The Gastric Microbiome Communities and Endoscopic Mucosal Morphologies Associated with Premalignant Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Cohort
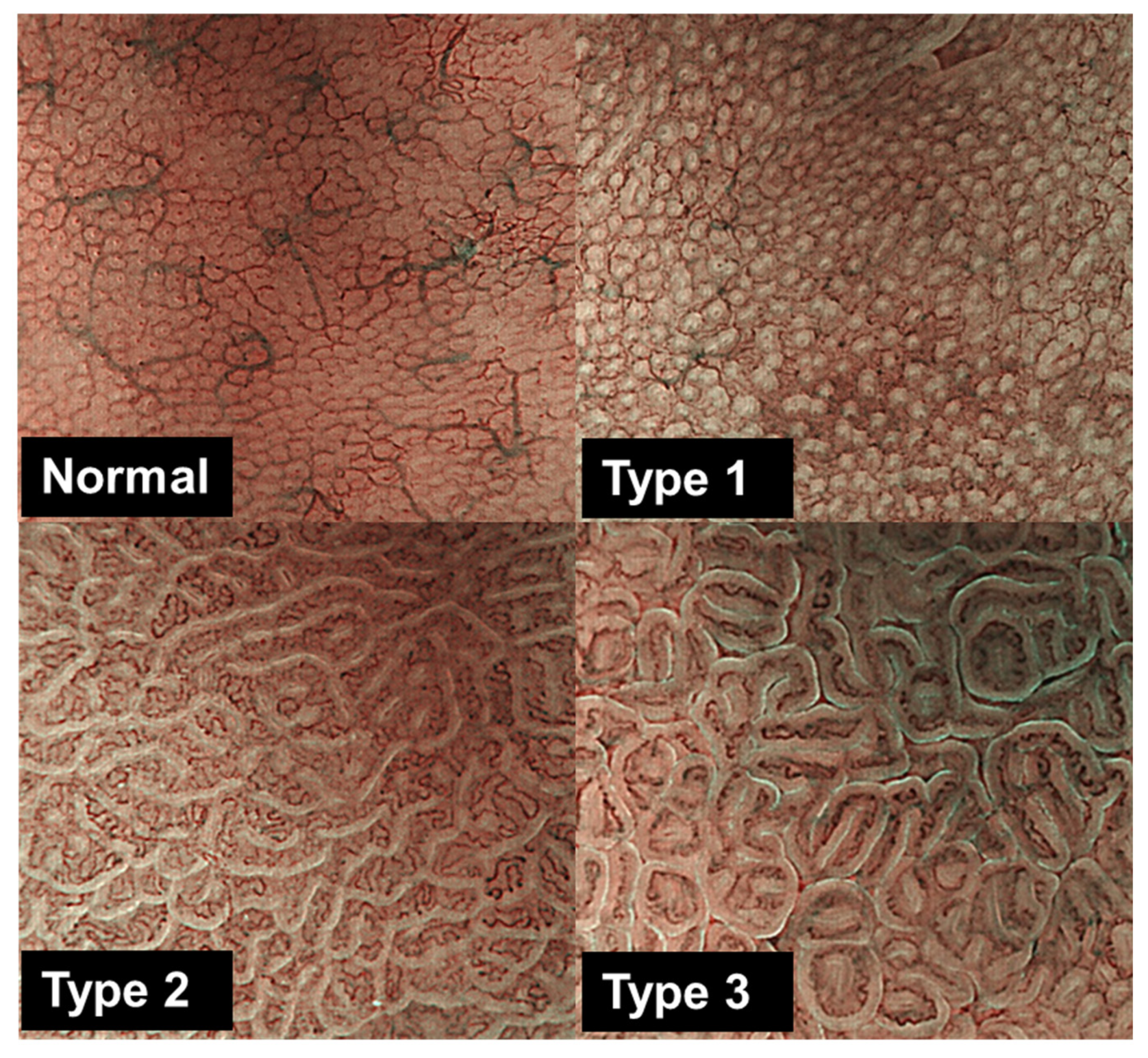
2.2. Endoscopy, Classification of Gastric Mucosa Morphology, and Sample Collection for Histological and Molecular Analyses
2.3. Detection of H. pylori Infection
2.4. 16S rRNA Gene Amplicon Sequencing
2.5. DNA Methylation Analysis
2.6. Statistical Analyses
3. Results
3.1. The Clinicopathological Characteristics of the Study Cohort
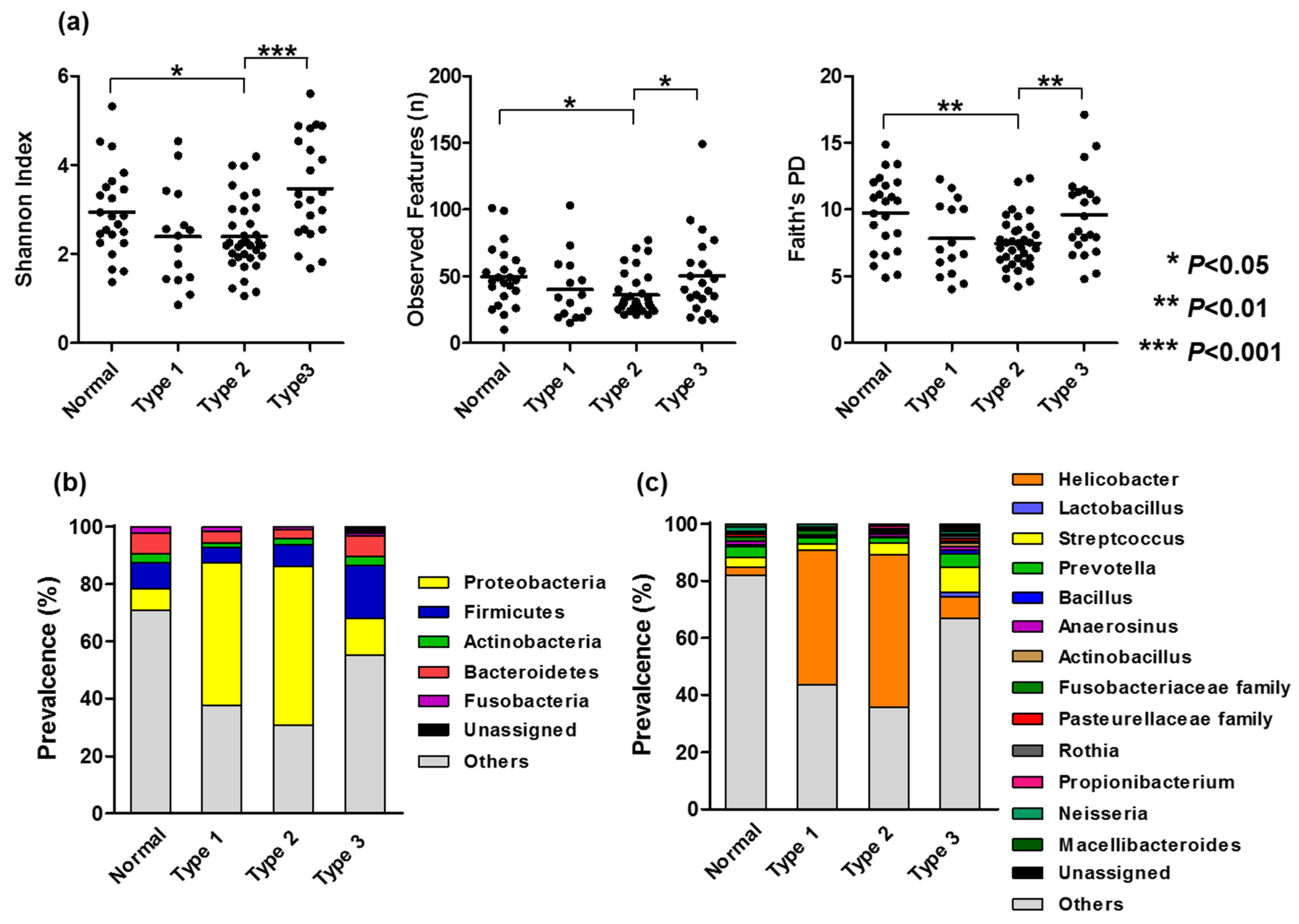
3.2. Bacterial Compositions Associated with Gastric Mucosal Morphology
3.3. Association Between Microbiome Dysbiosis and Clinicopathological Characteristics and DNA Methylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef]
- Huang, J.Q.; Sridhar, S.; Chen, Y.; Hunt, R.H. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998, 114, 1169–1179. [Google Scholar] [CrossRef]
- Blaser, M.J.; Parsonnet, J. Parasitism by the ‘‘slow’’ bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 1994, 94, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015, 148, 719–731.e3. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, A.; Kosunen, T.U.; Puolakkainen, P.; Sipponen, P.; Harkonen, M.; Laxen, F.; Virtamo, J.; Haapiainen, R.; Rautelin, H. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS 2003, 111, 619–624. [Google Scholar] [CrossRef]
- Tahara, T.; Yamazaki, J.; Tahara, S.; Okubo, M.; Kawamura, T.; Horiguchi, N.; Ishizuka, T.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; et al. Magnifying narrow-band imaging of gastric mucosal morphology predicts the H. pylori-related epigenetic field defect. Sci. Rep. 2017, 7, 3090. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiing reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process-fist American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Shibata, T.; Nakamura, M.; Yoshioka, D.; Okubo, M.; Arisawa, T.; Hirata, I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest. Endosc. 2009, 70, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gono, K.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; Hamamoto, Y.; Endo, T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J. Biomed. Opt. 2004, 9, 568–577. [Google Scholar] [CrossRef]
- Goda, K.; Tajiri, H.; Ikegami, M.; Urashima, M.; Nakayoshi, T.; Kaise, M. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett’s adenocarcinoma. Gastrointest. Endosc. 2007, 65, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Nakayoshi, T.; Tajiri, H.; Matsuda, K.; Kaise, M.; Ikegami, M.; Sasaki, H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: Correlation of vascular pattern with histopathology (including video). Endoscopy 2004, 36, 1080–1084. [Google Scholar] [CrossRef]
- Machida, H.; Sano, Y.; Hamamoto, Y.; Muto, M.; Kozu, T.; Tajiri, H.; Yoshida, S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: A pilot study. Endoscopy 2004, 36, 1094–1098. [Google Scholar] [CrossRef]
- Tahara, T.; Shibata, T.; Nakamura, M.; Okubo, M.; Yoshioka, D.; Arisawa, T.; Hirata, I. The mucosal pattern in the non-neoplastic gastric mucosa by using magnifying narrow-band imaging endoscopy significantly correlates with gastric cancer risk. Gastrointest. Endosc. 2010, 71, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis: The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kim, H.S.; Castoro, R.J.; Chung, W.; Estecio, M.R.; Kondo, K.; Guo, Y.; Ahmed, S.S.; Toyota, M.; Itoh, F.; et al. Sensitive and specific detection of early gastric cancer with DNA methylation analysis of gastric washes. Gastroenterology 2009, 136, 2149–2158. [Google Scholar] [CrossRef]
- Tahara, T.; Shibata, T.; Okubo, M.; Kawamura, T.; Horiguchi, N.; Ishizuka, T.; Nakano, N.; Nagasaka, M.; Nakagawa, Y.; Ohmiya, N. Demonstration of potential link between Helicobacter pylori related promoter CpG island methylation and telomere shortening in human gastric mucosa. Oncotarget 2016, 7, 43989–43996. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in newonset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, J.L.; Whary, M.T.; Ge, Z.; Muthupalani, S.; Taylor, N.S.; Mobley, M.; Potter, A.; Varro, A.; Eibach, D.; Suerbaum, S.; et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 2011, 140, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 2014, 63, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kekki, M.; Samloff, I.M.; Varis, K.; Ihamäki, T. Serum pepsinogen I and serum gastrin in the screening of severe atrophic corpus gastritis. Scand. J. Gastroenterol. 1991, 186, 109–116. [Google Scholar] [CrossRef]
- Asaka, M.; Kimura, T.; Kudo, M.; Takeda, H.; Mitani, S.; Miyazaki, T.; Miki, K.; Graham, D.Y. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 1992, 102, 760–766. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Xie, Y. Refractory Helicobacter pylori infection and the gastric microbiota. Front. Cells. Infect. Microbiol. 2022, 12, 976710. [Google Scholar] [CrossRef]
- Llorca, L.; Pérez-Pérez, G.; Urruzuno, P.; Martinez, M.J.; Iizumi, T.; Gao, Z.; Sohn, J.; Chung, J.; Cox, L.; Simón-Soro, A.; et al. Characterization of the Gastric Microbiota in a Pediatric Population According to Helicobacter pylori Status. Pediatr. Infect. Dis. J. 2017, 36, 173–178. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, T.; Lu, Y.; Wang, C.; Wu, Y.; Li, J.; Tao, Y.; Deng, L.; Zhang, X.; Ma, J. Helicobacter pylori infection affects the human gastric microbiome, as revealed by metagenomic sequencing. FEBS Open Bio 2022, 12, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Lehr, K.; Nikitina, D.; Vilchez-Vargas, R.; Steponaitiene, R.; Thon, C.; Skieceviciene, J.; Schanze, D.; Zenker, M.; Malfertheiner, P.; Kupcinskas, J.; et al. Microbial composition of tumorous and adjacent gastric tissue is associated with prognosis of gastric cancer. Sci. Rep. 2023, 13, 4640. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ji, X.; Wang, T.; Zhang, Y.; Jiang, W.; Meng, L.; Wu, H.J.; Xing, X.; Ji, J. Intratumoral microbiome is associated with gastric cancer prognosis and therapy efficacy. Gut Microbes 2024, 16, 2369336. [Google Scholar] [CrossRef]
- Okubo, M.; Tahara, T.; Shibata, T.; Nakamura, M.; Yoshioka, D.; Maeda, Y.; Yonemura, J.; Ishizuka, T.; Arisawa, T.; Hirata, I. Changes in gastric mucosal patterns seen by magnifying NBI during H. pylori eradication. J. Gastroenterol. 2011, 46, 175–182. [Google Scholar] [CrossRef] [PubMed]

| Correlated with the Changes in Mucosal Patterns from Normal to Types 1, 2, and 3. | ||
|---|---|---|
| Genus and Family | r | p-Value |
| Streptococcus | 0.34 | 0.0007 |
| Actinobacillus | 0.23 | 0.012 |
| Fusobacteriaceae family | −0.24 | 0.019 |
| Macellibacteroides | −0.226 | 0.028 |
| Variables | Dysbiosis Index (+/−SE) | p |
|---|---|---|
| Age | ||
| <60 y (37) | 0.98 +/− 0.21 | Reference |
| ≥60 y (57) | 1.34 +/− 0.18 | 0.02 |
| Gender | ||
| Male (66) | 1.29 +/− 0.17 | Reference |
| Female (28) | 0.98 +/− 0.20 | 0.31 |
| H. pylori test | ||
| Negative (41) | 1.06 +/− 0.23 | Reference |
| Positive (52) | 1.32 +/− 0.17 | 0.20 |
| Inflammatory mucosa | ||
| Negative (47) | 1.04 +/− 0.19 | Reference |
| Positive (47) | 1.36 +/− 0.20 | 0.01 |
| Atrophic mucosa | ||
| Negative (73) | 1.06 +/− 0.14 | Reference |
| Positive (21) | 1.68 +/− 0.37 | 0.01 |
| Cancer occurrence | ||
| Cancer-free (71) | 0.98 +/− 0.14 | Reference |
| Cancer (23) | 1.86 +/− 0.34 | 0.0007 |
| Methylation (Z-score) | ||
| Low (63) | 0.99 +/− 0.15 | Reference |
| High (31) | 1.63 +/− 0.28 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shijimaya, T.; Tahara, T.; Shimogama, T.; Yamazaki, J.; Kobayashi, S.; Nakamura, N.; Takahashi, Y.; Honzawa, Y.; Tomiyama, T.; Naganuma, M. The Gastric Microbiome Communities and Endoscopic Mucosal Morphologies Associated with Premalignant Conditions. Microorganisms 2025, 13, 2499. https://doi.org/10.3390/microorganisms13112499
Shijimaya T, Tahara T, Shimogama T, Yamazaki J, Kobayashi S, Nakamura N, Takahashi Y, Honzawa Y, Tomiyama T, Naganuma M. The Gastric Microbiome Communities and Endoscopic Mucosal Morphologies Associated with Premalignant Conditions. Microorganisms. 2025; 13(11):2499. https://doi.org/10.3390/microorganisms13112499
Chicago/Turabian StyleShijimaya, Takuya, Tomomitsu Tahara, Tsubasa Shimogama, Jumpei Yamazaki, Sanshiro Kobayashi, Naohiro Nakamura, Yu Takahashi, Yusuke Honzawa, Takashi Tomiyama, and Makoto Naganuma. 2025. "The Gastric Microbiome Communities and Endoscopic Mucosal Morphologies Associated with Premalignant Conditions" Microorganisms 13, no. 11: 2499. https://doi.org/10.3390/microorganisms13112499
APA StyleShijimaya, T., Tahara, T., Shimogama, T., Yamazaki, J., Kobayashi, S., Nakamura, N., Takahashi, Y., Honzawa, Y., Tomiyama, T., & Naganuma, M. (2025). The Gastric Microbiome Communities and Endoscopic Mucosal Morphologies Associated with Premalignant Conditions. Microorganisms, 13(11), 2499. https://doi.org/10.3390/microorganisms13112499


_Di_Marco.png)



