Role of IFN-γ from Different Immune Cells in Chlamydia Infection
Abstract
1. Introduction
2. The Role of Immune Cells in Host Resistance Against Chlamydial Infection
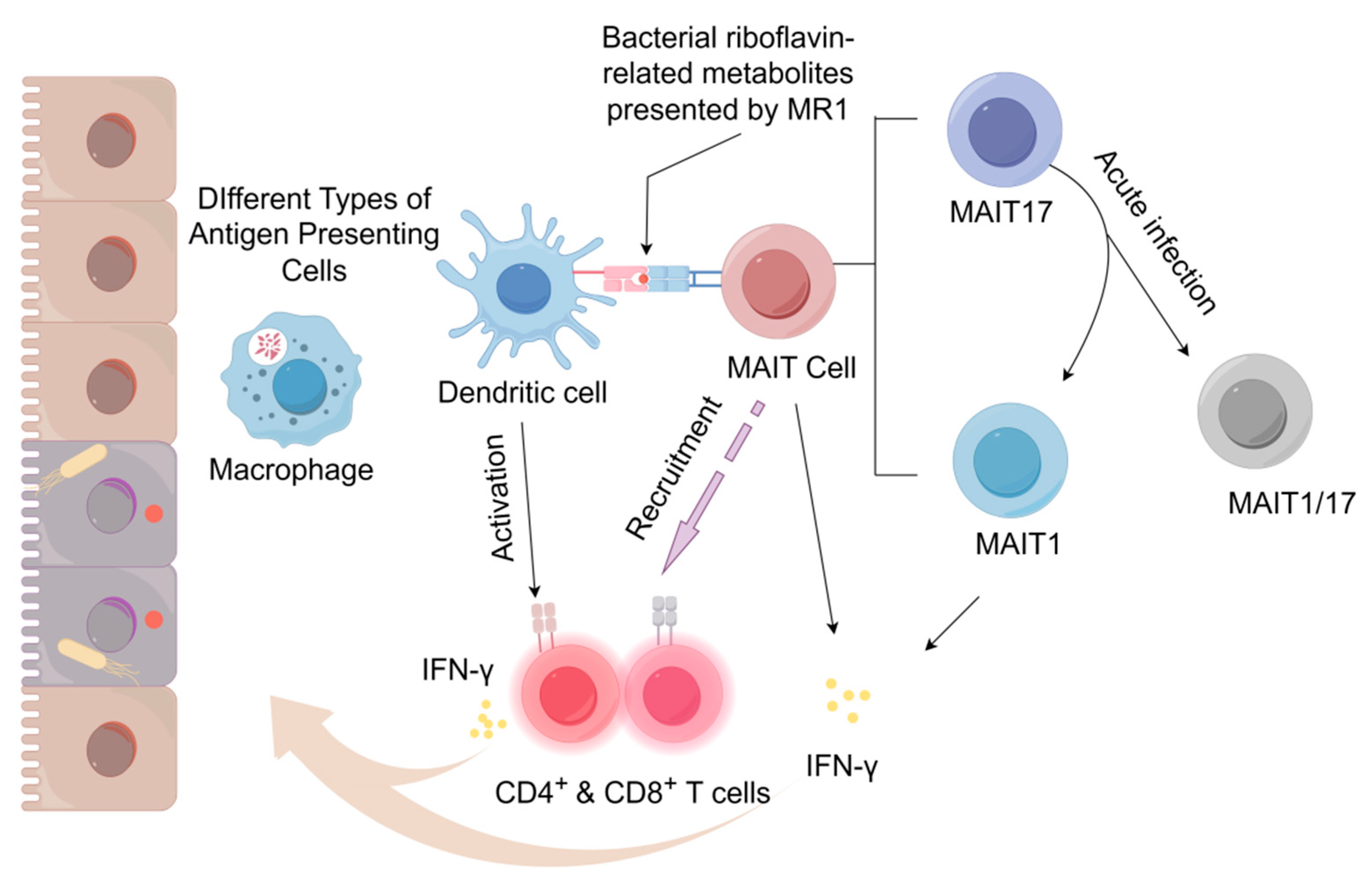
2.1. MAIT Cells
2.2. NKT Cells
2.3. NK Cells
2.4. The Role of Ex-ILC3s in Chlamydia Infection
2.5. T-Cell Resistance Against Chlamydial Infection
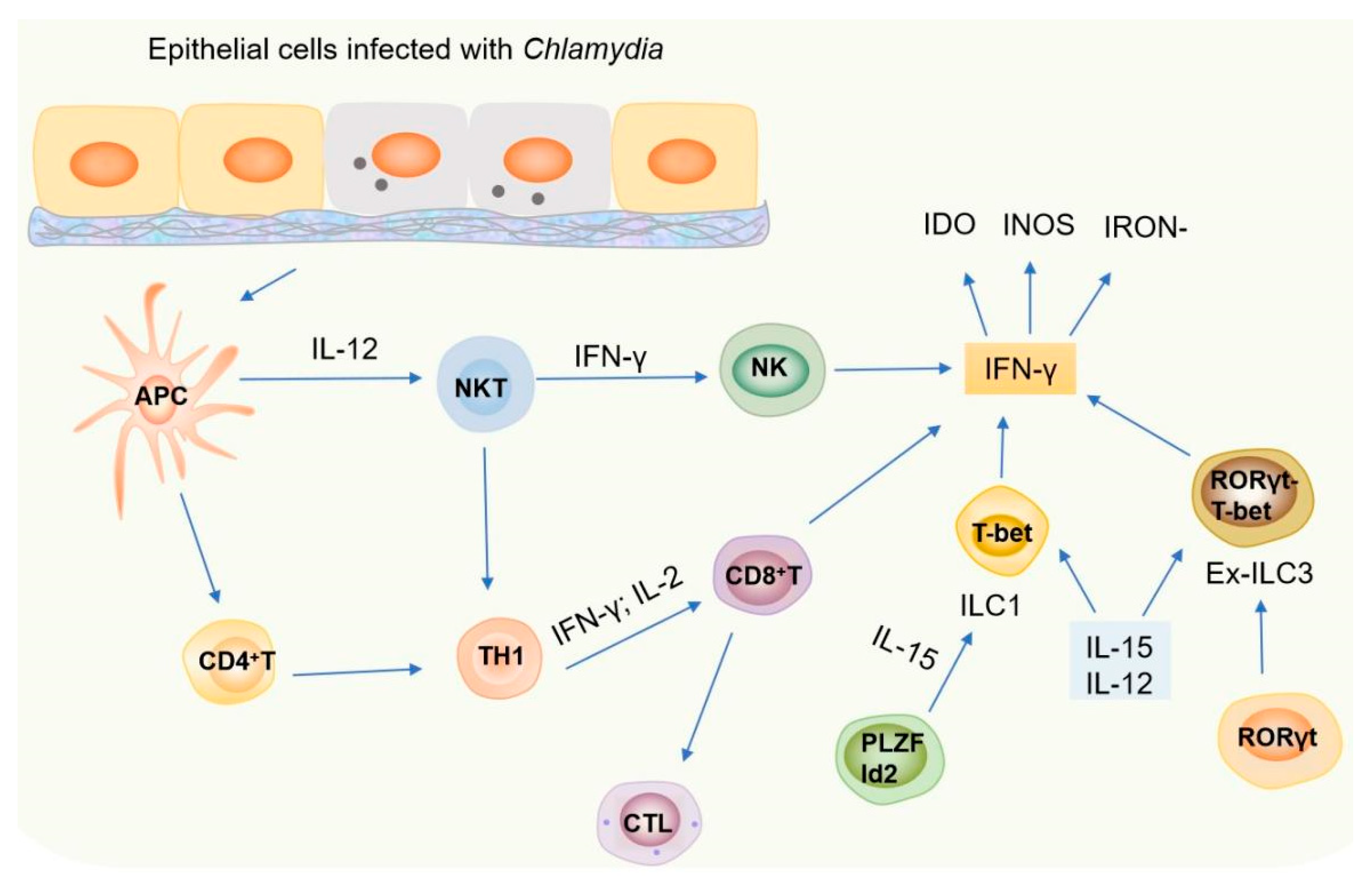
3. How Does the Induced IFN-γ Affect Chlamydia Infection and Pathogenesis
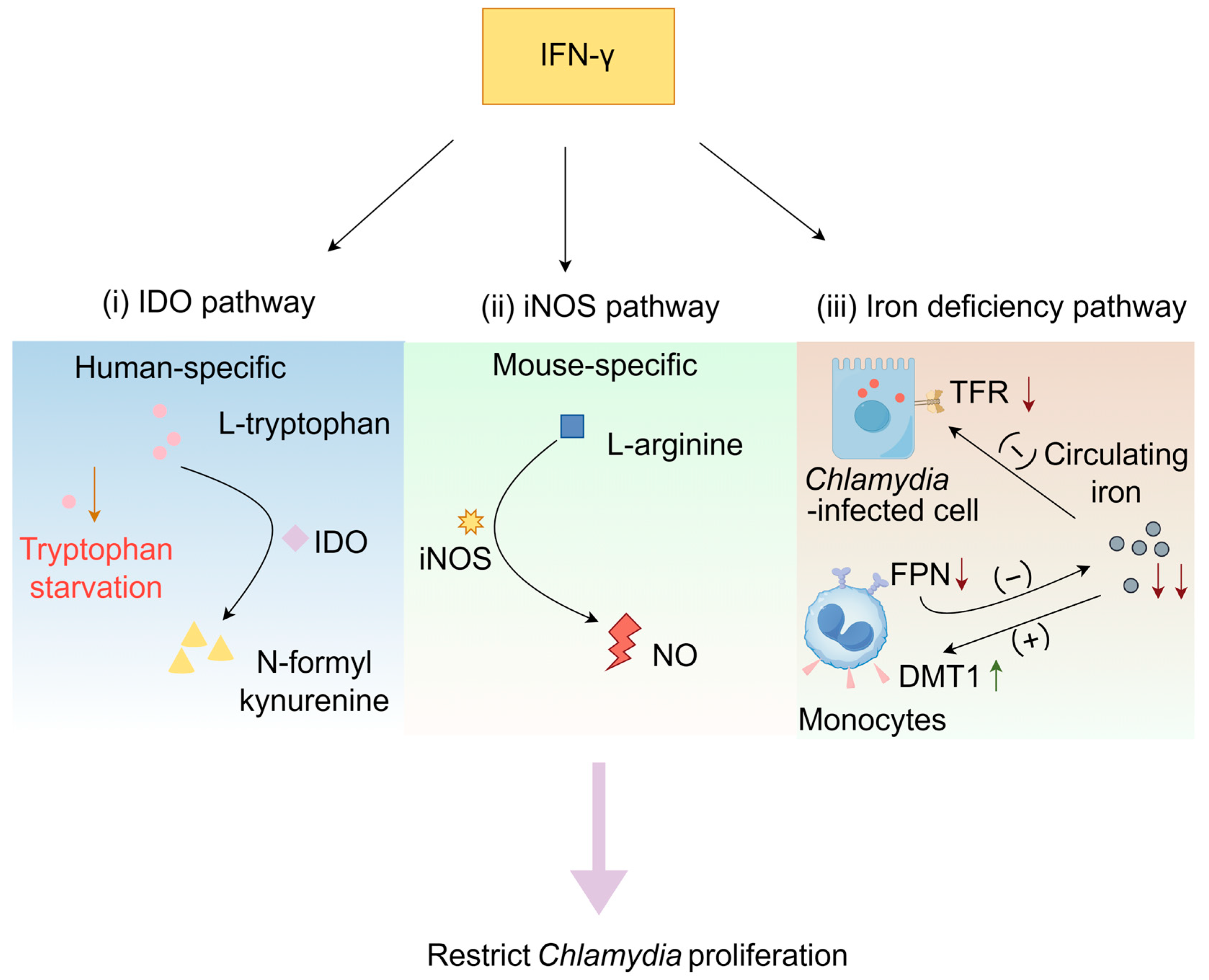
3.1. Mechanism of Action of the IFN-γ
3.1.1. IDO Pathway
3.1.2. IFN-γ-Induced Activation of the iNOS Pathway
3.1.3. IFN-γ-Induced Iron Deficiency Pathway
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howe, S.E.; Shillova, N.; Konjufca, V. Dissemination of Chlamydia from the Reproductive Tract to the Gastro-Intestinal Tract Occurs in Stages and Relies on Chlamydia Transport by Host Cells. PLoS Pathog. 2019, 15, e1008207. [Google Scholar] [CrossRef]
- Stephens, R.S.; Kalman, S.; Lammel, C.; Fan, J.; Marathe, R.; Aravind, L.; Mitchell, W.; Olinger, L.; Tatusov, R.L.; Zhao, Q.; et al. Genome Sequence of an Obligate Intracellular Pathogen of Humans: Chlamydia trachomatis. Science 1998, 282, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Teixeira da Costa, A.R.; Zietlow, R.; Meyer, T.F.; Rudel, T. Modulation of Host Cell Metabolism by Chlamydia trachomatis. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Onorini, D.; Borel, N.; Schoborg, R.V.; Leonard, C.A. Neisseria Gonorrhoeae Limits Chlamydia trachomatis Inclusion Development and Infectivity in a Novel In Vitro Co-Infection Model. Front. Cell Infect. Microbiol. 2022, 12, 911818. [Google Scholar] [CrossRef] [PubMed]
- Kalman, S.; Mitchell, W.; Marathe, R.; Lammel, C.; Fan, J.; Hyman, R.W.; Olinger, L.; Grimwood, J.; Davis, R.W.; Stephens, R. Comparative Genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 1999, 21, 385–389. [Google Scholar] [CrossRef]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirsalehian, A.; Bahador, A. Association of Chlamydia trachomatis with Infertility and Clinical Manifestations: A Systematic Review and Meta-Analysis of Case-Control Studies. Infect. Dis. 2016, 48, 517–523. [Google Scholar] [CrossRef]
- Rottenberg, M.E.; Gigliotti-Rothfuchs, A.; Wigzell, H. The Role of IFN-γ in the Outcome of Chlamydial Infection. Curr. Opin. Immunol. 2002, 14, 444–451. [Google Scholar] [CrossRef]
- Johnson, R.M. Murine Oviduct Epithelial Cell Cytokine Responses to Chlamydia muridarum Infection Include Interleukin-12-P70 Secretion. Infect. Immun. 2004, 72, 3951–3960. [Google Scholar] [CrossRef]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia Infection: Implications for a Chlamydia trachomatis Vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef]
- Mpiga, P.; Mansour, S.; Morisset, R.; Beaulieu, R.; Ravaoarinoro, M. Sustained Interleukin-6 and Interleukin-8 Expression Following Infection with Chlamydia trachomatis Serovar L2 in a HeLa/THP-1 Cell Co-culture Model. Scand. J. Immunol. 2006, 63, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Faris, R.; Andersen, S.E.; McCullough, A.; Gourronc, F.; Klingelhutz, A.J.; Weber, M.M. Chlamydia trachomatis Serovars Drive Differential Production of Proinflammatory Cytokines and Chemokines Depending on the Type of Cell Infected. Front. Cell. Infect. Microbiol. 2019, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Jendro, M.C.; Raum, E.; Schnarr, S.; Köhler, L.; Zeidler, H.; Kuipers, J.G.; Martin, M. Cytokine Profile in Serum and Synovial Fluid of Arthritis Patients with Chlamydia trachomatis Infection. Rheumatol. Int. 2005, 25, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.; Kothe, H.; Mueller, A.; Maass, M.; Dalhoff, K. Imbalanced Secretion of IL-1beta and IL-1RA in Chlamydia pneumoniae-Infected Mononuclear Cells from COPD Patients. Eur. Respir. J. 2003, 22, 274–279. [Google Scholar] [CrossRef]
- Xiang, W.; Yu, N.; Lei, A.; Li, X.; Tan, S.; Huang, L.; Zhou, Z. Insights Into Host Cell Cytokines in Chlamydia Infection. Front. Immunol. 2021, 12, 639834. [Google Scholar] [CrossRef]
- Yang, X. Role of Cytokines in Chlamydia trachomatis Protective Immunity and Immunopathology. CPD 2003, 9, 67–73. [Google Scholar] [CrossRef]
- Perfettini, J.-L.; Darville, T.; Dautry-Varsat, A.; Rank, R.G.; Ojcius, D.M. Inhibition of Apoptosis by Gamma Interferon in Cells and Mice Infected with Chlamydia muridarum (the Mouse Pneumonitis Strain of Chlamydia trachomatis). Infect. Immun. 2002, 70, 2559–2565. [Google Scholar] [CrossRef]
- Webster, S.J.; Brode, S.; Ellis, L.; Fitzmaurice, T.J.; Elder, M.J.; Gekara, N.O.; Tourlomousis, P.; Bryant, C.; Clare, S.; Chee, R.; et al. Detection of a Microbial Metabolite by STING Regulates Inflammasome Activation in Response to Chlamydia trachomatis Infection. PLoS Pathog. 2017, 13, e1006383. [Google Scholar] [CrossRef]
- Nagarajan, U.M.; Sikes, J.; Prantner, D.; Andrews, C.W.; Frazer, L.; Goodwin, A.; Snowden, J.N.; Darville, T. MyD88 Deficiency Leads to Decreased NK Cell Gamma Interferon Production and T Cell Recruitment during Chlamydia muridarum Genital Tract Infection, but a Predominant Th1 Response and Enhanced Monocytic Inflammation Are Associated with Infection Resolution. Infect. Immun. 2011, 79, 486–498. [Google Scholar] [CrossRef]
- Linhares, I.M.; Witkin, S.S. Immunopathogenic Consequences of Chlamydia trachomatis 60 kDa Heat Shock Protein Expression in the Female Reproductive Tract. Cell Stress Chaperones 2010, 15, 467–473. [Google Scholar] [CrossRef]
- Kinnunen, A.; Surcel, H.-M.; Halttunen, M.; Tiitinen, A.; Morrison, R.P.; Morrison, S.G.; Koskela, P.; Lehtinen, M.; Paavonen, J. Chlamydia trachomatis Heat Shock Protein-60 Induced Interferon-Gamma and Interleukin-10 Production in Infertile Women. Clin. Exp. Immunol. 2003, 131, 299–303. [Google Scholar] [CrossRef]
- Vollmuth, N.; Schlicker, L.; Guo, Y.; Hovhannisyan, P.; Janaki-Raman, S.; Kurmasheva, N.; Schmitz, W.; Schulze, A.; Stelzner, K.; Rajeeve, K.; et al. C-Myc Plays a Key Role in IFN-γ-Induced Persistence of Chlamydia trachomatis. eLife 2022, 11, e76721. [Google Scholar] [CrossRef]
- Burian, K.; Endresz, V.; Deak, J.; Kormanyos, Z.; Pal, A.; Nelson, D.; Virok, D.P. Transcriptome Analysis Indicates an Enhanced Activation of Adaptive and Innate Immunity by Chlamydia-Infected Murine Epithelial Cells Treated with Interferon γ. J. Infect. Dis. 2010, 202, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Frucht, D.M.; Fukao, T.; Bogdan, C.; Schindler, H.; O’Shea, J.J.; Koyasu, S. IFN-Gamma Production by Antigen-Presenting Cells: Mechanisms Emerge. Trends Immunol. 2001, 22, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Rothfuchs, A.G.; Gigliotti, D.; Palmblad, K.; Andersson, U.; Wigzell, H.; Rottenberg, M.E. IFN-Alpha Beta-Dependent, IFN-Gamma Secretion by Bone Marrow-Derived Macrophages Controls an Intracellular Bacterial Infection. J. Immunol. 2001, 167, 6453–6461. [Google Scholar] [CrossRef] [PubMed]
- Winner, H.; Friesenhahn, A.; Wang, Y.; Stanbury, N.; Wang, J.; He, C.; Zhong, G. Regulation of Chlamydial Colonization by IFNγ Delivered via Distinct Cells. Trends Microbiol. 2023, 31, 270–279. [Google Scholar] [CrossRef]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.-J.; Yu, Y.Y.L.; et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef]
- Sharma, P.K.; Wong, E.B.; Napier, R.J.; Bishai, W.R.; Ndung’u, T.; Kasprowicz, V.O.; Lewinsohn, D.A.; Lewinsohn, D.M.; Gold, M.C. High Expression of CD26 Accurately Identifies Human Bacteria-Reactive MR1-Restricted MAIT Cells. Immunology 2015, 145, 443–453. [Google Scholar] [CrossRef]
- Gracey, E.; Qaiyum, Z.; Almaghlouth, I.; Lawson, D.; Karki, S.; Avvaru, N.; Zhang, Z.; Yao, Y.; Ranganathan, V.; Baglaenko, Y.; et al. IL-7 Primes IL-17 in Mucosal-Associated Invariant T (MAIT) Cells, Which Contribute to the Th17-Axis in Ankylosing Spondylitis. Ann. Rheum. Dis. 2016, 75, 2124–2132. [Google Scholar] [CrossRef]
- Tilloy, F.; Treiner, E.; Park, S.-H.; Garcia, C.; Lemonnier, F.; De La Salle, H.; Bendelac, A.; Bonneville, M.; Lantz, O. An Invariant T Cell Receptor α Chain Defines a Novel TAP-Independent Major Histocompatibility Complex Class Ib–Restricted α/β T Cell Subpopulation in Mammals. J. Exp. Med. 1999, 189, 1907–1921. [Google Scholar] [CrossRef]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of Evolutionarily Conserved Mucosal-Associated Invariant T Cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Chengalroyen, M.D.; Mehaffy, C.; Lucas, M.; Bauer, N.; Raphela, M.L.; Oketade, N.; Warner, D.F.; Lewinsohn, D.A.; Lewinsohn, D.M.; Dobos, K.M.; et al. Modulation of Riboflavin Biosynthesis and Utilization in Mycobacteria. bioRxiv 2023. bioRxiv:2023.08.30.555301. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Akther, H.D.; Hackstein, C.-P.; Powell, K.; King, T.; Friedrich, M.; Christoforidou, Z.; McCuaig, S.; Neyazi, M.; Arancibia-Cárcamo, C.V.; et al. TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions. Cell Rep. 2019, 28, 3077-3091.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; D’Souza, C.; Sun, S.; Kostenko, L.; Eckle, S.B.G.; Meehan, B.S.; Jackson, D.C.; Strugnell, R.A.; Cao, H.; et al. Mucosal-Associated Invariant T-Cell Activation and Accumulation after in Vivo Infection Depends on Microbial Riboflavin Synthesis and Co-Stimulatory Signals. Mucosal Immunol. 2017, 10, 58–68. [Google Scholar] [CrossRef]
- Ussher, J.E.; Bilton, M.; Attwod, E.; Shadwell, J.; Richardson, R.; de Lara, C.; Mettke, E.; Kurioka, A.; Hansen, T.H.; Klenerman, P.; et al. CD161++ CD8+ T Cells, Including the MAIT Cell Subset, are Specifically Activated by IL-12+IL-18 in a TCR-Independent Manner. Eur. J. Immunol. 2014, 44, 195–203. [Google Scholar] [CrossRef]
- Brigl, M.; Tatituri, R.V.V.; Watts, G.F.M.; Bhowruth, V.; Leadbetter, E.A.; Barton, N.; Cohen, N.R.; Hsu, F.-F.; Besra, G.S.; Brenner, M.B. Innate and Cytokine-Driven Signals, Rather than Microbial Antigens, Dominate in Natural Killer T Cell Activation during Microbial Infection. J. Exp. Med. 2011, 208, 1163–1177. [Google Scholar] [CrossRef]
- Wang, H.; Souter, M.N.T.; De Lima Moreira, M.; Li, S.; Zhou, Y.; Nelson, A.G.; Yu, J.; Meehan, L.J.; Meehan, B.S.; Eckle, S.B.G.; et al. MAIT Cell Plasticity Enables Functional Adaptation That Drives Antibacterial Immune Protection. Sci. Immunol. 2024, 9, eadp9841. [Google Scholar] [CrossRef]
- Georgel, P.; Radosavljevic, M.; Macquin, C.; Bahram, S. The Non-Conventional MHC Class I MR1 Molecule Controls Infection by Klebsiella Pneumoniae in Mice. Mol. Immunol. 2011, 48, 769–775. [Google Scholar] [CrossRef]
- Szabo, P.A.; Anantha, R.V.; Shaler, C.R.; McCormick, J.K.; Haeryfar, S.M.M. CD1d- and MR1-Restricted T Cells in Sepsis. Front. Immunol. 2015, 6, 401. [Google Scholar] [CrossRef]
- Sakai, S.; Kauffman, K.D.; Oh, S.; Nelson, C.E.; Barry, C.E.; Barber, D.L. MAIT Cell-Directed Therapy of Mycobacterium Tuberculosis Infection. Mucosal Immunol. 2021, 14, 199–208. [Google Scholar] [CrossRef]
- Constantinides, M.G.; Link, V.M.; Tamoutounour, S.; Wong, A.C.; Perez-Chaparro, P.J.; Han, S.-J.; Chen, Y.E.; Li, K.; Farhat, S.; Weckel, A.; et al. MAIT Cells Are Imprinted by the Microbiota in Early Life and Promote Tissue Repair. Science 2019, 366, eaax6624. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Martin, E.; Kim, S.; Yu, L.; Soudais, C.; Fremont, D.H.; Lantz, O.; Hansen, T.H. MR1 Antigen Presentation to Mucosal-Associated Invariant T Cells Was Highly Conserved in Evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Veloso, P.; Lantz, O.; Legoux, F. MAIT Cells: Conserved Watchers on the Wall. J. Exp. Med. 2025, 222, e20232298. [Google Scholar] [CrossRef]
- Salerno-Goncalves, R.; Rezwan, T.; Sztein, M.B. B Cells Modulate Mucosal Associated Invariant T Cell Immune Responses. Front. Immunol. 2014, 4, 511. [Google Scholar] [CrossRef]
- Fraser, D.R.; Stadnyk, A.W. The Emerging Relationship between Mucosal-Associated Invariant T Cell Populations and the Onset and Progression of Type 1 Diabetes. Front. Immunol. 2025, 16, 1602934. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Zhang, X.-W. MAIT Cell Activation and Functions. Front. Immunol. 2020, 11, 1014. [Google Scholar] [CrossRef]
- Masina, N.; Bekiswa, A.; Shey, M. Mucosal-Associated Invariant T Cells in Natural Immunity and Vaccination against Infectious Diseases in Humans. Curr. Opin. Immunol. 2021, 71, 1–5. [Google Scholar] [CrossRef]
- Dlugosz, A.; Törnblom, H.; Mohammadian, G.; Morgan, G.; Veress, B.; Edvinsson, B.; Sandström, G.; Lindberg, G. Chlamydia trachomatis Antigens in Enteroendocrine Cells and Macrophages of the Small Bowel in Patients with Severe Irritable Bowel Syndrome. BMC Gastroenterol. 2010, 10, 19. [Google Scholar] [CrossRef]
- Kronenberg, M.; Gapin, L. The Unconventional Lifestyle of NKT Cells. Nat. Rev. Immunol. 2002, 2, 557–568. [Google Scholar] [CrossRef]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-Restricted and TCR-Mediated Activation of Valpha14 NKT Cells by Glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Kim, H.Y.; Albacker, L.A.; Lee, H.H.; Baumgarth, N.; Akira, S.; Savage, P.B.; Endo, S.; Yamamura, T.; Maaskant, J.; et al. Influenza Infection in Suckling Mice Expands an NKT Cell Subset That Protects against Airway Hyperreactivity. J. Clin. Invest. 2011, 121, 57–69. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Kim, H.Y.; Albacker, L.A.; Baumgarth, N.; McKenzie, A.N.J.; Smith, D.E.; Dekruyff, R.H.; Umetsu, D.T. Innate Lymphoid Cells Mediate Influenza-Induced Airway Hyper-Reactivity Independently of Adaptive Immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef]
- Kinjo, Y.; Tupin, E.; Wu, D.; Fujio, M.; Garcia-Navarro, R.; Benhnia, M.R.-E.-I.; Zajonc, D.M.; Ben-Menachem, G.; Ainge, G.D.; Painter, G.F.; et al. Natural Killer T Cells Recognize Diacylglycerol Antigens from Pathogenic Bacteria. Nat. Immunol. 2006, 7, 978–986. [Google Scholar] [CrossRef]
- Mattner, J.; Debord, K.L.; Ismail, N.; Goff, R.D.; Cantu, C.; Zhou, D.; Saint-Mezard, P.; Wang, V.; Gao, Y.; Yin, N.; et al. Exogenous and Endogenous Glycolipid Antigens Activate NKT Cells during Microbial Infections. Nature 2005, 434, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Joyee, A.G.; Gao, X.; Peng, Y.; Wang, S.; Yang, J.; Yang, X. Invariant Natural Killer T Cells Promote T Cell Immunity by Modulating the Function of Lung Dendritic Cells during Chlamydia pneumoniae Infection. J. Innate Immun. 2015, 7, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Bharhani, M.S.; Chiu, B.; Na, K.-S.; Inman, R.D. Activation of Invariant NKT Cells Confers Protection against Chlamydia trachomatis-Induced Arthritis. Int. Immunol. 2009, 21, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Bilenki, L.; Wang, S.; Yang, J.; Fan, Y.; Joyee, A.G.; Yang, X. NK T Cell Activation Promotes Chlamydia trachomatis Infection in Vivo. J. Immunol. 2005, 175, 3197–3206. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Peng, Y.; Liu, J.; Qi, M.; Chen, Q.; Yang, X.; Zhao, W. Protective Role of α-Galactosylceramide-Stimulated Natural Killer T Cells in Genital Tract Infection with Chlamydia muridarum. FEMS Immunol. Med. Microbiol. 2012, 65, 43–54. [Google Scholar] [CrossRef]
- Shekhar, S.; Joyee, A.G.; Yang, X. Dynamics of NKT-Cell Responses to Chlamydial Infection. Front. Immunol. 2015, 6, 233. [Google Scholar] [CrossRef]
- Joyee, A.G.; Qiu, H.; Wang, S.; Fan, Y.; Bilenki, L.; Yang, X. Distinct NKT Cell Subsets Are Induced by Different Chlamydia Species Leading to Differential Adaptive Immunity and Host Resistance to the Infections. J. Immunol. 2007, 178, 1048–1058. [Google Scholar] [CrossRef]
- Joyee, A.G.; Qiu, H.; Fan, Y.; Wang, S.; Yang, X. Natural Killer T Cells Are Critical for Dendritic Cells to Induce Immunity in Chlamydial pneumonia. Am. J. Respir. Crit. Care Med. 2008, 178, 745–756. [Google Scholar] [CrossRef]
- Joyee, A.G.; Uzonna, J.; Yang, X. Invariant NKT Cells Preferentially Modulate the Function of CD8 Alpha+ Dendritic Cell Subset in Inducing Type 1 Immunity against Infection. J. Immunol. 2010, 184, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.W.; Carey, A.J.; Bryan, E.R.; Kollipara, A.; Trim, L.K.; Beagley, K.W. Pathogenic NKT Cells Attenuate Urogenital Chlamydial Clearance and Enhance Infertility. Scand. J. Immunol. 2023, 97, e13263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, X.; Peng, Y.; Joyee, A.G.; Bai, H.; Wang, S.; Yang, J.; Zhao, W.; Yang, X. Differential Modulating Effect of Natural Killer (NK) T Cells on Interferon-γ Production and Cytotoxic Function of NK Cells and Its Relationship with NK Subsets in Chlamydia muridarum Infection: Differential Modulation of NKT on NK Function. Immunology 2011, 134, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Carnaud, C.; Lee, D.; Donnars, O.; Park, S.H.; Beavis, A.; Koezuka, Y.; Bendelac, A. Cutting Edge: Cross-Talk between Cells of the Innate Immune System: NKT Cells Rapidly Activate NK Cells. J. Immunol. 1999, 163, 4647–4650. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Volarevic, V. The Cross-Talk between Mesenchymal Stem Cells and Immune Cells in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 2472. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, J. Balancing Act: The Complex Role of NK Cells in Immune Regulation. Front. Immunol. 2023, 14, 1275028. [Google Scholar] [CrossRef]
- Martín-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced Recruitment of NK Cells to Lymph Nodes Provides IFN-γ for TH1 Priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef]
- Williams, D.M.; Grubbs, B.G.; Schachter, J.; Magee, D.M. Gamma Interferon Levels during Chlamydia trachomatis Pneumonia in Mice. Infect. Immun. 1993, 61, 3556–3558. [Google Scholar] [CrossRef]
- Hook, C.E.; Telyatnikova, N.; Goodall, J.C.; Braud, V.M.; Carmichael, A.J.; Wills, M.R.; Gaston, J.S.H. Effects of Chlamydia trachomatis Infection on the Expression of Natural Killer (NK) Cell Ligands and Susceptibility to NK Cell Lysis. Clin. Exp. Immunol. 2004, 138, 54–60. [Google Scholar] [CrossRef]
- Tseng, C.T.; Rank, R.G. Role of NK Cells in Early Host Response to Chlamydial Genital Infection. Infect. Immun. 1998, 66, 5867–5875. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Thakur, C.P.; Kumar, P.; Saini, S.; Kureel, A.K.; Kumari, S.; Seth, T.; Mitra, D.K. Decrease in the Frequency of Circulating CD56+CD161+ NK Cells in Human Visceral Leishmaniasis. Immunol. Investig. 2018, 47, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Schachter, J.; Grubbs, B. Role of Natural Killer Cells in Infection with the Mouse Pneumonitis Agent (Murine Chlamydia trachomatis). Infect. Immun. 1987, 55, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Dockterman, J.; Coers, J. Immunopathogenesis of Genital Chlamydia Infection: Insights from Mouse Models. Pathog. Dis. 2021, 79, ftab012. [Google Scholar] [CrossRef]
- Paust, S.; Senman, B.; von Andrian, U.H. Adaptive Immune Responses Mediated by Natural Killer Cells. Immunol. Rev. 2010, 235, 286–296. [Google Scholar] [CrossRef]
- Cai, J.; Lu, H.; Su, Z.; Mi, L.; Xu, S.; Xue, Z. Dynamic Changes of NCR-Type 3 Innate Lymphoid Cells and Their Role in Mice with Bronchopulmonary Dysplasia. Inflammation 2022, 45, 497–508. [Google Scholar] [CrossRef]
- Ryu, S.; Lim, M.; Kim, J.; Kim, H.Y. Versatile Roles of Innate Lymphoid Cells at the Mucosal Barrier: From Homeostasis to Pathological Inflammation. Exp. Mol. Med. 2023, 55, 1845–1857. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Rui, B.; Lei, Z.; Ning, X.; Liu, Y.; Li, M. Crosstalk between the Gut Microbiota and Innate Lymphoid Cells in Intestinal Mucosal Immunity. Front. Immunol. 2023, 14, 1171680. [Google Scholar] [CrossRef]
- Hernández-Torres, D.C.; Stehle, C. Embryonic ILC-Poiesis across Tissues. Front. Immunol. 2022, 13, 1040624. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The Biology of Innate Lymphoid Cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Seo, G.-Y.; Giles, D.A.; Kronenberg, M. The Role of Innate Lymphoid Cells in Response to Microbes at Mucosal Surfaces. Mucosal Immunol. 2020, 13, 399–412. [Google Scholar] [CrossRef]
- Fiancette, R.; Finlay, C.M.; Willis, C.; Bevington, S.L.; Soley, J.; Ng, S.T.H.; Baker, S.M.; Andrews, S.; Hepworth, M.R.; Withers, D.R. Reciprocal Transcription Factor Networks Govern Tissue-Resident ILC3 Subset Function and Identity. Nat. Immunol. 2021, 22, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Golebski, K.; Spits, H. Plasticity of Innate Lymphoid Cell Subsets. Nat. Rev. Immunol. 2020, 20, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Yomogida, K.; Trsan, T.; Sudan, R.; Rodrigues, P.F.; Ulezko Antonova, A.; Ingle, H.; Luccia, B.D.; Collins, P.L.; Cella, M.; Gilfillan, S.; et al. The Transcription Factor Aiolos Restrains the Activation of Intestinal Intraepithelial Lymphocytes. Nat. Immunol. 2024, 25, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human Type 1 Innate Lymphoid Cells Accumulate in Inflamed Mucosal Tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- De La Maza, L.M.; Pal, S.; Khamesipour, A.; Peterson, E.M. Intravaginal Inoculation of Mice with the Chlamydia trachomatis Mouse Pneumonitis Biovar Results in Infertility. Infect. Immun. 1994, 62, 2094–2097. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhou, Z.; Yang, Z.; Ding, Y.; Zhou, Z.; Zhong, E.; Arulanandam, B.; Baseman, J.; Zhong, G. Chlamydial Induction of Hydrosalpinx in 11 Strains of Mice Reveals Multiple Host Mechanisms for Preventing Upper Genital Tract Pathology. PLoS ONE 2014, 9, e95076. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Luo, L.; Min, S.; Wang, L.; Peng, L.; Hou, Y.; He, P.; He, S.; Tang, S.; et al. Characterization of Genital Chlamydia trachomatis Infection among Women Attending Infertility and Gynecology Clinics in Hunan, China. BMC Infect. Dis. 2024, 24, 405. [Google Scholar] [CrossRef]
- Yeruva, L.; Spencer, N.; Bowlin, A.K.; Wang, Y.; Rank, R.G. Chlamydial Infection of the Gastrointestinal Tract: A Reservoir for Persistent Infection. Pathog. Dis. 2013, 68, 88–95. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Zhang, T.; Zhang, Y.; Zhu, C.; Sun, X.; Zhang, N.; Xue, M.; Zhong, G. The Chlamydia muridarum Organisms Fail to Auto-Inoculate the Mouse Genital Tract after Colonization in the Gastrointestinal Tract for 70 Days. PLoS ONE 2016, 11, e0155880. [Google Scholar] [CrossRef]
- He, Y.; Xu, H.; Song, C.; Koprivsek, J.J.; Arulanandam, B.; Yang, H.; Tao, L.; Zhong, G. Adoptive Transfer of Group 3-Like Innate Lymphoid Cells Restores Mouse Colon Resistance to Colonization of a Gamma Interferon-Susceptible Chlamydia muridarum Mutant. Infect. Immun. 2021, 89, e00533-20. [Google Scholar] [CrossRef]
- Koprivsek, J.J.; Zhang, T.; Tian, Q.; He, Y.; Xu, H.; Xu, Z.; Zhong, G. Distinct Roles of Chromosome- versus Plasmid-Encoded Genital Tract Virulence Factors in Promoting Chlamydia muridarum Colonization in the Gastrointestinal Tract. Infect. Immun. 2019, 87, e00265-19. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Su, X.; Zhao, Y.; Tang, L.; Chen, J.; Zhong, G. Innate Lymphoid Cells Are Required for Endometrial Resistance to Chlamydia trachomatis Infection. Infect. Immun. 2020, 88, e00152-20. [Google Scholar] [CrossRef] [PubMed]
- Koprivsek, J.J.; He, Y.; Song, C.; Zhang, N.; Tumanov, A.; Zhong, G. Evasion of Innate Lymphoid Cell-Regulated Gamma Interferon Responses by Chlamydia muridarum To Achieve Long-Lasting Colonization in Mouse Colon. Infect. Immun. 2020, 88, e00798-19. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Kiss, E.A.; Schwierzeck, V.; Ebert, K.; Hoyler, T.; d’Hargues, Y.; Göppert, N.; Croxford, A.L.; Waisman, A.; Tanriver, Y.; et al. A T-Bet Gradient Controls the Fate and Function of CCR6−RORγt+ Innate Lymphoid Cells. Nature 2013, 494, 261–265. [Google Scholar] [CrossRef]
- Rank, R.G.; Soderberg, L.S.; Sanders, M.M.; Batteiger, B.E. Role of Cell-Mediated Immunity in the Resolution of Secondary Chlamydial Genital Infection in Guinea Pigs Infected with the Agent of Guinea Pig Inclusion Conjunctivitis. Infect. Immun. 1989, 57, 706–710. [Google Scholar] [CrossRef]
- Morrison, R.P.; Caldwell, H.D. Immunity to Murine Chlamydial Genital Infection. Infect. Immun. 2002, 70, 2741–2751. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Barrett, L.K.; Cosgrove Sweeney, Y.T.; Kuo, C.-C.; Patton, D.L. Analysis of Lymphocyte Phenotype and Cytokine Activity in the Inflammatory Infiltrates of the Upper Genital Tract of Female Macaques Infected with Chlamydia trachomatis. J. Infect. Dis. 1996, 174, 647–650. [Google Scholar] [CrossRef]
- Helble, J.D.; Starnbach, M.N. T Cell Responses to Chlamydia. Pathog. Dis. 2021, 79, ftab014. [Google Scholar] [CrossRef]
- Rescigno, M.; Borrow, P. The Host-Pathogen Interaction. Cell 2001, 106, 267–270. [Google Scholar] [CrossRef]
- Cain, T.K.; Rank, R.G. Local Th1-like Responses Are Induced by Intravaginal Infection of Mice with the Mouse Pneumonitis Biovar of Chlamydia trachomatis. Infect. Immun. 1995, 63, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.L.; Feilzer, K.; Caldwell, H.D. Immunity to Chlamydia trachomatis Is Mediated by T Helper 1 Cells through IFN-Gamma-Dependent and -Independent Pathways. J. Immunol. 1997, 158, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.L.; Dutton, R.W. Type 1 and Type 2: A Fundamental Dichotomy for All T-Cell Subsets. Curr. Opin. Immunol. 1996, 8, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.P. Chlamydial Hsp60 and the Immunopathogenesis of Chlamydial Disease. Semin. Immunol. 1991, 3, 25–33. [Google Scholar]
- Shaw, J.; Grund, V.; Durling, L.; Crane, D.; Caldwell, H.D. Dendritic Cells Pulsed with a Recombinant Chlamydial Major Outer Membrane Protein Antigen Elicit a CD4(+) Type 2 Rather than Type 1 Immune Response That Is Not Protective. Infect. Immun. 2002, 70, 1097–1105. [Google Scholar] [CrossRef]
- Thomas, S.M.; Garrity, L.F.; Brandt, C.R.; Schobert, C.S.; Feng, G.S.; Taylor, M.W.; Carlin, J.M.; Byrne, G.I. IFN-Gamma-Mediated Antimicrobial Response. Indoleamine 2,3-Dioxygenase-Deficient Mutant Host Cells No Longer Inhibit Intracellular Chlamydia Spp. or Toxoplasma Growth. J. Immunol. 1993, 150, 5529–5534. [Google Scholar] [CrossRef]
- Leonhardt, R.M.; Lee, S.-J.; Kavathas, P.B.; Cresswell, P. Severe Tryptophan Starvation Blocks Onset of Conventional Persistence and Reduces Reactivation of Chlamydia trachomatis. Infect. Immun. 2007, 75, 5105–5117. [Google Scholar] [CrossRef]
- Wyrick, P.B. Chlamydia trachomatis Persistence In Vitro: An Overview. J. Infect. Dis. 2010, 201, 88–95. [Google Scholar] [CrossRef]
- Jerchel, S.; Kaufhold, I.; Schuchardt, L.; Shima, K.; Rupp, J. Host Immune Responses after Hypoxic Reactivation of IFN-Î3 Induced Persistent Chlamydia trachomatis Infection. Front. Cell. Infect. Microbiol. 2014, 4, 43. [Google Scholar] [CrossRef]
- Beatty, W.L.; Belanger, T.A.; Desai, A.A.; Morrison, R.P.; Byrne, G.I. Tryptophan Depletion as a Mechanism of Gamma Interferon-Mediated Chlamydial Persistence. Infect. Immun. 1994, 62, 3705–3711. [Google Scholar] [CrossRef]
- Mayer, J.; Woods, M.L.; Vavrin, Z.; Hibbs, J.B. Gamma Interferon-Induced Nitric Oxide Production Reduces Chlamydia trachomatis Infectivity in McCoy Cells. Infect. Immun. 1993, 61, 491–497. [Google Scholar] [CrossRef]
- Al-Younes, H.M.; Rudel, T.; Brinkmann, V.; Szczepek, A.J.; Meyer, T.F. Low Iron Availability Modulates the Course of Chlamydia Pneumoniae Infection. Cell Microbiol. 2001, 3, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Roshick, C.; Wood, H.; Caldwell, H.D.; McClarty, G. Comparison of Gamma Interferon-Mediated Antichlamydial Defense Mechanisms in Human and Mouse Cells. Infect. Immun. 2006, 74, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Chandanala, S.; Mohan, G.; Manukonda, D.-L.; Kumar, A.; Prasanna, J. A Novel Role of CD73-IFNγ Signalling Axis in Human Mesenchymal Stromal Cell Mediated Inflammatory Macrophage Suppression. Regen. Ther. 2024, 26, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dong, T.; Eyvani, H.; Fang, Y.; Wang, X.; Zhang, X.; Lu, X. Metabolic Interventions: A New Insight into the Cancer Immunotherapy. Arch. Biochem. Biophys. 2021, 697, 108659. [Google Scholar] [CrossRef]
- Pfefferkorn, E.R.; Rebhun, S.; Eckel, M. Characterization of an Indoleamine 2,3-Dioxygenase Induced by Gamma-Interferon in Cultured Human Fibroblasts. J. Interferon Res. 1986, 6, 267–279. [Google Scholar] [CrossRef]
- Burke, S.J.; Updegraff, B.L.; Bellich, R.M.; Goff, M.R.; Lu, D.; Minkin, S.C.; Karlstad, M.D.; Collier, J.J. Regulation of iNOS Gene Transcription by IL-1β and IFN-γ Requires a Coactivator Exchange Mechanism. Mol. Endocrinol. 2013, 27, 1724–1742. [Google Scholar] [CrossRef]
- Shirey, K.A.; Jung, J.-Y.; Carlin, J.M. Up-Regulation of Gamma Interferon Receptor Expression Due to Chlamydia-Toll-like Receptor Interaction Does Not Enhance Signal Transducer and Activator of Transcription 1 Signaling. Infect. Immun. 2006, 74, 6877–6884. [Google Scholar] [CrossRef]
- Prantner, D.; Darville, T.; Sikes, J.D.; Andrews, C.W.; Brade, H.; Rank, R.G.; Nagarajan, U.M. Critical Role for Interleukin-1beta (IL-1beta) during Chlamydia muridarum Genital Infection and Bacterial Replication-Independent Secretion of IL-1beta in Mouse Macrophages. Infect. Immun. 2009, 77, 5334–5346. [Google Scholar] [CrossRef]
- Liew, F.Y.; Cox, F.E.G. Nonspecific Defence Mechanism: The Role of Nitric Oxide. Immunol. Today 1991, 12, A17–A21. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y. Regulation of Nitric Oxide Synthesis in Infectious and Autoimmune Diseases. Immunol. Lett. 1994, 43, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.A. IRG Proteins: Key Mediators of Interferon-Regulated Host Resistance to Intracellular Pathogens. Cell Microbiol. 2007, 9, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P.; Raffatellu, M. Metals in Infectious Diseases and Nutritional Immunity. Metallomics 2015, 7, 926–928. [Google Scholar] [CrossRef]
- Haschka, D.; Hoffmann, A.; Weiss, G. Iron in Immune Cell Function and Host Defense. Semin. Cell Dev. Biol. 2021, 115, 27–36. [Google Scholar] [CrossRef]
- Byrd, T.F.; Horwitz, M.A. Interferon Gamma-Activated Human Monocytes Downregulate Transferrin Receptors and Inhibit the Intracellular Multiplication of Legionella Pneumophila by Limiting the Availability of Iron. J. Clin. Investig. 1989, 83, 1457–1465. [Google Scholar] [CrossRef]
- Nairz, M.; Haschka, D.; Demetz, E.; Weiss, G. Iron at the Interface of Immunity and Infection. Front. Pharmacol. 2014, 5, 152. [Google Scholar] [CrossRef]
- Vander Haar, E.; Lee, S.-I.; Bandhakavi, S.; Griffin, T.J.; Kim, D.-H. Insulin Signalling to mTOR Mediated by the Akt/PKB Substrate PRAS40. Nat. Cell Biol. 2007, 9, 316–323. [Google Scholar] [CrossRef]
- Avivar-Valderas, A. Inhibition of PI3Kβ and mTOR Influence the Immune Response and the Defense Mechanism against Pathogens. Int. J. Infect. 2023, 7, 46–49. [Google Scholar]
- Rawal, Y.; Chereji, R.V.; Valabhoju, V.; Qiu, H.; Ocampo, J.; Clark, D.J.; Hinnebusch, A.G. Gcn4 Binding in Coding Regions Can Activate Internal and Canonical 5′ Promoters in Yeast. Mol. Cell 2018, 70, 297–311.e4. [Google Scholar] [CrossRef]
- Peng, Y.; Gao, X.; Yang, J.; Shekhar, S.; Wang, S.; Fan, Y.; Zhao, W.; Yang, X. Interleukin-22 Promotes T Helper 1 (Th1)/Th17 Immunity in Chlamydial Lung Infection. Mol. Med. 2014, 20, 109–119. [Google Scholar] [CrossRef]
- Quirk, S.; Agrawal, D.K. Immunobiology of IL-37: Mechanism of Action and Clinical Perspectives. Expert. Rev. Clin. Immunol. 2014, 10, 1703–1709. [Google Scholar] [CrossRef]
- Su, Z.; Tao, X. Current Understanding of IL-37 in Human Health and Disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Q.; Su, S.; Dong, W.; Zong, S.; Ma, Q.; Yang, X.; Zuo, D.; Zheng, S.; Meng, X.; et al. Interleukin 37 Suppresses M1 Macrophage Polarization Through Inhibition of the Notch1 and Nuclear Factor Kappa B Pathways. Front. Cell Dev. Biol. 2020, 8, 56. [Google Scholar] [CrossRef]
- Toniato, E. IL-37 Is an Inhibitory Cytokine That Could Be Useful for Treating Infections. Int. J. Infect. 2024, 8, 1–2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, W.; Hu, Y.; Zhou, Y.; Zhou, Z. Role of IFN-γ from Different Immune Cells in Chlamydia Infection. Microorganisms 2025, 13, 2374. https://doi.org/10.3390/microorganisms13102374
Chen X, Yang W, Hu Y, Zhou Y, Zhou Z. Role of IFN-γ from Different Immune Cells in Chlamydia Infection. Microorganisms. 2025; 13(10):2374. https://doi.org/10.3390/microorganisms13102374
Chicago/Turabian StyleChen, Xuan, Wenjing Yang, Yuchen Hu, Yang Zhou, and Zhou Zhou. 2025. "Role of IFN-γ from Different Immune Cells in Chlamydia Infection" Microorganisms 13, no. 10: 2374. https://doi.org/10.3390/microorganisms13102374
APA StyleChen, X., Yang, W., Hu, Y., Zhou, Y., & Zhou, Z. (2025). Role of IFN-γ from Different Immune Cells in Chlamydia Infection. Microorganisms, 13(10), 2374. https://doi.org/10.3390/microorganisms13102374






