Vulnerability of Walnut Pruning Wounds to Fungal Trunk Pathogens and Seasonal Conidial Dynamics of Botryosphaeriaceae in the Maule Region, Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Fungal Isolation and Inoculum Preparation
2.3. Susceptibility of Pruning Wound Age to Fungal Trunk Pathogen Infections
2.4. Spore Trapping and Monitoring
2.5. Weather Variables and Data Analysis
2.6. Experimental Setup and Statistical Analysis
3. Results
3.1. Pruning Wound Age on Infection by Botryosphaeriaceae and Diaporthaceae spp. in English Walnut
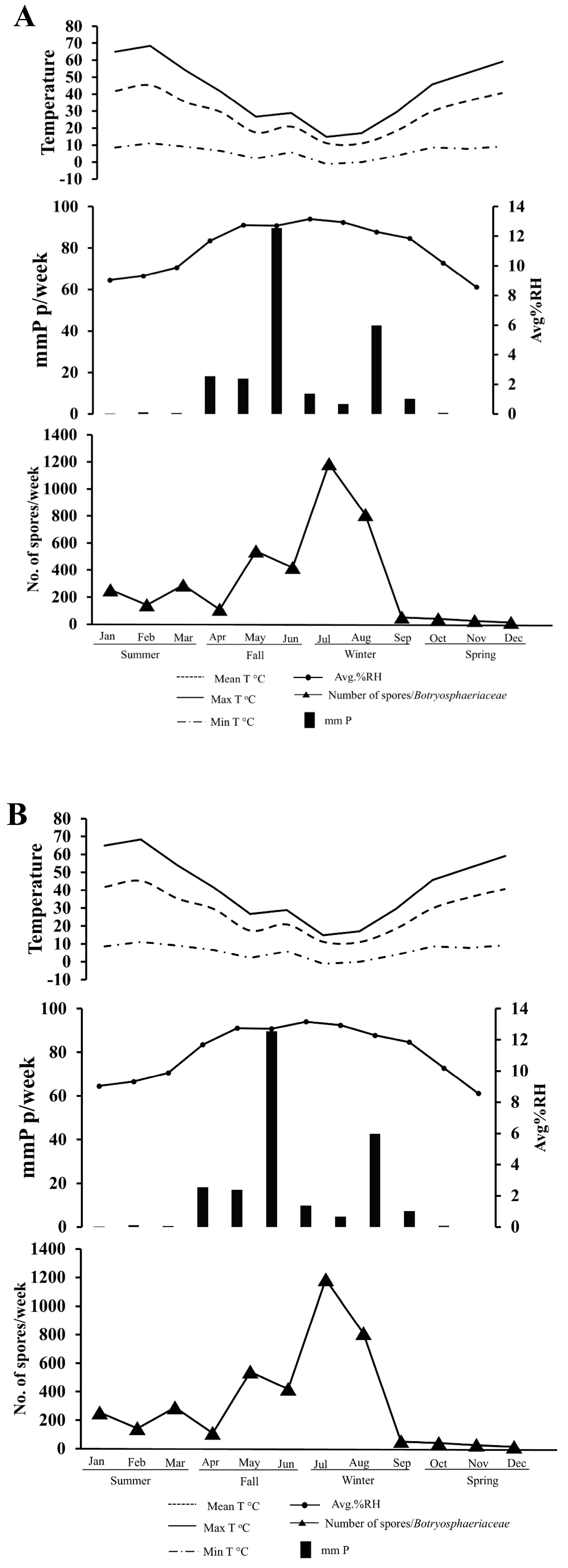
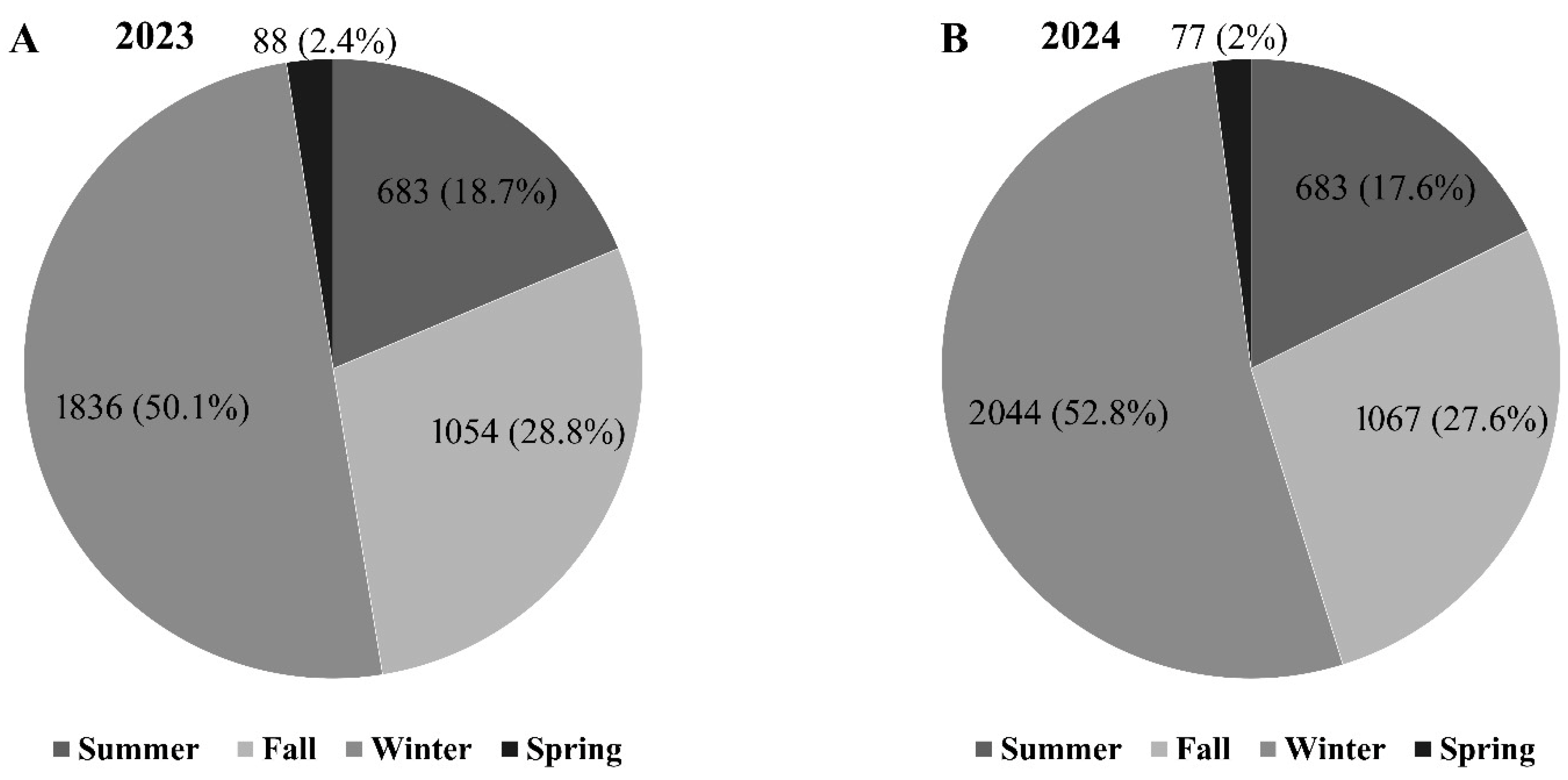
3.2. Monitoring of Botryosphaeriaceae Spore Dispersal and Weather Conditions
3.2.1. Climate-Spore Relationship-2023
3.2.2. Climate-Spore Relationship-2024
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Tree Nuts: World Markets and Trade–November 2024; United States Department of Agriculture, Foreign Agricultural Service: Washington, DC, USA, 2024. Available online: https://www.fas.usda.gov/sites/default/files/2024-11/TreeNuts.pdf (accessed on 31 August 2025).
- Odepa (Oficina de Estudios y Políticas Agrarias). Available online: https://www.odepa.gob.cl/estadisticas-del-sector/estadisticas-productivas (accessed on 31 August 2025).
- Chen, S.; Morgan, D.P.; Hasey, J.K.; Anderson, K.; Michailides, T.J. Phylogeny, morphology, distribution, and pathogenicity of Botryosphaeriaceae and Diaporthaceae from English walnut in California. Plant Dis. 2014, 98, 636–652. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Moral, J.; Felts, D.; Trapero, A.; Michailides, T.J. Interaction between Diaporthe rhusicola and Neofusicoccum mediterraneum causing branch dieback and fruit blight of English walnut in California, and the effect of pruning wounds on the infection. Plant Dis. 2019, 103, 1196–1205. [Google Scholar] [CrossRef]
- Eichmeier, A.; Pecenka, J.; Spetik, M.; Necas, T.; Ondrasek, I.; Armengol, J. Fungal trunk pathogens associated with Juglans regia in the Czech Republic. Plant Dis. 2020, 104, 761–771. [Google Scholar] [CrossRef]
- López-Moral, A.; Lovera, M.; Del Carmen Raya, M.; Cortés-Cosano, N.; Arquero, O.; Trapero, A. Etiology of branch dieback and shoot blight of English walnut caused by Botryosphaeriaceae and Diaporthe species in Southern Spain. Plant Dis. 2020, 104, 533–550. [Google Scholar] [CrossRef]
- Moral, J.; Morgan, D.; Trapero, A.; Michailides, T.J. Ecology and epidemiology of diseases of nut crops and olives caused by Botryosphaeriaceae fungi in California and Spain. Plant Dis. 2019, 103, 1809–1827. [Google Scholar] [CrossRef]
- Antony, S.; Billones-Baaijens, R.; Stodart, B.J.; Steel, C.C.; Lang, M.D.; Savocchia, S. Incidence and distribution of Botryosphaeriaceae species associated with dieback in walnut orchards in Australia. Plant Pathol. 2022, 72, 610–622. [Google Scholar] [CrossRef]
- Li, G.; Liu, F.; Li, J.; Liu, Q.; Chen, S. Characterization of Botryosphaeria dothidea and Lasiodiplodia pseudotheobromae from English Walnut in China. J. Phytopathol. 2015, 164, 348–353. [Google Scholar] [CrossRef]
- Belair, M.; Picot, A.; Masson, C.; Hébrard, M.-N.; Debled, M.; Moronvalle, A.; Pensec, F. Etiology and epidemiology of branch dieback and fruit blight and necrosis of English walnut in France. Plant Dis. 2025. [Google Scholar] [CrossRef]
- Sohrabi, M.; Mohammadi, H.; León Santana, M.; Armengol Fortí, J.; Banihashemi, Z. Fungal pathogens associated with branch and trunk cankers of nut crops in Iran. Eur. J. Plant Pathol. 2020, 157, 327–351. [Google Scholar] [CrossRef]
- Gusella, G.; Giambra, S.; Conigliaro, G.; Burruano, S.; Polizzi, G. Botryosphaeriaceae species causing canker and dieback of English walnut (Juglans regia) in Italy. For. Pathol. 2021, 51, e12661. [Google Scholar] [CrossRef]
- Çiftçi, O.; Özer, G.; Türkölmez, Ş.; Derviş, S. Lasiodiplodia theobromae and Neoscytalidium dimidiatum associated with grafted walnut (Juglans regia L.) decline in Turkey. J. Plant Dis. Prot. 2023, 130, 1117–1128. [Google Scholar] [CrossRef]
- Díaz, G.A.; Latorre, B.; Ferrada, E.; Gutiérrez, M.; Bravo, F.; Lolas, M. First report of Diplodia mutila causing branch dieback of English walnut cv. Chandler in the Maule Region, Chile. Plant Dis. 2018, 102, 1451. [Google Scholar] [CrossRef]
- Jiménez-Luna, I.; Cadiz, F.; Aravena, R.; Larach, A.; Besoain, X.; Ezcurra, E.; Rolshausen, P.E. First report of Diaporthe cynaroidis and D. australafricana associated with walnut branch canker in Chile. Plant Dis. 2020, 104, 2732. [Google Scholar] [CrossRef]
- Jiménez-Luna, I.; Besoain, X.; Saa, S.; Peach-Fine, E.; Cadiz Morales, F.; Riquelme, N.; Larach, A.; Morales, J.; Ezcurra, E.; Ashworth, V.E.T.M.; et al. Identity and pathogenicity of Botryosphaeriaceae and Diaporthaceae from Juglans regia in Chile. Phytopathol. Mediterr. 2022, 61, 79–94. [Google Scholar] [CrossRef]
- Barcos, J.; Farías, M.J.; Rebufel, P.; Soto, S.; Riquelme, D. First Report of Neofusicoccum australe Causing Branch Dieback of English Walnut (cv. Chandler) in Central Chile. Plant Dis. 2023, 107, 2543. [Google Scholar] [CrossRef]
- Iqbal, S.; Hernández, Y.; Lolas, M.; Elfar, K.; Eskalen, A.; Latorre, B.A. Dothiorella sarmentorum causing canker and branch dieback of English walnut in Maule Region, Chile. Plant Dis. 2023, 107, 1219. [Google Scholar] [CrossRef]
- Iqbal, S.; Díaz, G.A.; Ferrada, E. First report of Neofusicoccum nonquaesitum causing branch dieback of English walnut (Juglans regia) in Maule region, Chile. Plant Dis. 2024, 10, 1094. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Bostock, R.M.; Trouillas, F.P.; Michailides, T.J. A six-locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia 2010, 102, 1350–1368. [Google Scholar] [CrossRef]
- Díaz, G.A.; Valdez, A.; Halleen, F.; Ferrada, E.; Lolas, M.; Latorre, B.A. Characterization and pathogenicity of Diplodia, Lasiodiplodia, and Neofusicoccum species causing Botryosphaeria canker and dieback of apple trees in Central Chile. Plant Dis. 2022, 106, 925–937. [Google Scholar] [CrossRef]
- Bernal, V.V.; Pegahrad, Z.; Dashtarzhaneh, M.K.; Khodadadi, F. Identification, characterization, and fungicide sensitivity of Botryosphaeriaceae fungi associated with avocado branch canker disease in Southern California. Plant Dis. 2025, 109, 1690–1701. [Google Scholar] [CrossRef]
- Blagojević, J.; Aleksić, G.; Vučurović, I.; Starović, M.; Ristić, D. Exploring the phylogenetic diversity of Botryosphaeriaceae and Diaporthe species causing dieback and shoot blight of blueberry in Serbia. Phytopathology 2024, 114, 1333–1345. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Franceschini, A.; Alves, A.; Abdollahzadeh, J.; Phillips, A.J.L. Phylogeny, morphology, and pathogenicity of Botryosphaeriaceae, Diatrypaceae, and Gnomoniaceae associated with branch diseases of hazelnut in Sardinia (Italy). Eur. J. Plant Pathol. 2016, 146, 259–279. [Google Scholar] [CrossRef]
- Wiman, N.G.; Webber III, J.B.; Wiseman, M.; Merlet, L. Identity and pathogenicity of some fungi associated with hazelnut (Corylus avellana L.) trunk cankers in Oregon. PLoS ONE 2019, 14, e0223500. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A.; Ruiz, B.E.; Gambaro, J.R.; San Martín, J.G.; Lisperguer, M.J.; De Gregorio, T. First report of Diplodia mutila causing wood necrosis in European hazelnut in Chile. Plant Dis. 2023, 107, 565. [Google Scholar] [CrossRef]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef]
- Spetik, M.; Tekielska, D.A.; Berraf-Tebbal, A.; Pecenka, J.; Stuskova, K.; Mahamedi, A.E.; Eichmeier, A. Diversity of Botryosphaeriaceae species associated with grapevine trunk diseases in the Czech Republic. Diversity 2023, 15, 800. [Google Scholar] [CrossRef]
- Moral, J.; Muñoz-Díez, C.; González, N.; Trapero, A.; Michailides, T.J. Characterization and pathogenicity of Botryosphaeriaceae species collected from olive and other hosts in Spain and California. Phytopathology 2010, 100, 1340–1351. [Google Scholar] [CrossRef]
- Chen, S.F.; Morgan, D.P.; Michailides, T.J. Botryosphaeriaceae and Diaporthaceae associated with panicle and shoot blight of pistachio in California, USA. Fungal Divers. 2014, 67, 157–179. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Lombard, L.; Wingfield, M.J.; Crous, P.W. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Gomes, R.; Glienke, C.; Videira, S.; Lombard, L.; Groenewald, J.; Crous, P. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Vučković, N.; Vico, I.; Duduk, B.; Duduk, N. Diversity of Botryosphaeriaceae and Diaporthe species associated with postharvest apple fruit decay in Serbia. Phytopathology 2022, 112, 929–943. [Google Scholar] [CrossRef]
- Elfar, K.; Torres, R.; Díaz, G.A.; Latorre, B.A. Characterization of Diaporthe australafricana and Diaporthe spp. associated with stem canker of blueberry in Chile. Plant Dis. 2013, 97, 1042–1050. [Google Scholar] [CrossRef]
- Xiao, X.E.; Liu, Y.D.; Zheng, F.; Xiong, T.; Zeng, Y.T.; Wang, W.; Zheng, X.L.; Wu, Q.; Xu, J.P.; Crous, P.W.; et al. High species diversity in Diaporthe associated with citrus diseases in China. Persoonia 2023, 51, 229–256. [Google Scholar] [CrossRef]
- Waqas, M.; Guarnaccia, V.; Bardella, S.; Spadaro, D. Molecular characterization and pathogenicity of Diaporthe species causing nut rot of hazelnut in Italy. Plant Dis. 2024, 108, 1005–1013. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Woodhall, J.; Armengol, J.; Cinelli, T.; Eichmeier, A.; Crous, P.W. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia 2018, 40, 135–153. [Google Scholar] [CrossRef]
- Díaz, G.A.; Zoffoli, J.P.; Ferrada, E.E.; Lolas, M. Identification and pathogenicity of Diplodia, Neofusicoccum, Cadophora, and Diaporthe species associated with cordon dieback in kiwifruit cultivar Hayward in Central Chile. Plant Dis. 2021, 105, 1308–1319. [Google Scholar] [CrossRef]
- Cao, J.; Gao, W.; Yao, M.; Xie, S.; Yin, X.; Xu, C.; Wu, H.; Zhang, M.; Guo, Y. Diaporthe actinidiicola: A novel species causing branch canker or dieback of fruit trees in Henan Province, China. Plant Pathol. 2023, 72, 1236–1246. [Google Scholar] [CrossRef]
- Zabiák, A.; Kovács, C.; Takács, F.; Pál, K.; Peles, F.; Fekete, E.; Sándor, E. Diaporthe and Diplodia species associated with walnut (Juglans regia L.) in Hungarian orchards. Horticulturae 2023, 9, 205. [Google Scholar] [CrossRef]
- Jimenez-Luna, I.; Doll, D.; Ashworth, V.E.; Trouillas, F.P.; Rolshausen, P.E. Comparative profiling of wood canker pathogens from spore traps and symptomatic plant samples within California almond and walnut orchards. Plant Dis. 2022, 106, 2182–2190. [Google Scholar] [CrossRef]
- Valdez-Tenezaca, A.; Latorre, B.A.; Díaz, G.A. Susceptibility of pruning wounds of apple trees to Diplodia mutila, D. seriata, Lasiodiplodia theobromae, and Neofusicoccum arbuti infections and conidial release of Botryosphaeriaceae spp. in the Maule Region, Chile. Plant Dis. 2025, 109, 1121–1129. [Google Scholar] [CrossRef]
- Eskalen, A.; Faber, B.; Bianchi, M. Spore trapping and pathogenicity of fungi in the Botryosphaeriaceae and Diaporthaceae associated with avocado branch canker in California. Plant Dis. 2013, 97, 329–332. [Google Scholar] [CrossRef]
- Hernández, M.; Kc, A.N. Determining the timing of spore release by Botryosphaeriaceae species in Oregon Vineyards. Plant Dis. 2024, 108, 1033–1040. [Google Scholar] [CrossRef]
- Olmo, D.; Gramaje, D.; Armengol, J. Evaluation of fungicides to protect pruning wounds from Botryosphaeriaceae species infections on almond trees. Phytopathol. Mediterr. 2017, 56, 77–86. [Google Scholar]
- Holland, L.A.; Travadon, R.; Lawrence, D.P.; Jaime-Frias, R.; Nouri, M.T.; Sahtout, M.; Trouillas, F.P. Temporal susceptibility of almond pruning wounds to infection by fungal canker pathogens in California. Plant Pathol. 2023, 72, 489–498. [Google Scholar] [CrossRef]
- Brown-Rytlewski, D.E.; McManus, P.S. Virulence of Botryosphaeria dothidea and Botryosphaeria obtusa on apple and management of stem cankers with fungicides. Plant Dis. 2000, 84, 1031–1037. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Ayres, M.R.; Billones-Baaijens, R.; Savocchia, S.; Scott, E.S. Susceptibility of pruning wounds to grapevine trunk disease pathogens Eutypa lata and Diplodia seriata in three climatic conditions in Australia. Fungal Ecol. 2023, 64, 101260. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Gubler, W.D. Susceptibility of grapevine pruning wounds to infection by Lasiodiplodia theobromae and Neofusicoccum parvum. Plant Pathol. 2011, 60, 261–270. [Google Scholar] [CrossRef]
- López-Moral, A.; Lovera, M.; Antón-Domínguez, B.I.; Gámiz, A.M.; Michailides, T.J.; Arquero, O. Effects of cultivar susceptibility, branch age, and temperature on infection by Botryosphaeriaceae and Diaporthe fungi on English walnut (Juglans regia). Plant Dis. 2022, 106, 2920–2926. [Google Scholar] [CrossRef]
- Antony, S.; Billones-Baaijens, R.; Steel, C.C.; Stodart, B.J.; Savocchia, S. Pathogenicity and progression of Botryosphaeriaceae associated with dieback in walnut orchards in Australia. Eur. J. Plant Pathol. 2024, 168, 723–742. [Google Scholar] [CrossRef]
- Rosace, M.C.; Legler, S.E.; Salotti, I.; Rossi, V. Susceptibility of pruning wounds to grapevine trunk diseases: A quantitative analysis of literature data. Front. Plant Sci. 2023, 14, 1063932. [Google Scholar] [CrossRef] [PubMed]
- Twizeyimana, M.; McDonald, V.; Mayorquin, J.S.; Wang, D.H.; Na, F.; Akgül, D.S.; Eskalen, A.; Bostock, R.M. Effect of fungicide application on the management of avocado branch canker (formerly Dothiorella canker) in California. Plant Dis. 2013, 97, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Úrbez-Torres, J.R.; Battany, M.; Bettiga, L.J.; Gispert, C.; McGourty, G.; Roncoroni, J.; Gubler, W.D. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010, 94, 717–724. [Google Scholar] [CrossRef]
- Eskalen, A.; Feliciano, A.J.; Gubler, W.D. Susceptibility of grapevine pruning wounds and symptom development in response to infection by Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Plant Dis. 2007, 91, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Voegel, T.M.; Gubler, W.D. Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California. Plant Dis. 2006, 90, 1490–1503. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Dissanayake, A.J.; Li, X.; Liu, M.; Wanasinghe, D.N.; Xu, J.; Yan, J. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front. Microbiol. 2019, 10, 1936. [Google Scholar] [CrossRef]
- Amponsah, N.T.; Jones, E.E.; Ridgway, H.J.; Jaspers, M.V. Susceptibility of grapevine pruning wounds to infection by trunk disease pathogens. N. Z. Plant Prot. 2012, 65, 103–110. [Google Scholar]
- Serra, S.; Mannoni, M.A.; Ligios, V. Studies on the susceptibility of pruning wounds to infection by fungi involved in grapevine wood diseases in Italy. Phytopathol. Mediterr. 2008, 47, 234–246. [Google Scholar]
- Kuntzmann, P.; Villaume, S.; Bertsch, C. Conidia dispersal of Diplodia species in a French vineyard. Phytopathol. Mediterr. 2009, 48, 150–154. [Google Scholar]
- Mohankumar, V.; Guest, D.I.; Pegg, K.G. Seasonal dynamics of inoculum of Botryosphaeriaceae in macadamia orchards in Australia. Plant Pathol. 2023, 72, 123–134. [Google Scholar] [CrossRef]
- Valencia, D.; Torres, C.; Camps, R.; Lopez, E.; Celis-Diez, J.L.; Besoain, X. Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaíso Region of Chile. Phytopathol. Mediterr. 2015, 54, 394–402. [Google Scholar]
- Tapia, L.; Castillo-Novales, D.; Riquelme, N.; Valencia, A.L.; Larach, A.; Cautín, R.; Besoain, X. Rainfall and high humidity influence the seasonal dynamics of spores of Glomerellaceae and Botryosphaeriaceae genera in avocado orchards and their fruit rot association. Agronomy 2025, 15, 1453. [Google Scholar] [CrossRef]
- Amponsah, N.T.; Jones, E.E.; Ridgway, H.J.; Jaspers, M.V. Rain triggers the release of Botryosphaeriaceae conidia from infected grapevine canes. Australas. Plant Pathol. 2009, 38, 525–531. [Google Scholar]
- Ahimera, N.; Gisler, S.; Morgan, D.P.; Michailides, T.J. Effects of single-drop impactions and natural and simulated rains on the dispersal of Botryosphaeria dothidea conidia. Phytopathology 2004, 94, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Michailides, T.; Hasey, J. Botryosphaeria and Phomopsis cankers of walnuts in California. In Walnut Husk Fly Field Meeting; University of California Cooperative Extension: Half Moon Bay, CA, USA, 2010. [Google Scholar]
- Agustí-Brisach, C.; León, M.; García-Jiménez, J.; Armengol, J. Detection of grapevine fungal trunk pathogens on pruning shears and evaluation of their potential for spread of infection. Plant Dis. 2015, 99, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, P.E.; Úrbez-Torres, J.R.; Rooney-Latham, S.; Eskalen, A.; Smith, R.J.; Gubler, W.D. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 2010, 61, 113–119. [Google Scholar] [CrossRef]
- Pitt, W.M.; Sosnowski, M.R.; Huang, R.; Qiu, Y.; Steel, C.C.; Savocchia, S. Evaluation of fungicides for the management of Botryosphaeria canker of grapevines. Plant Dis. 2012, 96, 1303–1308. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, S.; Mubeen, I.; Lolas, M.; Moya-Elizondo, E.; Gundel, P.; Ortega-Farias, S.; Campillay-Llanos, W.; Díaz, G.A. Vulnerability of Walnut Pruning Wounds to Fungal Trunk Pathogens and Seasonal Conidial Dynamics of Botryosphaeriaceae in the Maule Region, Chile. Microorganisms 2025, 13, 2407. https://doi.org/10.3390/microorganisms13102407
Iqbal S, Mubeen I, Lolas M, Moya-Elizondo E, Gundel P, Ortega-Farias S, Campillay-Llanos W, Díaz GA. Vulnerability of Walnut Pruning Wounds to Fungal Trunk Pathogens and Seasonal Conidial Dynamics of Botryosphaeriaceae in the Maule Region, Chile. Microorganisms. 2025; 13(10):2407. https://doi.org/10.3390/microorganisms13102407
Chicago/Turabian StyleIqbal, Shehzad, Iqra Mubeen, Mauricio Lolas, Ernesto Moya-Elizondo, Pedro Gundel, Samuel Ortega-Farias, William Campillay-Llanos, and Gonzalo A. Díaz. 2025. "Vulnerability of Walnut Pruning Wounds to Fungal Trunk Pathogens and Seasonal Conidial Dynamics of Botryosphaeriaceae in the Maule Region, Chile" Microorganisms 13, no. 10: 2407. https://doi.org/10.3390/microorganisms13102407
APA StyleIqbal, S., Mubeen, I., Lolas, M., Moya-Elizondo, E., Gundel, P., Ortega-Farias, S., Campillay-Llanos, W., & Díaz, G. A. (2025). Vulnerability of Walnut Pruning Wounds to Fungal Trunk Pathogens and Seasonal Conidial Dynamics of Botryosphaeriaceae in the Maule Region, Chile. Microorganisms, 13(10), 2407. https://doi.org/10.3390/microorganisms13102407








