Prodigiosin as an Algicidal Agent: Inhibition of Pigment Accumulation and Photosynthetic Efficiency of Cyanobacteria Involved in Algal Blooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Prodigiosin-Producing Strain
2.2. Construction of Calibration Curve for Prodigiosin
2.3. Batch Prodigiosin Production Procedure
2.4. Algal Culture Preparation and Algicidal Activity of Prodigiosin
2.5. Analysis of Photosynthetic Inhibition Mechanisms of Prodigiosin on Algae Growth by Chlorophyll a Fluorescence Assay
2.6. Data Processing
3. Results
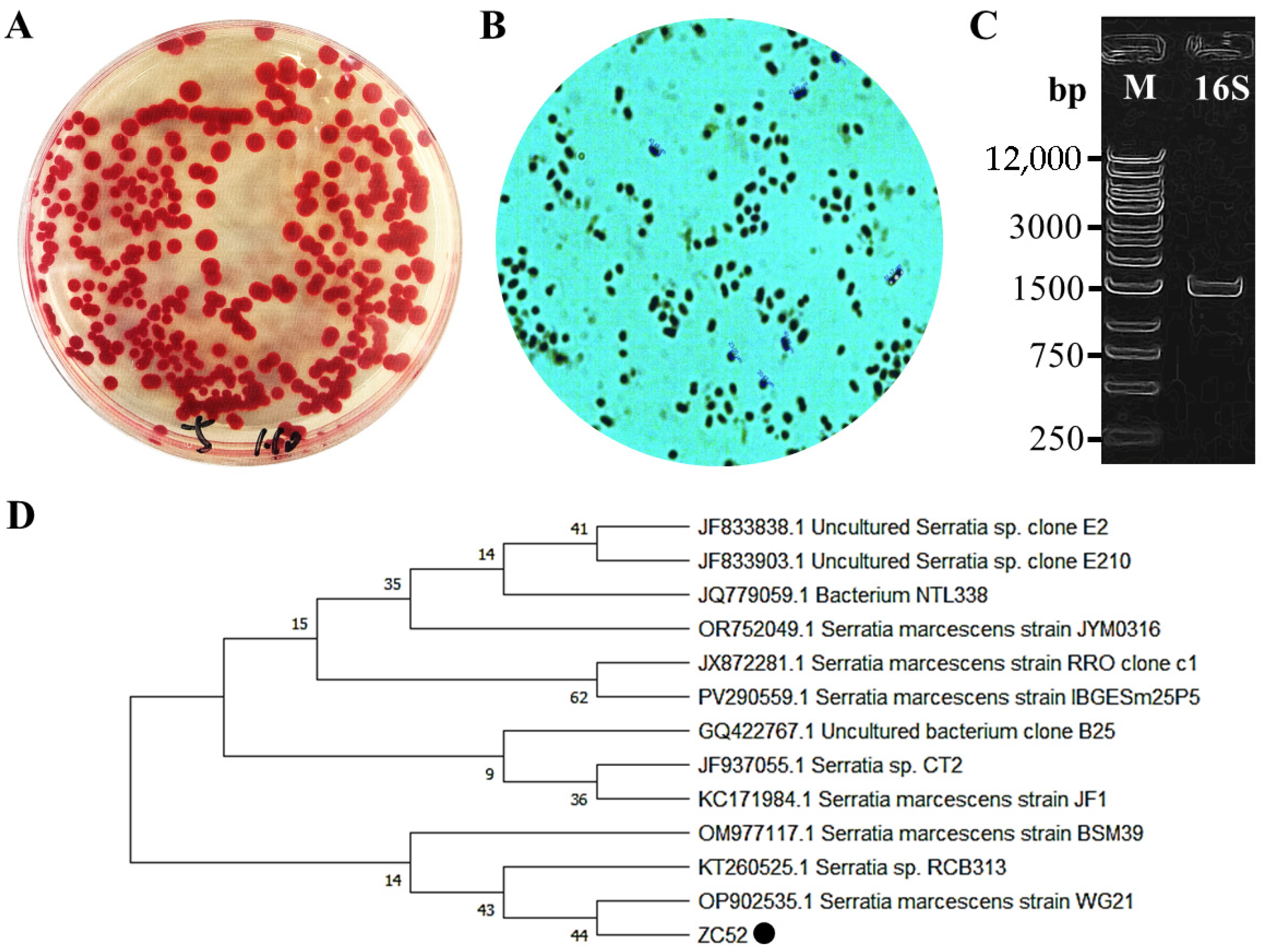
3.1. Identification of Newly Isolated S. marcescens Strain
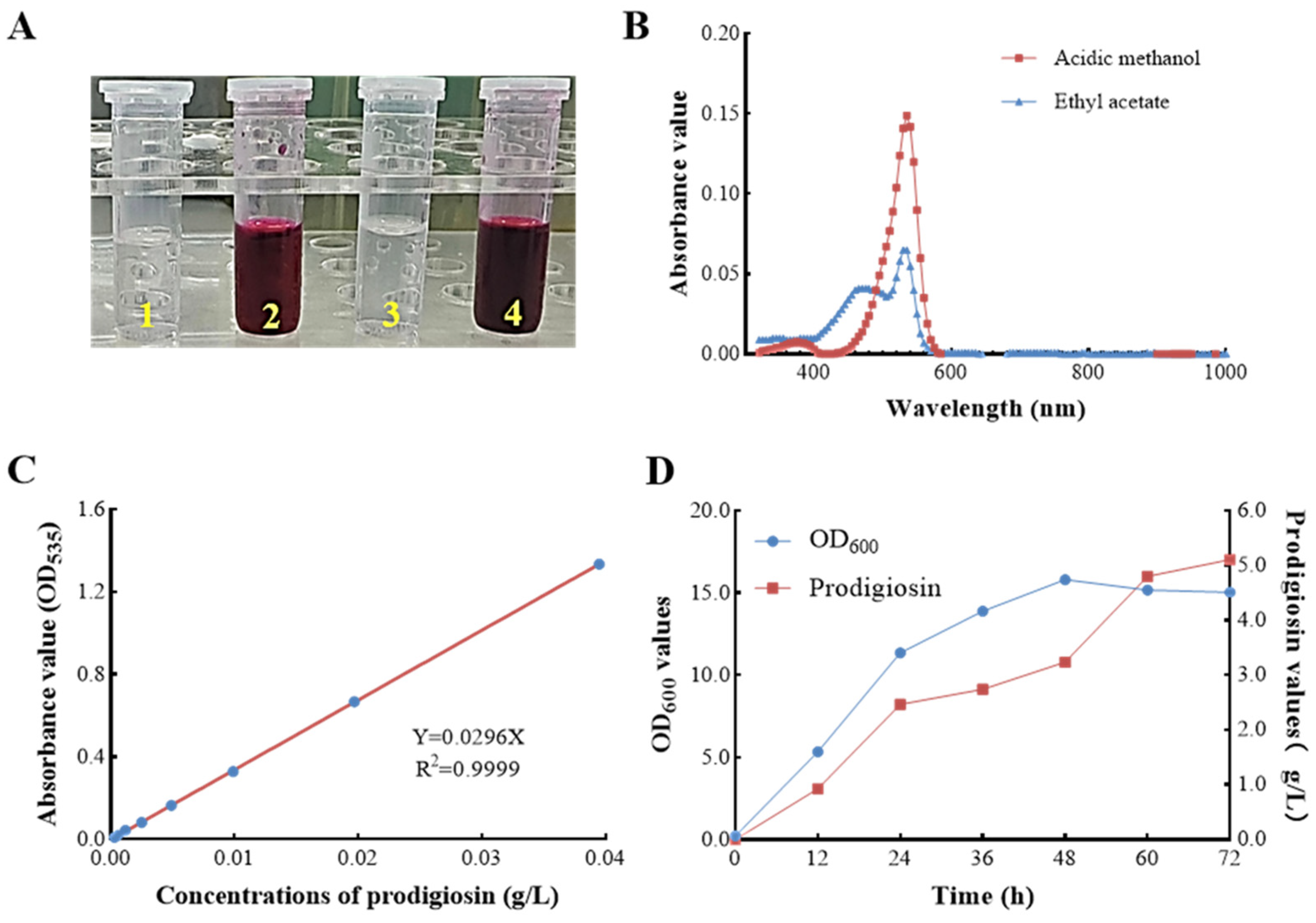
3.2. Prodigiosin Characterization and Preliminary Yield Evaluation in S. marcescens ZC52
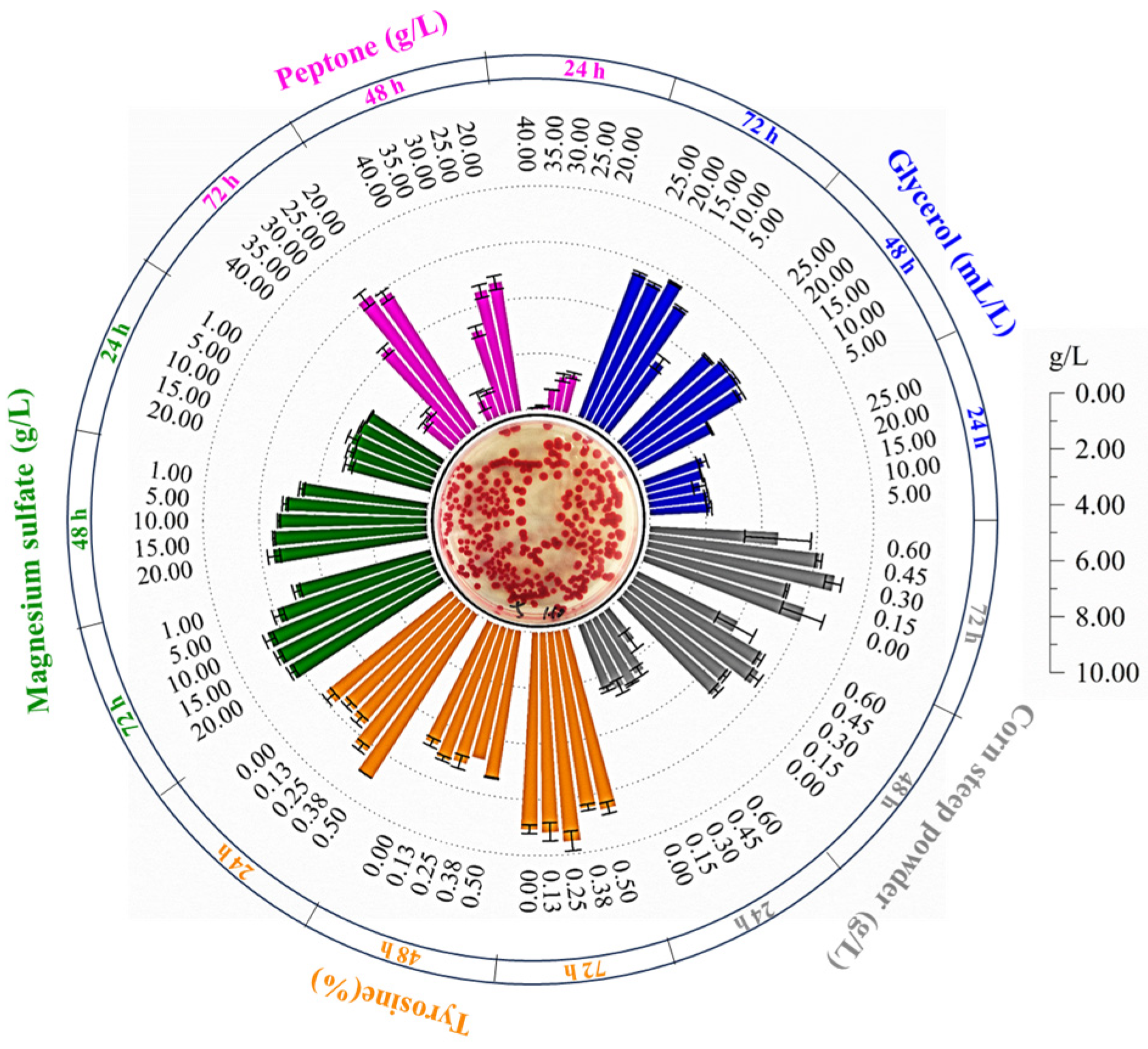
3.3. Optimizations of Medium Composition for Improving the Prodigiosin Yield
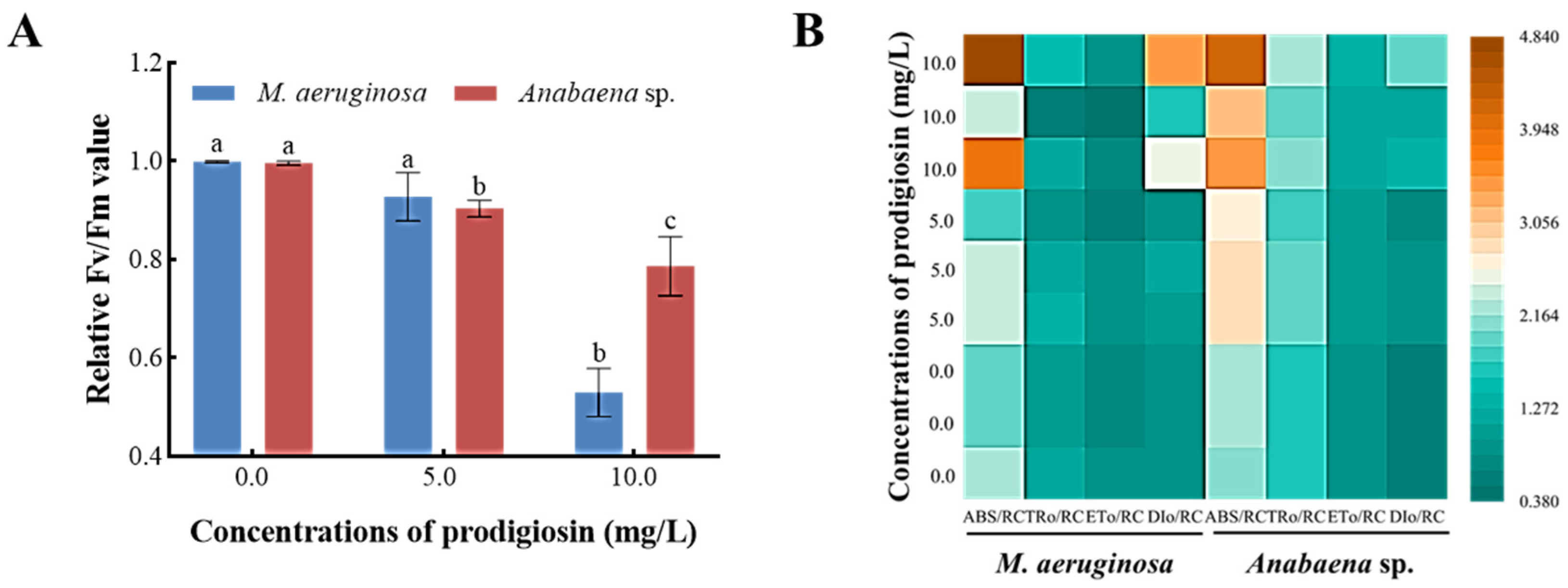
3.4. Algicidal Activity of Prodigiosin on M. aeruginosa and Anabaena sp.
3.5. Photosynthetic Inhibition Mechanisms of Prodigiosin on Algae Blooms
3.5.1. Effects of Prodigiosin on Chlorophyll Fluorescence Characteristics
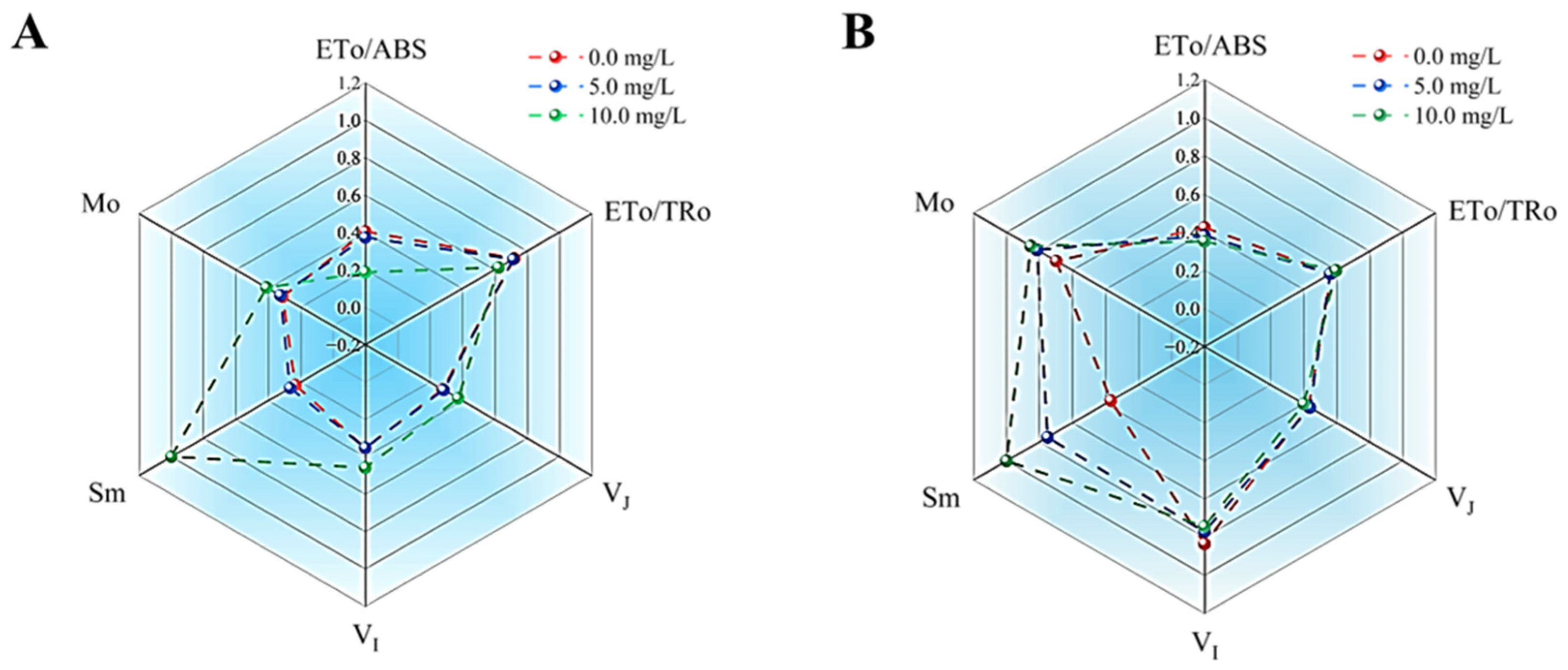
3.5.2. Effects of Prodigiosin on the Light Energy Conversion Efficiency and Energy Distribution in the PSII Reaction Center of M. aeruginosa and Anabaena sp.
3.5.3. Effect of Prodigiosin on the Photosynthetic Electron Transfer Efficiency of M. aeruginosa and Anabaena sp.
4. Discussion
4.1. Enhancing Prodigiosin Production by Optimizing Medium Composition
4.2. Evaluation of the Algicidal Mechanisms of Prodigiosin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.-A.; Tang, M.; Yang, J.; Yang, Q.-Y.; Dai, C.-C.; Chen, F. Highly efficient production of prodigiosin from corn stover hydrolysate in Serratia marcescens mutant RZ 21-6C generated by atmospheric and room temperature plasma mutagenesis. Bioprocess Biosyst. Eng. 2025, 48, 799–816. [Google Scholar] [CrossRef]
- Hu, D.X.; Withall, D.M.; Challis, G.L.; Thomson, R.J. Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem. Rev. 2016, 116, 7818–7853. [Google Scholar] [CrossRef]
- Pereira, R.F.S.; de Carvalho, C.C.C.R. Improving bioprocess conditions for the production of prodigiosin using a marine Serratia rubidaea strain. Mar. Drugs 2024, 22, 142. [Google Scholar] [CrossRef]
- Li, X.; Tan, X.; Chen, Q.; Zhu, X.; Zhang, J.; Zhang, J.; Jia, B. Prodigiosin of Serratia marcescens ZPG19 alters the gut microbiota composition of kunming mice. Molecules 2021, 26, 2156. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Candia, J.E.; Remonsellez, F.; Escudero, L.V.; Demergasso, C.S. The ecological coherence of temperature and salinity tolerance interaction and pigmentation in a non-marine Vibrio isolated from Salar de Atacama. Front. Microbiol. 2016, 7, 1943. [Google Scholar] [CrossRef]
- Williams, R.P. Biosynthesis of prodigiosin, a secondary metabolite of Serratia marcescens. Appl. Microbiol. 1973, 25, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef]
- Chen, W.-C.; Yu, W.-J.; Chang, C.-C.; Chang, J.-S.; Huang, S.-H.; Chang, C.-H.; Chen, S.-Y.; Chien, C.-C.; Yao, C.-L.; Chen, W.-M.; et al. Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem. Eng. J. 2013, 78, 93–100. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Zhu, T.; Li, D.; Gu, Q. Strain and culture medium optimization for production enhancement of prodiginines from marine-derived Streptomyces sp. GQQ-10. J. Ocean Univ. China 2012, 11, 361–365. [Google Scholar] [CrossRef]
- Shanks, R.M.Q.; Lahr, R.M.; Stella, N.A.; Arena, K.E.; Brothers, K.M.; Kwak, D.H.; Liu, X.; Kalivoda, E.J. A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS. PLoS ONE 2013, 8, e57634-21. [Google Scholar] [CrossRef]
- Solé, M.; Rius, N.; Francia, A.; Lorén, J.G. The effect of pH on prodigiosin production by non-proliferating cells of Serratia marcescens. Lett. Appl. Microbiol. 1994, 19, 341–344. [Google Scholar] [CrossRef]
- Kwon, S.K.; Park, Y.K.; Kim, J.F. Genome-Wide screening and identification of factors affecting the biosynthesis of prodigiosin by Hahella chejuensis, using Escherichia coli as a surrogate host. Appl. Environ. Microbiol. 2010, 76, 1661–1668. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, W.; Wang, H. Physiological response and morphological changes of Heterosigma akashiwo to an algicidal compound prodigiosin. J. Hazard. Mater. 2020, 385, 121530. [Google Scholar] [CrossRef]
- Nakashima, T.; Miyazaki, Y.; Matsuyama, Y.; Muraoka, W.; Yamaguchi, K.; Oda, T. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium γ-proteobacterium. Appl. Microbiol. Biotechnol. 2006, 73, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.F.; Yim, J.H.; Kwon, S.-K.; Lee, C.H.; Lee, H.K. Red to red—The marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zheng, W.; Yao, Z.; Peng, Y.; Zhang, S.; Hu, Z.; Tao, Z.; Zheng, T. Toxic effects of prodigiosin secreted by Hahella sp. KA22 on harmful alga Phaeocystis globosa. Front. Microbiol. 2017, 8, 999. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Wang, X.; Feng, J.; Yan, W.; Guo, L. A newly isolated Rhodobacter sphaeroides HY01 with high hydrogen production performance. Int. J. Hydrogen Energy 2014, 39, 10051–10060. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Zhang, L.; Guo, L. Enhanced hydrogen production performance of Rubrivivax gelatinosus M002 using mixed carbon sources. Int. J. Hydrogen Energy 2012, 37, 13296–13303. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- D’Aoust, J.Y.; Gerber, N.N. Isolation and purification of prodigiosin from Vibrio psychroerythrus. J. Bacteriol. 1974, 118, 756–757. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Tan, J.; Lin, S.; Li, D.; Yang, H. Isolation, identification and characterization of an algicidal bacterium from Lake Taihu and preliminary studies on its algicidal compounds. J. Environ. Sci. 2012, 24, 1823–1831. [Google Scholar] [CrossRef]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Gajdošik, M.Š.; Vicić, A.; Gvozdić, V.; Galić, V.; Begović, L.; Mlinarić, S. Effect of prolonged photoperiod on light-dependent photosynthetic reactions in cannabis. Int. J. Mol. Sci. 2022, 23, 9702. [Google Scholar] [CrossRef]
- Cheng, J.; Fan, W.; Zheng, L. Development of a mixotrophic cultivation strategy for simultaneous improvement of biomass and photosynthetic efficiency in freshwater microalga Scenedesmus obliquus by adding appropriate concentration of sodium acetate. Biochem. Eng. J. 2021, 176, 108177. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of porphyrins in antibacterial photodynamic therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Song, C.; Xu, W.; Wu, H.; Wang, X.; Gong, Q.; Liu, C.; Liu, J.; Zhou, L. Photodynamic therapy induces autophagy-mediated cell death in human colorectal cancer cells via activation of the ROS/JNK signaling pathway. Cell Death Dis. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]
- Gaultier, N.E.; Junqueira, A.C.M.; Uchida, A.; Purbojati, R.W.; Houghton, J.N.I.; Chénard, C.; Wong, A.; Clare, M.E.; Kushwaha, K.K.; Panicker, D.; et al. Complete genome sequence of the bacterium Serratia marcescens SGAir0764, isolated from Singapore air. Genome Announc. 2018, 6, e00637-18. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, A.; Nagaya, Y.; Pradel, E.; Ooka, T.; Ogura, Y.; Katsura, K.; Kurokawa, K.; Oshima, K.; Hattori, M.; Parkhill, J.; et al. Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol. Evol. 2014, 6, 2096–2110. [Google Scholar] [CrossRef]
- de Araújo, H.W.C.; Fukushima, K.; Takaki, G.M.C. Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 2010, 15, 6931–6940. [Google Scholar] [CrossRef] [PubMed]
- Naik, C.; Srisevita, J.M.; Shushma, K.N.; Noorin, F.; Shilpa, A.C.; Muttanna, C.D.; Darshan, N.; Sannadurgappa, D. Peanut oil cake: A novel substrate for enhanced cell growth and prodigiosin production from Serratia marcescens CF-53. J. Res. Biol. 2012, 2, 549–557. Available online: http://ojs.jresearchbiology.com/index.php/jrb/article/view/248 (accessed on 5 November 2025).
- Shahitha, S.; Poornima, K. Enhanced production of prodigiosin production in Serratia marcescens. J. Appl. Pharm. Sci. 2012, 2, 138–140. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Z.; Zhang, Z.; Zhao, J.; Liu, X.; Meng, G.; Zhang, J.; Zhang, J. Two-step optimization for improving prodigiosin production using a fermentation medium for Serratia marcescens and an extraction process. Fermentation 2024, 10, 85. [Google Scholar] [CrossRef]
- Fender, J.E.; Bender, C.M.; Stella, N.A.; Lahr, R.M.; Kalivoda, E.J.; Shanks, R.M.Q. Serratia marcescens quinoprotein glucose dehydrogenase activity mediates medium acidification and inhibition of prodigiosin production by glucose. Appl. Environ. Microbiol. 2012, 78, 6225–6235. [Google Scholar] [CrossRef]
- Jardak, M.; Atoissi, A.; Msalbi, D.; Atoui, D.; Bouizgarne, B.; Rigane, G.; Ben Salem, R.; Aifa, S.; Mnif, S. Antibacterial, antibiofilm and cytotoxic properties of prodigiosin produced by a newly isolated Serratia sp. C6LB from a milk collection center. Microb. Pathog. 2022, 164, 105449. [Google Scholar] [CrossRef]
- Zang, C.-Z.; Yeh, C.-W.; Chang, W.-F.; Lin, C.-C.; Kan, S.-C.; Shieh, C.-J.; Liu, Y.-C. Identification and enhanced production of prodigiosin isoform pigment from Serratia marcescens N10612. J. Taiwan Inst. Chem. Eng. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Wei, Y.H.; Chen, W.C. Enhanced production of prodigiosin-like pigment from Serratia marcescens SMΔR by medium improvement and oil-supplementation strategies. J. Biosci. Bioeng. 2005, 99, 616–622. [Google Scholar] [CrossRef]
- Su, W.T.; Tsou, T.Y.; Liu, H.L. Response surface optimization of microbial prodigiosin production from Serratia marcescens. J. Taiwan Inst. Chem. Eng. 2011, 42, 217–222. [Google Scholar] [CrossRef]
- Groisman, E.A.; Chan, C. Cellular adaptations to cytoplasmic Mg2+ limitation. Annu. Rev. Microbiol. 2021, 75, 649–672. [Google Scholar] [CrossRef]
- Romano, A.H.; Nickerson, W.J. Utilization of amino acids as carbon sources by Streptomyces fradiae. J. Bacteriol. 1958, 75, 161–166. [Google Scholar] [CrossRef]
- Halvorson, H. Utilization of single L-amino acids as sole source of carbon and nitrogen by bacteria. Can. J. Microbiol. 1972, 18, 1647–1650. [Google Scholar] [CrossRef]
- Wei, Y.H.; Yu, W.J.; Chen, W.C. Enhanced undecylprodigiosin production from Serratia marcescens SS-1 by medium formulation and amino-acid supplementation. J. Biosci. Bioeng. 2005, 100, 466–471. [Google Scholar] [CrossRef]
- Williams, R.P.; Gott, C.L.; Qadri, S.M.H. Induction of pigmentation in nonproliferating cells of Serratia marcescens by addition of single amino acids. J. Bacteriol. 1971, 106, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Qadri, S.M.H.; Williams, R.P. Role of methionine in biosynthesis of prodigiosin by Serratia marcescens. J. Bacteriol. 1973, 116, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Faraag, A.H.; El-Batal, A.I.; El-Hendawy, H.H. Characterization of prodigiosin produced by Serratia marcescens strain isolated from irrigation water in Egypt. Nat. Sci. 2017, 15, 55–68. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Yang, H.; Li, Y.; Wang, Y.; Chen, X.; Xu, L. Satellite data regarding the eutrophication response to human activities in the plateau lake Dianchi in China from 1974 to 2009. Sci. Total Environ. 2014, 485, 1–11. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Wei, J.; Xie, X.; Huang, F.; Xiang, L.; Wang, Y.; Han, T.; Massey, I.Y.; Liang, G.; Pu, Y.; Yang, F. Simultaneous Microcystis algicidal and microcystin synthesis inhibition by a red pigment prodigiosin. Environ. Pollut. 2020, 256, 113444. [Google Scholar] [CrossRef]
- Stirbet, A. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Ji, W.; Hong, E.; Chen, X.; Li, Z.; Lin, B.; Xia, X.; Li, T.; Song, X.; Jin, S.; Zhu, X. Photosynthetic and physiological responses of different peony cultivars to high temperature. Front. Plant Sci. 2022, 13, 969718. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, J.; Gong, D.; Zhao, X.; Qi, Y.; Su, Y.; Ma, W. The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci. Total Environ. 2018, 633, 593–599. [Google Scholar] [CrossRef]
- Kopperi, H.; Mohan, S.V. Comparative appraisal of nutrient recovery, bio-crude, and bio-hydrogen production using Coelestrella sp. in a closed-loop biorefinery. Front. Bioeng. Biotechnol. 2022, 10, 964070. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, J.; Tang, Z.; Hu, H.; Meng, F.; Li, A. Effects of polystyrene microplastics on growth and toxin production of Alexandrium pacificum. Toxins 2021, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Tan, L.; Wang, Y.; Gao, M.; Liu, F.; Wang, Q.; Xu, C.; Zhang, C.; Xu, W.; Hou, Y.; et al. Optimization of ultrasonic-assisted extraction of total flavonoids from Zanthoxylum bungeanum residue by response surface methodology and evaluation of its algicidal properties. Front. Microbiol. 2025, 16, 1540631. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, M.; Zhou, J.; Gao, X.; Zhu, S.; Yuan, L.; Hou, X.; Liu, T.; Chen, G.; Tang, X.; et al. Transcriptome analysis and differential gene expression profiling of wucai (Brassica campestris L.) in response to cold stress. BMC Genom. 2022, 23, 137. [Google Scholar] [CrossRef]


| Factors | Levels | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| A-Glycerol (mL·L−1) | 5.0 | 10.0 | 15.0 | 20.0 |
| B-Bovine bone peptone (g·L−1) | 15.0 | 20.0 | 25.0 | 30.0 |
| C-Magnesium sulfate heptahydrate(g·L−1) | 1.0 | 5.0 | 10.0 | 15.0 |
| D-Corn dry powder (g·L−1) | 0.00 | 0.15 | 0.30 | 0.45 |
| E-Tyrosine (%) | 0.000 | 0.125 | 0.250 | 0.375 |
| No. | Factors | Prodigiosin Yield | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | 24 h | 48 h | 72 h | |
| 1 | 1 | 1 | 1 | 1 | 1 | 2.115 | 2.340 | 2.404 |
| 2 | 1 | 2 | 2 | 2 | 2 | 0.785 | 1.784 | 1.871 |
| 3 | 1 | 3 | 3 | 3 | 3 | 1.156 | 2.190 | 2.254 |
| 4 | 1 | 4 | 4 | 4 | 4 | 1.062 | 1.926 | 1.997 |
| 5 | 2 | 1 | 2 | 3 | 4 | 2.104 | 3.560 | 3.706 |
| 6 | 2 | 2 | 1 | 4 | 3 | 0.454 | 1.883 | 2.064 |
| 7 | 2 | 3 | 4 | 1 | 2 | 0.260 | 1.555 | 1.373 |
| 8 | 2 | 4 | 3 | 2 | 1 | 0.245 | 0.967 | 0.920 |
| 9 | 3 | 1 | 3 | 4 | 2 | 0.920 | 2.183 | 2.707 |
| 10 | 3 | 2 | 4 | 3 | 1 | 0.367 | 0.742 | 1.058 |
| 11 | 3 | 3 | 1 | 2 | 4 | 0.379 | 2.036 | 2.313 |
| 12 | 3 | 4 | 2 | 1 | 3 | 0.288 | 2.376 | 2.644 |
| 13 | 4 | 1 | 4 | 2 | 3 | 2.644 | 6.461 | 7.645 |
| 14 | 4 | 2 | 3 | 1 | 4 | 1.389 | 3.793 | 4.132 |
| 15 | 4 | 3 | 2 | 4 | 1 | 0.375 | 1.492 | 1.772 |
| 16 | 4 | 4 | 1 | 3 | 2 | 0.268 | 1.480 | 1.654 |
| K1_24 h | 1.280 | 1.946 | 0.804 | 1.013 | 0.776 | |||
| K2_24 h | 0.766 | 0.749 | 0.888 | 1.013 | 0.558 | |||
| K3_24 h | 0.488 | 0.542 | 0.927 | 0.974 | 1.135 | |||
| K4_24 h | 1.169 | 0.466 | 1.083 | 0.703 | 1.234 | |||
| Range_24 h | 0.792 | 1.480 | 0.279 | 0.310 | 0.676 | |||
| K1_48 h | 2.060 | 3.636 | 1.935 | 2.516 | 1.385 | |||
| K2_48 h | 1.991 | 2.050 | 2.303 | 2.812 | 1.751 | |||
| K3_48 h | 1.834 | 1.818 | 2.283 | 1.993 | 3.228 | |||
| K4_48 h | 3.307 | 1.687 | 2.671 | 1.871 | 2.829 | |||
| Range_48 h | 1.473 | 1.949 | 0.736 | 0.941 | 1.843 | |||
| K1_72 h | 2.130 | 4.115 | 2.105 | 2.635 | 1.538 | |||
| K2_72 h | 2.015 | 2.280 | 2.498 | 3.185 | 1.900 | |||
| K3_72 h | 2.180 | 1.925 | 2.502 | 2.167 | 3.647 | |||
| K4_72 h | 3.797 | 1.803 | 3.018 | 2.135 | 3.037 | |||
| Range_72 h | 1.782 | 2.312 | 0.913 | 1.050 | 2.109 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Xu, C.; Zhu, Z.; Zhang, X.; Shao, Z.; Yu, Z.; Zheng, Z.; Wang, Y.; Wang, Y.; Chen, Y.; et al. Prodigiosin as an Algicidal Agent: Inhibition of Pigment Accumulation and Photosynthetic Efficiency of Cyanobacteria Involved in Algal Blooms. Microorganisms 2025, 13, 2569. https://doi.org/10.3390/microorganisms13112569
Zhang C, Xu C, Zhu Z, Zhang X, Shao Z, Yu Z, Zheng Z, Wang Y, Wang Y, Chen Y, et al. Prodigiosin as an Algicidal Agent: Inhibition of Pigment Accumulation and Photosynthetic Efficiency of Cyanobacteria Involved in Algal Blooms. Microorganisms. 2025; 13(11):2569. https://doi.org/10.3390/microorganisms13112569
Chicago/Turabian StyleZhang, Chaobo, Chengshuai Xu, Zhenxia Zhu, Xiu Zhang, Zhaoan Shao, Zhenhui Yu, Zhangdi Zheng, Yijie Wang, Yadong Wang, Yujie Chen, and et al. 2025. "Prodigiosin as an Algicidal Agent: Inhibition of Pigment Accumulation and Photosynthetic Efficiency of Cyanobacteria Involved in Algal Blooms" Microorganisms 13, no. 11: 2569. https://doi.org/10.3390/microorganisms13112569
APA StyleZhang, C., Xu, C., Zhu, Z., Zhang, X., Shao, Z., Yu, Z., Zheng, Z., Wang, Y., Wang, Y., Chen, Y., Xu, W., & Cheng, J. (2025). Prodigiosin as an Algicidal Agent: Inhibition of Pigment Accumulation and Photosynthetic Efficiency of Cyanobacteria Involved in Algal Blooms. Microorganisms, 13(11), 2569. https://doi.org/10.3390/microorganisms13112569






