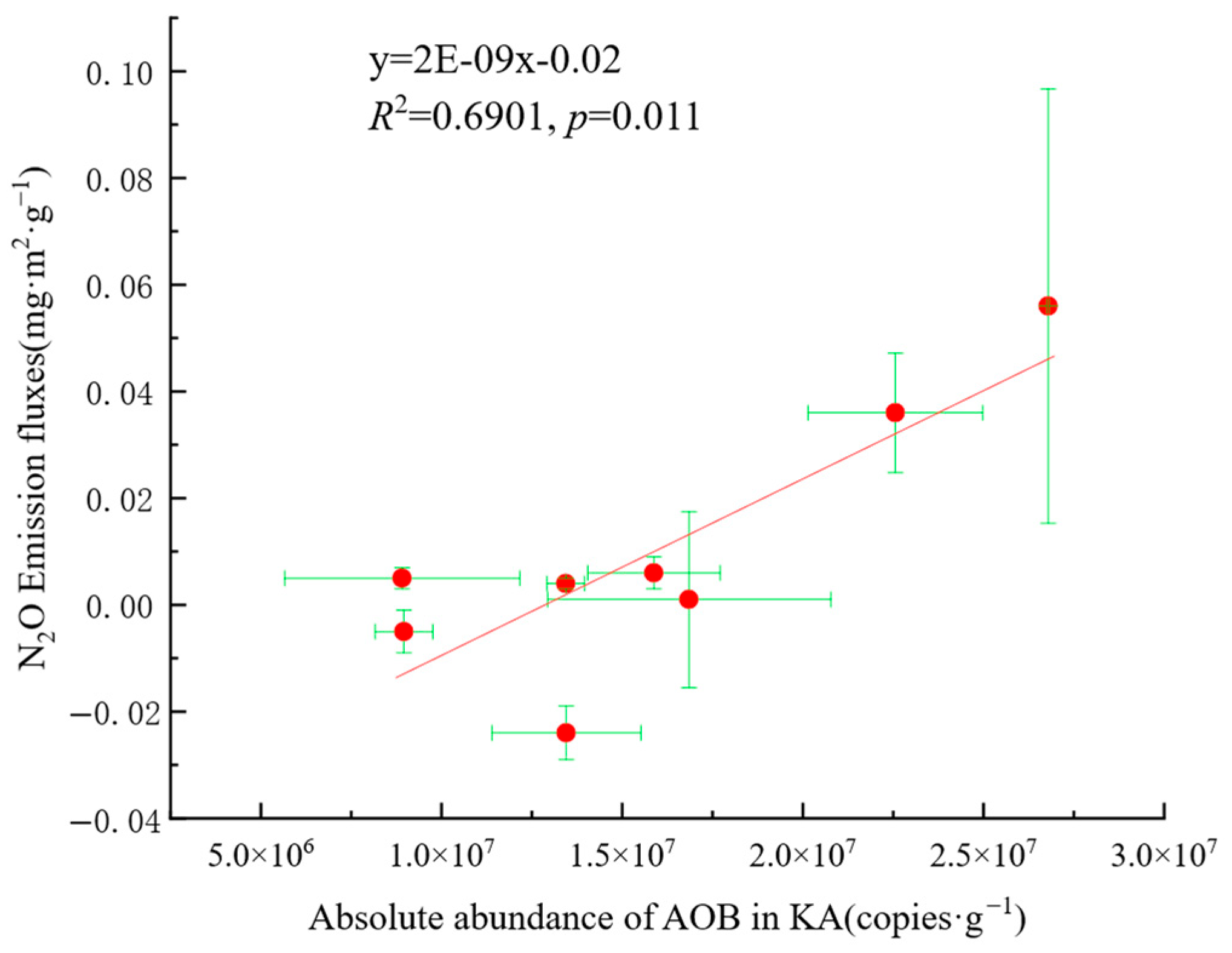
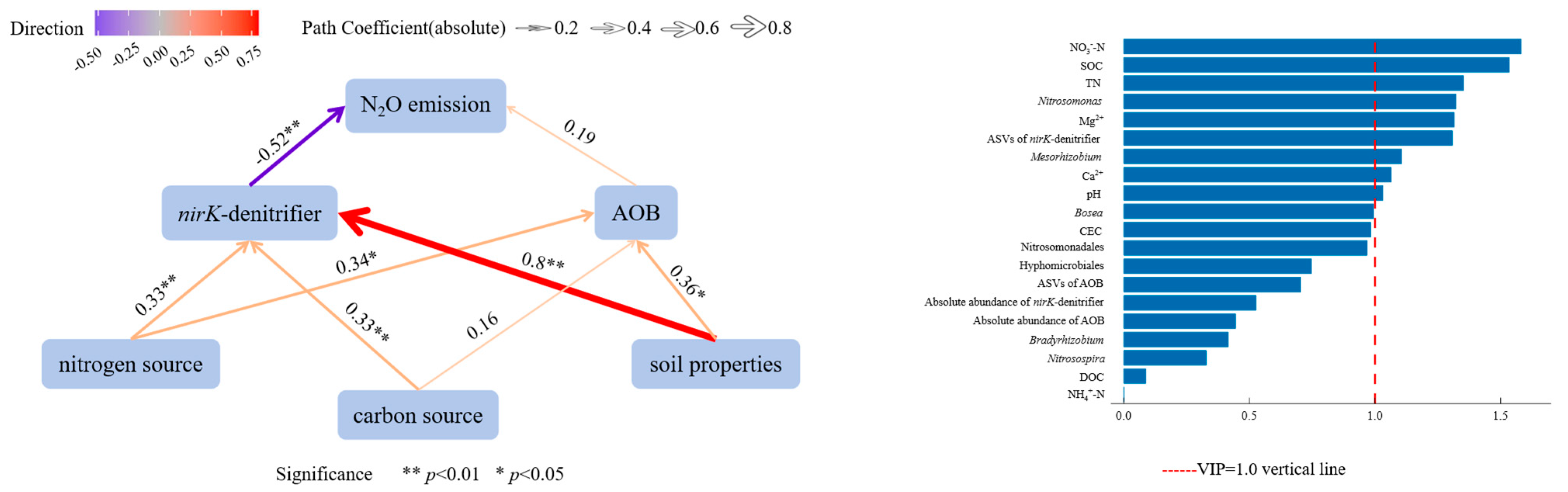
3.6. Correlation and Relative Importance of Soil Physicochemical Factors, AOB, nirK-Denitrifier, and N2O Emission Fluxes in KA and NKA
4. Discussion
4.1. Differences in N2O Emissions Between KA and NKA and Their Environmental Drivers
4.2. Dominant AOB Communities in KA and Their Underlying Causes
4.3. Relationship Between AOB and N2O Emissions
4.4. Differences in nirK-Denitrifier Community Structure Between KA and NKA and Their Causes
4.5. Relationship Between nirK-Denitrifiers and N2O Emissions
5. Conclusions
- (1)
- The cumulative N2O emissions were −0.054 kg·hm−2 in KA and 0.229 kg·hm−2 in the NKA throughout the rice growth period, respectively, indicating that karst rice fields are reservoirs of N2O.
- (2)
- The absolute abundance of AOB was significantly higher in KA than that in NKA, whereas the absolute abundance of nirK-denitrifier did not differ significantly between the two areas.
- (3)
- The dominant AOB in KA were Nitrosospira and Nitrosomonas, while the dominant AOB in NKA was an uncultured ammonia-oxidizing β-proteobacterium. The dominant nirK-denitrifiers in KA were Bradyrhizobium, Mesorhizobium, Aestuariivirga, and Bosea, whereas Rhizobium and Ensifer were dominant in NKA.
- (4)
- Soil properties, nitrogen sources, and carbon sources had positive effects on AOB, while soil properties, nitrogen sources, and phosphorus sources positively affected AOB. The nirK-denitrifiers had a negative effect on N2O emission fluxes. Environmental factors with high importance for N2O emission fluxes included NO3−-N, SOC, TN, Mg2+, Ca2+, and pH, and key microbial factors were Nitrosomonas, ASVs of nirK-denitrifiers, and Mesorhizobium, indicating that these factors significantly influence N2O emissions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Areas | 4 June | 12 June | 26 June | 10 July | 24 July | 7 August | 21 August | 4 September | Average | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | KA | 7.50 ± 0.04 a | 7.26 ± 0.61 a | 7.39 ± 0.13 a | 7.57 ± 0.29 a | 7.09 ± 0.11 a | 7.38 ± 0.14 a | 7.50 ± 0.21 a | 7.31 ± 0.25 a | 7.38 ± 0.16 a |
| NKA | 5.01 ± 0.26 b | 4.90 ± 0.23 b | 6.23 ± 0.5 b | 4.82 ± 0.11 b | 4.78 ± 0.06 b | 4.88 ± 0.27 b | 4.74 ± 0.14 b | 4.80 ± 0.31 b | 5.02 ± 0.50 b | |
| SOC (g/kg) | KA | 15.42 ± 0.37 a | 14.43 ± 1.10 a | 14.79 ± 0.90 a | 14.52 ± 1.72 a | 13.27 ± 1.76 a | 13.48 ± 0.42 a | 14.11 ± 0.95 a | 14.48 ± 2.09 a | 14.31 ± 0.69 a |
| NKA | 13.78 ± 1.01 b | 14.05 ± 1.27 a | 12.07 ± 1.56 b | 12.59 ± 0.37 b | 10.19 ± 1.42 b | 10.01 ± 0.94 b | 11.14 ± 0.73 b | 9.74 ± 3.39 b | 11.70 ± 1.69 b | |
| DOC (mg/kg) | KA | 0.23 ± 0.03 b | 1.06 ± 0.1 a | 0.21 ± 0.04 b | 0.70 ± 0.02 a | 0.90 ± 0.13 a | 0.56 ± 0.09 a | 0.24 ± 0.10 a | 0.25 ± 0.06 a | 0.52 ± 0.34 a |
| NKA | 0.38 ± 0.07 a | 0.32 ± 0.08 b | 1.65 ± 0.15 a | 0.38 ± 0.03 b | 0.21 ± 0.06 b | 0.44 ± 0.21 a | 0.17 ± 0.04 a | 0.26 ± 0.09 a | 0.48 ± 0.48 a | |
| TN (g/kg) | KA | 1.90 ± 0.03 a | 1.71 ± 0.03 a | 1.91 ± 0.05 a | 1.84 ± 0.03 a | 1.69 ± 0.03 a | 1.91 ± 0.31 a | 1.97 ± 0.00 a | 1.91 ± 0.10 a | 1.86 ± 0.10 a |
| NKA | 1.68 ± 0.03 b | 1.57 ± 0.02 b | 1.72 ± 0.03 b | 1.67 ± 0.06 b | 1.52 ± 0.14 a | 1.55 ± 0.04 a | 1.36 ± 0.04 b | 1.17 ± 0.02 b | 1.53 ± 0.19 b | |
| AN (mg/kg) | KA | 55.49 ± 5.67 b | 91.43 ± 2.04 a | 56.43 ± 10.30 b | 98.89 ± 2.13 a | 91.48 ± 9.15 a | 83.96 ± 18.16 a | 64.58 ± 2.01 a | 69.03 ± 3.83 a | 76.41 ± 17.10 a |
| NKA | 93.25 ± 8.11 a | 92.57 ± 1.47 a | 158.05 ± 7.10 a | 78.57 ± 5.33 b | 65.33 ± 1.58 b | 71.60 ± 7.16 a | 56.47 ± 2.01 b | 53.53 ± 1.76 b | 83.67 ± 33.51 a | |
| NH4+-N (mg/kg) | KA | 11.55 ± 0.37 b | 32.41 ± 0.15 b | 13.61 ± 0.31 b | 27.82 ± 1.38 b | 26.5 ± 0.45 b | 22.2 ± 0.17 b | 13.76 ± 0.45 b | 13.81 ± 0.15 b | 20.21 ± 8.03 b |
| NKA | 46.67 ± 0.60 a | 55.18 ± 1.32 a | 88.60 ± 4.00 a | 50.56 ± 0.55 b | 47.63 ± 0.27 b | 32.21 ± 0.32 a | 25.76 ± 0.44 a | 24.77 ± 0.36 a | 40.40 ± 12.51 a | |
| NO3−-N (mg/kg) | KA | 39.30 ± 0.77 a | 54.29 ± 10.65 a | 45.64 ± 1.11 a | 49.96 ± 6.95 a | 35.76 ± 13.72 a | 26.43 ± 2.33 a | 36.44 ± 0.41 a | 40.79 ± 0.17 a | 41.08 ± 8.80 a |
| NKA | 25.99 ± 1.18 b | 22.87 ± 1.09 b | 8.83 ± 0.62 b | 27.49 ± 1.79 b | 30.25 ± 1.64 b | 9.61 ± 0.95 b | 31.77 ± 0.82 b | 29.02 ± 0.52 b | 23.23 ± 9.06 b | |
| TP (g/kg) | KA | 0.32 ± 0.08 a | 0.21 ± 0.01 a | 0.15 ± 0.03 a | 0.18 ± 0.03 a | 0.33 ± 0.04 a | 0.21 ± 0.04 a | 0.24 ± 0.03 a | 0.26 ± 0.03 a | 0.24 ± 0.06 a |
| NKA | 0.08 ± 0.04 b | 0.14 ± 0.01 b | 0.07 ± 0.01 b | 0.08 ± 0.02 b | 0.13 ± 0.04 b | 0.11 ± 0.02 b | 0.12 ± 0.01 b | 0.13 ± 0.02 b | 0.11 ± 0.03 b | |
| AP (mg/kg) | KA | 30.53 ± 3.09 a | 12.82 ± 1.05 a | 27.76 ± 1.53 a | 20.09 ± 0.17 a | 24.14 ± 2.46 a | 42.38 ± 6.79 a | 23.47 ± 1.87 a | 33.73 ± 6.10 a | 26.87 ± 8.98 a |
| NKA | 13.05 ± 1.53 b | 7.60 ± 0.55 b | 15.99 ± 0.72 b | 15.89 ± 0.24 b | 14.60 ± 0.10 b | 12.88 ± 3.44 b | 11.60 ± 3.78 b | 12.59 ± 1.04 b | 13.03 ± 2.70 b | |
| CEC (cmol/kg) | KA | 6.51 ± 2.40 a | 6.47 ± 1.25 a | 6.97 ± 1.39 a | 7.30 ± 0.77 a | 7.87 ± 1.29 a | 6.86 ± 0.99 a | 8.34 ± 0.18 a | 6.98 ± 2.49 a | 7.16 ± 0.65 a |
| NKA | 4.14 ± 0.25 a | 3.97 ± 0.35 b | 4.58 ± 0.09 b | 3.92 ± 0.82 b | 3.55 ± 0.66 b | 4.05 ± 0.27 b | 2.84 ± 0.67 b | 3.58 ± 0.13 a | 3.83 ± 0.51 b | |
| Ca2+ (cmol/kg) | KA | 4.83 ± 0.16 a | 4.73 ± 0.33 a | 5.02 ± 0.14 a | 4.63 ± 0.19 a | 4.89 ± 0.25 a | 5.08 ± 0.27 a | 4.96 ± 0.17 a | 4.64 ± 0.29 a | 4.85 ± 0.17 a |
| NKA | 2.43 ± 0.22 b | 2.44 ± 0.3 b | 2.41 ± 0.03 b | 2.30 ± 0.01 b | 1.87 ± 0.32 b | 2.20 ± 0.16 b | 2.34 ± 0.22 b | 2.08 ± 0.03 b | 2.26 ± 0.20 b | |
| Mg2+ (cmol/kg) | KA | 0.26 ± 0.02 a | 0.24 ± 0.01 a | 0.23 ± 0.00 a | 0.23 ± 0.01 a | 0.24 ± 0.00 a | 0.24 ± 0.00 a | 0.24 ± 0.00 a | 0.23 ± 0.01 a | 0.24 ± 0.01 a |
| NKA | 0.21 ± 0.00 b | 0.22 ± 0.00 b | 0.22 ± 0.00 b | 0.21 ± 0.01 b | 0.15 ± 0.02 b | 0.17 ± 0.00 b | 0.20 ± 0.01 b | 0.16 ± 0.00 b | 0.19 ± 0.03 b |
| Diversity | Areas | 4 June | 12 June | 26 June | 10 July | 24 July | 7 August | 21 August | 4 September | Average |
|---|---|---|---|---|---|---|---|---|---|---|
| ASVs | KA | 268 ± 134 a | 536 ± 592 a | 268 ± 101 a | 416 ± 424 a | 475 ± 233 a | 25 ± 23 a | 28 ± 19 a | 78 ± 43 a | 262 ± 203 a |
| NKA | 212 ± 356 a | 177 ± 200 a | 936 ± 40 a | 83 ± 61 a | 288 ± 270 a | 2039 ± 3243 a | 52 ± 26 a | 132 ± 153 a | 490 ± 687 a |
| Diversity | Areas | 4 June | 12 June | 26 June | 10 July | 24 July | 7 August | 21 August | 4 September | Average |
|---|---|---|---|---|---|---|---|---|---|---|
| Chao1 Index | KA | 9661 ± 2658 a | 5916 ± 1475 a | 9990 ± 2730 a | 8417 ± 2614 a | 6964 ± 2732 a | 7501 ± 1210 a | 6999 ± 2269 a | 5462 ± 2007 a | 7614 ± 1639 a |
| NKA | 1988 ± 1230 b | 2461 ± 2050 a | 2105 ± 864 b | 1642 ± 550 b | 2176 ± 371 b | 2207 ± 1117 b | 1447 ± 583 b | 1318 ± 695 b | 1918 ± 404 b | |
| ACE Index | KA | 43.27 ± 5.72 a | 70.01 ± 52.05 a | 68.04 ± 16.88 a | 42.88 ± 6.04 a | 42.80 ± 4.93 a | 48.56 ± 7.51 a | 70.48 ± 32.23 a | 46.05 ± 17.83 a | 54.01 ± 12.99 a |
| NKA | 52.67 ± 17.47 a | 40.06 ±1.42 a | 40.46 ± 4.46 a | 39.31 ± 8.00 a | 49.44 ± 22.15 a | 41.92 ± 6.42 a | 34.87 ± 9.42 a | 47.50 ± 30.64 a | 43.28 ± 5.98 a | |
| Shannon Index | KA | 47.19 ± 2.85 a | 53.98 ±16.25 a | 87.36 ± 24.70 a | 47.77 ± 9.54 a | 50.68 ± 3.65 a | 50.77 ± 5.37 a | 66.42 ± 23.25 a | 50.28 ± 21.76 a | 56.81 ± 13.76 a |
| NKA | 51.21 ± 11.81 a | 43.77 ± 3.16 a | 43.86 ± 5.34 b | 43.65 ± 7.80 a | 53.52 ± 24.72 a | 45.06 ± 5.97 a | 35.45 ± 10.42 a | 37.72 ± 13.76 a | 44.28 ± 6.05 a | |
| Simpson Index | KA | 2.19 ± 0.20 a | 2.36 ± 0.19 a | 2.29 ± 0.15 a | 2.41 ± 0.07 a | 2.40 ± 0.47 a | 2.46 ± 0.23 a | 1.95 ±0.12 a | 1.73 ± 0.69 a | 2.22 ± 0.26 a |
| NKA | 1.32 ± 0.10 b | 1.41 ± 0.29 b | 1.58 ±0.12 b | 1.61 ± 0.18 b | 1.41 ± 0.43 b | 1.70 ± 0.18 b | 1.79 ±0.12 a | 1.68 ± 0.43 a | 1.56 + 0.17 b | |
| Chao1 Index | KA | 0.79 ±0.05 a | 0.85 ± 0.04 a | 0.82 ± 0.04 a | 0.87 ± 0.01 a | 0.83 ± 0.08 a | 0.86 ± 0.04 a | 0.74 ± 0.06 a | 0.62 ±0.25 a | 0.80 ± 0.08 a |
| NKA | 0.52 ± 0.05 b | 0.56 ± 0.12 b | 0.61 ± 0.03 b | 0.63 ± 0.06 b | 0.55 ± 0.19 b | 0.67 ± 0.03 b | 0.69 ± 0.02 a | 0.65 ± 0.11 a | 0.61 ± 0.06 b |
Appendix B
References
- Barnes, P.W.; Lucas, R.M.; Williamson, C.E.; Lucas, R.M.; Robinson, S.A.; Zepp, R.G. Ozone Depletion, Ultraviolet Radiation, Climate Change and Prospects for A Sustainable Future. Nat. Sustain. 2019, 2, 569–579. [Google Scholar] [CrossRef]
- Prather, M.J.; Hsu, J.; DeLuca, N.M.; Jackman, C.H.; Oman, L.D.; Douglass, A.R.; Fleming, E.L.; Strahan, S.E.; Steenrod, S.D.; Søvde, O.A. Measuring and Modeling the Lifetime of Nitrous Oxide Including Its Variability. J. Geophys. Res. Atmos. 2015, 120, 5693–5705. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Qin, D.; Manning, M.; Marquis, M.; Averyt, K.; Tignor, M.M.B.; Miller, H.R., Jr.; Chen, Z. Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2007. [Google Scholar]
- Stocker, T.F.; Plattner, G.K.; Qin, D.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Huang, S.; Sun, Y.N.; Zhang, W.J. Changes in Soil Organic Carbon Stocks as Affected by Cropping Systems and Cropping Duration in China’s Paddy Fields: A Meta-Analysis. Clim. Chang. 2012, 112, 847–858. [Google Scholar] [CrossRef]
- Wu, J.S. Carbon Accumulation in Paddy Ecosystems in Subtropical China: Evidence from Landscape Studies. Eur. J. Soil Sci. 2011, 62, 29–34. [Google Scholar] [CrossRef]
- Raushan, K.; Bipradeep, M.; Nirmali, B. Application of Straw-Derived Biochar: A Sustainable Approach to Improve Soil Quality and Crop Yield and Reduce N2O Emissions in Paddy Soil. Environ. Sci. Pollut. Res. Int. 2024, 31, 60804–60818. [Google Scholar]
- Liu, H.Y.; Ding, Y.; Zhang, Q.C.; Liu, X.M.; Xu, J.M.; Li, Y.; Di, H.J.l. Heterotrophic Nitrification and Denitrification Are the Main Sources of Nitrous Oxide in Two Paddy Soils. Plant Soil Int. J. Plant-Soil Relatsh. 2019, 445, 39–53. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Li, T.; Lu, X.; Hu, Q.; Qin, Z. GloRice, a global rice database (v1.0): I. Gridded paddy rice annual distribution from 1961 to 2021. Sci. Data 2025, 12, 182. [Google Scholar] [CrossRef]
- Hei, Z.; Peng, Y.; Hao, S.; Li, Y.; Yang, X.; Zhu, T.; Müller, C.; Zhang, H.; Hu, H.; Chen, Y. Full Substitution of Chemical Fertilizer by Organic Manure Decreases Soil N2O Emissions Driven by Ammonia Oxidizers and Gross Nitrogen Transformations. Glob. Change Biol. 2023, 29, 7117–7130. [Google Scholar] [CrossRef]
- Jäger, N.; Stange, C.F.; Ludwig, B.; Flessa, H. Emission Rates of N2O and CO2 from Soils with Different Organic Matter Content from Three Long-Term Fertilization Experiments-a Laboratory Study. Biol. Fertil. Soils 2011, 47, 483–494. [Google Scholar] [CrossRef]
- Deng, N.; Cecile, G.R.; Song, X.; Ju, X.T.; Liu, S.Y.; Shen, J.P.; Di, H.J.; Han, L.; Zhang, L.M. AOB Nitrosospira Cluster 3a.2 (D11) Dominates N2O Emissions in Fertilised Agricultural Soils. J. Environ. Manag. 2024, 355, 120504. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, W.; Zhao, X.; Li, J.; Chu, G.; Tao, R. The Cooperative Interaction of AOB and Comammox Clade A Drives Nitrification and N2O Emissions in a Long-Term Organic Fertilized Paddy Soil. Appl. Soil Ecol. 2024, 200, 105451. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, H.; Wang, J.; Ji, C.; Liu, Y.; Chen, D.; Zhang, H.; Wang, J.; Zhang, Y. Fertilizer N Triggers Native Soil N-Derived N2O Emissions by Priming Gross N Mineralization. Soil Biol. Biochem. 2023, 178, 108961. [Google Scholar] [CrossRef]
- Sheng, M.; Xiong, K.; Wang, L.; Li, X.; Li, R.; Tian, X. Response of Soil Physical and Chemical Properties to Rocky Desertification Succession in South China karst. Carbonates Evaporites 2018, 33, 15–28. [Google Scholar] [CrossRef]
- Jerray, A.; Rumpel, C.; Le, R.X.; Massad, R.S. N2O Emissions from Cropland and Grassland Management Systems are Determined by Soil Organic Matter Quality and Soil Physical Parameters rather than Carbon Stock and Denitrifier Abundances. Soil Biol. Biochem. 2024, 190, 109274. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Zhao, Q.; Gao, X.; He, T.; Yang, S. Response of GHG Emissions to Interactions of Temperature and Drying in the Karst Wetland of the Yunnan-Guizhou Plateau. Front. Environ. Sci. 2022, 10, 973900. [Google Scholar] [CrossRef]
- Lin, F.; Wang, H.; Shaghaleh, H.; Hamad, A.; Zhang, Y.; Yang, B.; Hamoud, Y.A. Effects of Biochar Amendment on N2O Emissions from Soils with Different pH Levels. Atmosphere 2024, 15, 68. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, M.; Wang, S.; Luo, W.; Peng, T.; Zhu, B.; Wang, T. Effects of Afforestation on Soil CH4 and N2O Fluxes in a Nsubtropical Karst Landscape. Sci. Total Environ. 2020, 705, 135974. [Google Scholar] [CrossRef]
- Wei, L.; Liu, X.; Qin, C.; Xing, W.; Gu, Y.; Wang, X.; Bai, L.; Li, J. Impacts of Soil Moisture and Fertilizer on N2O Emissions from Cornfield Soil in a Karst Watershed, SW China. Atmosphere 2022, 13, 1200. [Google Scholar] [CrossRef]
- Han, B.; Yao, Y.; Liu, B.; Wang, Y.; Su, X.; Ma, L.; Liu, D.; Niu, S.; Chen, X.; Li, Z. Relative Importance between Nitrification and Denitrification to N2O from a Global Perspective. Glob. Change Biol. 2023, 30, e17082. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, Structure, and Size of N2O Producing Microbial Communities in Soils-What Matters for Their Functioning. Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar]
- Yang, L.; Zhu, G.; Ju, X.; Liu, R. How Nitrification-Related N2O is Associated with Soil Ammonia Oxidizers in Two Contrasting Soils in China. Sci. Total Environ. 2020, 770, 143212. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Xing, Y.; Bodington, D.; Huang, X.; Ding, C.; Ge, T.; Di, H.; Xu, J.; Li, Y.; et al. Organic Fertilizer Significantly Mitigates N2O Emissions While Increase Contributed of Comammox Nitrospira in Paddy Soils. Sci. Total Environ. 2024, 954, 176578. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, G.; Rees, R.M.; Cao, W. Green Manuring Inhibits Nitrification in a Typical Paddy Soil by Changing the Contributions of Ammonia-Oxidizing Archaea and Bacteria. Appl. Soil Ecol. 2020, 156, 103698. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; He, Y.; Liu, H.; Dumont, M.G.; Brookes, P.C.; Xu, J. Nitrosospira Cluster 3-Like Bacterial Ammonia Oxidizers and Nitrospira-Like Nitrite Oxidizers Dominate Nitrification Activity in Acidic Terrace Paddy Soils. Soil Biol. Biochem. 2019, 131, 229–237. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; He, Y.; Brookes, P.C.; Xu, J. Elevated Temperature Increased Nitrification Activity by Stimulating AOB Growth and Activity in an Acidic Paddy Soil. Plant Soil 2019, 445, 71–83. [Google Scholar] [CrossRef]
- Zuo, J.; Hu, H.; Fu, Q.; Zhu, J.; Zheng, H.; Mo, M.; Tu, A. Responses of N2O Production and Abundances of Associated Microorganisms to Soil Profiles and Water Regime in Two Paddy Soils. Agronomy 2022, 12, 743. [Google Scholar] [CrossRef]
- Xiang, H.; Hong, Y.; Wu, J.; Wang, Y.; Ye, F.; Ye, J.; Lu, J.; Long, A. Denitrification Contributes to N2O Emission in Paddy Soils. Front. Microbiol. 2023, 14, 1218207. [Google Scholar] [CrossRef]
- Li, H.; Meng, J.; Liu, Z.; Lan, Y.; Yang, X.; Huang, Y.; He, T.; Chen, W. Effects of Biochar on N2O Emission in Denitrification Pathway from Paddy Soil: A Drying Incubation Study. Sci. Total Environ. 2021, 787, 147591. [Google Scholar] [CrossRef]
- Yang, L.; Barnard, R.; Kuzyakov, Y.; Tian, J. Bacterial Communities Drive the Resistance of Soil Multifunctionality to Land-Use Change in Karst Soils. Eur. J. Soil Biol. 2021, 104, 103313. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, P.; Zhou, G.; Chang, D.; Liang, H.; Chai, Q.; Cao, W. Co-Incorporation of Wheat Straw and Hairy Vetch Reduced Soil N2O Emission Via Regulating Nitrifier and Denitrifier Structure on the Qinghai Plateau. Appl. Soil Ecol. 2024, 202, 105574. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, C.; Wei, X.; Liu, Y.; Chen, X.; Qin, H.; Wu, J.; Su, Y.; Ge, T.; Hu, Y. Characterization of NirS- and nirK-Containing Communities and Potential Denitrification Activity in Paddy Soil from Eastern China. Agric. Ecosyst. Environ. 2021, 319, 107561. [Google Scholar] [CrossRef]
- Jin, W.; Cao, W.; Liang, F.; Wen, Y.; Wang, F.; Dong, Z.; Song, H. Water Management Impact on Denitrifier Community and Denitrification Activity in a Paddy Soil at Different Growth Stages of Rice. Agric. Water Manag. 2020, 241, 106354. [Google Scholar] [CrossRef]
- Hendra, G.W.L.; Suman, L.; Dou, F.G.; Gentry, T. The Environmental Trade-Offs of Applying Soil Amendments Microbial Biomass and Greenhouse Gas Emission Dynamics in Organic Rice Paddy Soils. Appl. Soil Ecol. 2025, 208, 105977. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Zhang, Y.; Qin, S.; Wei, S.; Wang, S.; Hu, C.; Liu, B. Organic Carbon Availability Limiting Microbial Denitrification in The Deep Vadose Zone. Environ. Microbiol. 2018, 20, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, X.; Zhang, W.; Hu, P.; Sun, M.; Wang, K. Comparison of Bacterial and Fungal Diversity and Network Connectivity in Karst and Non-Karst Forests in Southwest China. Sci. Total Environ. 2022, 822, 153179. [Google Scholar] [CrossRef] [PubMed]
- Luo, D. The Characteristics of Soil Ca and Mg Leakage in a Karst Depression. Sustainability 2022, 14, 9627. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, H.; Zheng, C.; Wu, X.; Zhao, Y.; Li, X.; Liu, H.; Dong, L.; Lu, Z.; Zhou, J.; et al. Bacteria Life-History Strategies and The Linkage of Soil C-N-P Stoichiometry to Microbial Resource Limitation Differed in Karst and Non-Karst Plantation Forests in Southwest China. Catena 2023, 231, 107341. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Wang, K.; Hou, Y. Soil Organic Carbon and Total Nitrogen as Affected by Land Use Types in Karst and Non-Karst of Northwest Guangxi, China. J. Sci. Food Agric. 2012, 92, 1086–1093. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Wen, D.; Liu, L.; Ni, K.; Cao, J.; Zhu, T.; Müller, C. Soil Calcium Constrains Nitrogen Mineralization and Nitrification Rates in Subtropical Karst Regions. Soil Biol. Biochem. 2023, 186, 109176. [Google Scholar] [CrossRef]
- Chen, C.; Ai, J.; Chen, L.; Li, Y.; Tang, X.; Li, J. Nitrogen Metabolism Pathways and Functional Microorganisms in Typical Karst Wetlands. Environ. Sci. Pollut. Res. 2024, 31, 22494–22506. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Gao, G.; Peng, Y.; Ning, Y.; Huang, Z.; Chen, Z.; Xu, X.; Wu, Z. Karst Ecosystem Moso Bamboo Intercropping Enhances Soil Fertility and Microbial Diversity in the Rhizosphere of Giant Lily (Cardiocrinum giganteum). Forests 2024, 15, 2004. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Z.; Yuan, W.; Chen, W.; Li, X.; Xiong, L.; Cheng, G. Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas. Agronomy 2023, 13, 2001. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Xiong, L.; Tong, L.; Zhu, H.; Zhang, X.; Qin, G. Effects of Land Reclamation on Soil Bacterial Community and Potential Functions in Bauxite Mining Area. Int. J. Environ. Res. Public Health 2022, 19, 16921. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Analysis Methods of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; Volume 107, pp. 147–150. [Google Scholar]
- Wang, Z.; Hasi, E.; Han, X.; Qingda, M. Fractal Characterization of Soil Particle Size Distribution under Different Land Use Patterns on the North Slope of Wula Mountain in China. J. Soils Sediments 2024, 24, 1148–1164. [Google Scholar] [CrossRef]
- Bi, X.; Chu, H.; Fu, M.; Xu, D.; Zhao, W.; Zhong, Y.; Wang, M.; Li, K.; Zhang, Y. Distribution Characteristics of Organic Carbon (Nitrogen) Content, Cation Exchange Capacity, and Specific Surface Area in Different Soil Particle Sizes. Sci. Rep. 2023, 13, 12242. [Google Scholar] [CrossRef]
- Hou, H.; Peng, S.; Xu, J.; Yang, S.; Mao, Z. Seasonal Variations of CH4 and N2O Emissions in Response to Water Management of Paddy Fields Located in Southeast China. Chemosphere 2012, 89, 884–892. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Stockholm, Sweden, 2013. [Google Scholar]
- Rotthauwe, J.H.; Witael, K.P.; Liesack, W. The Ammonia Monooxygenase Structural Gene AmoA as a Functional Marker: Molecular Fine-scale Analysis of Aatural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Zhang, X.; Agogué, H.; Dupuy, C.; Gong, J. Relative Abundance of Ammonia Oxidizers, Denitrifiers, and Anammox Bacteria in Sediments of Hyper-Nutrified Estuarine Tidal Flats and in Relation to Environmental Conditions. Clean-Soil Air Water 2014, 42, 815–823. [Google Scholar] [CrossRef]
- Shang, Z.; Cui, X.; Kees, J.V.G.; Matthias, K.; Mohamed, A.; Luo, J.; Zhang, W.; Song, Z.; Jiang, Y.; Pete, S.; et al. Global Cropland Nitrous Oxide Emissions in Fallow Period are Comparable to Growing-Season Emissions. Glob. Change Biol. 2024, 30, e17165. [Google Scholar] [CrossRef]
- Tang, Y.; Su, X.; Wen, T.; McBratney, A.B.; Zhou, S.; Huang, F.; Zhu, Y. Soil Properties Shape the Heterogeneity of Denitrification and N2O Emissions Across Large-Scale Flooded Paddy Soils. Glob. Change Biol. 2024, 30, e17176. [Google Scholar] [CrossRef]
- Wu, L.; Tang, S.; Hu, R.; Wang, J.; Duan, P.; Xu, C.; Zhang, W.; Xu, M. Increased N2O Emission due to Paddy Soil Drainage is Regulated by Carbon and Nitrogen Availability. Geoderma 2023, 432, 116422. [Google Scholar] [CrossRef]
- Song, X.; Parker, J.; Jones, S.K.; Zhang, L.; Lan, B.; Rees, R.M.; Ju, X. Labile Carbon from Artificial Roots Alters the Patterns of N2O and N2 Production in Agricultural Soils. Environ. Sci. Technol. 2024, 58, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Ros, G.; Chen, Y.; Yang, X.; Cui, Z.; Liu, X.; Jiang, R.; Zhang, F.; Vries, W.D. Global Meta-Analysis of Terrestrial Nitrous Oxide Emissions and Associated Functional Genes under Nitrogen Addition. Soil Biol. Biochem. 2022, 165, 108523. [Google Scholar] [CrossRef]
- Wang, J.; Lv, L.; Hu, R.G.; Ma, H.Y.; Liu, B.; Zhang, W.J.; Wu, L. Patterns and determinants of nitrification and denitrification potentials across 24 rice paddy soils in subtropical China. Agric. Ecosyst. Environ. 2024, 361, 108799. [Google Scholar] [CrossRef]
- Zheng, C.H.; Wan, L.; Wang, R.S.; Wang, G.; Dong, L.; Yang, T.; Yang, Q.L.; Zhou, J.X. Effects of Rock Lithology and Soil Nutrients on Nitrogen and Phosphorus Mobility in Trees in Non-karst and Karst Forests of Southwest China. For. Ecol. Manag. 2023, 548, 121392. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Zhan, Y.; Zheng, X.; Zhou, M.; Yan, G.; Wang, L.; Werner, C.; Klaus, B. Potential Benefits of Liming to Acid Soils on Climate Change Mitigation and Food Security. Glob. Change Biol. 2021, 27, 2807–2821. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, X.; Wang, X.; Xie, C.; Liu, J.; Cheng, Y.; Yue, Y.; You, X.; Li, Y. Modified Biochar Affects CO2 and N2O Emissions from Coastal Saline Soil by Altering Soil pH and Elemental Stoichiometry. Sci. Total Environ. 2024, 954, 176283. [Google Scholar] [CrossRef]
- Bera, T.; Inglett, K.S.; Liu, G. Effects of Solid Oxygen Fertilizers and Biochars on Nitrous Oxide Production from Agricultural Soils in Florida. Sci. Rep. 2020, 10, 21754. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Diego, A.; Luo, Y.; Hui, D.; Hungate, B.A.; Pablo, G.P.; Kuzyakov, Y.; Olesen, J.E.; Chen, J.; et al. Stimulation of Ammonia Oxidizer and Denitrifier Abundances by Nitrogen Loading Poor Predictability for Increased Soil N2O Emission. Glob. Change Biol. 2022, 28, 2158–2168. [Google Scholar] [CrossRef]
- Dong, Y.; Scharffe, D.; Domroes, M.; Qi, Y.; Zhang, S. N2O Emissions from Agricultural Soils in the North China Plain: The Effect of Chemical Nitrogen Fertilizer and Organic Manure. J. Environ. Sci. 2000, 12, 463–468. [Google Scholar]
- Liao, B.; Cai, T.; Wu, X.; Luo, Y.; Liao, P.; Zhang, B.; Zhang, Y.; Wei, G.; Hu, R.; Luo, Y.; et al. A Combination of Organic Fertilizers Partially Substitution with Alternate Wet and Dry Irrigation Could Further Reduce Greenhouse Gases Emission in Rice Field. J. Environ. Manag. 2023, 344, 118372. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Lu, Y. Adaptation of ammonia-oxidizing microorganisms to environment shift of paddy field soil. FEMS Microbiol. Ecol. 2012, 80, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Le, X.; Zhou, Z.; Wang, B.; Cheng, H. Nitrous Oxide Emissions from Different Land Use Patterns in a Typical Karst Region, Southwest China. Chin. J. Geochem. 2013, 32, 137–145. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community Structure of Ammonia-Oxidizing Bacteria under Long-Term Application of Mineral Fertilizer and Organic Manure in a Sandy Loam Soil. Appl. Environ. Microbiol. 2007, 73, 485–491. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, D.; Zhang, W.; Wang, K. Ammonia—Oxidizing Bacteria Rather than Ammonia—Oxidizing Archaea Dominates Soil Nitrification during Vegetation Restoration in Karst. Land Degrad. Dev. 2024, 35, 4304–4313. [Google Scholar] [CrossRef]
- Groeneweg, J.; Sellner, B.; Tappe, W. Ammonia Oxidation in Nitrosomonas at NH3 Concentrations Near km: Effects of pH and Temperature. Water Res. 1994, 28, 2561–2566. [Google Scholar] [CrossRef]
- Chisholm, C.; Di, H.; Cameron, K.; Podolyan, A.; Shen, J.; Zhang, L.; Sirisena, K.; Godsoe, W. Contrasting Response of Comammox Nitrospira, Ammonia Oxidising Bacteria, and Archaea to Soil pH and Nitrogen Inputs. Sci. Total Environ. 2024, 924, 171627. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Zeng, M.; Qin, Z.; Liang, J.; Chen, Y.; Wu, Y.; Chen, S.; Yu, F.; Li, Y. Soil Aggregate Size Mediates the Variations in the Abundance and Function of Ammonia Oxidizers in Heavy Metal-Contaminated Soil Under Different Nitrogen Fertilization Regimes. Appl. Soil Ecol. 2024, 200, 105448–105460. [Google Scholar] [CrossRef]
- Jiang, Y.; Jin, C.; Sun, B. Soil Aggregate Stratification of Nematodes and Ammonia Oxidizers Affects Nitrification in an Acid Soil. Environ. Microbiol. 2014, 16, 3083–3094. [Google Scholar] [CrossRef]
- Hou, Q.; Zuo, T.; Wang, J.; Huang, S.; Wang, X.; Yao, L.; Ni, W. Responses of Nitrification and Bacterial Community in Three Size Aggregates of Paddy Soil to both of Initial Fertility and Biochar Addition. Appl. Soil Ecol. 2021, 166, 104004. [Google Scholar] [CrossRef]
- Duan, P.P.; Yang, X.Y.; He, X.Y.; Jiang, Y.L.; Xiao, K.C.; Wang, K.L.; Li, D.J. Topography-Driven Soil Properties Modulate Effects of Nitrogen Deposition on Soil Nitrous Oxide Sources in A Subtropical Forest. Biol. Fertil. Soils 2022, 58, 707–720. [Google Scholar] [CrossRef]
- Bock, E.; Schmidt, I.; Stüven, R.; Zart, D. Nitrogen Loss Caused by Denitrifying Nitrosomonas Cells Using Ammonium or Hydrogen as Electron Donors and Nitrite as Electron Acceptor. Arch. Microbiol. 1995, 163, 16–20. [Google Scholar] [CrossRef]
- Poth, M.; Focht, D.D. 15N Kinetic Analysis of N2O Production by Nitrosomonas europaea: An Examination of Nitrifier Denitrification. Appl. Environ. Microbiol. 1985, 49, 1134–1141. [Google Scholar] [PubMed]
- Schmidt, I.; Bock, E. Anaerobic Ammonia Oxidation with Nitrogen Dioxide by Nitrosomonas eutropha. Arch. Microbiol. 1997, 167, 106–111. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Lieberei, R. Arbuscular Mycorrhizal Infection Changes the Bacterial 16S rDNA Community Composition in the Rhizosphere of Maize. Mycorrhiza 2001, 11, 297–302. [Google Scholar] [CrossRef]
- Leite, R.D.A.; Martins, L.C.; Ferreira, L.V.D.S.F.; Barbosa, E.S.; Alves, B.J.R.; Zilli, J.E.; Araújo, A.P.; Jesus, E.D.C. Co-Inoculation of Rhizobium and Bradyrhizobium Promotes Growth and Yield of Common Beans. Appl. Soil Ecol. 2022, 172, 104356. [Google Scholar] [CrossRef]
- Shi, J.; Gong, J.; Li, X.; Zhang, Z.; Zhang, W.; Li, Y.; Song, L.; Zhang, S.; Dong, J.; Baoyin, T. Phosphorus Application Promoted the Sequestration of Orthophosphate within Soil Microorganisms and Regulated the Soil Solution P Supply in a Temperate Grassland in Northern China: A 31P NMR Study. Soil Tillage Res. 2023, 127, 105612. [Google Scholar] [CrossRef]
- Deng, X.; Xu, T.; Zhang, F.; Xue, L.; Yang, L.; Hou, P. Effects of Warming and Fertilization on nirK-, NirS- and NosZ-Type Denitrifier Communities in Paddy Soil. Sci. Total Environ. 2024, 955, 177057. [Google Scholar] [CrossRef]
- Shi, Y.; Rahaman, M.A.; Zhang, Q.; Zhan, X.; Zheng, L. Effects of Partial Substitution of Chemical Fertilizer with Biogas Slurry on Nitrous Oxide Emissions and the Related Nitrifier and Denitrifier in a Saline–Alkali Soil. Environ. Technol. Innov. 2022, 28, 102900. [Google Scholar] [CrossRef]
- Li, X.; Salam, N.; Li, J.; Chen, Y.; Yang, Z.; Han, M.; Mou, X.; Xiao, M.; Li, W. Aestuariivirga litoralis Gen. Nov., Sp. Nov., a Proteobacterium Isolated from a Water Sample, and Proposal of Aestuariivirgaceae Fam. Nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 299–306. [Google Scholar] [CrossRef]
- Caroline, F.H.L.; Fuzinatto, D.R.; Marçon, D.J.R.; Hungria, M. Mesorhizobium atlanticum Sp. Nov., a New Nnitrogen-Fixing Species from Soils of the Brazilian Atlantic Forest Biome. Int. J. Syst. Evol. Microbiol. 2019, 69, 1800–1806. [Google Scholar]
- Cao, C.; Zhang, Y.; Cui, Z.; Li, H.; Wang, T.; Ren, Q. Recovery of Soil-Denitrifying Community along a Chronosequence of Sand-Fixation Forest in a Semi-Arid Desertified Grassland. Forests 2021, 12, 354. [Google Scholar] [CrossRef]
| Diversity | pH | SOC | DOC | TN | AN | NH4+-N | NO3−-N | TP | AP | CEC | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASVs | 0.915 ** | 0.749 ** | −0.015 | 0.773 ** | −0.168 | −0.187 | 0.551 * | 0.725 ** | 0.723 ** | 0.935 ** | 0.739 ** | 0.879 ** |
| Chao1 Index | 0.466 * | 0.371 | −0.044 | 0.405 | −0.193 | −0.161 | 0.518 * | 0.189 | 0.168 | 0.496 * | 0.249 | 0.506 * |
| ACE Index | 0.522 * | 0.466 * | −0.144 | 0.600 ** | −0.197 | −0.19 | 0.405 | 0.194 | 0.379 | 0.554 * | 0.314 | 0.569 * |
| Shannon Index | −0.793 ** | −0.568 * | −0.038 | −0.389 | 0.25 | 0.209 | −0.648 ** | −0.792 ** | −0.559 * | −0.837 ** | −0.634 ** | −0.733 ** |
| Simpson Index | −0.746 ** | −0.500 * | −0.036 | −0.318 | 0.27 | 0.215 | −0.632 ** | −0.783 ** | −0.514 * | −0.784 ** | −0.555 * | −0.689 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Chen, W.; Yuan, W.; Sun, Y.; Xiao, X.; Liang, H.; Yang, C.; Dong, B. Linking N2O Emission with AOB and nirK-Denitrifier in Paddy Fields of Karst and Non-Karst Areas. Microorganisms 2025, 13, 2633. https://doi.org/10.3390/microorganisms13112633
Jin Z, Chen W, Yuan W, Sun Y, Xiao X, Liang H, Yang C, Dong B. Linking N2O Emission with AOB and nirK-Denitrifier in Paddy Fields of Karst and Non-Karst Areas. Microorganisms. 2025; 13(11):2633. https://doi.org/10.3390/microorganisms13112633
Chicago/Turabian StyleJin, Zhenjiang, Weijian Chen, Wu Yuan, Yunlong Sun, Xiaoyi Xiao, Heyao Liang, Chengxi Yang, and Bin Dong. 2025. "Linking N2O Emission with AOB and nirK-Denitrifier in Paddy Fields of Karst and Non-Karst Areas" Microorganisms 13, no. 11: 2633. https://doi.org/10.3390/microorganisms13112633
APA StyleJin, Z., Chen, W., Yuan, W., Sun, Y., Xiao, X., Liang, H., Yang, C., & Dong, B. (2025). Linking N2O Emission with AOB and nirK-Denitrifier in Paddy Fields of Karst and Non-Karst Areas. Microorganisms, 13(11), 2633. https://doi.org/10.3390/microorganisms13112633








