Antimicrobial Peptides: Current Status, Mechanisms of Action, and Strategies to Overcome Therapeutic Limitations
Abstract
1. Introduction
2. Antimicrobial Peptide
2.1. Classification of AMPs by Origin
2.1.1. Mammalian AMPs
2.1.2. Insects AMPs
2.1.3. Plants AMPs
2.1.4. Microorganism AMPs
| Origin | AMP Family/ Peptide | Structure | Mechanism of Action | Activity | Characteristics and Function | Reference |
|---|---|---|---|---|---|---|
| Mammalian | Cathelicidins | Cationic, amphipathic peptides | Disrupt microbial membranes | Broad antimicrobial and antibiofilm activity (Gram-positive and -negative) | Possess a conserved N-terminal propeptide (cathelin domain). Performs dual roles in antimicrobial defense and immune modulation | [33] |
| LL-37 | Curved amphipathic α-helix structure | membrane disruption (pore formation) and non-membranolytic mechanisms | Broad antimicrobial and antibiofilm activity (Gram-positive and -negative) | Immunomodulatory functions | [26,34,35,36,37,38,39] | |
| Defensins | Cationic peptides, rigid, triple-stranded antiparallel β-sheet structure by disulfide bonds | Increases permeability of bacterial membranes | Antibacterial Antifungal Antiviral | Recruits immune system components to the site of infection. Links innate and adaptive immunity through chemotactic activity on T cells and immature dendritic cells | [40,41,42,43,44] | |
| Insect | Gallerimycin and Galiomicin | Cysteine-rich defensin-like peptides | Induce membrane depolarization | Antifungal | Increased expression during Immune priming in G. mellonella | [48,69,70] |
| Drosophila AMPs | includes various structures | Act by disrupting microbial membranes | Diptericin, Attacin, Drosocin, Cecropin: Gram-negative bacteria Defensin: Gram-positive bacteria Drosomycin: fungi | A cocktail of ~20 different AMPs is secreted from the fat body of Drosophila upon pathogen attack | [47,49,50,51,52,53,54,71] | |
| Plant | Thionins | 45–47 amino acid, 6–8 cysteines, 3–4 disulfide bonds cyclic structure | Interacts with membrane lipid, leading to increased cell membrane permeability and lysis | Antibacterial Antifungal | Found in various parts of plants High stability | [28,55,56,57,72] |
| Plant defensins | Cysteine-rich motifs, multiple disulfide bonds | Interacts with specific membrane components to trigger intracellular signaling cascades that hinder pathogen growth | Antibacterial Antifungal | Found in wheat, barley, etc. | [28,55,73,74] | |
| Snakins | Cysteine-rich motifs, multiple disulfide bonds | Act by disrupting microbial membranes | Antibacterial Antifungal | Found in potato tubers, etc. | [28,55,73,74,75,76] | |
| Cyclotide | Head-to-tail cyclized peptide backbone, cysteine-rich with multiple disulfide bonds rigid and stable structure | Disrupts microbial membranes by forming pores or through a detergent-like effect | Antibacterial Antifungal Insecticidal, nematocidal activity | Highly resistant to heat, chemicals and proteases | [28,73,74] | |
| Microorganism | Nisin | Post-translationally modified structure containing lanthionine rings | Binds to Lipid Ⅱ, a precursor for peptidoglycan synthesis, to inhibit cell wall synthesis, and then forms pores in the bacterial membrane | Inhibit or kill closely related bacterial species | Derived from Lactococcus lactis | [60,61,62] |
| Pediocin-like bacteriocins | Lack post-translational modification | Binds to Man-PTS of target bacteria, forming a permanently open pore that disrupts ion balance | Inhibit or kill closely related bacterial species | Uses Man-PTS as a receptor | [64,66] | |
| Klebsazolicin | Linear 23 amino acid peptide containing four azle heterocycle and an N-terminal lactamidine ring | Blocks the ribosome exit tunnel to inhibit protein synthesis | Inhibit or kill closely related bacterial species | Acts intracellularly | [67] | |
| Micocin J25 | Lasso peptide | Enter the cell and inhibits transcription by interacting with the secondary channel of RNA polymerase | Inhibit or kill closely related bacterial species | Acts intracellularly | [68] | |
| Gramicidin | Linear pentadecapeptide with alternation L- and D-amino acids. Forms a helical dimer that functions as an ion channel | Forms a transmembrane ion channel via head-to-head dimerization | Inhibit or kill closely related bacterial species | A representative AMP derived from Bacillus brevis | [63,64,65,77,78,79] |
2.2. Classification of AMPs by Structure
2.2.1. Net Positive Charge and Amphiphilicity
2.2.2. α-Helix
2.2.3. β-Sheet
2.2.4. Atypical Structure
2.3. Classification of AMPs by Activity
2.3.1. Antibacterial Activity
2.3.2. Antifungal Activity
2.3.3. Antiviral Activity
3. Mechanism of Antimicrobial Activity of AMPs
3.1. Membrane Targeting Mechanisms
3.2. Non-Membrane Targeting Mechanisms
4. Bioengineered AMPs for Therapeutic Application
4.1. Clinical Applications and Limitations of AMPs
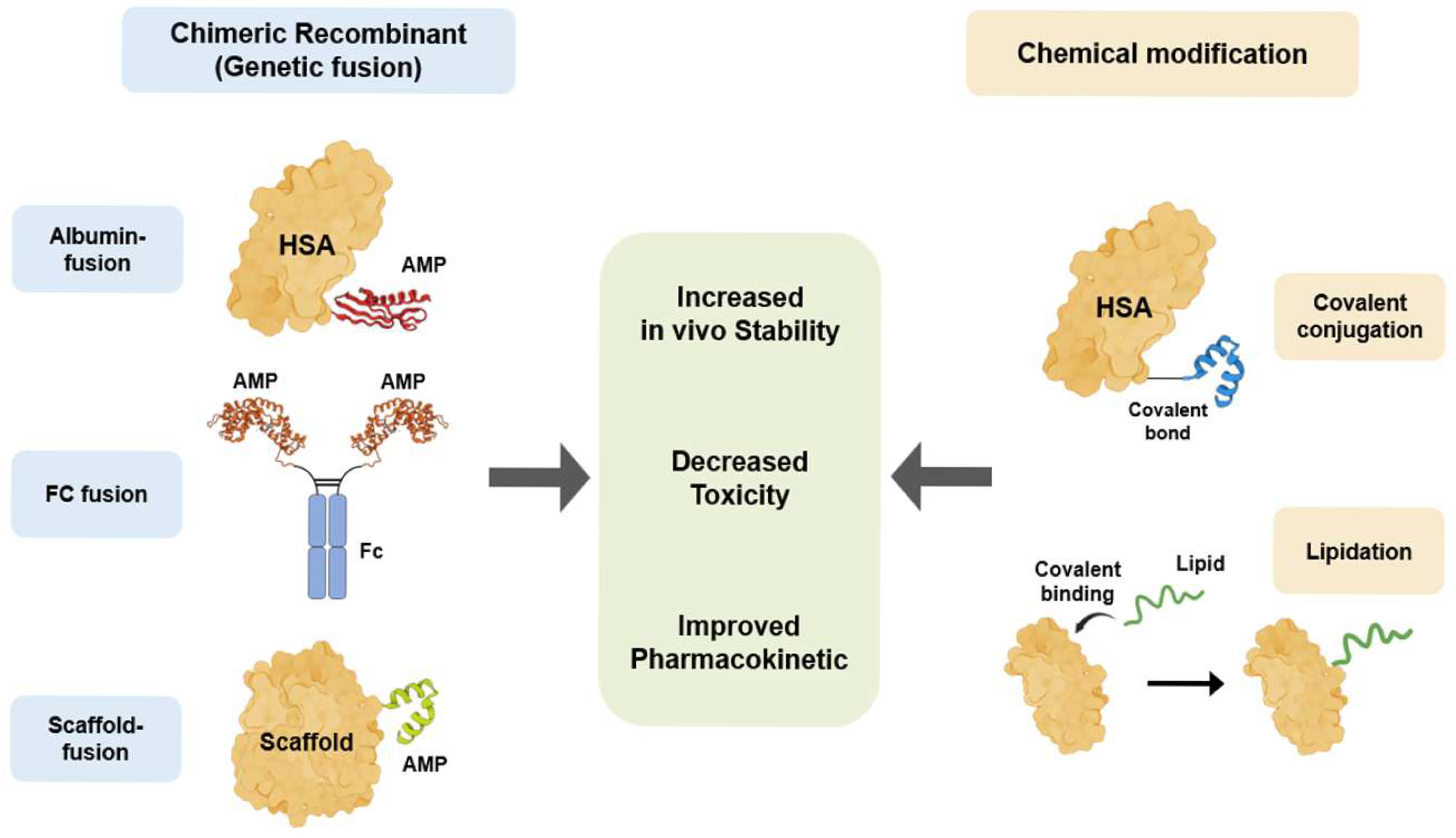
4.2. Bioengineering Technology to Overcome the Limitations of Therapeutic Peptides
4.2.1. Bioengineered AMPs with Human Serum Albumin
4.2.2. Fc-Fusion Recombinant
4.2.3. Scaffold-Fusion Recombinant
4.2.4. Lipidation
| Bioengineering Technology | Characteristics | Advantages | Disadvantages/ Challenges | Reference |
|---|---|---|---|---|
| Albumin-fusion recombinant | Extends half-life by utilizing the FcRn receptor recycling pathway and increasing molecular size to evade renal filtration. | Long in vivo half-life (albumin half-life: approx. 19–23 days) | Low immunogenicity and excellent safety property | [106,130,132,178,179] |
| Fc-fusion recombinant | Extends half-life by evading lysosomal degradation through the FcRn recycling pathway, similar to albumin. | Long half-life and high stability | A proven platform that has produced numerous blockbuster drugs | [144,145,146,178,180,181,182,183,184] |
| Scaffold-fusion recombinant | Genetic fusion of two or more functional peptide/protein domains to create a new therapeutic mechanism (e.g., bispecific antibodies, immunotoxins). | Implements new therapeutic paradigms with a single molecule (e.g., linking T-cells and cancer cells) | Increases efficacy and specificity through multi-targeting | [148,185,186] |
| Lipidation | Extends half-life through a dual mechanism: (1) non-covalent binding to circulating albumin; (2) formation of a depot at the subcutaneous injection site via self-assembly. | Minimal increase in molecular size, which is favorable for preserving drug activity | Utilizes the natural transport protein (albumin) | [151,152,153,154,155,156,187,188,189,190] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morrison, L.; Zembower, T.R. Antimicrobial resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar]
- World Bank. Antimicrobial Resistance (AMR). 2023. Available online: https://www.worldbank.org/en/topic/health/brief/antimicrobial-resistance-amr (accessed on 29 August 2025).
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Eiamphungporn, W.; Schaduangrat, N.; Malik, A.A.; Nantasenamat, C. Tackling the Antibiotic Resistance Caused by Class A β-Lactamases through the Use of β-Lactamase Inhibitory Protein. Int. J. Mol. Sci. 2018, 19, 2222. [Google Scholar] [CrossRef]
- Rice, L.B. Mechanisms of resistance and clinical relevance of resistance to β-lactams, glycopeptides, and fluoroquinolones. Mayo Clin. Proc. 2012, 87, 198–208. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Meng, Y.; Wang, W.; Hu, J.; Wang, Y.; Li, G.; Wang, Y.; Liu, Y.; Zhang, W.; et al. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Hetta, H.F.; Alanazi, F.E.; Ali, M.A.S.; Alatawi, A.D.; Aljohani, H.M.; Ahmed, R.; Alansari, N.A.; Alkhathami, F.M.; Albogmi, A.; Alharbi, B.M.; et al. Hypervirulent Klebsiella pneumoniae: Insights into Virulence, Antibiotic Resistance, and Fight Strategies Against a Superbug. Pharmaceuticals 2025, 18, 724. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial peptides-Mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.L.; Macedo, A.J.; Tasca, T. Therapeutic potential of antimicrobial peptides against pathogenic protozoa. Parasitol. Res. 2024, 123, 122. [Google Scholar] [CrossRef]
- Meng, S.; Xu, H.; Wang, F. Research advances of antimicrobialpeptides and applications in food industry and agriculture. Curr. Protein Pept. Sci. 2010, 11, 264–273. [Google Scholar] [CrossRef]
- Kocagoz, T.; Temur, B.Z.; Unubol, N.; Acikel Elmas, M.; Kanlidere, Z.; Cilingir, S.; Acar, D.; Boskan, G.; Akcelik Deveci, S.; Aybakan, E.; et al. Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity. Life 2025, 15, 242. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Werle, M.; Bernkop-Schnürch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Chan, L.W.; Fleming, H.E.; Bhatia, S.N. Conditional Antimicrobial Peptide Therapeutics. ACS Nano 2022, 16, 15779–15791. [Google Scholar] [CrossRef]
- Johnson, K.; Delaney, J.C.; Guillard, T.; Reffuveille, F.; Varin-Simon, J.; Li, K.; Wollacott, A.; Frapy, E.; Mong, S.; Tissire, H.; et al. Development of an antibody fused with an antimicrobial peptide targeting Pseudomonas aeruginosa: A new approach to prevent and treat bacterial infections. PLoS Pathog. 2023, 19, e1011612. [Google Scholar] [CrossRef]
- Sun, J.; Xia, Y.; Li, D.; Du, Q.; Liang, D. Relationship between peptide structure and antimicrobial activity as studied by de novo designed peptides. Biochim. Biophys. Acta 2014, 1838, 2985–2993. [Google Scholar] [CrossRef]
- Tajer, L.; Paillart, J.C.; Dib, H.; Sabatier, J.M.; Fajloun, Z.; Abi Khattar, Z. Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms 2024, 12, 1259. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. The human cathelicidin hCAP18/LL-37: A multifunctional peptide involved in mycobacterial infections. Peptides 2010, 31, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sørensen, O.; Borregaard, N.; Ståhle-Bäckdahl, M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Daly, N.L.; Bond, T.; Waine, C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 1999, 294, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- APD3. The Antimicrobial Peptide Database. 2023. Available online: https://aps.unmc.edu (accessed on 3 August 2025).
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Dürr, U.H.N. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- van Harten, R.M.; van Woudenbergh, E.; van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines 2018, 6, 63. [Google Scholar] [CrossRef]
- Hancock, R.E.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 1996, 238, 325–332. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Zhang, Z.; Ramamoorthy, A. LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases. Biomolecules 2024, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- Duplantier, A.J.; van Hoek, M.L. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front. Immunol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef]
- Nagib, M.; Sayed, A.M.; Korany, A.H.; El-Kersh, D.H. Human Defensins: Structure, Function, and Potential as Therapeutic Antimicrobial Agents with Highlights Against SARS CoV-2. Probiotics Antimicrob. Proteins 2025, 17, 1563–1583. [Google Scholar] [CrossRef]
- Mercuri, L.G. Prevention and detection of prosthetic temporomandibular joint infections-update. Int. J. Oral Maxillofac. Surg. 2019, 48, 217–224. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. Host Defenses against Viral Infection and Viral Counterdefenses. In Viruses and Human Disease, 2nd ed.; Strauss, J.H., Strauss, E.G., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 369–421. ISBN 978-0-12-373741-0. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.; Chen, J. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. J. Insect Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef]
- Fallon, J.P.; Troy, N.; Kavanagh, K. Pre-exposure of Galleria mellonella larvae to different doses of Aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence 2011, 2, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.; Murphy, L.; Keenan, J.; Clynes, M.; Kavanagh, K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006, 8, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Wicker, C.; Reichhart, J.M.; Hoffmann, D.; Hultmark, D.; Samakovlis, C.; Hoffmann, J.A. Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J. Biol. Chem. 1990, 265, 22493–22498. [Google Scholar] [CrossRef]
- Asling, B.; Dushay, M.S.; Hultmark, D. Identification of early genes in the Drosophila immune response by PCR-based differential display: The Attacin A gene and the evolution of attacin-like proteins. Insect Biochem. Mol. Biol. 1995, 25, 511–518. [Google Scholar] [CrossRef]
- Bulet, P.; Dimarcq, J.L.; Hetru, C.; Lagueux, M.; Charlet, M.; Hegy, G.; Van Dorsselaer, A.; Hoffmann, J.A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J. Biol. Chem. 1993, 268, 14893–14897. [Google Scholar] [CrossRef]
- Dimarcq, J.L.; Hoffmann, D.; Meister, M.; Bulet, P.; Lanot, R.; Reichhart, J.M.; Hoffmann, J.A. Characterization and transcriptional profiles of a Drosophila gene encoding an insect defensin. A study in insect immunity. Eur. J. Biochem. 1994, 221, 201–209. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Bulet, P.; Michaut, L.; Lagueux, M.; Broekaert, W.F.; Hetru, C.; Hoffmann, J.A. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J. Biol. Chem. 1994, 269, 33159–33163. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Sirag, N.; Alsharif, S.M.; Alharbi, A.A.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; Ramadan, Y.N.; Rashed, Z.I.; Alanazi, F.E. Antimicrobial Peptides: The Game-Changer in the Epic Battle Against Multidrug-Resistant Bacteria. Pharmaceuticals 2024, 17, 1555. [Google Scholar] [CrossRef] [PubMed]
- Stec, B. Plant thionins--the structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Daly, N.L.; Rosengren, K.J.; Craik, D.J. Discovery, structure and biological activities of cyclotides. Adv. Drug Deliv. Rev. 2009, 61, 918–930. [Google Scholar] [CrossRef]
- Kido, E.A.; Pandolfi, V.; Houllou-Kido, L.M.; Andrade, P.P.; Marcelino, F.C.; Nepomuceno, A.L.; Abdelnoor, R.V.; Burnquist, W.L.; Benko-Iseppon, A.M. Plant antimicrobial peptides: An overview of SuperSAGE transcriptional profile and a functional review. Curr. Protein Pept. Sci. 2010, 11, 220–230. [Google Scholar] [CrossRef]
- Salas, C.E.; Badillo-Corona, J.A.; Ramírez-Sotelo, G.; Oliver-Salvador, C. Biologically Active and Antimicrobial Peptides from Plants. Biomed Res. Int. 2015, 2015, 102129. [Google Scholar] [CrossRef]
- Shwaiki, L.N.; Arendt, E.K.; Lynch, K.M. Study on the characterisation and application of synthetic peptide Snakin-1 derived from potato tubers—Action against food spoilage yeast. Food Control 2020, 118, 107362. [Google Scholar] [CrossRef]
- Kiba, A.; Saitoh, H.; Nishihara, M.; Omiya, K.; Yamamura, S. C-terminal domain of a hevein-like protein from Wasabia japonica has potent antimicrobial activity. Plant Cell Physiol. 2003, 44, 296–303. [Google Scholar] [CrossRef]
- Vanzolini, T.; Bruschi, M.; Rinaldi, A.C.; Magnani, M.; Fraternale, A. Multitalented Synthetic Antimicrobial Peptides and Their Antibacterial, Antifungal and Antiviral Mechanisms. Int. J. Mol. Sci. 2022, 23, 545. [Google Scholar] [CrossRef]
- Mihaylova-Garnizova, R.; Davidova, S.; Hodzhev, Y.; Satchanska, G. Antimicrobial Peptides Derived from Bacteria: Classification, Sources, and Mechanism of Action against Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2024, 25, 10788. [Google Scholar] [CrossRef] [PubMed]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Field, D.; Fernandez de Ullivarri, M.; Ross, R.P.; Hill, C. After a century of nisin research—Where are we now? FEMS Microbiol. Rev. 2023, 47, fuad023. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, J.; Wang, J. Structural Basis of the Immunity Mechanisms of Pediocin-like Bacteriocins. Appl. Environ. Microbiol. 2022, 88, e00481-22. [Google Scholar] [CrossRef]
- Metelev, M.; Osterman, I.A.; Ghilarov, D.; Khabibullina, N.F.; Yakimov, A.; Shabalin, K.; Utkina, I.; Travin, D.Y.; Komarova, E.S.; Serebryakova, M.; et al. Klebsazolicin inhibits 70S ribosome by obstructing the peptide exit tunnel. Nat. Chem. Biol. 2017, 13, 1129–1136. [Google Scholar] [CrossRef]
- Braffman, N.R.; Piscotta, F.J.; Hauver, J.; Campbell, E.A.; Link, A.J.; Darst, S.A. Structural mechanism of transcription inhibition by lasso peptides microcin J25 and capistruin. Proc. Natl. Acad. Sci. USA 2019, 116, 1273–1278. [Google Scholar] [CrossRef]
- Langen, G.; Imani, J.; Altincicek, B.; Kieseritzky, G.; Kogel, K.H.; Vilcinskas, A. Transgenic expression of gallerimycin, a novel antifungal insect defensin from the greater wax moth Galleria mellonella, confers resistance to pathogenic fungi in tobacco. Biol. Chem. 2006, 387, 549–557. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Tonk, M.; Schreiber, C.; Salzig, D.; Czermak, P.; Vilcinskas, A.; Rahnamaeian, M. The potential of the Galleria mellonella innate immune system is maximized by the co-presentation of diverse antimicrobial peptides. Biol. Chem. 2016, 397, 939–945. [Google Scholar] [CrossRef]
- Hetru, C.; Troxler, L.; Hoffmann, J.A. Drosophila melanogaster antimicrobial defense. J. Infect. Dis. 2003, 187, S327–S334. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; de Souza, C.M.; de Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The role of antimicrobial peptides in plant immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.S.S.; Cherene, M.B.; Taveira, G.B.; de Oliveira Mello, É.; de Oliveira Carvalho, A.; Gomes, V.M. Plant Antimicrobial Peptides and Their Main Families and Roles: A Review of the Literature. Curr. Issues Mol. Biol. 2024, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, B.M.; Li, N.; Langs, D.A.; Pangborn, W.A.; Duax, W.L. The conducting form of gramicidin A is a right-handed double-stranded double helix. Proc. Natl. Acad. Sci. USA 1998, 95, 12950–12955. [Google Scholar] [CrossRef]
- Liou, J.W.; Hung, Y.J.; Yang, C.H.; Chen, Y.C. The antimicrobial activity of gramicidin A is associated with hydroxyl radical formation. PLoS ONE 2015, 10, e0117065. [Google Scholar] [CrossRef]
- David, J.M.; Rajasekaran, A.K. Gramicidin A: A New Mission for an Old Antibiotic. J. Kidney Cancer VHL 2015, 2, 15–24. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Deslouches, B.; Phadke, S.M.; Lazarevic, V.; Cascio, M.; Islam, K.; Montelaro, R.C.; Mietzner, T.A. De novo generation of cationic antimicrobial peptides: Influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 2005, 49, 316–322. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms: My Perspective. In Antimicrobial Peptides; Matsuzaki, K., Ed.; Springer: Singapore, 2019; Volume 1117. [Google Scholar] [CrossRef]
- Wang, G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Cabi: Wallingford, UK, 2017; ISBN 9781845936570. [Google Scholar]
- Chen, N.; Jiang, C. Antimicrobial peptides: Structure, mechanism, and modification. Eur. J. Med. Chem. 2023, 255, 115377. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, R.; Zanetti, M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers 2000, 55, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Uggerhøj, L.E.; Poulsen, T.J.; Munk, J.K.; Fredborg, M.; Sondergaard, T.E.; Frimodt-Moller, N.; Hansen, P.R.; Wimmer, R. Rational Design of Alpha-Helical Antimicrobial Peptides: Do’s and Don’ts. ChemBioChem 2015, 16, 242–253. [Google Scholar] [CrossRef]
- Mura, M.; Wang, J.; Zhou, Y.; Schöne, S.; Titz, A.; De Simone, A.; Raap, J.; Hoffmann, R. The effect of amidation on the behaviour of antimicrobial peptides. Eur. Biophys. J. 2016, 45, 195–207. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Mohanram, H.; Bhattacharjya, S. Cysteine deleted protegrin-1 (CDP-1): Anti-bacterial activity, outer-membrane disruption and selectivity. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3006–3016. [Google Scholar] [CrossRef]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1499–1512. [Google Scholar] [CrossRef]
- Wedemeyer, W.J.; Welker, E.; Narayan, M.; Scheraga, H.A. Disulfide bonds and protein folding. Biochemistry 2000, 39, 4207–4216. [Google Scholar] [CrossRef]
- Lee, T.H.; Hall, K.N.; Aguilar, M.I. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Rodziewicz-Motowidło, S.; Mickiewicz, B.; Greber, K.; Sikorska, E.; Szultka, L.; Kamysz, E.; Kamysz, W. Antimicrobial and conformational studies of the active and inactive analogues of the protegrin-1 peptide. FEBS J. 2010, 277, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Mandard, N.; Sodano, P.; Labbe, H.; Bonmatin, J.M.; Bulet, P.; Hetru, C.; Ptak, M.; Vovelle, F. Solution structure of thanatin, a potent bactericidal and fungicidal insect peptide, determined from proton two-dimensional nuclear magnetic resonance data. Eur. J. Biochem. 1998, 256, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Laederach, A.; Andreotti, A.H.; Fulton, D.B. Solution and micelle-bound structures of tachyplesin I and its active aromatic linear derivatives. Biochemistry 2002, 41, 12359–12368. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.; Rozek, A.; Hancock, R.E. Structure-activity relationships for the beta-hairpin cationic antimicrobial peptide polyphemusin I. Biochim. Biophys. Acta Proteins Proteom. 2004, 1698, 239–250. [Google Scholar] [CrossRef]
- Mandard, N.; Bulet, P.; Caille, A.; Daffre, S.; Vovelle, F. The solution structure of gomesin, an antimicrobial cysteine-rich peptide from the spider. Eur. J. Biochem. 2002, 269, 1190–1198. [Google Scholar] [CrossRef]
- Sitaram, N.; Nagaraj, R. Interaction of antimicrobial peptides with biological and model membranes: Structural and charge requirements for activity. Biochim. Biophys. Acta Biomembr. 1999, 1462, 29–54. [Google Scholar] [CrossRef]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schibli, D.J.; Vogel, H.J. Structural studies and model membrane interactions of two peptides derived from bovine lactoferricin. J. Pept. Sci. 2005, 11, 379–389. [Google Scholar] [CrossRef]
- Li, W.; Tailhades, J.; O’Brien-Simpson, N.M.; Separovic, F.; Otvos, L., Jr.; Hossain, M.A.; Wade, J.D. Proline-rich antimicrobial peptides: Potential therapeutics against antibiotic-resistant bacteria. Amino Acids 2014, 46, 2287–2294. [Google Scholar] [CrossRef]
- Krizsan, A.; Volke, D.; Weinert, S.; Sträter, N.; Knappe, D.; Hoffmann, R. Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew. Chem. Int. Ed. Engl. 2014, 53, 12236–12239. [Google Scholar] [CrossRef]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The host antimicrobial peptide Bac71-35 binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, A.C.; Nguyen, F.; Antunes, S.; Pérébaskine, N.; Graf, M.; Arenz, S.; Inampudi, K.K.; Douat, C.; Guichard, G.; Wilson, D.N.; et al. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat. Struct. Mol. Biol. 2015, 22, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Zhang, L.; Rozek, A.; Hancock, R.E. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 2001, 276, 35714–35722. [Google Scholar] [CrossRef]
- Hetta, H.F.; Melhem, T.; Aljohani, H.M.; Salama, A.; Ahmed, R.; Elfadil, H.; Alanazi, F.E.; Ramadan, Y.N.; Battah, B.; Rottura, M.; et al. Beyond Conventional Antifungals: Combating Resistance Through Novel Therapeutic Pathways. Pharmaceuticals 2025, 18, 364. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Bastian, A.; Schäfer, H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 2001, 101, 157–161. [Google Scholar] [CrossRef]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef]
- Sinha, S.; Cheshenko, N.; Lehrer, R.I.; Herold, B.C. NP-1, a rabbit alpha-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 2003, 47, 494–500. [Google Scholar] [CrossRef]
- Fillion, M.; Valois-Paillard, G.; Lorin, A.; Chatenay, D.; Dufourc, E.J.; Aussenac, F.; Grelard, A.; Manigand, C.; Burlina, F.; Bolbach, G.; et al. Membrane Interactions of Synthetic Peptides with Antimicrobial Potential: Effect of Electrostatic Interactions and Amphiphilicity. Probiotics Antimicrob. Proteins 2015, 7, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Ulmschneider, J.P.; Ulmschneider, M.B. Melittin can permeabilize membranes via large transient pores. Nat. Commun. 2024, 15, 7281. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, R.B.; Lazaridis, T. Implicit Membrane Investigation of the Stability of Antimicrobial Peptide β-Barrels and Arcs. J. Membr. Biol. 2015, 248, 469–486. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Balandin, S.V.; Trunov, K.I.; Paramonov, A.S.; Sukhanov, S.V.; Barsukov, L.I.; Arseniev, A.S.; Ovchinnikova, T.V. Molecular mechanism of action of β-hairpin antimicrobial peptide arenicin: Oligomeric structure in dodecylphosphocholine micelles and pore formation in planar lipid bilayers. Biochemistry 2011, 50, 6255–6265. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Le Clair, S.V.; Ye, S.; Chen, Z. Molecular interactions between magainin 2 and model membranes in situ. J. Phys. Chem. B 2009, 113, 12358–12363. [Google Scholar] [CrossRef]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Evangelista, A.G.; Nazareth, T.M.; Luciano, F.B. Fundamentals on the Molecular Mechanism of Action of Antimicrobial Peptides. Materialia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Hasper, H.E.; Kramer, N.E.; Smith, J.L.; Hillman, J.D.; Zachariah, C.; Kuipers, O.P.; de Kruijff, B.; Breukink, E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 2006, 313, 1636–1637. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Le, C.F.; Gudimella, R.; Razali, R.; Manikam, R.; Sekaran, S.D. Transcriptome analysis of Streptococcus pneumoniae treated with the designed antimicrobial peptides, DM3. Sci. Rep. 2016, 6, 26828. [Google Scholar] [CrossRef]
- Boman, H.G.; Agerberth, B.; Boman, A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 1993, 61, 2978–2984. [Google Scholar] [CrossRef]
- Graf, M.; Mardirossian, M.; Nguyen, F.; Seefeldt, A.C.; Guichard, G.; Scocchi, M.; Innis, C.A.; Wilson, D.N. Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep. 2017, 34, 702–711. [Google Scholar] [CrossRef]
- Knappe, D.; Zahn, M.; Sauer, U.; Schiffer, G.; Sträter, N.; Hoffmann, R. Rational design of oncocin derivatives with superior protease stabilities and antibacterial activities based on the high-resolution structure of the oncocin-DnaK complex. ChemBioChem 2011, 12, 874–876. [Google Scholar] [CrossRef]
- Hussein, M.; Barclay, J.; Baker, M.; Wu, Y.; Thombare, V.J.; Patil, N.; Murthy, A.B.; Sharma, R.; Rao, G.G.; Blaskovich, M.A.; et al. A Comparative Review of the Pharmacology of Dalbavancin and Oritavancin for Gram-Positive Infections: Birds of a Feather or Apples and Oranges? Infect. Dis. Ther. 2025, 14, 2221–2246. [Google Scholar] [CrossRef]
- Birlutiu, R.M.; Birlutiu, V. Oritavancin a Therapeutic Option for Periprosthetic Joint Infections in Selected Cases: A Comprehensive Review. Pharmaceuticals 2025, 18, 1217. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Ma, X.; Peng, F.; Wen, J.; Allahou, L.W.; Williams, G.R.; Knowles, J.C.; Poma, A. Advances in antimicrobial peptides: From mechanistic insights to chemical modifications. Biotechnol. Adv. 2025, 81, 108570. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.; Indrakumar, S.; Sønderby Tuelung, P.; Johansson, J.; Kikhney, A.; Jeffries, C.; Svergun, D.; Andreasen, M.; Andersen, C.B.F.; Arleth, L. Albumin-neprilysin fusion protein: Understanding stability using small angle X-ray scattering and molecular dynamic simulations. Sci. Rep. 2020, 10, 10089. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L.; Cai, Y.; Yang, Y.; Qiu, L.; Shen, Y.; Jin, J.; Zhou, J.; Chen, J. Bioengineered Human Serum Albumin Fusion Protein as Target/Enzyme/pH Three-Stage Propulsive Drug Vehicle for Tumor Therapy. ACS Nano 2020, 14, 17405–17418. [Google Scholar] [CrossRef]
- Weimer, T.; Metzner, H.J.; Schulte, S. Recombinant Albumin Fusion Proteins. In Fusion Protein Technologies for Biopharmaceuticals; Schmidt, S.R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Andersen, J.T.; Sandlie, I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: Implications for development of new diagnostics and therapeutics. Drug Metab. Pharmacokinet. 2009, 24, 318–332. [Google Scholar] [CrossRef]
- Kontermann, R.E. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs 2009, 23, 93–109. [Google Scholar] [CrossRef]
- Chuang, V.T.; Kragh-Hansen, U.; Otagiri, M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm. Res. 2002, 19, 569–577. [Google Scholar] [CrossRef]
- Anderson, C.L.; Chaudhury, C.; Kim, J.; Bronson, C.L.; Wani, M.A.; Mohanty, S. Perspective—FcRn transports albumin: Relevance to immunology and medicine. Trends Immunol. 2006, 27, 343–348. [Google Scholar] [CrossRef]
- Chaudhury, C.; Mehnaz, S.; Robinson, J.M.; Hayton, W.L.; Pearl, D.K.; Roopenian, D.C.; Anderson, C.L. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 2003, 197, 315–322. [Google Scholar] [CrossRef]
- Maruyama, T.; Chuang, V.T.G.; Otagiri, M. Albumin Fusion Protein. In Albumin in Medicine; Otagiri, M., Chuang, V., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Subramanian, G.M.; Fiscella, M.; Lamousé-Smith, A.; Zeuzem, S.; McHutchison, J.G. Albinterferon alpha-2b: A genetic fusion protein for the treatment of chronic hepatitis C. Nat. Biotechnol. 2007, 25, 1411–1419. [Google Scholar] [CrossRef]
- Rustgi, V.K. Albinterferon alfa-2b, a novel fusion protein of human albumin and human interferon alfa-2b, for chronic hepatitis C. Curr. Med. Res. Opin. 2009, 25, 991–1002. [Google Scholar] [CrossRef]
- Zeuzem, S.; Sulkowski, M.S.; Lawitz, E.J.; Rustgi, V.K.; Rodriguez-Torres, M.; Bacon, B.R.; Grigorescu, M.; Tice, A.D.; Lurie, Y.; Cianciara, J.; et al. Albinterferon Alfa-2b was not inferior to pegylated interferon-α in a randomized trial of patients with chronic hepatitis C virus genotype 1. Gastroenterology 2010, 139, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, G.; Liu, Y.; Cheng, Z.; Lin, H.; Liu, J.; Wu, Z.; Xue, J.; Hong, W.; Huang, M.; et al. Using porphyrins as albumin-binding molecules to enhance antitumor efficacies and reduce systemic toxicities of antimicrobial peptides. Eur. J. Med. Chem. 2021, 217, 113382. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Caspersen, M.B.; Robinson, E.; Morais, M.; Maruani, A.; Nunes, J.P.; Nicholls, K.; Saxton, M.J.; Caddick, S.; Baker, J.R.; et al. A platform for efficient, thiol-stable conjugation to albumin’s native single accessible cysteine. Org. Biomol. Chem. 2015, 13, 7946–7949. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Baggio, L.L.; Bridon, D.P.; Castaigne, J.P.; Robitaille, M.F.; Jetté, L.; Benquet, C.; Drucker, D.J. Development and characterization of a glucagon-like peptide 1-albumin conjugate: The ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes 2003, 52, 751–759. [Google Scholar] [CrossRef]
- Menacho-Melgar, R.; Decker, J.S.; Hennigan, J.N.; Lynch, M.D. A review of lipidation in the development of advanced protein and peptide therapeutics. J. Control. Release 2019, 295, 1–12. [Google Scholar] [CrossRef]
- Rosenstock, J.; Balas, B.; Charbonnel, B.; Bolli, G.B.; Boldrin, M.; Ratner, R.; Balena, R. The fate of taspoglutide, a weekly GLP-1 receptor agonist, versus twice-daily exenatide for type 2 diabetes: The T-emerge 2 trial. Diabetes Care 2013, 36, 498–504. [Google Scholar] [CrossRef]
- Přáda Brichtová, E.; Edu, I.A.; Li, X.; Becher, F.; Gomes Dos Santos, A.L.; Jackson, S.E. Effect of Lipidation on the Structure, Oligomerization, and Aggregation of Glucagon-like Peptide 1. Bioconjug. Chem. 2025, 36, 401–414. [Google Scholar] [CrossRef]
- Binder, U.; Skerra, A. Strategies for extending the half-life of biotherapeutics: Successes and complications. Expert Opin. Biol. Ther. 2025, 25, 93–118. [Google Scholar] [CrossRef]
- Bech, E.M.; Pedersen, S.L.; Jensen, K.J. Chemical Strategies for Half-Life Extension of Biopharmaceuticals: Lipidation and Its Alternatives. ACS Med. Chem. Lett. 2018, 9, 577–580. [Google Scholar] [CrossRef]
- Kalra, S.; Sahay, R. A Review on Semaglutide: An Oral Glucagon-Like Peptide 1 Receptor Agonist in Management of Type 2 Diabetes Mellitus. Diabetes Ther. 2020, 11, 1965–1982. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yao, C.; Wang, L.; Min, W.; Xu, J.; Xiao, J.; Huang, M.; Chen, B.; Liu, B.; Li, X.; et al. An albumin-conjugated peptide exhibits potent anti-HIV activity and long in vivo half-life. Antimicrob. Agents Chemother. 2010, 54, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Zolbanin, N.M.; Rafatpanah, H.; Majidi, J.; Kazemi, T. Fc-fusion Proteins in Therapy: An Updated View. Curr. Med. Chem. 2017, 24, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Chamow, S.M.; Ryll, T.; Lowman, H.B.; Farson, D. Therapeutic Fc-Fusion Proteins. ChemMedChem 2014, 9, 2623–2624. [Google Scholar] [CrossRef]
- Cavaco, M.; Castanho, M.A.R.B.; Neves, V. Peptibodies: An elegant solution for a long-standing problem. Biopolymers 2017, 108, e23095. [Google Scholar] [CrossRef]
- Baldo, B.A. Safety of Biologics Therapy. In Drug-Induced Hypersensitivity; Baldo, B.A., Pham, N.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 263–307. [Google Scholar]
- Abbasi, M.; Behmard, E.; Yousefi, M.H.; Shekarforoush, S.S.; Mahmoodi, S. Expression, purification and investigation of antibacterial activity of a novel hybrid peptide LL37/hBD-129 by applied comprehensive computational and experimental approaches. Arch. Microbiol. 2023, 205, 199. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef]
- Kim, D.Y.; Oh, Y.B.; Park, J.S.; Min, Y.H.; Park, M.C. Anti-Microbial Activities of Mussel-Derived Recombinant Proteins against Gram-Negative Bacteria. Antibiotics 2024, 13, 239. [Google Scholar] [CrossRef]
- Rounds, T.; Straus, S.K. Lipidation of Antimicrobial Peptides as a Design Strategy for Future Alternatives to Antibiotics. Int. J. Mol. Sci. 2020, 21, 9692. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, P.; Li, J.; Lin, J.; Lu, Y.; Liu, D.; Wu, S.; Huang, Y.; Zhang, X.; Xu, F. Protein lipidation in health and disease: Molecular basis, physiological function and pathological implication. Signal Transduct. Target. Ther. 2024, 9, 60. [Google Scholar] [CrossRef]
- Kamysz, E.; Sikorska, E.; Jaśkiewicz, M.; Bauer, M.; Neubauer, D.; Bartoszewska, S.; Barańska-Rybak, W.; Kamysz, W. Lipidated Analogs of the LL-37-Derived Peptide Fragment KR12—Structural Analysis, Surface-Active Properties and Antimicrobial Activity. Int. J. Mol. Sci. 2020, 21, 887. [Google Scholar] [CrossRef]
- Bellavita, R.; Falanga, A.; Buommino, E.; Merlino, F.; Casciaro, B.; Cappiello, F.; Mangoni, M.L.; Novellino, E.; Catania, M.R.; Paolillo, R.; et al. Novel temporin L antimicrobial peptides: Promoting self-assembling by lipidic tags to tackle superbugs. J. Enzym. Inhib. Med. Chem. 2020, 35, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Malina, A.; Shai, Y. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem. J. 2005, 390, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Bellavita, R.; Braccia, S.; Galdiero, S.; Falanga, A. Glycosylation and Lipidation Strategies: Approaches for Improving Antimicrobial Peptide Efficacy. Pharmaceuticals 2023, 16, 439. [Google Scholar] [CrossRef] [PubMed]
- Húmpola, M.V.; Rey, M.C.; Carballeira, N.M.; Simonetta, A.C.; Tonarelli, G.G. Biological and structural effects of the conjugation of an antimicrobial decapeptide with saturated, unsaturated, methoxylated and branched fatty acids. J. Pept. Sci. 2017, 23, 45–55. [Google Scholar] [CrossRef]
- Bellavita, R.; Falanga, A.; Merlino, F.; D’Auria, G.; Molfetta, N.; Saviano, A.; Maione, F.; Galdiero, U.; Catania, M.R.; Galdiero, S.; et al. Unveiling the mechanism of action of acylated temporin L analogues against multidrug-resistant Candida albicans. J. Enzym. Inhib. Med. Chem. 2023, 38, 36–50. [Google Scholar] [CrossRef]
- Roscetto, E.; Bellavita, R.; Paolillo, R.; Merlino, F.; Molfetta, N.; Grieco, P.; Buommino, E.; Catania, M.R. Antimicrobial Activity of a Lipidated Temporin L Analogue against Carbapenemase-Producing Klebsiella pneumoniae Clinical Isolates. Antibiotics 2021, 10, 1312. [Google Scholar] [CrossRef]
- Mak, P.; Pohl, J.; Dubin, A.; Reed, M.S.; Bowers, S.E.; Fallon, M.T.; Shafer, W.M. The increased bactericidal activity of a fatty acid-modified synthetic antimicrobial peptide of human cathepsin G correlates with its enhanced capacity to interact with model membranes. Int. J. Antimicrob. Agents 2003, 21, 13–19. [Google Scholar] [CrossRef]
- Chionis, K.; Krikorian, D.; Koukkou, A.I.; Sakarellos-Daitsiotis, M.; Panou-Pomonis, E. Synthesis and biological activity of lipophilic analogs of the cationic antimicrobial active peptide anoplin. J. Pept. Sci. 2016, 22, 731–736. [Google Scholar] [CrossRef]
- Tan, H.; Su, W.; Zhang, W.; Wang, P.; Sattler, M.; Zou, P. Recent Advances in Half-life Extension Strategies for Therapeutic Peptides and Proteins. Curr. Pharm. Des. 2018, 24, 4932–4946. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.Z.; Ge, G.F.; Yue, Z.R.; Wang, R.Y.; Zhang, Q.; Gu, Y.; Song, M.J.; Li, W.B.; Ma, M.Z.; et al. Albumin Fusion at the N-Terminus or C-Terminus of HM-3 Leads to Improved Pharmacokinetics and Bioactivities. Biomedicines 2021, 9, 1084. [Google Scholar] [CrossRef] [PubMed]
- Graf, L. Extended Half-Life Factor VIII and Factor IX Preparations. Transfus. Med. Hemother. 2018, 45, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Reichert, J.M. Therapeutic Fc-fusion proteins and peptides as successful alternatives to antibodies. mAbs 2011, 3, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Misini, G.; Nebija, D.; Petkovska, R.; Nakov, N. Fc-fusion proteins: Therapeutic relevance and quality assessment. Maced. Pharm. Bull. 2022, 68, 65–66. [Google Scholar] [CrossRef]
- Czajkowsky, D.M.; Hu, J.; Shao, Z.; Pleass, R.J. Fc-fusion proteins: New developments and future perspectives. EMBO Mol. Med. 2012, 4, 1015–1028. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; McFarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies. Antibodies 2019, 8, 43. [Google Scholar] [CrossRef]
- Sawant, M.S.; Streu, C.N.; Wu, L.; Tessier, P.M. Toward Drug-Like Multispecific Antibodies by Design. Int. J. Mol. Sci. 2020, 21, 7496. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef]
- Giannetti, M.; Palleschi, A.; Ricciardi, B.; Venanzi, M. A Spectroscopic and Molecular Dynamics Study on the Aggregation Properties of a Lipopeptide Analogue of Liraglutide, a Therapeutic Peptide against Diabetes Type 2. Molecules 2023, 28, 7536. [Google Scholar] [CrossRef]
- Myšková, A.; Sýkora, D.; Kuneš, J.; Maletínská, L. Lipidization as a tool toward peptide therapeutics. Drug Deliv. 2023, 30, 2284685. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Harris, P.W.R.; Williams, G.M.; Yang, S.H.; Brimble, M.A. Peptide Lipidation—A Synthetic Strategy to Afford Peptide Based Therapeutics. Adv. Exp. Med. Biol. 2017, 1030, 185–227. [Google Scholar] [CrossRef]
- Zorzi, A.; Linciano, S.; Angelini, A. Non-covalent albumin-binding ligands for extending the circulating half-life of small biotherapeutics. MedChemComm 2019, 10, 1068–1081. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Min, Y.-H.; Park, M.C. Antimicrobial Peptides: Current Status, Mechanisms of Action, and Strategies to Overcome Therapeutic Limitations. Microorganisms 2025, 13, 2574. https://doi.org/10.3390/microorganisms13112574
Kim SH, Min Y-H, Park MC. Antimicrobial Peptides: Current Status, Mechanisms of Action, and Strategies to Overcome Therapeutic Limitations. Microorganisms. 2025; 13(11):2574. https://doi.org/10.3390/microorganisms13112574
Chicago/Turabian StyleKim, Seong Hwan, Yu-Hong Min, and Min Chul Park. 2025. "Antimicrobial Peptides: Current Status, Mechanisms of Action, and Strategies to Overcome Therapeutic Limitations" Microorganisms 13, no. 11: 2574. https://doi.org/10.3390/microorganisms13112574
APA StyleKim, S. H., Min, Y.-H., & Park, M. C. (2025). Antimicrobial Peptides: Current Status, Mechanisms of Action, and Strategies to Overcome Therapeutic Limitations. Microorganisms, 13(11), 2574. https://doi.org/10.3390/microorganisms13112574






