Advances in Pseudomonas aeruginosa-Induced Programmed Cell Death and Potential Targeted Treatment Strategies
Abstract
1. Introduction
2. P. aeruginosa-Induced PCD
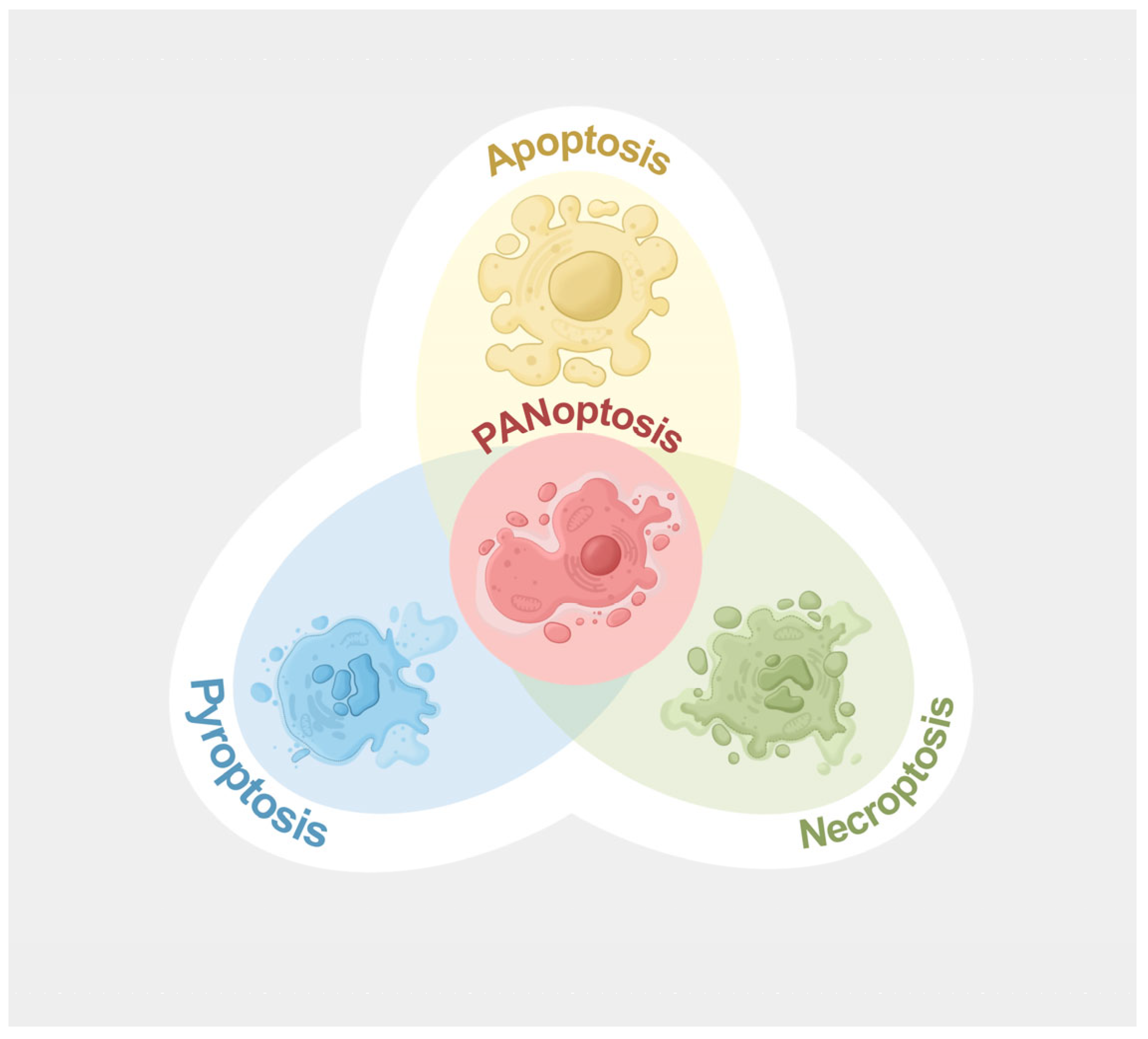
2.1. P. aeruginosa-Induced PANoptosis
2.1.1. Composition of PANoptosome Complexes
2.1.2. Key Upstream Regulators of PANoptosome Complexes
2.1.3. Progress in P. aeruginosa-Induced PANoptosis
Upregulation and/or Activation of PANoptosome Sensor Proteins Upon P. aeruginosa Infection
Upregulation and/or Activation of Key Upstream Regulators of PANoptosome Complexes Upon P. aeruginosa Infection
2.1.4. Outstanding Questions in P. aeruginosa-Induced PANoptosis
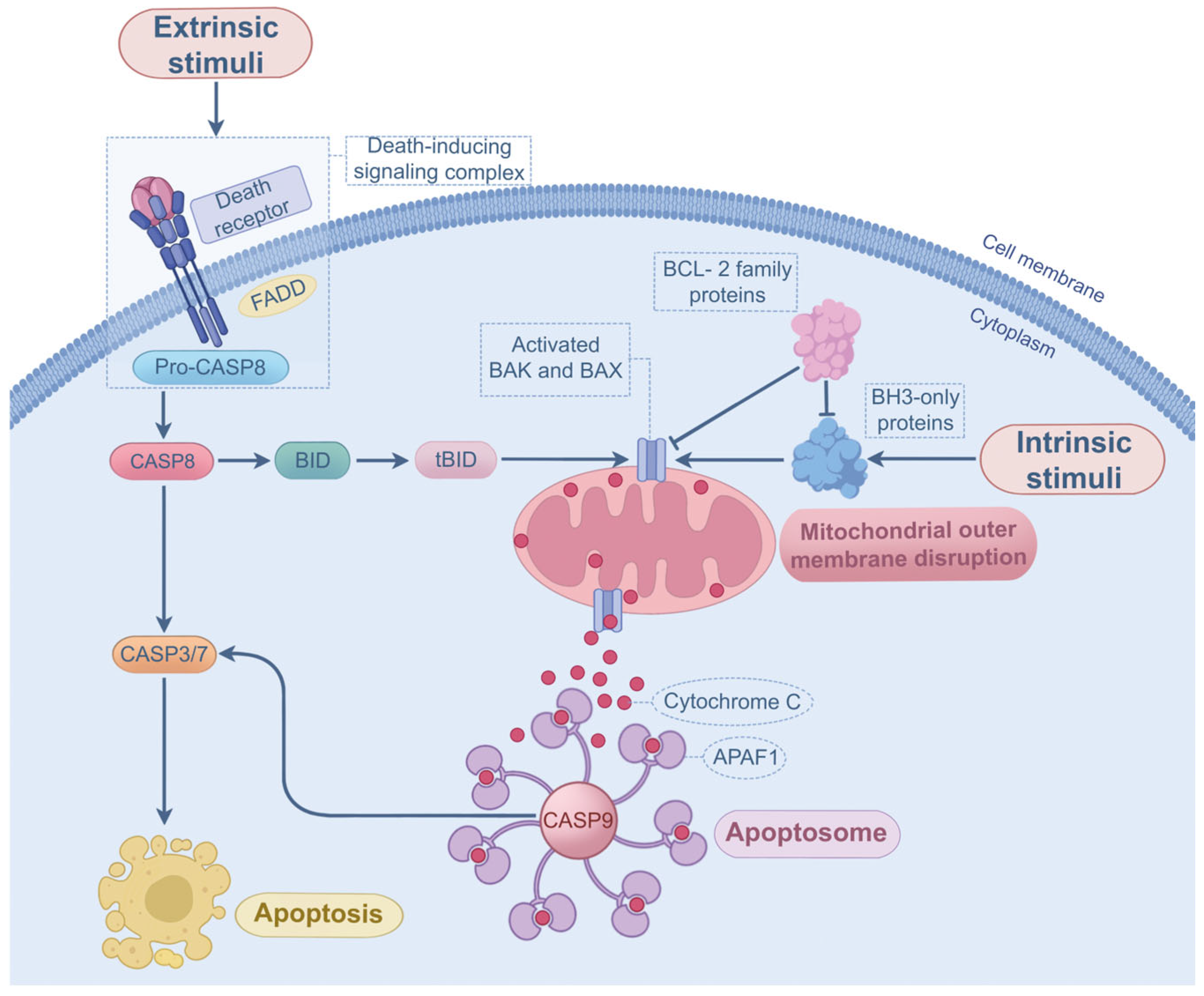
2.2. P. aeruginosa-Induced Apoptosis
Progress and Outstanding Questions in P. aeruginosa-Induced Apoptosis
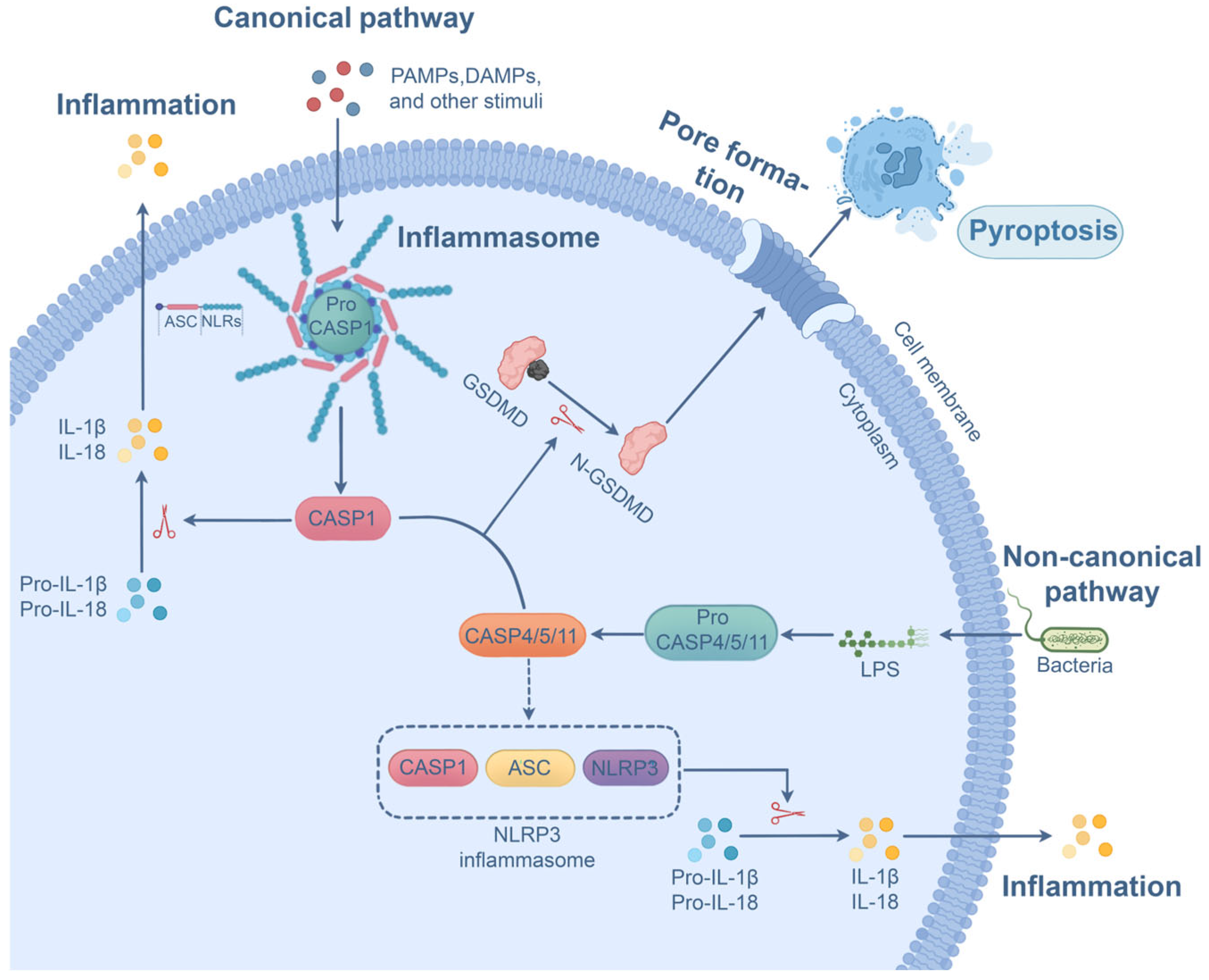
2.3. P. aeruginosa-Induced Pyroptosis
Progress and Outstanding Questions in P. aeruginosa-Induced Pyroptosis
2.4. P. aeruginosa-Induced Necroptosis
Progress and Outstanding Questions in P. aeruginosa-Induced Necroptosis
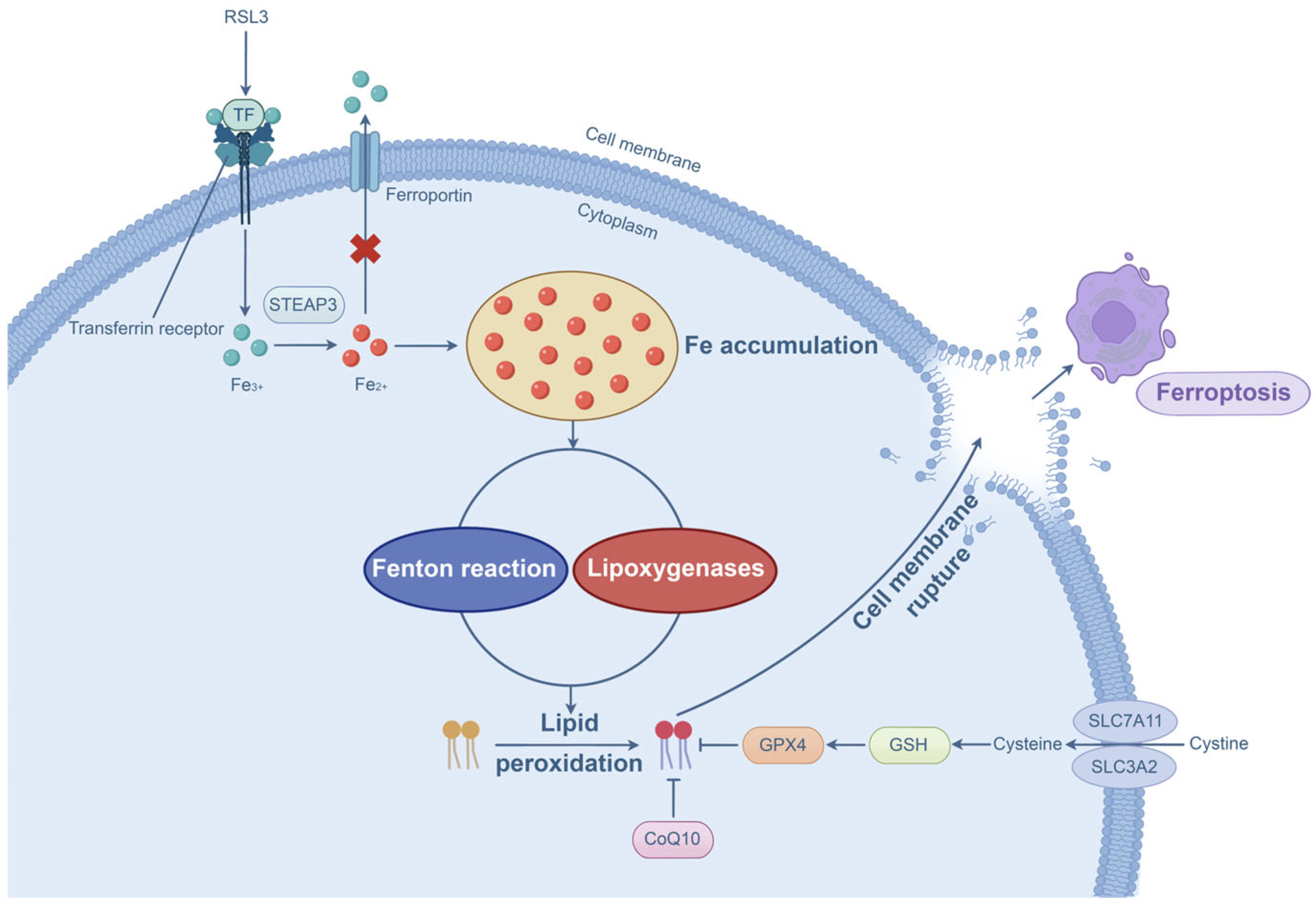
2.5. P. aeruginosa-Induced Ferroptosis
Progress in P. aeruginosa-Induced Ferroptosis
3. Potential Targeted Treatment Strategies for P. aeruginosa-Induced PCD
3.1. Targeting Key Upstream Regulators
3.2. Targeting Sensor Proteins
3.3. Targeting Other Molecular Components of PANoptosome Complexes or Downstream Executioners
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Edelstein, M.V.; Skleenova, E.N.; Shevchenko, O.V.; D’SOuza, J.W.; Tapalski, D.V.; Azizov, I.S.; Sukhorukova, M.V.; Pavlukov, R.A.; Kozlov, R.S.; Toleman, M.A.; et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: A longitudinal epidemiological and clinical study. Lancet Infect. Dis. 2013, 13, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Difrancesco, L.F.; Liapikou, A.; Rinaudo, M.; Carbonara, M.; Li Bassi, G.; Gabarrus, A.; Torres, A. Polymicrobial intensive care unit-acquired pneumonia: Prevalence, microbiology and outcome. Crit. Care 2015, 19, 450. [Google Scholar] [CrossRef]
- Bauer, K.A.; Puzniak, L.A.; Yu, K.C.; Finelli, L.; Moise, P.; Ai, C.; Watts, J.A.; Gupta, V. Epidemiology and outcomes of culture-positive bloodstream pathogens prior to and during the SARS-CoV-2 pandemic: A multicenter evaluation. BMC Infect. Dis. 2022, 22, 841. [Google Scholar] [CrossRef]
- Karaba, S.M.; Cosgrove, S.E.; Lee, J.H.; Fiawoo, S.; Heil, E.L.; Quartuccio, K.S.; Shihadeh, K.C.; Tamma, P.D. Extended-Infusion beta-Lactam Therapy, Mortality, and Subsequent Antibiotic Resistance Among Hospitalized Adults With Gram-Negative Bloodstream Infections. JAMA Netw. Open 2024, 7, e2418234. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Catena, F.; Ansaloni, L.; Coccolini, F.; Corbella, D.; Moore, E.E.; Malangoni, M.; Velmahos, G.; Coimbra, R.; Koike, K.; et al. Complicated intra-abdominal infections worldwide: The definitive data of the CIAOW Study. World J. Emerg. Surg. 2014, 9, 37. [Google Scholar] [CrossRef]
- Rhee, C.; Chen, T.; Kadri, S.S.; Lawandi, A.; Yek, C.; Walker, M.; Warner, S.; Fram, D.; Chen, H.C.; Shappell, C.N.; et al. Trends in Empiric Broad-Spectrum Antibiotic Use for Suspected Community-Onset Sepsis in US Hospitals. JAMA Netw. Open 2024, 7, e2418923. [Google Scholar] [CrossRef]
- Johnson, L.E.; D’Agata, E.M.; Paterson, D.L.; Clarke, L.; Qureshi, Z.A.; Potoski, B.A.; Peleg, A.Y. Pseudomonas aeruginosa bacteremia over a 10-year period: Multidrug resistance and outcomes in transplant recipients. Transpl. Infect. Dis. 2009, 11, 227–234. [Google Scholar] [CrossRef]
- Montero, M.; Sala, M.; Riu, M.; Belvis, F.; Salvado, M.; Grau, S.; Horcajada, J.P.; Alvarez-Lerma, F.; Terradas, R.; Orozco-Levi, M.; et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 335–339. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Yayan, J.; Ghebremedhin, B.; Rasche, K. Antibiotic Resistance of Pseudomonas aeruginosa in Pneumonia at a Single University Hospital Center in Germany over a 10-Year Period. PLoS ONE 2015, 10, e0139836. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Komarow, L.; Chen, L.; Ge, L.; Hanson, B.M.; Cober, E.; Herc, E.; Alenazi, T.; Kaye, K.S.; Garcia-Diaz, J.; et al. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): A prospective cohort study. Lancet Microbe 2023, 4, e159–e170. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Seu, M.Y.; Dorafshar, A.H.; Shafikhani, S.H. Pseudomonas aeruginosa Cytotoxins: Mechanisms of Cytotoxicity and Impact on Inflammatory Responses. Cells 2023, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, A.; Gupta, K.H.; Goldufsky, J.W.; Lee, H.W.; Gupta, V.; Shafikhani, S.H. Pseudomonas aeruginosa ExoS Induces Intrinsic Apoptosis in Target Host Cells in a Manner That is Dependent on its GAP Domain Activity. Sci. Rep. 2018, 8, 14047. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.H.; Indramohan, M.; Ratsimandresy, R.A.; Gangopadhyay, A.; Morris, E.P.; Monack, D.M.; Dorfleutner, A.; Stehlik, C. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 2018, 9, 996. [Google Scholar] [CrossRef]
- Basso, P.; Wallet, P.; Elsen, S.; Soleilhac, E.; Henry, T.; Faudry, E.; Attree, I. Multiple Pseudomonas species secrete exolysin-like toxins and provoke Caspase-1-dependent macrophage death. Environ. Microbiol. 2017, 19, 4045–4064. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Kesavardhana, S.; Karki, R.; Kancharana, B.; Burton, A.R.; Kanneganti, T.D. RIPK1 Distinctly Regulates Yersinia-Induced Inflammatory Cell Death, PANoptosis. Immunohorizons 2020, 4, 789–796. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Lee, S.; Karki, R.; Wang, Y.; Nguyen, L.N.; Kalathur, R.C.; Kanneganti, T.D. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 2021, 597, 415–419. [Google Scholar] [CrossRef]
- Sundaram, B.; Karki, R.; Kanneganti, T.D. NLRC4 Deficiency Leads to Enhanced Phosphorylation of MLKL and Necroptosis. Immunohorizons 2022, 6, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, L.; Li, L.; Miao, E.A.; Amer, A.O.; Wozniak, D.J.; Wen, H. Pseudomonas aeruginosa Mediates Host Necroptosis through Rhl-Pqs Quorum Sensing Interaction. Immunohorizons 2024, 8, 721–728. [Google Scholar] [CrossRef]
- Yue, L.; Cao, H.; Qi, J.; Yuan, J.; Wang, X.; Wang, Y.; Shan, B.; Ke, H.; Li, H.; Luan, N.; et al. Pretreatment with 3-methyladenine ameliorated Pseudomonas aeruginosa-induced acute pneumonia by inhibiting cell death of neutrophils in a mouse infection model. Int. J. Med. Microbiol. 2023, 313, 151574. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front. Cell Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Karki, R.; Lee, S.; Mall, R.; Pandian, N.; Wang, Y.; Sharma, B.R.; Malireddi, R.S.; Yang, D.; Trifkovic, S.; Steele, J.A.; et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 2022, 7, eabo6294. [Google Scholar] [CrossRef]
- Takaoka, A.; Wang, Z.; Choi, M.K.; Yanai, H.; Negishi, H.; Ban, T.; Lu, Y.; Miyagishi, M.; Kodama, T.; Honda, K.; et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007, 448, 501–505. [Google Scholar] [CrossRef]
- Maelfait, J.; Liverpool, L.; Bridgeman, A.; Ragan, K.B.; Upton, J.W.; Rehwinkel, J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017, 36, 2529–2543. [Google Scholar] [CrossRef]
- Kuriakose, T.; Kanneganti, T.D. ZBP1: Innate Sensor Regulating Cell Death and Inflammation. Trends Immunol. 2018, 39, 123–134. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Malireddi, R.K.; Neale, G.; Vogel, P.; Yamamoto, M.; Lamkanfi, M.; Kanneganti, T.D. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 2015, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Pandian, N.; Mall, R.; Wang, Y.; Sarkar, R.; Kim, H.J.; Malireddi, R.K.S.; Karki, R.; Janke, L.J.; Vogel, P.; et al. NLRP12-PANoptosome activates PANoptosis and pathology in response to heme and PAMPs. Cell 2023, 186, 2783–2801.e20. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef]
- Tummers, B.; Mari, L.; Guy, C.S.; Heckmann, B.L.; Rodriguez, D.A.; Ruhl, S.; Moretti, J.; Crawford, J.C.; Fitzgerald, P.; Kanneganti, T.D.; et al. Caspase-8-Dependent Inflammatory Responses Are Controlled by Its Adaptor, FADD, and Necroptosis. Immunity 2020, 52, 994–1006.e8. [Google Scholar] [CrossRef]
- Schwarzer, R.; Jiao, H.; Wachsmuth, L.; Tresch, A.; Pasparakis, M. FADD and Caspase-8 Regulate Gut Homeostasis and Inflammation by Controlling MLKL- and GSDMD-Mediated Death of Intestinal Epithelial Cells. Immunity 2020, 52, 978–993.e6. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Pandian, N.; Kanneganti, T.D. PANoptosis: A Unique Innate Immune Inflammatory Cell Death Modality. J. Immunol. 2022, 209, 1625–1633. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Kuriakose, T.; Zheng, M.; Neale, G.; Kanneganti, T.D. IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection. J. Immunol. 2018, 200, 1489–1495. [Google Scholar] [CrossRef]
- Sharma, B.R.; Karki, R.; Rajesh, Y.; Kanneganti, T.D. Immune regulator IRF1 contributes to ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosome activation and inflammatory cell death (PANoptosis). J. Biol. Chem. 2023, 299, 105141. [Google Scholar] [CrossRef]
- Varfolomeev, E.E.; Schuchmann, M.; Luria, V.; Chiannilkulchai, N.; Beckmann, J.S.; Mett, I.L.; Rebrikov, D.; Brodianski, V.M.; Kemper, O.C.; Kollet, O.; et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.; Gunther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Pierini, R.; Juruj, C.; Perret, M.; Jones, C.L.; Mangeot, P.; Weiss, D.S.; Henry, T. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012, 19, 1709–1721. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Vogel, P.; Kanneganti, T.D. Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell 2020, 181, 674–687.e13. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.L.; He, J.R.; Jin, S.M.; Yang, X.; Bai, H.M.; Liu, C.B.; Ma, Y.B. P. aeruginosa Mediated Necroptosis in Mouse Tumor Cells Induces Long-Lasting Systemic Antitumor Immunity. Front. Oncol. 2020, 10, 610651. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Han, Y.; Ge, C.; Ye, J.; Li, R.; Zhang, Y. Demethyleneberberine alleviates Pseudomonas aeruginosa-induced acute pneumonia by inhibiting the AIM2 inflammasome and oxidative stress. Pulm. Pharmacol. Ther. 2023, 83, 102259. [Google Scholar] [CrossRef]
- Ulland, T.K.; Jain, N.; Hornick, E.E.; Elliott, E.I.; Clay, G.M.; Sadler, J.J.; Mills, K.A.; Janowski, A.M.; Volk, A.P.; Wang, K.; et al. Nlrp12 mutation causes C57BL/6J strain-specific defect in neutrophil recruitment. Nat. Commun. 2016, 7, 13180. [Google Scholar] [CrossRef]
- Penaranda, C.; Chumbler, N.M.; Hung, D.T. Dual transcriptional analysis reveals adaptation of host and pathogen to intracellular survival of Pseudomonas aeruginosa associated with urinary tract infection. PLoS Pathog. 2021, 17, e1009534. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, Y.; Zhou, X.; Huang, C.; Wu, M. Atg7 enhances host defense against infection via downregulation of superoxide but upregulation of nitric oxide. J. Immunol. 2015, 194, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Ahn, S.H.; Kim, S.H.; Oh, D.J. N-3-oxododecanoyl homoserine lactone exacerbates endothelial cell death by inducing receptor-interacting protein kinase 1-dependent apoptosis. Am. J. Physiol. Cell Physiol. 2021, 321, C644–C653. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284, 555–556. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Martinon, F.; Tschopp, J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004, 117, 561–574. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27 (Suppl. S1), S2–S19. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, N.; Marassi, F.M.; Newmeyer, D.D.; Hanein, D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014, 21, 206–215. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Vanamee, E.S.; Faustman, D.L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal 2018, 11, eaao4910. [Google Scholar] [CrossRef] [PubMed]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011, 18, 538–548. [Google Scholar] [CrossRef]
- Alaoui-El-Azher, M.; Jia, J.; Lian, W.; Jin, S. ExoS of Pseudomonas aeruginosa induces apoptosis through a Fas receptor/caspase 8-independent pathway in HeLa cells. Cell Microbiol. 2006, 8, 326–338. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Bello, D.; Masood, S.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT Induces Mitochondrial Apoptosis in Target Host Cells in a Manner That Depends on Its GTPase-activating Protein (GAP) Domain Activity. J. Biol. Chem. 2015, 290, 29063–29073. [Google Scholar] [CrossRef] [PubMed]
- Kloth, C.; Schirmer, B.; Munder, A.; Stelzer, T.; Rothschuh, J.; Seifert, R. The Role of Pseudomonas aeruginosa ExoY in an Acute Mouse Lung Infection Model. Toxins 2018, 10, 185. [Google Scholar] [CrossRef]
- Buommino, E.; Morelli, F.; Metafora, S.; Rossano, F.; Perfetto, B.; Baroni, A.; Tufano, M.A. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect. Immun. 1999, 67, 4794–4800. [Google Scholar] [CrossRef]
- Liu, X.; Shao, K.; Sun, T. SIRT1 regulates the human alveolar epithelial A549 cell apoptosis induced by Pseudomonas aeruginosa lipopolysaccharide. Cell Physiol. Biochem. 2013, 31, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Manago, A.; Becker, K.A.; Carpinteiro, A.; Wilker, B.; Soddemann, M.; Seitz, A.P.; Edwards, M.J.; Grassme, H.; Szabo, I.; Gulbins, E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid. Redox Signal. 2015, 22, 1097–1110. [Google Scholar] [CrossRef]
- Song, D.; Meng, J.; Cheng, J.; Fan, Z.; Chen, P.; Ruan, H.; Tu, Z.; Kang, N.; Li, N.; Xu, Y.; et al. Pseudomonas aeruginosa quorum-sensing metabolite induces host immune cell death through cell surface lipid domain dissolution. Nat. Microbiol. 2019, 4, 97–111. [Google Scholar] [CrossRef]
- Jacobi, C.A.; Schiffner, F.; Henkel, M.; Waibel, M.; Stork, B.; Daubrawa, M.; Eberl, L.; Gregor, M.; Wesselborg, S. Effects of bacterial N-acyl homoserine lactones on human Jurkat T lymphocytes-OdDHL induces apoptosis via the mitochondrial pathway. Int. J. Med. Microbiol. 2009, 299, 509–519. [Google Scholar] [CrossRef]
- Du, X.; Youle, R.J.; FitzGerald, D.J.; Pastan, I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol. Cell Biol. 2010, 30, 3444–3452. [Google Scholar] [CrossRef]
- Jenkins, C.E.; Swiatoniowski, A.; Issekutz, A.C.; Lin, T.J. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and -3-dependent mechanism. J. Biol. Chem. 2004, 279, 37201–37207. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Dockrell, D.H.; Pattery, T.; Lee, D.G.; Cornelis, P.; Hellewell, P.G.; Whyte, M.K. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 2005, 174, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.L.; Kowalski, M.P.; Stopak, K.S.; Pier, G.B. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Cell Mol. Biol. 2003, 29, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, W.T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Sborgi, L.; Ruhl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Muller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef]
- Ryu, J.C.; Kim, M.J.; Kwon, Y.; Oh, J.H.; Yoon, S.S.; Shin, S.J.; Yoon, J.H.; Ryu, J.H. Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 2017, 10, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Santoni, K.; Pericat, D.; Gorse, L.; Buyck, J.; Pinilla, M.; Prouvensier, L.; Bagayoko, S.; Hessel, A.; Leon-Icaza, S.A.; Bellard, E.; et al. Caspase-1-driven neutrophil pyroptosis and its role in host susceptibility to Pseudomonas aeruginosa. PLoS Pathog. 2022, 18, e1010305. [Google Scholar] [CrossRef]
- Minns, M.S.; Liboro, K.; Lima, T.S.; Abbondante, S.; Miller, B.A.; Marshall, M.E.; Tran Chau, J.; Roistacher, A.; Rietsch, A.; Dubyak, G.R.; et al. NLRP3 selectively drives IL-1beta secretion by Pseudomonas aeruginosa infected neutrophils and regulates corneal disease severity. Nat. Commun. 2023, 14, 5832. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Karki, R.; Berwin, B.; Yamamoto, M.; Kanneganti, T.D. Guanylate binding proteins facilitate caspase-11-dependent pyroptosis in response to type 3 secretion system-negative Pseudomonas aeruginosa. Cell Death Discov. 2018, 4, 66, Erratum in Cell Death Discov. 2019, 5, 116. https://doi.org/10.1038/s41420-019-0186-2. [Google Scholar] [CrossRef] [PubMed]
- Virreira Winter, S.; Zychlinsky, A. The bacterial pigment pyocyanin inhibits the NLRP3 inflammasome through intracellular reactive oxygen and nitrogen species. J. Biol. Chem. 2018, 293, 4893–4900. [Google Scholar] [CrossRef]
- Gao, P.; Guo, K.; Pu, Q.; Wang, Z.; Lin, P.; Qin, S.; Khan, N.; Hur, J.; Liang, H.; Wu, M. oprC Impairs Host Defense by Increasing the Quorum-Sensing-Mediated Virulence of Pseudomonas aeruginosa. Front. Immunol. 2020, 11, 1696. [Google Scholar] [CrossRef]
- Tan, Q.; Ai, Q.; He, Y.; Li, F.; Yu, J. P. aeruginosa biofilm activates the NLRP3 inflammasomes in vitro. Microb. Pathog. 2022, 164, 105379. [Google Scholar] [CrossRef]
- Faure, E.; Mear, J.B.; Faure, K.; Normand, S.; Couturier-Maillard, A.; Grandjean, T.; Balloy, V.; Ryffel, B.; Dessein, R.; Chignard, M.; et al. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am. J. Respir. Crit. Care Med. 2014, 189, 799–811. [Google Scholar] [CrossRef]
- Cohen, T.S.; Prince, A.S. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Invest. 2013, 123, 1630–1637. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Yang, M.; Liu, S.B. Research progress on morphology and mechanism of programmed cell death. Cell Death Dis. 2024, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.J.; Upton, J.W.; Long, A.B.; Livingston-Rosanoff, D.; Daley-Bauer, L.P.; Hakem, R.; Caspary, T.; Mocarski, E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 2011, 471, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, D.; Ehrenschwender, M.; Wajant, H. TNFR2 unlocks a RIPK1 kinase activity-dependent mode of proinflammatory TNFR1 signaling. Cell Death Dis. 2018, 9, 921. [Google Scholar] [CrossRef]
- Siegmund, D.; Kums, J.; Ehrenschwender, M.; Wajant, H. Activation of TNFR2 sensitizes macrophages for TNFR1-mediated necroptosis. Cell Death Dis. 2016, 7, e2375. [Google Scholar] [CrossRef]
- He, S.; Liang, Y.; Shao, F.; Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 20054–20059. [Google Scholar] [CrossRef]
- Kim, S.J.; Li, J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013, 4, e716. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Matsuda, M.; Mizokami, A.; Kitagawa, N.; Takeuchi, H.; Jimi, E.; Inai, T.; Hirata, M. Osteocalcin triggers Fas/FasL-mediated necroptosis in adipocytes via activation of p300. Cell Death Dis. 2018, 9, 1194. [Google Scholar] [CrossRef]
- Jouan-Lanhouet, S.; Arshad, M.I.; Piquet-Pellorce, C.; Martin-Chouly, C.; Le Moigne-Muller, G.; Van Herreweghe, F.; Takahashi, N.; Sergent, O.; Lagadic-Gossmann, D.; Vandenabeele, P.; et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012, 19, 2003–2014. [Google Scholar] [CrossRef]
- Paudel, S.; Ghimire, L.; Jin, L.; Baral, P.; Cai, S.; Jeyaseelan, S. NLRC4 suppresses IL-17A-mediated neutrophil-dependent host defense through upregulation of IL-18 and induction of necroptosis during Gram-positive pneumonia. Mucosal Immunol. 2019, 12, 247–257. [Google Scholar] [CrossRef]
- Thapa, R.J.; Nogusa, S.; Chen, P.; Maki, J.L.; Lerro, A.; Andrake, M.; Rall, G.F.; Degterev, A.; Balachandran, S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl. Acad. Sci. USA 2013, 110, E3109–E3118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, D.; Liu, J.; Wang, M.; Duan, L.J.; Liu, M.; Meng, H.; Zhuang, Y.; Wang, H.; Wang, Y.; et al. Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation. Nat. Cell Biol. 2023, 25, 950–962. [Google Scholar] [CrossRef]
- Kitur, K.; Wachtel, S.; Brown, A.; Wickersham, M.; Paulino, F.; Penaloza, H.F.; Soong, G.; Bueno, S.; Parker, D.; Prince, A. Necroptosis Promotes Staphylococcus aureus Clearance by Inhibiting Excessive Inflammatory Signaling. Cell Rep. 2016, 16, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Xie, T.; Peng, W.; Yan, C.; Wu, J.; Gong, X.; Shi, Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013, 5, 70–78. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013, 288, 31268–31279. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012, 11, 290–297. [Google Scholar] [CrossRef]
- Yun, M.; Park, S.H.; Kang, D.H.; Kim, J.W.; Kim, J.D.; Ryu, S.; Lee, J.; Jeong, H.M.; Hwang, H.R.; Song, K.S. Inhibition of Pseudomonas aeruginosa LPS-Induced airway inflammation by RIPK3 in human airway. J. Cell Mol. Med. 2022, 26, 5506–5516. [Google Scholar] [CrossRef]
- Mossine, V.V.; Waters, J.K.; Chance, D.L.; Mawhinney, T.P. Transient Proteotoxicity of Bacterial Virulence Factor Pyocyanin in Renal Tubular Epithelial Cells Induces ER-Related Vacuolation and Can Be Efficiently Modulated by Iron Chelators. Toxicol. Sci. 2016, 154, 403–415. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Guan, J.; Chen, J.; Sun, W.; Chen, H.; Wen, Y.; Chen, Q.; Xie, S.; Zhang, X.; Tao, A.; et al. Necroptosis signaling and NLRP3 inflammasome cross-talking in epithelium facilitate Pseudomonas aeruginosa mediated lung injury. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166613. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mo, J.; Dai, J.; Ye, C.; Cen, W.; Zheng, X.; Jiang, L.; Ye, L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021, 12, 1079. [Google Scholar] [CrossRef]
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Zhou, N.; He, Y.; Zhong, J.; Ma, C.; Zeng, M.; Ji, J.; Huang, J.D.; Ke, Y.; et al. Engineered Salmonella inhibits GPX4 expression and induces ferroptosis to suppress glioma growth in vitro and in vivo. J. Neurooncol 2023, 163, 607–622. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Che, Y.; Liang, H.; Guo, Y.; Xiao, C. A respiratory Streptococcus strain inhibits Acinetobacter baumannii from causing inflammatory damage through ferroptosis. BMC Microbiol. 2024, 24, 437. [Google Scholar] [CrossRef]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.C.; Tyurin, V.A.; Krieger, J.; St Croix, C.M.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018, 128, 4639–4653. [Google Scholar] [CrossRef]
- Dar, H.H.; Anthonymuthu, T.S.; Ponomareva, L.A.; Souryavong, A.B.; Shurin, G.V.; Kapralov, A.O.; Tyurin, V.A.; Lee, J.S.; Mallampalli, R.K.; Wenzel, S.E.; et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(*) sabotage of theft-ferroptosis. Redox Biol. 2021, 45, 102045. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.H.; Epperly, M.W.; Tyurin, V.A.; Amoscato, A.A.; Anthonymuthu, T.S.; Souryavong, A.B.; Kapralov, A.A.; Shurin, G.V.; Samovich, S.N.; St Croix, C.M.; et al. P. aeruginosa augments irradiation injury via 15-lipoxygenase-catalyzed generation of 15-HpETE-PE and induction of theft-ferroptosis. JCI Insight 2022, 7, e156013. [Google Scholar] [CrossRef]
- Tong, J.; Lan, X.T.; Zhang, Z.; Liu, Y.; Sun, D.Y.; Wang, X.J.; Ou-Yang, S.X.; Zhuang, C.L.; Shen, F.M.; Wang, P.; et al. Ferroptosis inhibitor liproxstatin-1 alleviates metabolic dysfunction-associated fatty liver disease in mice: Potential involvement of PANoptosis. Acta Pharmacol. Sin. 2023, 44, 1014–1028. [Google Scholar] [CrossRef]
- Wu, M.F.; Peng, X.; Zhang, M.C.; Guo, H.; Xie, H.T. Ferroptosis and PANoptosis under hypoxia pivoting on the crosstalk between DHODH and GPX4 in corneal epithelium. Free Radic. Biol. Med. 2025, 228, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Du, X.; Ji, L.; Han, Y.; Dang, J.; Wen, J.; Wang, Y.; Pu, Q.; Wu, M.; Liang, H. Pseudomonas aeruginosa T6SS-mediated molybdate transport contributes to bacterial competition during anaerobiosis. Cell Rep. 2021, 35, 108957. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Chen, J.; Song, B.; Tang, Z.; Li, X.; Zhang, S.; Yang, T.; Liu, Y.; Mo, W.; Zhang, Y.; et al. Chaperone- and PTM-mediated activation of IRF1 tames radiation-induced cell death and the inflammatory response. Cell Mol. Immunol. 2024, 21, 856–872. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Lin, F.; Li, W.; Zhao, Y.; Zhu, F.; Yang, H.; Rao, M.; Li, Y.; Liang, H.; et al. MiR-29a-3p Improves Acute Lung Injury by Reducing Alveolar Epithelial Cell PANoptosis. Aging Dis. 2022, 13, 899–909. [Google Scholar] [CrossRef]
- Lentini, G.; Fama, A.; De Gaetano, G.V.; Coppolino, F.; Mahjoub, A.K.; Ryan, L.; Lien, E.; Espevik, T.; Beninati, C.; Teti, G. Caspase-8 inhibition improves the outcome of bacterial infections in mice by promoting neutrophil activation. Cell Rep. Med. 2023, 4, 101098. [Google Scholar] [CrossRef]
- Monnier, P.P.; D’Onofrio, P.M.; Magharious, M.; Hollander, A.C.; Tassew, N.; Szydlowska, K.; Tymianski, M.; Koeberle, P.D. Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J. Neurosci. 2011, 31, 10494–10505. [Google Scholar] [CrossRef]
- de Reuver, R.; Verdonck, S.; Dierick, E.; Nemegeer, J.; Hessmann, E.; Ahmad, S.; Jans, M.; Blancke, G.; Van Nieuwerburgh, F.; Botzki, A.; et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature 2022, 607, 784–789. [Google Scholar] [CrossRef]
- Ren, L.; Yang, Y.; Li, W.; Zheng, X.; Liu, J.; Li, S.; Yang, H.; Zhang, Y.; Ge, B.; Zhang, S.; et al. CDK1 serves as a therapeutic target of adrenocortical carcinoma via regulating epithelial-mesenchymal transition, G2/M phase transition, and PANoptosis. J. Transl. Med. 2022, 20, 444. [Google Scholar] [CrossRef]
- Wang, P.H.; Ye, Z.W.; Deng, J.J.; Siu, K.L.; Gao, W.W.; Chaudhary, V.; Cheng, Y.; Fung, S.Y.; Yuen, K.S.; Ho, T.H.; et al. Inhibition of AIM2 inflammasome activation by a novel transcript isoform of IFI16. EMBO Rep. 2018, 19, e45737. [Google Scholar] [CrossRef]
- Jiao, Y.; Nan, J.; Mu, B.; Zhang, Y.; Zhou, N.; Yang, S.; Zhang, S.; Lin, W.; Wang, F.; Xia, A.; et al. Discovery of a novel and potent inhibitor with differential species-specific effects against NLRP3 and AIM2 inflammasome-dependent pyroptosis. Eur. J. Med. Chem. 2022, 232, 114194. [Google Scholar] [CrossRef]
- Green, J.P.; El-Sharkawy, L.Y.; Roth, S.; Zhu, J.; Cao, J.; Leach, A.G.; Liesz, A.; Freeman, S.; Brough, D. Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome. iScience 2023, 26, 106758. [Google Scholar] [CrossRef] [PubMed]
- Delehouze, C.; Leverrier-Penna, S.; Le Cann, F.; Comte, A.; Jacquard-Fevai, M.; Delalande, O.; Desban, N.; Baratte, B.; Gallais, I.; Faurez, F.; et al. 6E11, a highly selective inhibitor of Receptor-Interacting Protein Kinase 1, protects cells against cold hypoxia-reoxygenation injury. Sci. Rep. 2017, 7, 12931. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, H.; Xie, H.; Zhang, B.; Nie, T.; Fan, C.; Yang, T.; Xu, Y.; Su, H.; Tang, W.; et al. Potent and Selective RIPK1 Inhibitors Targeting Dual-Pockets for the Treatment of Systemic Inflammatory Response Syndrome and Sepsis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114922. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Su, Y.; Sun, L.; He, S.; Meng, L.; Liao, D.; Liu, X.; Ma, Y.; Liu, C.; Li, S.; et al. Discovery of a Highly Potent, Selective, and Metabolically Stable Inhibitor of Receptor-Interacting Protein 1 (RIP1) for the Treatment of Systemic Inflammatory Response Syndrome. J. Med. Chem. 2017, 60, 972–986. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Fang, S.; Jiao, C.; Gao, J.; Zhang, A.; Wu, T.; Tan, R.; Xu, Q.; Guo, W. Spirodalesol analog 8A inhibits NLRP3 inflammasome activation and attenuates inflammatory disease by directly targeting adaptor protein ASC. J. Biol. Chem. 2022, 298, 102696. [Google Scholar] [CrossRef]
- Peukert, K.; Fox, M.; Schulz, S.; Feuerborn, C.; Frede, S.; Putensen, C.; Wrigge, H.; Kummerer, B.M.; David, S.; Seeliger, B.; et al. Inhibition of Caspase-1 with Tetracycline Ameliorates Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2021, 204, 53–63. [Google Scholar] [CrossRef]
- Cocco, M.; Garella, D.; Di Stilo, A.; Borretto, E.; Stevanato, L.; Giorgis, M.; Marini, E.; Fantozzi, R.; Miglio, G.; Bertinaria, M. Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis. J. Med. Chem. 2014, 57, 10366–10382. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Qin, X.; Chen, X.; Cong, H.; Hu, L.; Chen, L.; Miao, Z.; Zhang, W.; Cai, Z.; et al. N-(7-Cyano-6-(4-fluoro-3-(2-(3-(trifluoromethyl)phenyl)acetamido)phenoxy)benzo[d]thiazol-2-yl)cyclopropanecarboxamide (TAK-632) Analogues as Novel Necroptosis Inhibitors by Targeting Receptor-Interacting Protein Kinase 3 (RIPK3): Synthesis, Structure-Activity Relationships, and in Vivo Efficacy. J. Med. Chem. 2019, 62, 6665–6681. [Google Scholar] [CrossRef]
- Xu, C.H.; Wang, J.N.; Suo, X.G.; Ji, M.L.; He, X.Y.; Chen, X.; Zhu, S.; He, Y.; Xie, S.S.; Li, C.; et al. RIPK3 inhibitor-AZD5423 alleviates acute kidney injury by inhibiting necroptosis and inflammation. Int. Immunopharmacol. 2022, 112, 109262. [Google Scholar] [CrossRef]
- Rathkey, J.K.; Zhao, J.; Liu, Z.; Chen, Y.; Yang, J.; Kondolf, H.C.; Benson, B.L.; Chirieleison, S.M.; Huang, A.Y.; Dubyak, G.R.; et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 2018, 3, eaat2738. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, K.; Wang, Y.; Huang, Y.; Duan, C.; Wang, T.; Li, C.; Zhang, P.; Yin, Y.; Gao, J.; et al. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem. Pharmacol. 2022, 206, 115338. [Google Scholar] [CrossRef]
- Ramos, R.; Vale, N. Dual Drug Repurposing: The Example of Saracatinib. Int. J. Mol. Sci. 2024, 25, 4565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Liu, Y.; Huang, F.; Liang, J.; Lin, Y.; Hu, F.; Feng, J.; Han, Z.; Chen, Y.; et al. Saracatinib inhibits necroptosis and ameliorates psoriatic inflammation by targeting MLKL. Cell Death Dis. 2024, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Pipitone, G.; Venanzi Rullo, E.; Cosentino, F.; Ippolito, R.; Costa, R.; Bagarello, S.; Russotto, Y.; Iaria, C.; Cacopardo, B.; et al. Restoring Control: Real-World Success with Imipenem-Relebactam in Critical MDR Infections-A Multicenter Observational Study. Pathogens 2025, 14, 685. [Google Scholar] [CrossRef]


| Compound/ Small Molecule | Target and Mechanism | Disease/Condition | Outcome | Refs |
|---|---|---|---|---|
| I-2 | IRF1 Forms hydrogen bonds and hydrophobic contacts with IRF; reduces IRF1 transcriptional activity | Radiation-induced inflammatory cell death and tissue injury | Inhibits cleavage of PANoptotic molecules and mitigates cell death and tissue injury | [138] |
| I-19 | IRF1 Targets DNA-binding domain of IRF1; reduces IRF1 transcriptional activity | Radiation-induced inflammatory cell death and tissue injury | Inhibits cleavage of PANoptotic molecules and mitigates cell death and tissue injury | [138] |
| Necrostatin-1 | caspase-8 Decreases caspase-8 cleavage | Cell death induced by P. aeruginosa-derived 3-oxo-C12-HSL | Suppresses cell death in 3-oxo-C12-HSL-treated cells | [53] |
| miR-29a-3p | caspase-8 and ZBP-1 Inhibits caspase-8 activation and ZBP-1 expression | Acute lung injury | Reduces alveolar epithelial cell PANoptosis and alleviates disease severity | [139] |
| Z-IETD-FMK | caspase-8 Inhibits caspase-8 cleavage | Bacterial peritonitis, pneumonia, and endotoxin shock | Enhances neutrophil antimicrobial activity in the absence of increased cell death | [140] |
| Z-VEID-FMK | caspase-6 Selectively and irreversibly inhibits caspase-6 | Optic nerve injury | Reduces apoptotic cell death and promotes cell survival | [141] |
| ADAR1 | ZBP-1 Zα domain of ADAR1 limits ZBP1 activation | Embryonic lethality, intestinal cell death, and skin inflammation | Prevents ZBP1-induced cell death and autoinflammation | [142] |
| Cucurbitacin E | CDK1/ZBP-1 Inhibits CDK1, a protein mediating PANoptosis via interaction with ZBP-1 PANoptosome | Adrenocortical carcinoma | Regulates PANoptosis in cancer cells | [143] |
| IFI16-β | AIM2 Impairs AIM2-ASC interaction and AIM2-dsDNA sensing | Bacterial and viral infection | Suppresses AIM2 inflammasome activation and pro-inflammatory cytokine release | [144] |
| J114 | AIM2 Disturbs AIM2-ASC interaction | AIM2-dependent inflammation | Reduces PANoptotic molecule expression and pro-inflammatory cytokine release | [145] |
| 4-Sulfonic calix[6]arene/suramin | AIM2 Blocks DNA-binding HIN domain of AIM2 | Post-stroke immunosuppression | Prevents AIM2 activation and AIM2-dependent cell death | [146] |
| 6E11 | RIPK1 Competes with RIPK1-ligand complexes | TNF-α-induced cell death and cold hypoxia/reoxygenation injury | Protects against RIPK1-dependent cell death | [147] |
| ZB-R-55 | RIPK1 Occupies allosteric and ATP binding pockets of RIPK1 | Sepsis and systemic inflammatory response syndrome | Attenuates systemic inflammation and disease severity | [148] |
| RIPA-56 | RIPK1 Inhibits RIPK1 phosphorylation and its kinase activity | Systemic inflammatory response syndrome | Prevents multiorgan damage and reduces mortality | [149] |
| Spirodalesol analog 8A | ASC Suppresses ASC speck formation and oligomerization | Endotoxemia, peritonitis, and gouty arthritis | Reduces mortality and multiorgan damage | [150] |
| Tetracycline | caspase-1 Reduces cleavage and activation of caspase-1 | Acute lung injury | Attenuates lung injury and pulmonary inflammation; improves survival | [151] |
| Unsaturated ester derivative-compound 9 | caspase-1 Blocks caspase-1 activity | Caspase-1-dependent cell death | Protects against caspase-1-dependent cell death | [152] |
| Compound 42 (an analog of TAK-632) | RIPK3 Inhibits RIPK3 phosphorylation and blocks necrosome formation | Systemic inflammatory response syndrome | Alleviates disease symptoms and improves survival | [153] |
| AZD5423 | RIPK3 Binds to the kinase domain of RIPK3 and decreases its activation | RIPK3-mediated cell injury and acute kidney injury | Restores cell viability and attenuates kidney injury and inflammation | [154] |
| Necrosulfonamide (NSA) | GSDMD Binds to the Cys191 of GSDMD MLKL Binds to the Cys86 of human MLKL; inhibits MLKL phosphorylation | LPS-induced endotoxicity and sepsis | Prevents cell death and reduces mortality | [155] |
| Acute colitis | Alleviates intestinal inflammation | [38,157] | ||
| Disulfiram | GSDMD Modifies the Cys191 of human GSDMD AIM2 Mechanism unknown | Sepsis | Improves survival and attenuates systemic inflammation | [156] |
| Saracatinib | MLKL Disrupts the phosphorylation, translocation, and oligomerization of MLKL | Psoriasiform dermatitis | Attenuates skin inflammation | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; Luo, Y. Advances in Pseudomonas aeruginosa-Induced Programmed Cell Death and Potential Targeted Treatment Strategies. Microorganisms 2025, 13, 2560. https://doi.org/10.3390/microorganisms13112560
Tan C, Luo Y. Advances in Pseudomonas aeruginosa-Induced Programmed Cell Death and Potential Targeted Treatment Strategies. Microorganisms. 2025; 13(11):2560. https://doi.org/10.3390/microorganisms13112560
Chicago/Turabian StyleTan, Chunjiang, and Yifeng Luo. 2025. "Advances in Pseudomonas aeruginosa-Induced Programmed Cell Death and Potential Targeted Treatment Strategies" Microorganisms 13, no. 11: 2560. https://doi.org/10.3390/microorganisms13112560
APA StyleTan, C., & Luo, Y. (2025). Advances in Pseudomonas aeruginosa-Induced Programmed Cell Death and Potential Targeted Treatment Strategies. Microorganisms, 13(11), 2560. https://doi.org/10.3390/microorganisms13112560







