Pine Shoot Blight Driven Seasonal Variations in Fungal Assembly of Pinus elliottii Rhizosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Sample Settings and Soil Sampling
2.3. Testing of Soil Physicochemical Properties
2.4. DNA Extraction and Sequencing of PCR Amplicons
2.5. Statistical Analyses
3. Results
3.1. Soil Physicochemical Analyses
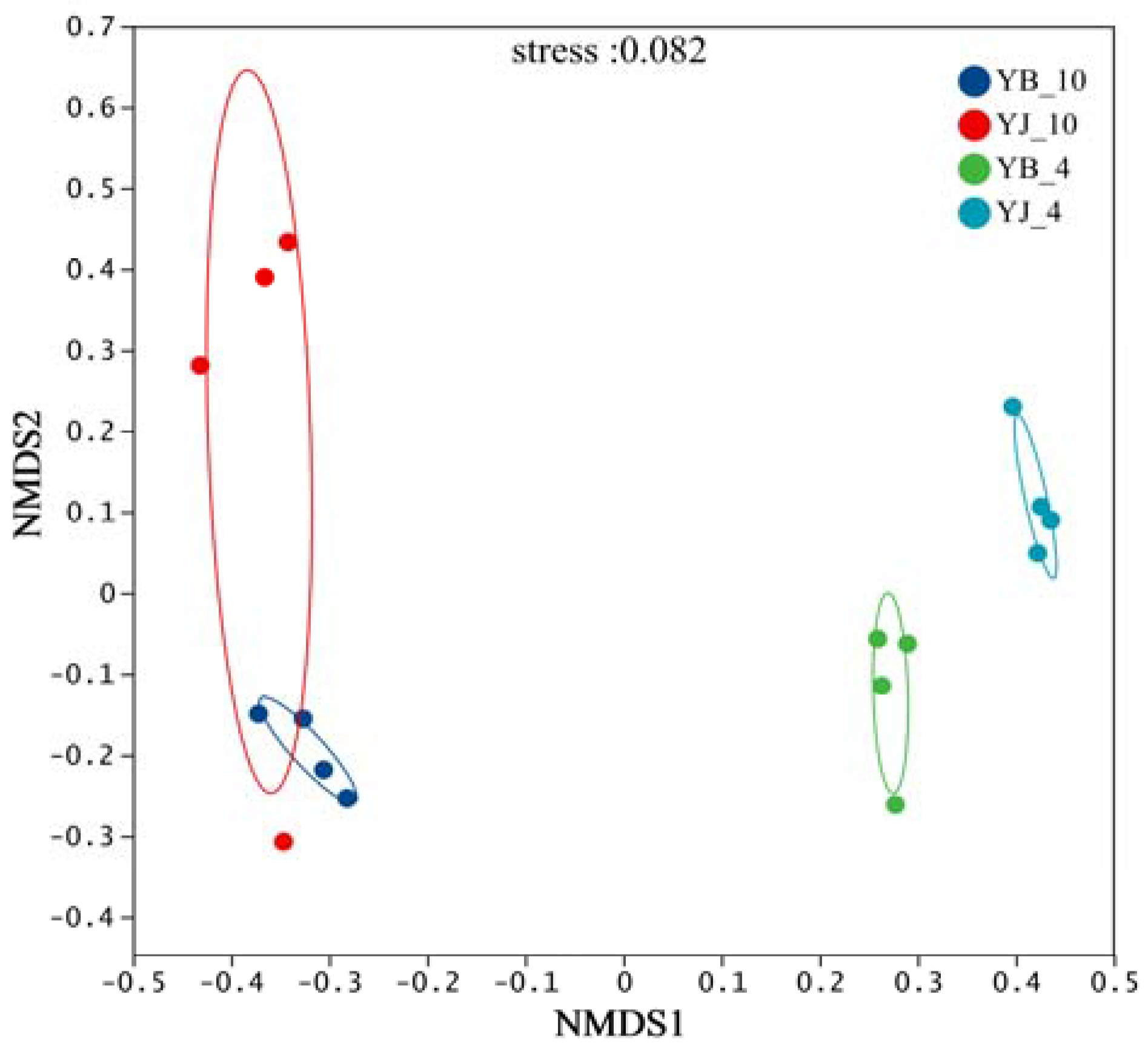
3.2. Community Diversity Analysis
3.3. Analysis of Fungal Community Structure and Composition
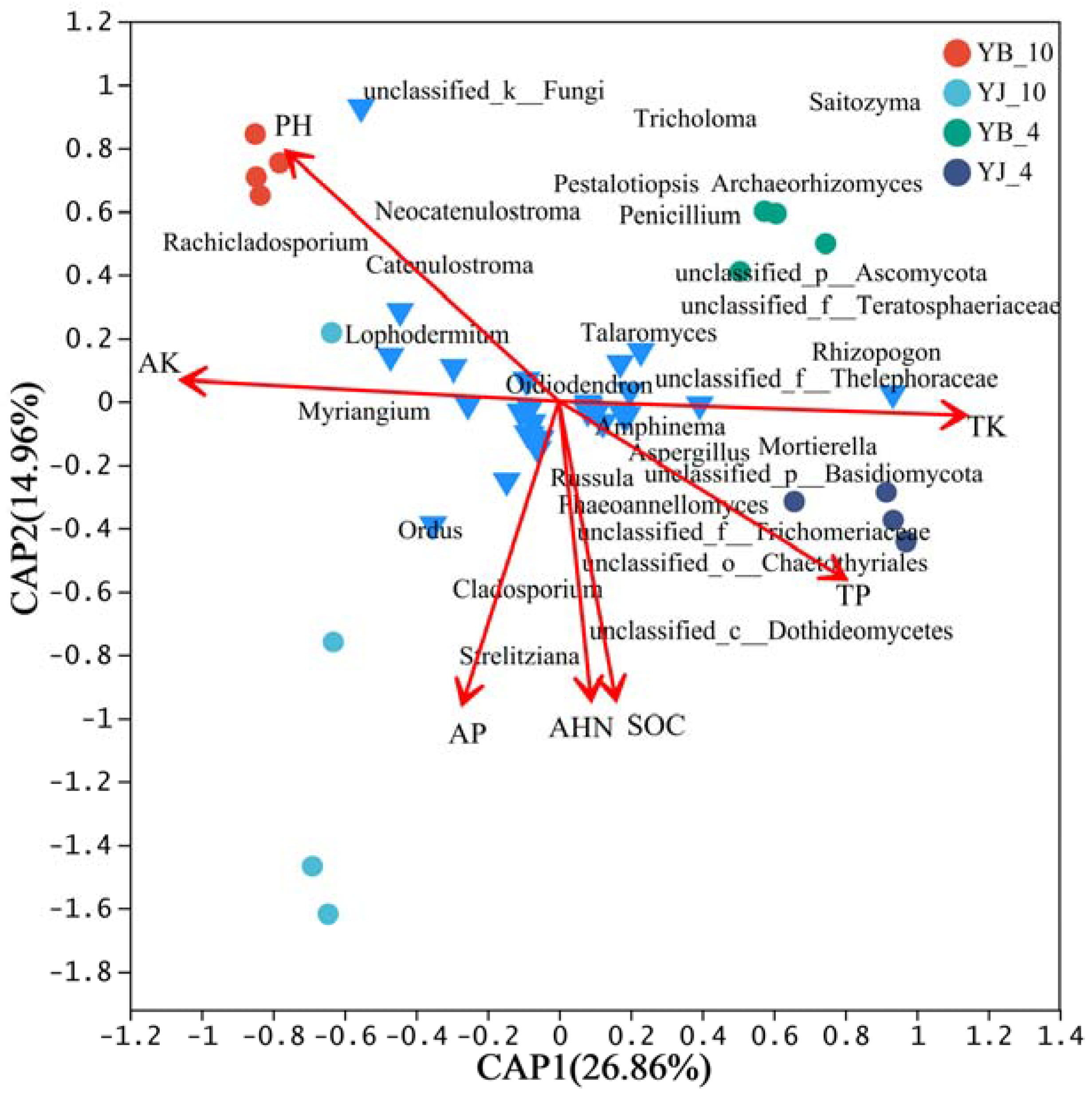
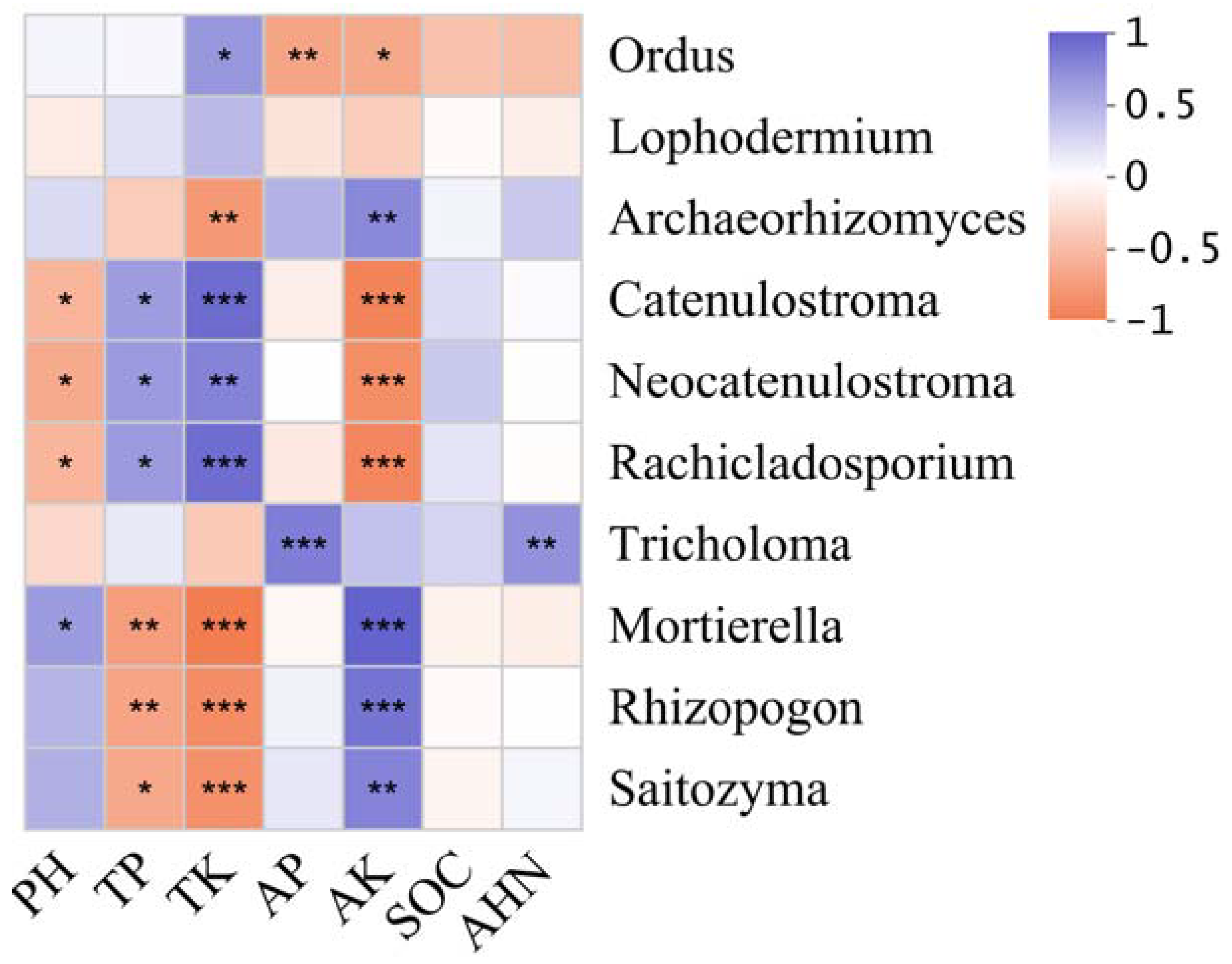
3.4. Effects of Environmental Factors on Fungal Community Structure
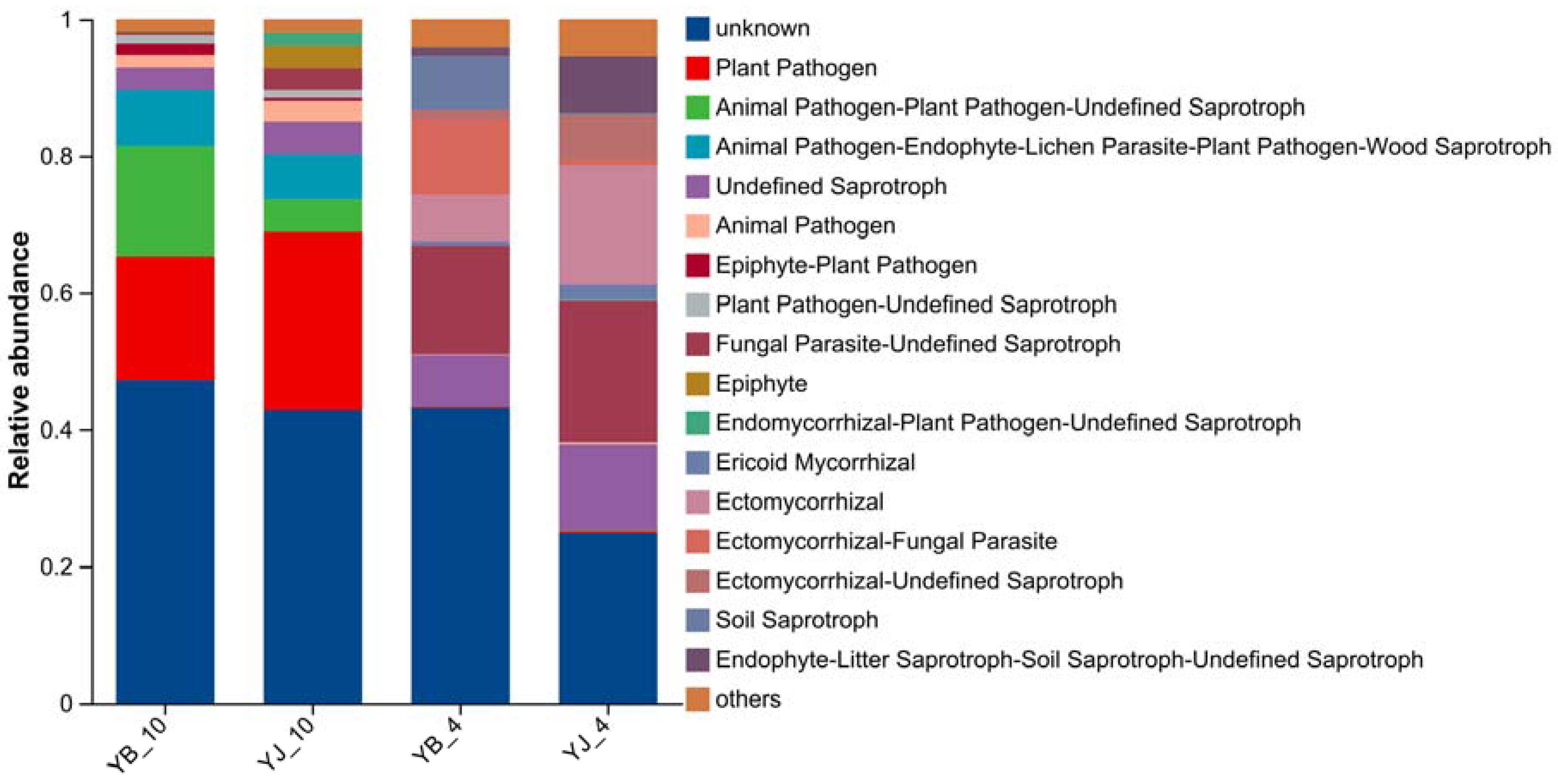
3.5. Fungal Functional Prediction Analysis
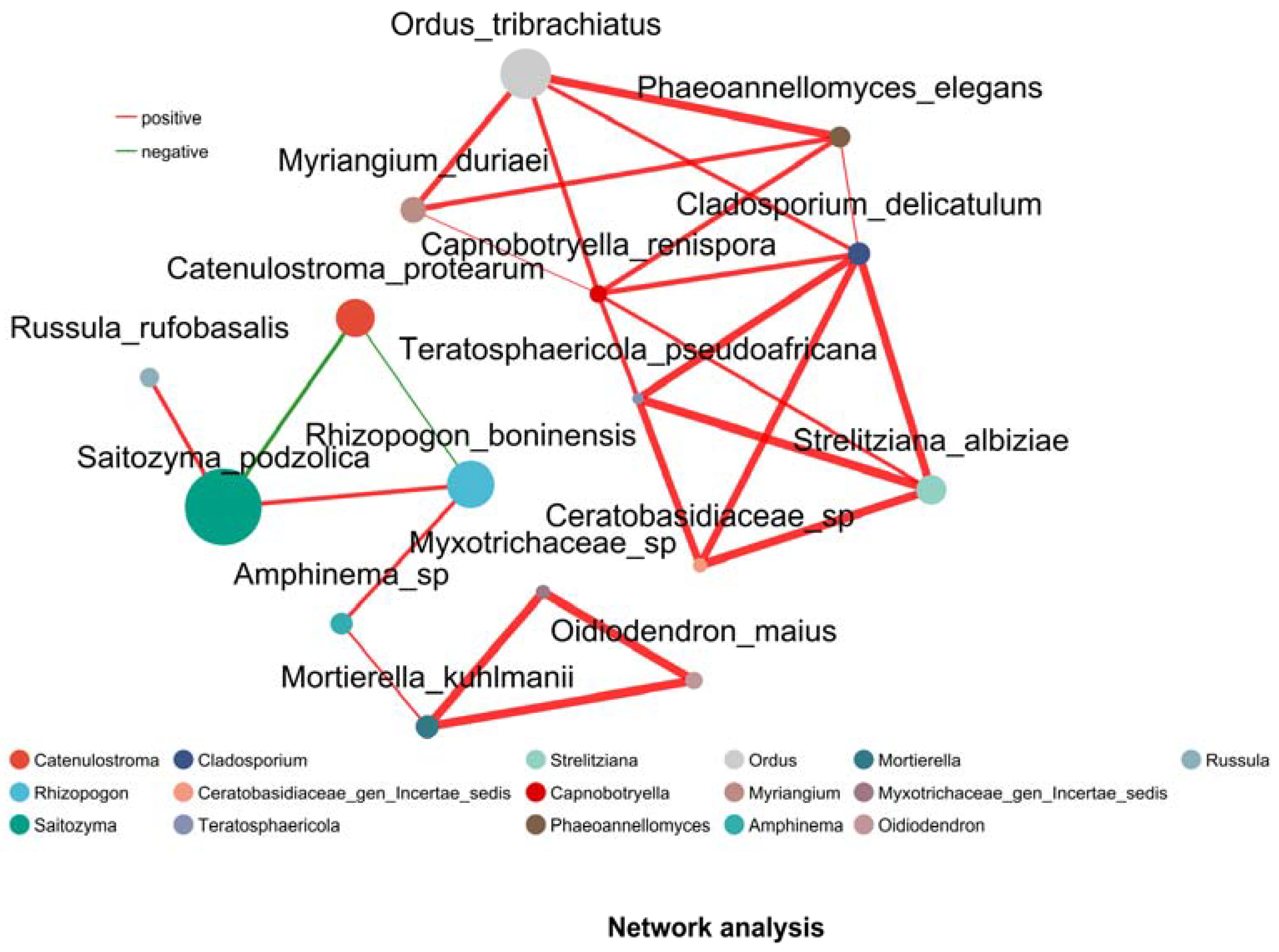
3.6. Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and Common Traits in Mycorrhizal Symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Deng, M.F.; Hu, S.J.; Guo, L.L.; Jiang, L.; Huang, Y.Y.; Schmid, B.; Liu, C.J.; Chang, P.F.; Li, S.; Liu, X.J.; et al. Tree Mycorrhizal Association Types Control Biodiversity-Productivity Relationship in A Subtropical Forest. Sci. Adv. 2023, 9, eadd4468. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Zhang, L.Z.; Tang, Y.; Zou, F.; Xiong, H.; Zhu, J.L.; Tang, J. Effects of Inoculation with Ectomycorrhizal Fungi on Growth and Physiological Characteristics of Quercus variabilis Seedlings from Different Provenances. J. Cent. South. Univ. For. Technol. 2022, 42, 63–70. [Google Scholar]
- Wen, Z.G.; Zhu, X.M.; Liu, C.; Zhao, B.Q.; Dong, J.; Xing, J.C.; Zhao, X.H.; Hong, L.Z. Effects of Two Ectomycorrhizal Fungi Strains on Growth of Pinus thunbergii Seedlings in Saline Soil. J. Cent. South. Univ. For. Technol. 2019, 39, 22–27. [Google Scholar]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Leake, J.R. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 1994, 127, 171–216. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The Mycorrhiza Helper Bacteria Revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Fang, X.M.; Yu, D.P.; Zhou, W.M.; Zhou, L.; Dai, L.M. Effects of Forest Type on Soil Microbial Activity in Changbai Mountain, Northeast China. Ann. For. Sci. 2016, 73, 473–482. [Google Scholar] [CrossRef]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-Soil Feedbacks and Mycorrhizal Type Influence Temperate Forest Population Dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Sebastiana, M.; da Silva, A.B.; Matos, A.R.; Alcântara, A.; Silvestre, S.; Malhó, R. Ectomycorrhizal Inoculation with Pisolithus tinctorius Reduces Stress Induced by Drought in Cork Oak. Mycorrhiza 2018, 28, 247–258. [Google Scholar] [CrossRef]
- Courty, P.E.; Franc, A.; Pierrat, J.C.; Garbaye, J. Temporal Changes in the Ectomycorrhizal Community in Two Soil Horizons of A Temperate Oak Forest. Appl. Environ. Microbiol. 2008, 74, 5792–5801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.S.; Gao, G.L.; Ding, G.D.; Zhang, Y.; Ren, Y.; Wang, J.Y. Biogeography and Ecological Functions of Root-Associated and Soil Fungi of Pinus sylvestris var. mongolica Across Different Afforestation Areas in Desertified Northern China. Land Degrad. Dev. 2022, 34, 313–326. [Google Scholar]
- Yuan, Z.L.; Pan, X.Y.; Jin, W. Tree Symbiotic Fungi System and Its Mechanism: A Case Study of Poplar. Acta Ecol. Sin. 2019, 39, 381–397. [Google Scholar]
- Cairney, J.W.G. Ectomycorrhizal Fungi: The Symbiotic Route to the Root for Phosphorus in Forest Soils. Plant Soil. 2011, 344, 51–71. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Shang, H. Effects of Ectomycorrhizal Fungi (Pisolithus tinctorius) of Masson Pine (Pinus massoniana) on Soil Microbial Metabolic Function under Simulated Acid Rain. Sci. Silvae Sin. 2014, 50, 99–104. [Google Scholar]
- Miyamoto, Y.; Terashima, Y.; Nara, K. Temperature Niche Position and Breadth of Ectomycorrhizal Fungi: Reduced Diversity under Warming Predicted by A Nested Community Structure. Glob. Change Biol. 2018, 24, 5724–5737. [Google Scholar] [CrossRef]
- Arraiano-Castilho, R.; Bidartondo, M.I.; Niskanen, T.; Clarkson, J.J.; Brunner, I.; Zimmermann, S.; Senn-Irlet, B.; Frey, B.; Peintner, U.; Mrak, T.; et al. Habitat Specialization Controls Ectomycorrhizal Fungi above the Treeline in the European Alps. New Phytol. 2021, 229, 2901–2916. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, M.S.; Ding, G.D.; Wang, Y. Ectomycorrhizal Fungi Associated with Pinus sylvestris var. mongolica were Altered by Soil Environments with Aging Plantation in A Semi-Arid Desert. Front. Environ. Sci. 2022, 10, 858452. [Google Scholar]
- Han, Q.S.; Huang, J.; Long, D.F.; Wang, X.B.; Liu, J.J. Diversity and Community Structure of Ectomycorrhizal Fungi Associated with Larix chinensis Across the Alpine Treeline Ecotone of Taibai Mountain. Mycorrhiza 2017, 27, 487–497. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.D.; Knelman, J.E.; Darcy, J.L.; Lync, R.C.; Wickey, P.; et al. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Shinohara, N.; Nakadai, R.; Suzuki, Y.; Terui, A. Spatiotemporal Dimensions of Community Assembly. Popul. Ecol. 2023, 65, 5–16. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Rosendahl, S.; Michelsen, A.; Olsson, P.A. Seasonal Carbon Allocation to Arbuscular Mycorrhizal Fungi Assessed by Microscopic Examination, Stable Isotope Probing and Fatty Acid Analysis. Plant Soil. 2013, 368, 547–555. [Google Scholar] [CrossRef]
- Feng, W.Y.; Zhao, Y.Z.; Tan, J.H.; Yang, J.Q.; Sun, X.J. Formation Process of Mycorrhizal Symbionts between Pinus massoniana and Suillus bovinus. Mycosystema 2019, 38, 1620–1630. [Google Scholar]
- Oliva, J.; Boberg, J.; Stenlid, J. First Report of Sphaeropsis sapinea on Scots Pine (Pinus sylvestris) and Austrian Pine (P. nigra) in Sweden. New Dis. Rep. 2013, 27, 23. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Bonello, P. The Aggressiveness of Sphaeropsis sapinea on Austrian Pine Varies with Isolate Group and Site of Infection. For. Pathol. 2003, 33, 15–19. [Google Scholar] [CrossRef]
- Flowers, J.; Nuckles, E.; Hartman, J.; Vaillancourt, L. Latent Infection of Austrian and Scots Pine Tissues by Sphaeropsis sapinea. Plant Dis. 2007, 85, 1107–1112. [Google Scholar] [CrossRef]
- Pedersen, C.T.; Sylvia, D.M.; Shilling, D.G. Pisolithus arhizus Ectomycorrhiza Affects Plant Competition for Phosphorus between Pinus elliottii and Panicum chamaelonche. Mycorrhiza 1999, 9, 199–204. [Google Scholar] [CrossRef]
- Chen, Y.L.; Kang, L.H.; Malajczuk, N.; Dell, B. Selecting Ectomycorrhizal Fungi for Inoculating Plantations in South China: Effect of Scleroderma on Colonization and Growth of Exotic Eucalyptus globulus, E. urophylla, Pinus elliottii, and P. radiata. Mycorrhiza 2006, 16, 251–259. [Google Scholar]
- Wang, Y.; Ding, G.J. Effects of Ectomycorrhizal on Growth, Physiological Characteristics and Nutrition in Pinus massoniana Seedlings. J. Nanjing For. Univ. Nat. Sci. Ed. 2013, 37, 97–102. [Google Scholar]
- Deng, X.; Song, X.S.; Yin, D.C.; Cui, W.F.; Song, R.Q. Research Advances in Improving Host Plant Resistance by Dark Septate Endophytes. J. Anhui Agri. Sci. 2015, 43, 10–17. [Google Scholar]
- Bödeker, I.; Lindahl, B.D.; Olson, Å.; Dell, B. Mycorrhizal and Saprotrophic Fungal Guilds compete for the Same Organic Substrates but Affect Decomposition Differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Li, S.; Tang, M.; Huang, L.L. Effect of Inoculating Suillus lactifluus and Pseudomonas fluorescens to Pinus tabulaeformis Growth and Resistant of Damping-off. Acta Bot. Boreali Occident. Sin. 2011, 31, 1384–1389. [Google Scholar]
- Liu, J.F.; Yuan, Y.Q.; Guo, S.X.; Li, M. Characteristics and Mechanisms of Plant Disease Resistance Induced by Mycorrhizal Fungi. J. Qingdao Agric. Univ. Nat. 2015, 32, 83–91. [Google Scholar]
- Tedersoo, L.; Suvi, T.; Larsson, E.; Kõljalg, U. Diversity and Community Structure of Ectomycorrhizal Fungi in A Wooded Meadow. Mycol. Res. 2006, 110, 734–748. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.; Tisserand, E.; Cébron, A.; Turpault, M.P.; Buée, M.; Boer, W.D.; Leveau, J.H.J.; Frey-Klett, P. Specific Impacts of Beech and Norway Spruce on The Structure and Diversity of The Rhizosphere and Soil Microbial Communities. Sci. Rep. 2016, 6, 27756. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.C.; Ru, J.L.; Zhao, J.H.; Xiao, S. Long-Term and High-Concentration Heavy-Metal Contamination Strongly Influences The Microbiome and Functional Genes in Yellow River Sediments. Sci. Total Environ. 2018, 637–638, 1400–1412. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.Q.; Yan, Q.W.; Ning, D.L.; Qin, Y.J.; Xue, K.; Wu, L.Y.; He, Z.L.; et al. Temperature Mediates Continental-Scale Diversity of Microbes in Forest Soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, X.; Xu, Y.; Zhao, Y.L.; Wang, J.Q.; Zhang, Y.J.; Yang, Y.C. Diversity and Community Assembly Mechanism of Soil Ectomycorrhizal Fungi in Urban Parks of Baotou City, China. J. Appl. Ecol. 2023, 34, 1225–1234. [Google Scholar]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Heij, G.J.; de Vries, W.; Posthumus, A.C.; Mohren, G.M.J. Effects of Air Pollution and Acid Deposition on Forests and Forest Soils. Stud. Environ. Sci. 1991, 46, 97–137. [Google Scholar]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in The Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, G.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into The New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhou, C.; Liu, G.Z.; Di, L.; Yang, H.; Luo, Y.C. Changes in Arbuscular Mycorrhizal Fungal Communities in Rhizosphere Soil of Phoebe bournei Young Plantations Based on High-Throughput Sequencing. Microbiol. China 2021, 48, 1461–1472. [Google Scholar]
- Chen, R.J.; Xiong, D.; Ou, J.; Long, H.Y.; Xiong, X.R.; He, Y.J.; Li, Z.C. Influences of ERM Strain on Endogenous Hormones of Rhododendron annae in Continuous Drought. J. Southwest Univ. Nat. Sci. Ed. 2018, 40, 26–33. [Google Scholar]
- Halifu, S.; Deng, X.; Song, X.S.; Song, R.Q. Seasonal Changes in Rhizosphere Soil Nutrients and Fungal Community Structure of Pinus sylvestris var. mongolica Infected and Uninfected by Shoot Blight. J. Jilin Agric. Univ. 2022, 44, 572–585. [Google Scholar]
- Cruywagen, E.M.; Crous, P.W.; Roux, J.; Slippers, B.; Wingfield, M.J. Fungi Associated with Black Mould on Baobab Trees in Southern Africa. Anto. Leeuw. Int. J. G. 2015, 108, 85–95. [Google Scholar] [CrossRef]
- Piatek, M.; Stryjak-Bogacka, M.; Czachura, P. The Genus Rachicladosporium: Introducing New Species from Sooty Mould Communities and Excluding Cold Adapted Species. Sci. Rep. 2023, 13, 22795. [Google Scholar] [CrossRef]
- Markovskaja, S.; Kacergius, A.; Davydenko, K.; Fraser, S. First Record of Neocatenulostroma germanicum on Pines in Lithuania and Ukraine and Its Cooccurrence with Dothistroma spp. and other pathogens. For. Pathol. 2016, 46, 522–533. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, J.; An, M.N.; Li, B.; Xie, Y.B.; Xu, C.T.; Jiang, L.Q.; Yan, F.F.; Wang, Z.P.; Wu, Y.H. Bacillus velezensis SYL-3 Suppresses Alternaria alternata and Tobacco Mosaic Virus Infecting Nicotiana Tabacum by Regulating the Phyllosphere Microbial Community. Front. Microbiol. 2022, 13, 840318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Liu, L.J.; Yang, S.; Zeng, Q.; He, Z.R.; Liu, Y.G. Cloning, Characterization and Expression of the Phenylalanine Ammonia-Lyase Gene (PaPAL) from Spruce Picea asperata. Forests 2019, 10, 613. [Google Scholar] [CrossRef]
- Zhao, W.D.; Liu, L.J.; Li, C.S.; Yang, C.L.; Li, S.J.; Han, S.; Lin, T.T.; Liu, Y.G. Cloning and Characterization of Two Novel PR4 Genes from Picea asperata. Int. J. Mol. Sci. 2022, 23, 14906. [Google Scholar] [CrossRef]
- Aggangan, N.S.; Moon, H.K. Characterization of Ectomycorrhizal Fungi in Association with Eucalyptus pellita F. Muell Seedlings. Philipp. J. Crop Sci. 2020, 44, 48–58. [Google Scholar]
- Guerin-Laguette, A.; Shindo, K.; Matsushita, N.; Suzuki, K.; Lapeyrie, F. Mycorrhizal Fungus Tricholoma Matsutake Stimulates Pinus densiflora Seedling Growth In Vitro. Mycorrhiza 2004, 14, 397–400. [Google Scholar] [CrossRef]
- Murata, H.; Nakano, S.; Yamanaka, T.; Shimokawa, T.; Abe, T.; Ichida, H.; Hayashi, Y.; Tahara, K.; Ohta, A. Conversion from Mutualism to Parasitism: A Mutant of The Ectomycorrhizal Agaricomycete Tricholoma matsutake that Induces Stunting, Wilting, and Root Degeneration in Seedlings of Its Symbiotic Partner, Pinus densiflora, In Vitro. Botany 2019, 97, 463–474. [Google Scholar] [CrossRef]
- Suárez, J.O.; Villada, D.; de Rueda, J.A.O.; Alves-Santos, M.F.; Diez, J.J. Effects of Lactarius deliciosus and Rhizopogon roseolus Ectomycorrhyzal Fungi on Seeds and Seedlings of Scots and Stone Pines Inoculated with Fusarium oxysporum and Fusarium verticillioides. For. Chron. 2018, 94, 126–134. [Google Scholar] [CrossRef]
- Dalong, M.; Luhe, W.; Guoting, Y.; Liqiang, M.; Chun, L. Growth Response of Pinus Densiflora Seedlings Inoculated with Three Indigenous Ectomycorrhizal Fungi in Combination. Braz. J. Microbiol. 2011, 42, 1196–1202. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Linderman, R.G.; Boersma, L. Carbon-sink stimulation of photosynthesis in Douglas fir seedlings by some ectomycorrhizas. New Phytol. 1990, 115, 269–274. [Google Scholar] [CrossRef]
- Kang, T.T. Control of Pine Wilt Disease. Agric. Technol. Serv. 2012, 29, 1227. [Google Scholar]
- Awad, A.; Majcherczyk, A.; Schall, P.; Schröter, K.; Schöning, I.; Schrumpf, M.; Ehbrecht, M.; Boch, S.; Kahl, T.; Bauhus, J.; et al. Ectomycorrhizal and Saprotrophic Soil Fungal Biomass are Driven by Different Factors and Vary among Broadleaf and Coniferous Temperate Forests. Soil. Biol. Biochem. 2019, 131, 9–18. [Google Scholar] [CrossRef]
- Perrenoud, S. Potassium and Plant Health, 2nd ed.; International Potash Institute: Bern, Switzerland, 1990. [Google Scholar]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; Corato, U.G.; Sansinenea, E. Plant Mineral Nutrition and Disease Resistance: A Significant Linkage for Sustainable Crop Protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Campos-Soriano, L.; Bundó, M.; Bach-Pages, M.; Chiang, S.F.; Chiou, T.J.; Segundo, B.S. Phosphate Excess Increases Susceptibility to Pathogen Infection in Rice. Mol. Plant Pathol. 2020, 21, 555–570. [Google Scholar] [CrossRef]
- Ogo, S.; Yamanaka, T.; Akama, K.; Ota, Y.; Tahara, K.; Nagakura, J.; Kinoshita, A.; Yamaji, K. Growth and Uptake of Caesium, Rubidium, and Potassium by Ectomycorrhizal and Saprotrophic Fungi Grown on Either Ammonium or Nitrate as The N Source. Mycol. Prog. 2017, 16, 801–809. [Google Scholar] [CrossRef]

| Sample | pH | SOC (g/kg) | AP (mg/kg) | TP (g/kg) | AK (mg/kg) | TK (g/kg) | AHN (mg/kg) |
|---|---|---|---|---|---|---|---|
| YJ-4 | 4.66 ± 0.05 a | 18.8 ± 0.85 c | 1.38 ± 0.06 c | 0.20 ± 0.00 c | 80.65 ± 4.50 a | 5.48 ± 0.60 d | 64.09 ± 2.87 c |
| YB-4 | 4.20 ± 0.05 c | 36.6 ± 1.75 b | 5.61 ± 0.18 a | 0.25 ± 0.01 bc | 71.42 ± 11.08 ab | 9.73 ± 3.17 c | 102.47 ± 3.88 a |
| YJ-10 | 4.30 ± 0.02 b | 14.56 ± 0.33 d | 1.74 ± 0.64 c | 0.23 ± 0.02 b | 55.23 ± 0.93 bc | 30.32 ± 0.73 ab | 61.56 ± 9.98 c |
| YB-10 | 4.05 ± 0.03 d | 40.29 ± 0.53 a | 2.91 ± 0.64 b | 0.34 ± 0.02 a | 45.08 ± 1.61 c | 33.59 ± 1.99 a | 99.52 ± 2.43 ab |
| Sample | Shannon Index | Simpson Index | Chao Index | Coverage Index |
|---|---|---|---|---|
| YJ-4 | 3.90 ± 0.33 a | 0.08 ± 0.03 bc | 514.10 ± 87.62 b | 99.98 ± 0.03 a |
| YB-4 | 3.84 ± 0.43 ab | 0.08 ± 0.04 c | 616.66 ± 11.97 a | 99.81 ± 0.04 b |
| YJ-10 | 2.99 ± 0.67 bc | 0.12 ± 0.10 ab | 109.28 ± 82.37 c | 99.98 ± 0.03 a |
| YB-10 | 2.47 ± 0.33 c | 0.18 ± 0.08 a | 89.74 ± 83.43 c | 99.98 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Li, W.; Zhou, J.; Chen, X.; Chen, P.; Zhou, G. Pine Shoot Blight Driven Seasonal Variations in Fungal Assembly of Pinus elliottii Rhizosphere. Microorganisms 2025, 13, 2476. https://doi.org/10.3390/microorganisms13112476
Duan X, Li W, Zhou J, Chen X, Chen P, Zhou G. Pine Shoot Blight Driven Seasonal Variations in Fungal Assembly of Pinus elliottii Rhizosphere. Microorganisms. 2025; 13(11):2476. https://doi.org/10.3390/microorganisms13112476
Chicago/Turabian StyleDuan, Xiang, Wenhao Li, Jiechen Zhou, Xingzhou Chen, Pingan Chen, and Guoying Zhou. 2025. "Pine Shoot Blight Driven Seasonal Variations in Fungal Assembly of Pinus elliottii Rhizosphere" Microorganisms 13, no. 11: 2476. https://doi.org/10.3390/microorganisms13112476
APA StyleDuan, X., Li, W., Zhou, J., Chen, X., Chen, P., & Zhou, G. (2025). Pine Shoot Blight Driven Seasonal Variations in Fungal Assembly of Pinus elliottii Rhizosphere. Microorganisms, 13(11), 2476. https://doi.org/10.3390/microorganisms13112476







