History and Evolution of the Hypervirulent Clostridioides difficile Ribotype 027 Lineage
Abstract
1. Introduction
2. Methods
3. A Brief Review of C. difficile Disease
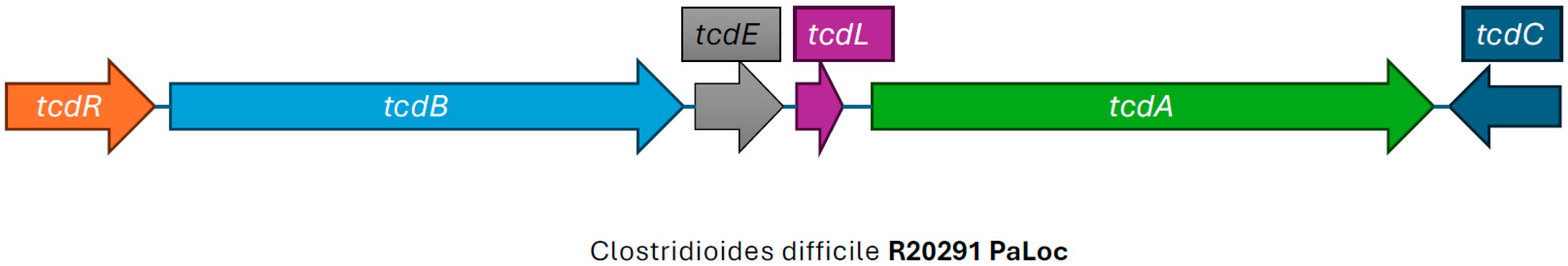
4. The Pathogenicity Locus and Its Relationship to CDI
5. The Origin of the PaLoc
6. The Large Clostridial Glucosylating Toxins: TcdA and TcdB
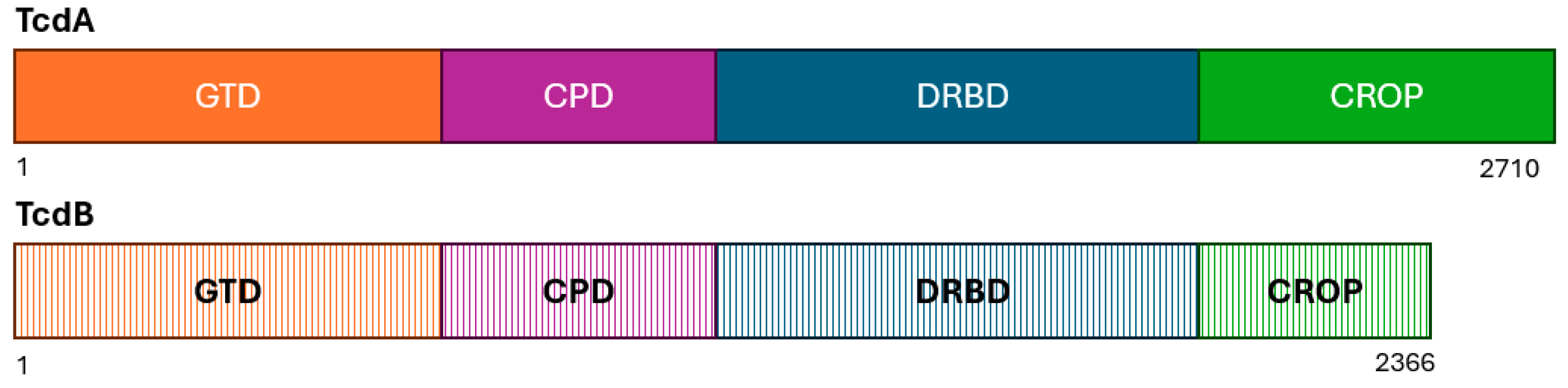
7. Variants of TcdA and TcdB and Their Relationship to RT027
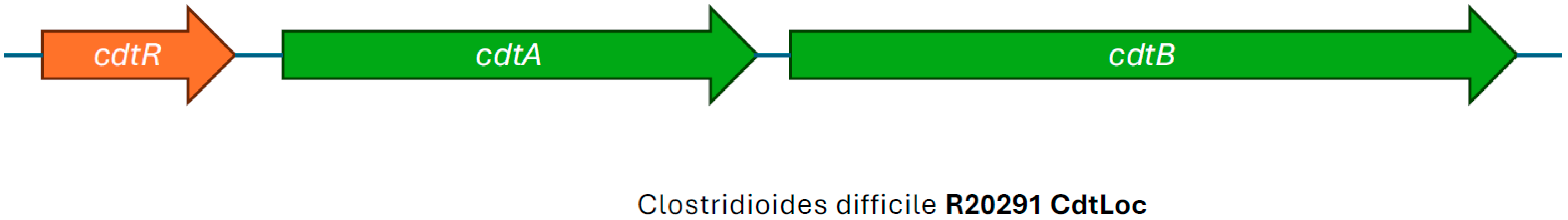
8. Binary Toxin—The C. difficile Transferase (cdt) Locus
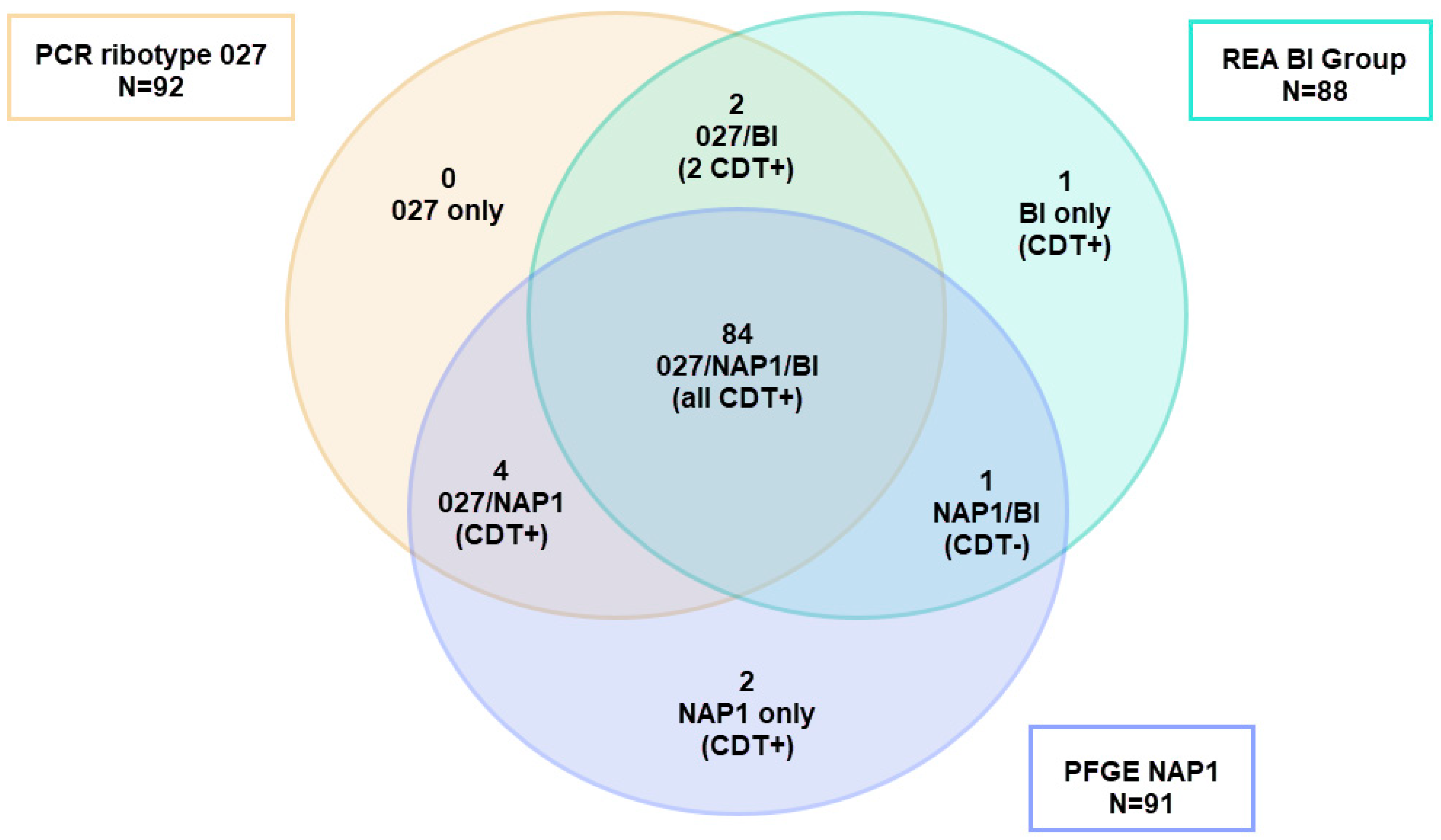
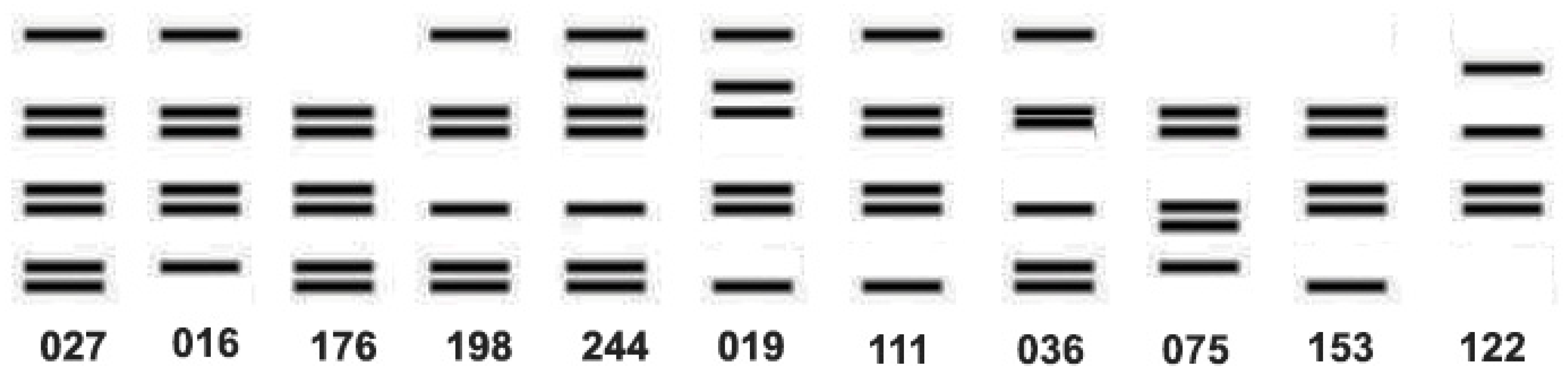
9. The Ambiguities of Traditional C. difficile Typing Methods
10. C. difficile Lineages and Phylogeny
11. The Emergence of C. difficile RT027
| Year | Location | Event | Type of Change | References |
|---|---|---|---|---|
| 1999 | United States and Europe | Introduction of moxifloxacin (4th gen fluoroquinolone) |  | [90] |
| 2000–2003 | United States | Increase in CDI cases from 25,000 in year 2000 to 54,000 in year 2003 |  | [80] |
| 2003 | Canada | Increase in CDI cases from 102 per 100,000 patients in 1991–92 to 866 per 100,000 patients in 2003 |  | [8] |
| 2001–2004 | United Kingdom | A 98% rise in CDI cases |  | [84] |
| 2006 | United States and United Kingdom | Peak fluoroquinolone use |  | [90,93] |
| 2007 | United Kingdom | Restriction of fluoroquinolone prescriptions |  | [93] |
| 2009–2011 | United States and Europe (part) | Decline in CDI cases caused by RT027 |  | [101,106,107,108] |
| 2016–2017 | Europe | RT027 was the third frequent ribotype overall in Europe, except for Czech Republic, Hungary, Poland, and Slovakia |  | [28] |
| 2018–2020 | Europe | ECDC survey: further decline of RT027 (now 11th most frequently reported) |  | [28] |
| 2020 | Greece | Outbreak of CDI due to a 027-like PCR ribotype 181 |  | [109] |
| 2024 | Greece | RT181 is the predominant ribotype in a rehabilitation center (76.6%) |  | [110] |
| 2024 | Poland | Study reported RT027 (77.8%) still dominant ribotype, emergence of RT955 (12.7%) |  | [111] |
12. The Potential Impact of Other Antimicrobial Resistance Determinants
13. Did Traditional Typing Methods Obscure Epidemiologic Changes in C. difficile Strains?
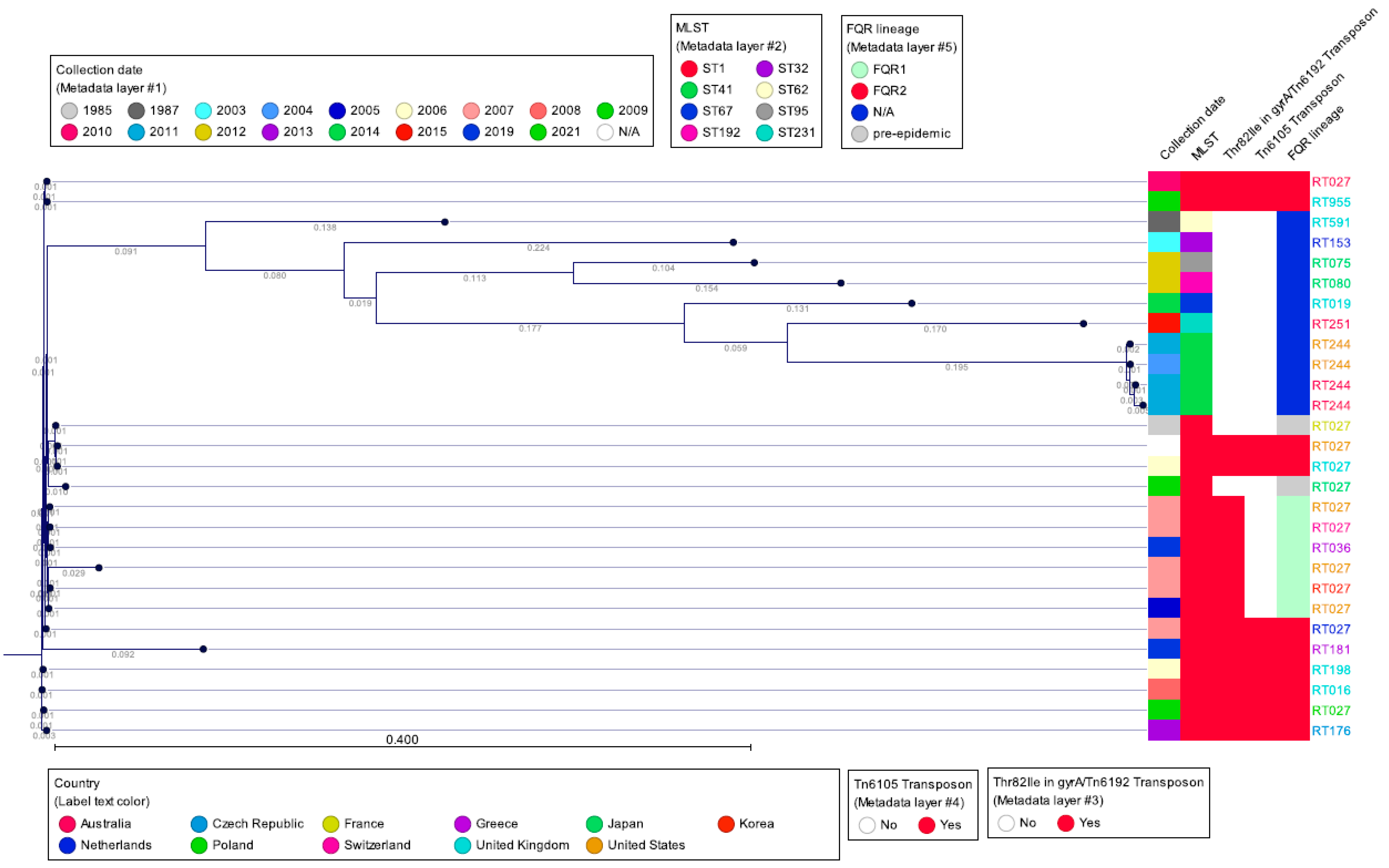
14. Clade 2: C. difficile Strains Genetically Related to RT027
15. Single Nucleotide Polymorphism Sequence Data and wgMLST
16. The Decline of RT027; Epidemiological Data

17. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDI | C. difficile infection |
| WGS | Whole genome sequencing |
| NGS | Next-generation sequencing |
| PCR | Polymerase chain reaction |
| PFGE | Pulsed-field gel electrophoresis |
| REA | Restriction endonuclease analysis |
| MLVA | Multilocus variable-number tandem-repeat analysis |
| PaLoc | Pathogenicity locus |
| CDT | C. difficile transferase |
| MGE | Mobile genetic elements |
| SNP | Single-nucleotide polymorphism |
References
- Di Bella, S.; Sanson, G.; Monticelli, J.; Zerbato, V.; Principe, L.; Giuffrè, M.; Pipitone, G.; Luzzati, R. Clostridioides difficile infection: History, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin. Microbiol. Rev. 2024, 37, e0013523. [Google Scholar] [CrossRef]
- Hall, I.C.; O’Toole, E. Intestinal flora in new-born infants: With a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 1935, 49, 390–402. [Google Scholar] [CrossRef]
- Kwon, J.H.; Olsen, M.A.; Dubberke, E.R. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect. Dis. Clin. North Am. 2015, 29, 123–134. [Google Scholar] [CrossRef]
- Sahrmann, J.M.; Olsen, M.A.; Stwalley, D.; Yu, H.; Dubberke, E.R. Costs Attributable to Clostridioides difficile Infection Based on the Setting of Onset. Clin. Infect. Dis. 2023, 76, 809–815. [Google Scholar] [CrossRef]
- McDonald, L.C.; Killgore, G.E.; Thompson, A.; Owens RCJr Kazakova, S.V.; Sambol, S.P.; Johnson, S.; Gerding, D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005, 353, 2433–2441. [Google Scholar] [CrossRef]
- Tenover, F.C.; Akerlund, T.; Gerding, D.N.; Goering, R.V.; Boström, T.; Jonsson, A.-M.; Wong, E.; Wortman, A.T.; Persing, D.H. Comparison of strain typing results for Clostridium difficile isolates from North America. J. Clin. Microbiol. 2011, 49, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Killgore, G.; Thompson, A.; Johnson, S.; Brazier, J.; Kuijper, E.; Pepin, J.; Frost, E.H.; Savelkoul, P.; Nicholson, B.; van den Berg, R.J.; et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: Restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J. Clin. Microbiol. 2008, 46, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Pepin, J.; Fang, A.; Killgore, G.; Thompson, A.; Brazier, J.; Frost, E.; McDonald, L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005, 366, 1079–1084. [Google Scholar] [CrossRef]
- Norén, T. Outbreak from a high-toxin intruder: Clostridium difficile. Lancet 2005, 366, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Muto, C.A.; Blank, M.K.; Marsh, J.W.; Vergis, E.N.; O’Leary, M.M.; Shutt, K.A.; Pasculle, A.W.; Pokrywka, M.; Garcia, J.G.; Posey, K.; et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin. Infect. Dis. 2007, 45, 1266–1273. [Google Scholar] [CrossRef]
- Markovska, R.; Dimitrov, G.; Gergova, R.; Boyanova, L. Clostridioides difficile, a New “Superbug”. Microorganisms 2023, 11, 845. [Google Scholar] [CrossRef]
- Gentry, C.A.; Williams, R.J.; Campbell, D. Continued decline in the prevalence of the Clostridioides difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Diagn. Microbiol. Infect. Dis. 2021, 100, 115308. [Google Scholar] [CrossRef]
- Liu, C.; Monaghan, T.; Yadegar, A.; Louie, T.; Kao, D. Insights into the Evolving Epidemiology of Clostridioides difficile Infection and Treatment: A Global Perspective. Antibiotics 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Clostridium difficile: History of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis 1994, 18 (Suppl. S4), S265–S272. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, F.J.; Barton, R.W.; Alpers, D.H. Clindamycin-associated colitis. A prospective study. Ann. Intern. Med. 1974, 81, 429–433. [Google Scholar] [CrossRef]
- Tougas, S.R.; Lodha, N.; Vandermeer, B.; Lorenzetti, D.L.; Tarr, P.I.; Tarr, G.A.M.; Chui, L.; Vanderkooi, O.G.; Freedman, S.B. Prevalence of Detection of Clostridioides difficile Among Asymptomatic Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021, 175, e212328. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Chang, T.W.; Gurwith, M.; Gorbach, S.L.; Onderdonk, A.B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 1978, 298, 531–534. [Google Scholar] [CrossRef]
- George, R.H.; Symonds, J.M.; Dimock, F.; Brown, J.D.; Arabi, Y.; Shinagawa, N.; Keighley, M.R.; Alexander-Williams, J.; Burdon, D.W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. BMJ 1978, 1, 695. [Google Scholar] [CrossRef]
- Borriello, S.P.; Ketley, J.M.; Mitchell, T.J.; Barclay, F.E.; Welch, A.R.; Price, A.B.; Stephen, J. Clostridium difficile—A spectrum of virulence and analysis of putative virulence determinants in the hamster model of antibiotic-associated colitis. J. Med. Microbiol. 1987, 24, 53–64. [Google Scholar] [CrossRef]
- Lyras, D.; O’Connor, J.R.; Howarth, P.M.; Sambol, S.P.; Carter, G.P.; Phumoonna, T.; Poon, R.; Adams, V.; Vedantam, G.; Johnson, S.; et al. Toxin B is essential for virulence of. Nature 2009, 458, 1176–1179. [Google Scholar] [CrossRef]
- Carroll, K.C.; Mizusawa, M. Laboratory Tests for the Diagnosis of Clostridium difficile. Clin. Colon Rectal Surg. 2020, 33, 73–81. [Google Scholar] [CrossRef]
- Braun, V.; Hundsberger, T.; Leukel, P.; Sauerborn, M.; von Eichel-Streiber, C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 1996, 181, 29–38. [Google Scholar] [CrossRef]
- Popoff, M.R.; Rubin, E.J.; Gill, D.M.; Boquet, P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 1988, 56, 2299–2306. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/?CDC_AAref_Val=https://www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 19 December 2022).
- Feuerstadt, P.; Theriault, N.; Tillotson, G. The burden of CDI in the United States: A multifactorial challenge. BMC Infect. Dis. 2023, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Coia, C.W.; Banks, A.-L.; Cottom, L.; Fitzpatrick, F. The need for European surveillance of CDI. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2024; Volume 1435, pp. 13–31. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Emerging Infections Program, Healthcare-Associated Infections—Community Interface Surveillance Report, Clostridioides Difficile Infection (CDI) 2022. Available online: https://www.cdc.gov/healthcare-associated-infections/media/pdfs/2022-CDI-Report-508.pdf (accessed on 19 September 2025).
- Clostridioides (Clostridium) Difficile Infections—Annual Epidemiological Report for 2016–2017 n.d. Available online: https://www.ecdc.europa.eu/en/publications-data/clostridiodes-difficile-infections-annual-epidemiological-report-2016-2017 (accessed on 11 June 2024).
- Carter, G.P.; Chakravorty, A.; Pham Nguyen, T.A.; Mileto, S.; Schreiber, F.; Li, L.; Howarth, P.; Clare, S.; Cunningham, B.; Sambol, S.P.; et al. Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections. mBio 2015, 6, e00551. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Madan, R. Clostridioides difficile Toxins: Host Cell Interactions and Their Role in Disease Pathogenesis. Toxins 2024, 16, 241. [Google Scholar] [CrossRef] [PubMed]
- Dingle, K.E.; Elliott, B.; Robinson, E.; Griffiths, D.; Eyre, D.W.; Stoesser, N.; Vaughan, A.; Golubchik, T.; Fawley, W.N.; Wilcox, M.; et al. Evolutionary history of the Clostridium difficile pathogenicity locus. Genome Biol. Evol. 2014, 6, 36–52. [Google Scholar] [CrossRef]
- Awad, M.M.; Suraweera, C.D.; Vidor, C.J.; Ye-Lin, A.Y.; Williams, G.C.; Mileto, S.J.; Barlow, C.K.; McGowan, S.; Lyras, D. A Clostridioides difficile endolysin modulates toxin secretion without cell lysis. Commun. Biol. 2024, 7, 1044. [Google Scholar] [CrossRef]
- García-Saura, A.G.; Zapata-Pérez, R.; Hidalgo, J.F.; Sánchez-Ferrer, Á. Comparative inhibitory profile and distribution of bacterial PARPs, using Clostridioides difficile CD160 PARP as a model. Sci. Rep. 2018, 8, 8056. [Google Scholar] [CrossRef]
- Ramírez-Vargas, G.; López-Ureña, D.; Badilla, A.; Orozco-Aguilar, J.; Murillo, T.; Rojas, P.; Riedel, T.; Overmann, J.; González, G.; Chaves-Olarte, E.; et al. Novel Clade C-I Clostridium difficile strains escape diagnostic tests, differ in pathogenicity potential and carry toxins on extrachromosomal elements. Sci. Rep. 2018, 8, 13951. [Google Scholar] [CrossRef]
- Brouwer, M.S.M.; Roberts, A.P.; Hussain, H.; Williams, R.J.; Allan, E.; Mullany, P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat. Commun. 2013, 4, 2601. [Google Scholar] [CrossRef] [PubMed]
- Mullany, P.; Allan, E.; Roberts, A.P. Mobile genetic elements in Clostridium difficile and their role in genome function. Res. Microbiol. 2015, 166, 361–367. [Google Scholar] [CrossRef]
- Monot, M.; Eckert, C.; Lemire, A.; Hamiot, A.; Dubois, T.; Tessier, C.; Dumoulard, B.; Hamel, B.; Petit, A.; Lalande, V.; et al. Clostridium difficile: New Insights into the Evolution of the Pathogenicity Locus. Sci. Rep. 2015, 5, 15023. [Google Scholar] [CrossRef] [PubMed]
- Fettucciari, K.; Dini, F.; Marconi, P.; Bassotti, G. Role of the Alteration in Calcium Homeostasis in Cell Death Induced by Clostridioides difficile Toxin A and Toxin, B. Biology 2023, 12, 1117. [Google Scholar] [CrossRef]
- Janezic, S.; Dingle, K.; Alvin, J.; Accetto, T.; Didelot, X.; Crook, D.W.; Lacy, D.B.; Rupnik, M. Comparative genomics of Clostridioides difficile toxinotypes identifies module-based toxin gene evolution. Microb. Genom. 2020, 6, e000449. [Google Scholar] [CrossRef]
- Orrell, K.E.; Melnyk, R.A. Large clostridial toxins: Mechanisms and roles in disease. Microbiol. Mol. Biol. Rev. 2021, 85, e0006421. [Google Scholar] [CrossRef]
- Mastrantonio, P.; Rupnik, M. (Eds.) Updates on Clostridioides difficile in Europe: Advances in Microbiology, Infectious Diseases and Public Health Volume 18; Springer International Publishing: Cham, Switzerland, 2024; Volume 1435. [Google Scholar] [CrossRef]
- Lyerly, D.M.; Lockwood, D.E.; Richardson, S.H.; Wilkins, T.D. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. 1982, 35, 1147–1150. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Ketley, J.M.; Haslam, S.C.; Stephen, J.; Burdon, D.W.; Candy, D.C.; Daniel, R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut 1986, 27, 78–85. [Google Scholar] [CrossRef]
- Lyerly, D.M.; Krivan, H.C.; Wilkins, T.D. Clostridium difficile: Its disease and toxins. Clin. Microbiol. Rev. 1988, 1, 1–18. [Google Scholar] [CrossRef]
- Moncrief, J.S.; Zheng, L.; Neville, L.M.; Lyerly, D.M. Genetic characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates by PCR. J. Clin. Microbiol. 2000, 38, 3072–3075. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.P.; Rood, J.I.; Lyras, D. The role of toxin A and toxin B in Clostridium difficile-associated disease: Past and present perspectives. Gut Microbes 2010, 1, 58–64. [Google Scholar] [CrossRef]
- Couture-Cossette, A.; Carignan, A.; Ilangumaran, S.; Valiquette, L. Bezlotoxumab for the prevention of Clostridium difficile recurrence. Expert. Opin. Biol. Ther. 2017, 17, 1439–1445. [Google Scholar] [CrossRef]
- Fettucciari, K.; Marguerie, F.; Fruganti, A.; Marchegiani, A.; Spaterna, A.; Brancorsini, S.; Marconi, P.; Bassotti, G. Clostridioides difficile toxin B alone and with pro-inflammatory cytokines induces apoptosis in enteric glial cells by activating three different signalling pathways mediated by caspases, calpains and cathepsin B. Cell. Mol. Life Sci. 2022, 79, 442. [Google Scholar] [CrossRef]
- Pourliotopoulou, E.; Karampatakis, T.; Kachrimanidou, M. Exploring the Toxin-Mediated Mechanisms in Clostridioides difficile Infection. Microorganisms 2024, 12, 1004. [Google Scholar] [CrossRef]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile toxins: Mechanisms of action and antitoxin therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef]
- Shen, E.; Zhu, K.; Li, D.; Pan, Z.; Luo, Y.; Bian, Q.; He, L.; Song, X.; Zhen, Y.; Jin, D.; et al. Subtyping analysis reveals new variants and accelerated evolution of Clostridioides difficile toxin B. Commun. Biol. 2020, 3, 347. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, Y.; Luo, J.; Li, D.; Zhou, Y.; He, L.; Yang, Q.; Dong, M.; Tao, L. Functional analyses of epidemic Clostridioides difficile toxin B variants reveal their divergence in utilizing receptors and inducing pathology. PLoS Pathog. 2021, 17, e1009197. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Tremblay, B.J.-M.; Zeng, J.; Wei, X.; Hodgins, H.; Worley, J.; Bry, L.; Dong, M.; Doxey, A.C. Phylogenomics of 8,839 Clostridioides difficile genomes reveals recombination-driven evolution and diversification of toxin A and B. PLoS Pathog. 2020, 16, e1009181. [Google Scholar] [CrossRef] [PubMed]
- Janezic, S.; Rupnik, M. Genomic diversity of Clostridium difficile strains. Res. Microbiol. 2015, 166, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ducarmon, Q.R.; van der Bruggen, T.; Harmanus, C.; Sanders, I.M.J.G.; Daenen, L.G.M.; Fluit, A.C.; Vossen, R.H.A.M.; Kloet, S.L.; Kuijper, E.J.; Smits, W.K. Clostridioides difficile infection with isolates of cryptic clade C-II: A genomic analysis of polymerase chain reaction ribotype 151. Clin. Microbiol. Infect. 2023, 29, 538.e1–538.e6. [Google Scholar] [CrossRef]
- Landenberger, M.; Nieland, J.; Roeder, M.; Nørgaard, K.; Papatheodorou, P.; Ernst, K.; Barth, H. The cytotoxic effect of Clostridioides difficile pore-forming toxin CDTb. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183603. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef]
- Rupnik, M.; Grabnar, M.; Geric, B. Binary toxin producing Clostridium difficile strains. Anaerobe 2003, 9, 289–294. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Lacy, D.B. The role of toxins in Clostridium difficile infection. FEMS Microbiol. Rev. 2017, 41, 723–750. [Google Scholar] [CrossRef]
- Eckert, C.; Emirian, A.; Le Monnier, A.; Cathala, L.; De Montclos, H.; Goret, J.; Berger, P.; Petit, A.; De Chevigny, A.; Jean-Pierre, H.; et al. Prevalence and pathogenicity of binary toxin-positive Clostridium difficile strains that do not produce toxins A and B. New Microbes New Infect. 2015, 3, 12–17. [Google Scholar] [CrossRef]
- Androga, G.O.; Knight, D.R.; Lim, S.-C.; Foster, N.F.; Riley, T.V. Antimicrobial resistance in large clostridial toxin-negative, binary toxin-positive Clostridium difficile ribotypes. Anaerobe 2018, 54, 55–60. [Google Scholar] [CrossRef]
- Stiles, B.G.; Pradhan, K.; Fleming, J.M.; Samy, R.P.; Barth, H.; Popoff, M.R. Clostridium and bacillus binary enterotoxins: Bad for the bowels, and eukaryotic being. Toxins 2014, 6, 2626–2656. [Google Scholar] [CrossRef] [PubMed]
- López-Cárdenas, S.; Torres-Martos, E.; Mora-Delgado, J.; Sánchez-Calvo, J.M.; Santos-Peña, M.; Zapata López, Á.; Dolores López-Prieto, M.; Pérez-Cortés, S.; Carlos Alados, J. The prognostic value of toxin B and binary toxin in Clostridioides difficile infection. Gut Microbes 2021, 13, 1884516. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Pham, N.V.S.; Hays, R.A.; Bolick, D.T.; Goldbeck, S.M.; Poulter, M.D.; Hoang, S.C.; Shin, J.H.; Wu, M.; Warren, C.A. Influence of Binary Toxin Gene Detection and Decreased Susceptibility to Antibiotics among Clostridioides difficile Strains on Disease Severity: A Single-Center Study. Antimicrob. Agents Chemother. 2022, 66, e0048922. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S.; Mølbak, K.; Kjeldsen, M.K.; Olsen, K.E.P. Binary toxin and death after Clostridium difficile infection. Emerg. Infect. Dis. 2011, 17, 976–982. [Google Scholar] [CrossRef]
- Huber, C.A.; Foster, N.F.; Riley, T.V.; Paterson, D.L. Challenges for standardization of Clostridium difficile typing methods. J. Clin. Microbiol. 2013, 51, 2810–2814. [Google Scholar] [CrossRef]
- Abad-Fau, A.; Sevilla, E.; Martín-Burriel, I.; Moreno, B.; Bolea, R. Update on Commonly Used Molecular Typing Methods for Clostridioides difficile. Microorganisms 2023, 11, 1752. [Google Scholar] [CrossRef]
- Baktash, A.; Corver, J.; Harmanus, C.; Smits, W.K.; Fawley, W.; Wilcox, M.H.; Kumar, N.; Eyre, D.W.; Indra, A.; Mellmann, A.; et al. Comparison of Whole-Genome Sequence-Based Methods and PCR Ribotyping for Subtyping of Clostridioides difficile. J. Clin. Microbiol. 2021, 60, e01737-21. [Google Scholar] [CrossRef] [PubMed]
- Bogiel, T.; Dura, A.; Woźniak, M.; Mikucka, A.; Kanarek, P. Usefulness of Capillary Gel Electrophoresis-Based PCR for Detection of Clostridioides difficile Strains with Hypervirulent Ribotypes. Gels 2024, 10, 343. [Google Scholar] [CrossRef]
- Knight, D.R.; Imwattana, K.; Kullin, B.; Guerrero-Araya, E.; Paredes-Sabja, D.; Didelot, X.; Dingle, K.E.; Eyre, D.W.; Rodríguez, C.; Riley, T.V. Major genetic discontinuity and novel toxigenic species in Clostridioides difficile taxonomy. ELife 2021, 10, e64325. [Google Scholar] [CrossRef]
- He, M.; Sebaihia, M.; Lawley, T.D.; Stabler, R.A.; Dawson, L.F.; Martin, M.J.; Holt, K.E.; Seth-Smith, H.M.B.; Quail, M.A.; Rance, R.; et al. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc. Natl. Acad. Sci. USA 2010, 107, 7527–7532. [Google Scholar] [CrossRef]
- Williamson, C.H.D.; Stone, N.E.; Nunnally, A.E.; Roe, C.C.; Vazquez, A.J.; Lucero, S.A.; Hornstra, H.; Wagner, D.M.; Keim, P.; Rupnik, M.; et al. Identification of novel, cryptic Clostridioides species isolates from environmental samples collected from diverse geographical locations. Microb. Genom. 2022, 8, 742. [Google Scholar] [CrossRef]
- Mengoli, M.; Barone, M.; Fabbrini, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Make It Less difficile: Understanding Genetic Evolution and Global Spread of Clostridioides difficile. Genes 2022, 13, 2200. [Google Scholar] [CrossRef]
- Shaw, H.A.; Preston, M.D.; Vendrik, K.E.W.; Cairns, M.D.; Browne, H.P.; Stabler, R.A.; Crobach, M.J.T.; Corver, J.; Pituch, H.; Ingebretsen, A.; et al. The recent emergence of a highly related virulent Clostridium difficile clade with unique characteristics. Clin. Microbiol. Infect. 2020, 26, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.R.; Kullin, B.; Androga, G.O.; Barbut, F.; Eckert, C.; Johnson, S.; Spigaglia, P.; Tateda, K.; Tsai, P.-J.; Riley, T.V. Evolutionary and Genomic Insights into Clostridioides difficile Sequence Type 11: A Diverse Zoonotic and Antimicrobial-Resistant Lineage of Global One Health Importance. mBio 2019, 10, e00446-19. [Google Scholar] [CrossRef] [PubMed]
- Razaq, N.; Sambol, S.; Nagaro, K.; Zukowski, W.; Cheknis, A.; Johnson, S.; Gerding, D.N. Infection of hamsters with historical and epidemic BI types of Clostridium difficile. J. Infect. Dis. 2007, 196, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsson, M.; Samore, M.H.; Gerding, D.N.; DeGirolami, P.C.; Bettin, K.M.; Karchmer, A.W.; Arbeit, R.D. Comparison of restriction endonuclease analysis, ribotyping, and pulsed-field gel electrophoresis for molecular differentiation of Clostridium difficile strains. J. Clin. Microbiol. 1994, 32, 1963–1969. [Google Scholar] [CrossRef]
- Loo, V.G.; Poirier, L.; Miller, M.A.; Oughton, M.; Libman, M.D.; Michaud, S.; Bourgault, A.M.; Nguyen, T.; Frenette, C.; Kelly, M.; et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 2005, 353, 2442–2449, Erratum in N. Engl. J. Med. 2006, 354, 2200. [Google Scholar] [CrossRef] [PubMed]
- Archibald, L.K.; Banerjee, S.N.; Jarvis, W.R. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987-2001. J. Infect. Dis. 2004, 189, 1585–1589. [Google Scholar] [CrossRef]
- McDonald, L.C.; Owings, M.; Jernigan, D.B. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg. Infect. Dis. 2006, 12, 409–415. [Google Scholar] [CrossRef]
- Kuijper, E.J.; Coignard, B.; Tüll, P.; ESCMID Study Group for Clostridium difficile; EU Member States, European Centre for Disease Prevention and Control. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 2006, 12 (Suppl. S6), 2–18. [Google Scholar] [CrossRef]
- Eggertson, L.; Sibbald, B. Hospitals battling outbreaks of C. difficile. CMAJ 2004, 171, 19–21. [Google Scholar] [CrossRef][Green Version]
- MacCannell, D.R.; Louie, T.J.; Gregson, D.B.; Laverdiere, M.; Labbe, A.-C.; Laing, F.; Henwick, S. Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from Eastern and Western Canada. J. Clin. Microbiol. 2006, 44, 2147–2152. [Google Scholar] [CrossRef]
- Health Protection Agency. Voluntary reporting of Clostridium difficile, England, Wales and Northern Ireland: 2004. CDR Weekly 2005, 15, 1–3. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/+/http://www.hpa.org.uk/cdr/archives/2005/cdr2005.pdf (accessed on 29 May 2024).
- Kuijper, E.J.; Barbut, F.; Brazier, J.S.; Kleinkauf, N.; Eckmanns, T.; Lambert, M.L.; Drudy, D.; Fitzpatrick, F.; Wiuff, C.; Brown, D.J.; et al. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Eurosurveillance 2008, 13, 18942. [Google Scholar] [CrossRef] [PubMed]
- Spigaglia, P.; Barbanti, F.; Louie, T.; Barbut, F.; Mastrantonio, P. Molecular analysis of the gyrA and gyrB quinolone resistance-determining regions of fluoroquinolone-resistant Clostridium difficile mutants selected in vitro. Antimicrob. Agents Chemother. 2009, 53, 2463–2468. [Google Scholar] [CrossRef]
- Wasels, F.; Kuehne, S.A.; Cartman, S.T.; Spigaglia, P.; Barbanti, F.; Minton, N.P.; Mastrantonio, P. Fluoroquinolone resistance does not impose a cost on the fitness of Clostridium difficile in vitro. Antimicrob. Agents Chemother. 2015, 59, 1794–1796. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, R.; Rimland, D.; Killum, E.; Lowery, H.K.; Johnson, T.M.; Killgore, G.; Tenover, F.C. Outbreak of Clostridium difficile infection in a long-term care facility: Association with gatifloxacin use. Clin. Infect. Dis. 2004, 38, 640–645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ly, N.F.; Flach, C.; Lysen, T.S.; Markov, E.; van Ballegooijen, H.; Rijnbeek, P.; Duarte-Salles, T.; Reyes, C.; John, L.H.; Karimi, L.; et al. Impact of European Union Label Changes for Fluoroquinolone-Containing Medicinal Products for Systemic and Inhalation Use: Post-Referral Prescribing Trends. Drug Saf. 2023, 46, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Brant, A.; Lewicki, P.; Wu, X.; Sze, C.; Johnson, J.P.; Ponsky, L.; Kaye, K.S.; Wise, G.J.; Shoag, J.E. Antibiotic use in hospital urinary tract infections after FDA regulation. J. Gen. Intern. Med. 2024, 39, 1414–1422. [Google Scholar] [CrossRef]
- Redmond, S.N.; Silva, S.Y.; Wilson, B.M.; Cadnum, J.L.; Donskey, C.J. Impact of Reduced Fluoroquinolone Use on Clostridioides difficile Infections Resulting From the Fluoroquinolone-Resistant Ribotype 027 Strain in a Veterans Affairs Medical Center. Pathog. Immun. 2019, 4, 251–259. [Google Scholar] [CrossRef]
- Wieczorkiewicz, J.T.; Skinner, A.M.; Cheknis, A.; Petrella, L.A.; Stevens, V.W.; Wright, L.M.; Gerding, D.N.; Johnson, S. Epidemiology of Clostridioides difficile infection at one hospital 10 years after an outbreak of the epidemic C. difficile strain BI/027: Changing strain prevalence, antimicrobial susceptibilities, and patient antibiotic exposures. Antimicrob. Agents Chemother. 2024, 68, e0069824. [Google Scholar] [CrossRef]
- Dingle, K.E.; Didelot, X.; Quan, T.P.; Eyre, D.W.; Stoesser, N.; Golubchik, T.; Harding, R.M.; Wilson, D.J.; Griffiths, D.; Vaughan, A.; et al. Effects of control interventions on Clostridium difficile infection in England: An observational study. Lancet Infect. Dis. 2017, 17, 411–421. [Google Scholar] [CrossRef]
- He, M.; Miyajima, F.; Roberts, P.; Ellison, L.; Pickard, D.J.; Martin, M.J.; Connor, T.R.; Harris, S.R.; Fairley, D.; Bamford, K.B.; et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 2013, 45, 109–113. [Google Scholar] [CrossRef]
- Emmerson, A.M.; Jones, A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 13–20. [Google Scholar] [CrossRef]
- Freeman, J.; Sanders, I.M.J.G.; Harmanus, C.; Clark, E.V.; Berry, A.M.; Smits, W.K. Antimicrobial susceptibility testing of Clostridioides difficile: A dual-site study of three different media and three therapeutic antimicrobials. Clin Microbiol Infect 2025, 31, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Vernon, J.; Pilling, S.; Morris, K.; Nicholson, S.; Shearman, S.; Longshaw, C.; Wilcox, M.H.; Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes Study Group. The ClosER study: Results from a three-year pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes, 2011-2014. Clin. Microbiol. Infect. 2018, 24, 724–731. [Google Scholar] [CrossRef]
- Fitzpatrick, F.; Brennan, R.; van Prehn, J.; Skally, M.; Brady, M.; Burns, K.; Rooney, C.; Wilcox, M.H. European practice for CDI treatment. Adv. Exp. Med. Biol. 2024, 1435, 57–84. [Google Scholar] [CrossRef]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. 2), S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Tickler, I.A.; Obradovich, A.E.; Goering, R.V.; Fang, F.C.; Tenover, F.C.; The HAI Consortium. Changes in molecular epidemiology and antimicrobial resistance profiles of Clostridioides (Clostridium) difficile strains in the United States between 2011 and 2017. Anaerobe 2019, 60, 102050. [Google Scholar] [CrossRef]
- Abdrabou, A.M.M.; Ul Habib Bajwa, Z.; Halfmann, A.; Mellmann, A.; Nimmesgern, A.; Margardt, L.; Bischoff, M.; von Müller, L.; Gärtner, B.; Berger, F.K. Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in Germany, 2014-2019. Int. J. Med. Microbiol. 2021, 311, 151507. [Google Scholar] [CrossRef]
- Kabała, M.; Gofron, Z.; Aptekorz, M.; Sacha, K.; Harmanus, C.; Kuijper, E.; Martirosian, G. Clostridioides difficile Ribotype 027 (RT027) Outbreak Investigation Due to the Emergence of Rifampicin Resistance Using Multilocus Variable-Number Tandem Repeat Analysis (MLVA). Infect. Drug Resist. 2021, 14, 3247–3254. [Google Scholar] [CrossRef]
- Adams, R.A.; Leon, G.; Miller, N.M.; Reyes, S.P.; Thantrong, C.H.; Thokkadam, A.M.; Lemma, A.S.; Sivaloganathan, D.M.; Wan, X.; Brynildsen, M.P. antibiotics and the mechanisms of their failure. J. Antibiot. 2021, 74, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.K.; Mellmann, A.; Färber, J.; von Müller, L.; Gärtner, B. Advantages and disadvantages of rifampicin use in orthopedic patients to avoid Clostridium difficile infections. J. Orthop. Ther. JORT-170 2018. [Google Scholar] [CrossRef]
- Hensgens, M.P.; Goorhuis, A.; Notermans, D.W.; van Benthem, B.H.; Kuijper, E.J. Decrease of hypervirulent Clostridium difficile PCR ribotype 027 in the Netherlands. Euro Surveill. 2009, 14, 19402. [Google Scholar] [CrossRef] [PubMed]
- Fawley, W.N.; Davies, K.A.; Morris, T.; Parnell, P.; Howe, R.; Wilcox, M.H.; Clostridium Difficile Ribotyping Network (CDRN) Working Group. Enhanced surveillance of Clostridium difficile infection occurring outside hospital, England, 2011 to 2013. Euro Surveill 2016, 21, 30295. [Google Scholar] [CrossRef]
- Giancola, S.E.; Williams, R.J.; Gentry, C.A. Prevalence of the Clostridium difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Clin. Microbiol. Infect. 2018, 24, 877–881. [Google Scholar] [CrossRef]
- Kachrimanidou, M.; Baktash, A.; Metallidis, S.; Tsachouridou, O.; Netsika, F.; Dimoglou, D.; Kassomenaki, A.; Mouza, E.; Haritonidou, M.; Kuijper, E. An outbreak of Clostridioides difficile infections due to a 027-like PCR ribotype 181 in a rehabilitation centre: Epidemiological and microbiological characteristics. Anaerobe 2020, 65, 102252. [Google Scholar] [CrossRef]
- Charisi, E.; Tsioka, K.; Karampatakis, T.; Kachrimanidou, M. Epidemiology of Clostridioides difficile PCR ribotype 181 after the COVID-19 pandemic in Northern Greece. Acta Microbiol. Immunol. Hung. 2024, 71, 315–323. [Google Scholar] [CrossRef]
- Szarek, K.; Smits Wiep, K.; Kabala, M.; Sanders, I.M.J.G.; Krutova, M.; Frankowska, N.; Iwanicki, A.; Wultanska, D.; Hinc, K.; Pituch, H.; et al. Prevalence Binary Toxin Producing Strains of Clostridioides difficile—Varied Ribotypes, Including RT955, in the Silesia Region of Southern Poland. In Abstract Book ICDS 2024, Proceedings of the 8th International C. Difficile Symposium, Bled, Slovenia, 17–19 September 2024; Nacionalni Laboratorij Za Zdravje, Okolje in Hrano: Maribor, Slovenia, 2024. [Google Scholar]
- Kartalidis, P.; Skoulakis, A.; Tsilipounidaki, K.; Florou, Z.; Petinaki, E.; Fthenakis, G.C. Clostridioides difficile as a Dynamic Vehicle for the Dissemination of Antimicrobial-Resistance Determinants: Review and In Silico Analysis. Microorganisms 2021, 9, 1383. [Google Scholar] [CrossRef]
- Curry, S.R.; Marsh, J.W.; Shutt, K.A.; Muto, C.A.; O’Leary, M.M.; Saul, M.I.; Pasculle, A.W.; Harrison, L.H. High frequency of rifampin resistance identified in an epidemic Clostridium difficile clone from a large teaching hospital. Clin. Infect. Dis. 2009, 48, 425–429. [Google Scholar] [CrossRef]
- Miller, M.A.; Blanchette, R.; Spigaglia, P.; Barbanti, F.; Mastrantonio, P. Divergent rifamycin susceptibilities of Clostridium difficile strains in Canada and Italy and predictive accuracy of rifampin Etest for rifamycin resistance. J. Clin. Microbiol. 2011, 49, 4319–4321. [Google Scholar] [CrossRef] [PubMed]
- Zikova, I.; Kabala, M.; Wultanska, D.; Frankowska, N.; Iwanicki, A.; Hinc, K.; Mucha, A.; Kuijper, E.; Pituch, H. The Recognition and Characterisation OF C. Difficile Ribotype 955 in Poland. In Abstract Book ICDS 2024, Proceedings of the 8th International C. Difficile Symposium, Bled, Slovenia, 17–19 September 2024; Nacionalni Laboratorij Za Zdravje, Okolje in Hrano: Maribor, Slovenia, 2024. [Google Scholar]
- Olaitan, A.O.; Dureja, C.; Youngblom, M.A.; Topf, M.A.; Shen, W.-J.; Gonzales-Luna, A.J.; Deshpande, A.; Hevener, K.E.; Freeman, J.; Wilcox, M.H.; et al. Decoding a cryptic mechanism of metronidazole resistance among globally disseminated fluoroquinolone-resistant Clostridioides difficile. Nat. Commun. 2023, 14, 4130. [Google Scholar] [CrossRef]
- Boekhoud, I.M.; Sidorov, I.; Nooij, S.; Harmanus, C.; Bos-Sanders, I.M.J.G.; Viprey, V.; Spittal, W.; Clark, E.; Davies, K.; Freeman, J.; et al. Haem is crucial for medium-dependent metronidazole resistance in clinical isolates of Clostridioides difficile. J. Antimicrob. Chemother. 2021, 76, 1731–1740. [Google Scholar] [CrossRef]
- Kolte, B.; Nübel, U. Genetic determinants of resistance to antimicrobial therapeutics are rare in publicly available Clostridioides difficile genome sequences. J. Antimicrob. Chemother. 2024, 79, 1320–1328. [Google Scholar] [CrossRef]
- Goyal, M.; Hauben, L.; Pouseele, H.; Jaillard, M.; De Bruyne, K.; van Belkum, A.; Goering, R. Retrospective Definition of Clostridioides difficile PCR Ribotypes on the Basis of Whole Genome Polymorphisms: A Proof of Principle Study. Diagnostics 2020, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Valiente, E.; Dawson, L.F.; Cairns, M.D.; Stabler, R.A.; Wren, B.W. Emergence of new PCR ribotypes from the hypervirulent Clostridium difficile 027 lineage. J. Med. Microbiol. 2012, 61, 49–56. [Google Scholar] [CrossRef]
- Valiente, E.; Cairns, M.D.; Wren, B.W. The Clostridium difficile PCR ribotype 027 lineage: A pathogen on the move. Clin. Microbiol. Infect. 2014, 20, 396–404. [Google Scholar] [CrossRef]
- Zikova, J.; Szarek, K.; Kabała, M.; Wultańska, D.; Frankowska, N.; Iwanicki, A.; Hinc, K.; Mucha, A.; Komarnicka, J.; Jagielska, A.; et al. Newly emerging metronidazole-resistant Clostridioides difficile PCR ribotype 955 identified in Poland, 2021 to 2023 but not in Czechia, 2012 to 2023 and Slovakia, 2015 to 2023. Eurosurveillance 2025, 30, 2400675. [Google Scholar] [CrossRef] [PubMed]
- Knetsch, C.W.; Terveer, E.M.; Lauber, C.; Gorbalenya, A.E.; Harmanus, C.; Kuijper, E.J.; Corver, J.; van Leeuwen, H.C. Comparative analysis of an expanded Clostridium difficile reference strain collection reveals genetic diversity and evolution through six lineages. Infect. Genet. Evol. 2012, 12, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Stabler, R.A.; He, M.; Dawson, L.; Martin, M.; Valiente, E.; Corton, C.; Lawley, T.D.; Sebaihia, M.; Quail, M.A.; Rose, G.; et al. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 2009, 10, R102. [Google Scholar] [CrossRef]
- Dingle, K.E.; Freeman, J.; Didelot, X.; Quan, T.P.; Eyre, D.W.; Swann, J.; Spittal, W.D.; Clark, E.V.; Jolley, K.A.; Walker, A.S.; et al. Penicillin Binding Protein Substitutions Cooccur with Fluoroquinolone Resistance in Epidemic Lineages of Multidrug-Resistant Clostridioides difficile. mBio 2023, 14, e0024323. [Google Scholar] [CrossRef]
- Freeman, J.; Vernon, J.; Pilling, S.; Morris, K.; Nicolson, S.; Shearman, S.; Clark, E.; Palacios-Fabrega, J.A.; Wilcox, M.; Pan-European Longitudinal Surveillance of Antibiotic Resistance Among Prevalent Clostridium Difficile Ribotypes’ Study Group. Five-year Pan-European, longitudinal surveillance of Clostridium difficile ribotype prevalence and antimicrobial resistance: The extended ClosER study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 169–177. [Google Scholar] [CrossRef]
- Polivkova, S.; Krutova, M.; Petrlova, K.; Benes, J.; Nyc, O. Clostridium difficile ribotype 176—A predictor for high mortality and risk of nosocomial spread? Anaerobe 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Toporová, A.; Čurová, K.; Novotný, M.; Lovayová, V.; Nagyová, M.; Siegfried, L.; Takáčová, V.; Lišková, A.; Longauerová, A.; Vukušičová Uhrinová, M.B.; et al. Characteristics of Clostridioides difficile isolates circulating in the Slovak hospitals. Biologia 2023, 78, 3287–3294. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (RIVM). Annual report C. difficile Reference Laboratory May 2019–Jan 2021, RIVM. Available online: https://www.rivm.nl/documenten/annual-report-c-difficile-reference-laboratory-may-2019-jan-2021 (accessed on 22 January 2024).
- Diniz, A.N.; Moura, L.N.F.; Cruz, D.S.G.; Oliveira Junior, C.A.; Figueiredo, H.C.P.; Cunha, J.L.R.; Vilela, E.G.; Kuijper, E.J.; Wilcox, M.H.; Lobato, F.C.F.; et al. Characterization of the virulence of three novel clade 2 Clostridioides (Clostridium) difficile strains and a two-year screening in animals and humans in Brazil. PLoS ONE 2022, 17, e0273013. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Stuart, R.L.; Mackin, K.E.; Carter, G.P.; Kotsanas, D.; Francis, M.J.; Easton, M.; Dimovski, K.; Elliott, B.; Riley, T.V.; et al. Emergence of a ribotype 244 strain of Clostridium difficile associated with severe disease and related to the epidemic ribotype 027 strain. Clin. Infect. Dis. 2014, 58, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Hertfordshire Care Providers Association. Emergence of New Clostridioides difficile Ribotype (955)—HCPA [Internet]. 2024. Available online: https://www.hcpa.info/gov-uk/emergence-of-new-clostridioides-difficile-ribotype-955/ (accessed on 9 October 2025).
- Tóth, J.; Urbán, E.; Osztie, H.; Benczik, M.; Indra, A.; Nagy, E.; Allerberger, F. Distribution of PCR ribotypes among recent Clostridium difficile isolates collected in two districts of Hungary using capillary gel electrophoresis and review of changes in the circulating ribotypes over time. J. Med. Microbiol. 2016, 65, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Clostridioides Difficile Infections—Annual Epidemiological Report for 2018−2020. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/clostridioides-difficile-infections-annual-epidemiological-report-2018-2020 (accessed on 2 April 2025).
- Plankaova, A.; Brajerova, M.; Capek, V.; Balikova Novotna, G.; Kinross, P.; Skalova, J.; Soltesova, A.; Drevinek, P.; Krutova, M. Clostridioides difficile infections were predominantly driven by fluoroquinolone-resistant Clostridioides difficile ribotypes 176 and 001 in Slovakia in 2018-2019. Int. J. Antimicrob. Agents 2023, 62, 106824. [Google Scholar] [CrossRef]
- Dirks, E.E.; Pfiffer, V.; Sohl, G.; Berger, F.K.; Friesen, I.; Friesen, J.; Ignatius, R.; Elias, J.; Mellmann, A.; Arvand, M. Whole genome sequence analysis reveals limited diversity among Clostridioides difficile ribotype 027 and 078 isolates collected in 22 hospitals in Berlin and Brandenburg, Germany. Antimicrob. Resist. Infect. Control 2025, 14, 56. [Google Scholar] [CrossRef]
- Szarek, K.; Frankowska, N.; Kabała, M.; Smits, W.K.; Wultańska, D.; Lalowski, P.; Pituch, H.; Iwanicki, A.; Hinc, K.; Harmanus, C.; et al. Dominance of toxigenic Clostridioides difficile strains and the appearance of the emerging PCR ribotype 955 in hospitals in Silesia, Poland. Front. Microbiol. 2025, 16, 1644051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tickler, I.A.; Goering, R.V.; Tenover, F.C. History and Evolution of the Hypervirulent Clostridioides difficile Ribotype 027 Lineage. Microorganisms 2025, 13, 2376. https://doi.org/10.3390/microorganisms13102376
Tickler IA, Goering RV, Tenover FC. History and Evolution of the Hypervirulent Clostridioides difficile Ribotype 027 Lineage. Microorganisms. 2025; 13(10):2376. https://doi.org/10.3390/microorganisms13102376
Chicago/Turabian StyleTickler, Isabella A., Richard V. Goering, and Fred C. Tenover. 2025. "History and Evolution of the Hypervirulent Clostridioides difficile Ribotype 027 Lineage" Microorganisms 13, no. 10: 2376. https://doi.org/10.3390/microorganisms13102376
APA StyleTickler, I. A., Goering, R. V., & Tenover, F. C. (2025). History and Evolution of the Hypervirulent Clostridioides difficile Ribotype 027 Lineage. Microorganisms, 13(10), 2376. https://doi.org/10.3390/microorganisms13102376






