Associations Between the Gut Microbiome and Outcomes in Autologous Stem Cell Transplantation: A Systematic Review
Abstract
1. Introduction
1.1. Overview of Autologous Stem Cell Transplantation
1.2. The Gut Microbiome in Health and Disease
1.3. The Gut Microbiome and Immune Homeostasis
1.4. Methods Used for Gut Microbiome Analysis
- 1.
- 16S rRNA Gene Amplicon Sequencing: provides taxonomic information to the genus level. However, it has limitations such as lacking direct functional details and not capturing horizontal gene transfer between organisms.
- 2.
- Shotgun Metagenomic Sequencing (SMGS): which is capable of multi-level, rapid profiling of individual microorganisms, including taxonomic classification and functional potential. This method can infer functional capabilities of microbial communities, addressing some of the limitations of 16S rRNA sequencing.
- 3.
- Whole Genome Sequencing (WGS): analyses the entire genome of a single bacterial colony, offering detailed genetic information but at a higher resolution compared to other methods.
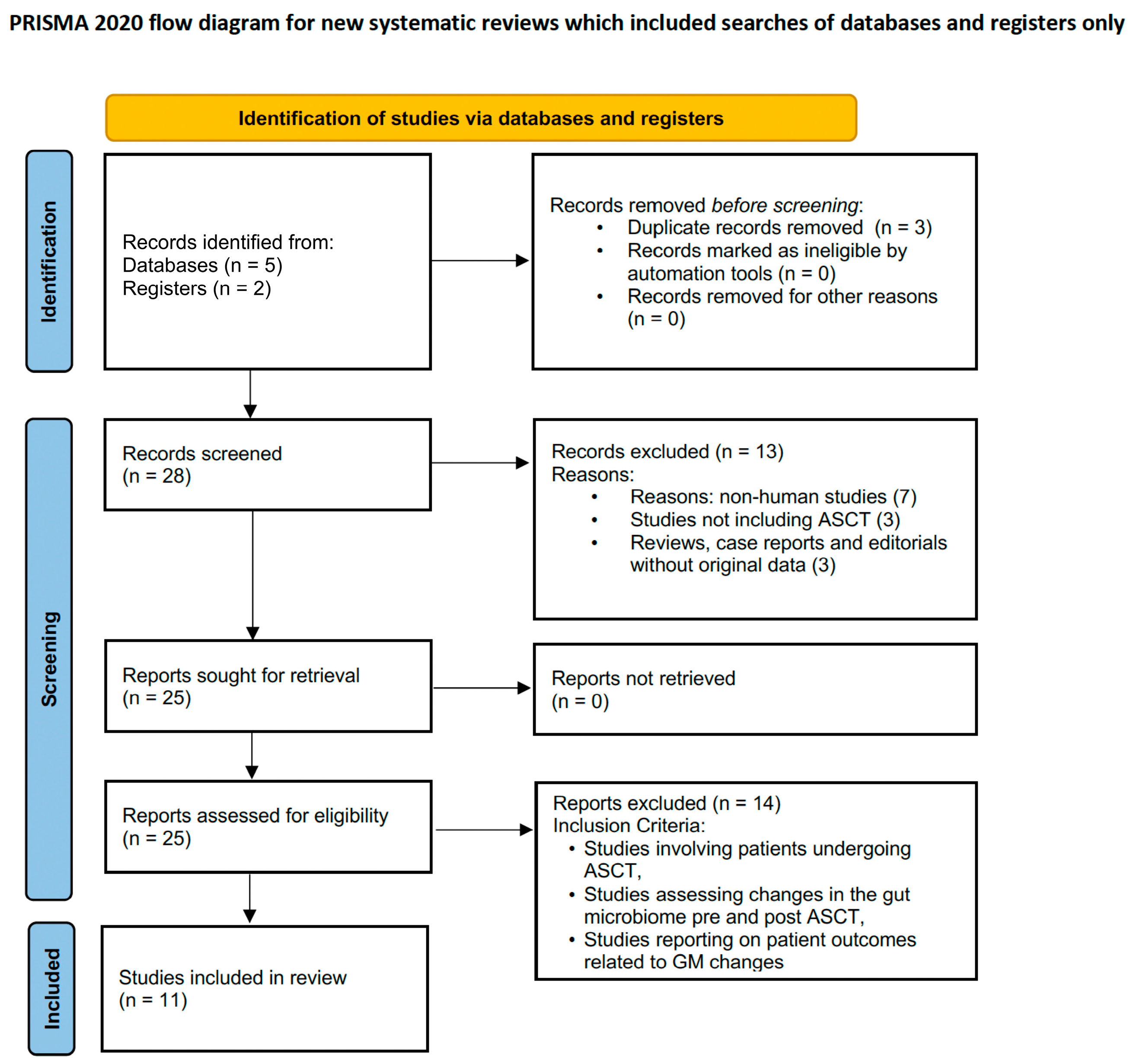
2. Materials and Methods
- Study Design: Studies were assessed based on design quality using both the Centre for Evidence-Based Medicine (CEBM) critical appraisal tool and the Newcastle-Ottawa Scale (NOS) for assessing the risk of bias and overall quality of included studies.
- Sample Size: Although larger sample sizes were preferred for the reliability and generalizability of results, most of the studies included were limited by small sample sizes.
- Microbiome Assessment Methods: The methods used for sample collection and microbiome assessment were scrutinised for validity and reliability (i.e., 16S rRNA sequencing, shotgun metagenomic sequencing, whole-genome sequencing).
- Key Findings: The key findings of each study were reviewed to determine the relevance and significance of reported changes in the GM and their associations with study outcomes.
- Reported Outcomes: Study outcomes were assessed for clinical relevance, including how changes in the GM were linked to treatment responses, adverse events, and overall patient outcomes.
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. Results
3.1. Impact of Gut Microbiota Diversity on Patient Outcomes
3.2. Associations Between Specific Bacterial Taxa and Clinical Outcomes
3.3. Gut Microbiota and Mucositis
3.4. Comparison of Study Designs and Methods
3.5. Study Strengths and Weaknesses
| Study, Year of Publication | Focus Question | Study Design | Participants | Methods of GM Analysis | Statistical Analysis | Level of Evidence | Quality Assessment Method | Strengths | Weaknesses | Main Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Khan et al. (2021) [42] | Does gut microbiota diversity impact overall survival (OS) and progression-free survival (PFS) in ASCT patients? | Prospective observational study | N = 534 (ASCT = 534, Myeloma = 272, Lymphoma = 227 [NHL = 200, HL = 27], Amyloidosis = 35) | 16S rRNA sequencing | Kaplan-Meier curves and Cox proportional hazards regression for survival; Generalized estimating equation modelling for microbiota associations. Small sample size limits inference and statistical power. | 2b | CEBM | Prospective design; detailed microbiota profiling. | Small sample size; limited to one institution. | Higher microbiota diversity at engraftment associated with better OS and PFS. Positive: Higher abundance of Faecalibacterium prausnitzii associated with improved OS and PFS. Negative: Enterococcus abundance linked to increased mortality (OS HR 5.76). |
| Laheij et al. (2019) [49] | What are the changes in oral and gut microbiota during ASCT, and how are these linked to oral mucositis development? | Prospective observational study | N = 51 (ASCT = 51, Allo-HCT = 0) | 16S rRNA sequencing | Mixed-effects models for microbiota changes; non-parametric tests for subgroup analysis. | 2b | CEBM | Focus on oral and gut microbiota dynamics; clinical relevance. | Short follow-up duration; no longitudinal outcomes. | Oral and gut microbiota shifts linked to ulcerative oral mucositis development. Positive: Bacteroidetes on day +7 reduced gastrointestinal toxicity. Negative: Blautia and Ruminococcus correlated with increased vomiting severity. |
| Kusakabe et al. (2020) [44] | How does gut microbiota composition differ pre- and post-transplant in auto- and allo-HCT recipients? | Prospective observational study | N = 24 (ASCT = 8, Allo-HCT = 16) | 16S rRNA sequencing and UniFrac analysis | Alpha diversity metrics and UniFrac distances for microbiota variability. Descriptive results lacked inferential depth. | 2b | CEBM | Longitudinal data collection; robust sequencing methods. | Small cohort; exploratory nature limits conclusions. | Stable gut microbiota composition linked to fewer complications post-HSCT. |
| Märtson et al. (2023) [50] | What is the impact of severe mucositis on gut microbiota composition and drug exposure during ASCT? | Prospective pilot study | N = 21 (ASCT-HCT = 21, Allo-HCT = 0) | 16S rRNA sequencing | Correlation analysis for drug exposure and microbiota; descriptive statistics for mucositis severity. Limited inferential statistics. | 2b | Newcastle-Ottawa | Exploratory pilot study with novel focus on drug-microbiota interplay. | Non-randomised design; limited sample size. | Severe mucositis disrupts gut microbiota and alters drug exposure (e.g., ciprofloxacin). |
| D’Angelo et al. (2023) [45] | How does gut microbiota diversity at engraftment correlate with therapeutic responses in multiple myeloma patients? | Prospective cohort study | N = 30 (ASCT = 30, Allo-HCT = 0) | 16S rRNA sequencing and dietary analysis | ANOVA and linear regressions for diversity metrics; no causal modelling included. | 2b | Newcastle-Ottawa | Detailed microbiota and dietary analysis across multiple time points. Comprehensive integration of microbiota and dietary data. | Correlational design; no mechanistic insights. Limited exploration of functional GM changes. | Loss of bacterial diversity at engraftment linked to partial response in MM patients. Highlights dietary impacts on GM diversity and potential for dietary interventions in improving outcomes. |
| Pianko et al. (2019) [47] | How does MRD negativity correlate with specific gut microbiota taxa in multiple myeloma patients? | Prospective cohort study | N = 34 (ASCT = 34, Allo-HCT = 0) | 16S rRNA sequencing | Logistic regression to associate MRD negativity with specific microbiota taxa. No analysis of causality or interaction effects. | 2b | CEBM | Rigorous focus on MRD status as a key clinical endpoint. | Lack of longitudinal microbiota tracking; no randomisation. | Higher abundance of E. hallii associated with MRD negativity in MM patients. |
| Jian et al. (2020) [52] | What are the roles of nitrogen-recycling bacteria in promoting multiple myeloma progression? | Prospective observational study with mechanistic validation | N = 19 (ASCT = 19, Allo-HCT = 0) | Shotgun metagenomic sequencing | Shotgun metagenomics for microbiota profiling; mouse models used to establish mechanistic links. | 2a | CEBM | Integration of human data and mechanistic validation in preclinical models. | Limited generalisability from mouse models; cross-sectional human cohort. | Nitrogen-recycling bacteria enriched in MM promote disease progression via glutamine biosynthesis. Negative: Enrichment of Klebsiella spp. and nitrogen-recycling bacteria promoted tumour progression. |
| Bansal et al. (2022) [53] | How does antibiotic use influence gut microbiota composition in ASCT and allo-HCT recipients? | Longitudinal observational study | N = 35 (ASCT = 17, Allo-HCT = 18) | 16S rRNA sequencing and alpha diversity metrics | Alpha diversity metrics pre- and post-HCT; regression analysis for antibiotic effects. Focused heavily on descriptive changes. | 2b | Newcastle-Ottawa | Clear comparison of antibiotic effects in auto- and allo-HCT populations. Direct comparison between ASCT and allo-HCT effects on GM diversity over time. | Lack of intervention analysis; observational design. Lacks intervention testing for microbiota preservation. | Antibiotic exposure, not conditioning intensity, major driver of microbiota dysbiosis. Provides evidence for microbiota recovery by day 100 post-HCT with important implications for timing interventions. Positive: Firmicutes and Streptococcus increases on day +7 were protective. Negative: Proteobacteria abundance correlated with nausea severity. |
| El Jurdi et al. (2019) [51] | What are the longitudinal dynamics of bacteriome and mycobiome recovery post-ASCT? | Prospective non-randomized pilot study | N = 15 (ASCT = 15, Allo-HCT = 0) | 16S rRNA sequencing for bacteriome and mycobiome | Correlation of bacteriome/mycobiome changes with clinical outcomes. Limited statistical rigour for small sample size. | 2b | Newcastle-Ottawa | Focus on both bacteriome and mycobiome dynamics; clinical applicability. | Small sample size; limited follow-up period. | Bacteriome recovers within 1 month; mycobiome diversity remains disrupted longer. |
| Shah et al. (2022) [46] | Can dietary interventions and stool butyrate levels predict sustained MRD negativity in multiple myeloma? | Cross-sectional observational study | N = 48 (ASCT = 48, Allo-HCT = 0) | 16S rRNA sequencing and dietary data integration | Logistic regression for microbiota markers and MRD status; inclusion of dietary data for broader relevance. | 2b | CEBM | Novel integration of dietary factors and microbiota composition. | Cross-sectional design; observational correlations lack mechanistic insights. | Plant-based diets and higher stool butyrate linked to sustained MRD negativity in MM. Positive: Butyrate-producing taxa (Eubacterium hallii and Faecalibacterium prausnitzii) associated with sustained MRD negativity and better outcomes. |
| Schwabkey et al. (2022) [48] | What bacterial taxa are associated with febrile neutropenia during severe neutropenia post-ASCT? | Single-centre observational study with preclinical models | N = 119 (ASCT = 63, Allo-HCT = 56) | 16S rRNA sequencing and mouse model validation | Permutational MANOVA for beta diversity; mouse models validated human data findings. | 2a | Newcastle-Ottawa | Combined human and preclinical models; robust microbiota-metabolite analyses. | Observational study design for human data; potential overinterpretation of mouse results. | A. muciniphila abundance correlates with febrile neutropenia in post-HCT patients. |
| Bacterial Taxa | Key Role | Impact on Clinical Outcomes | Proposed Mechanism |
|---|---|---|---|
| Eubacterium hallii | Butyrate production | Improved PFS, MRD negativity | SCFA production, T-reg induction, gut barrier integrity. |
| Akkermansia muciniphila | Mucin degradation | Higher infection risk, febrile neutropenia | Excess mucin degradation weakens gut barrier, increasing inflammation. |
| Faecalibacterium prausnitzii | Anti-inflammatory, butyrate producer | Poor outcomes with depletion | SCFA production, anti-inflammatory effects, gut barrier protection. |
| Blautia spp. | SCFA production | Better PFS and OS | Supports epithelial health, anti-inflammatory signalling. |
| Klebsiella spp. | Nitrogen recycling | Tumour progression in MM | Promotes glutamine biosynthesis, enhancing tumour survival. |
| Ruminococcus spp. | SCFA production | Suboptimal therapeutic responses when depleted | Supports epithelial integrity and anti-inflammatory signalling. |
| Prevotella spp. | Pro-inflammatory | Tumour-promoting inflammation | IL-17-mediated inflammatory pathways linked to disease progression. |
4. Discussion
4.1. Challenges in Synthesising ASCT Studies
4.2. Associations Between the GM and ASCT Outcomes
4.2.1. Beneficial Bacteria
4.2.2. Pathogenic Bacteria
4.3. Mechanistic Pathways
4.4. Probiotic Interventions in ASCT
4.5. Critical Evaluation of Statistical Models
4.6. Strengths in Statistical Approaches
4.7. Limitations in Statistical Approaches
4.8. Comparisons Across Study Designs
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.; Avet-Loiseau, H.; Anderson, K.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A large meta analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef] [PubMed]
- Medical Scientific Advisory Group (MSAG) to Myeloma Australia (MA). Available online: https://myeloma.org.au/health-professional-resources/ (accessed on 4 February 2024).
- International Myeloma Working Group (IMWG). Available online: https://www.myeloma.org/imwg-publications (accessed on 4 February 2024).
- Benson, A.B.; Ajani, J.A.; Catalano, R.B.; Engelking, C.; Kornblau, S.M.; Martenson, J.A., Jr.; McCallum, R.; Mitchell, E.P.; O’DOrisio, T.M.; Vokes, E.E.; et al. Recommended guidelines for the treatment of cancer treatment induced diarrhea. J. Clin. Oncol. 2004, 22, 2918–2926. [Google Scholar] [CrossRef]
- Rashidi, A.; Weisdorf, D.J. Microbiota-based approaches to mitigate infectious complications of intensive chemotherapy in patients with acute leukemia. Transl. Res. 2020, 220, 167–181. [Google Scholar] [CrossRef]
- Perrot, A.; Lauwers-Cances, V.; Cazaubiel, T.; Facon, T.; Caillot, D.; Clement-Filliatre, L.; Macro, M.; Decaux, O.; Belhadj, K.; Mohty, M.; et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the IFM 2009 Trial. Blood 2020, 136, 39. [Google Scholar] [CrossRef]
- Leone, G.; Pagano, L.; Ben-Yehuda, D.; Voso, M.T. Therapy-related leukemia and myelodysplasia: Susceptibility and incidence. Haematologica 2007, 92, 1389–1398. [Google Scholar] [CrossRef]
- Lederberg, J. Ome Sweet’Omics–A Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- Barone, M.; D’Amico, F.; Fabbrini, M.; Rampelli, S.; Brigidi, P.; Turroni, S. Over-feeding the gut microbiome: A scoping review on health implications and therapeutic perspectives. World J. Gastroenterol. 2021, 27, 7041–7064. [Google Scholar] [CrossRef]
- Yi, M.; Qin, S.; Chu, Q.; Wu, K. The role of gut microbiota in immune checkpoint inhibitor therapy. Hepatobiliary Surg. Nutr. 2018, 7, 481–483. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2018, 11, E51. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Alharbi, A.A.; Alsharef, S.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; El Amin, H. Gut Microbiome as a Target of Intervention in Inflammatory Bowel Disease Pathogenesis and Therapy. Immuno 2024, 4, 400–425. [Google Scholar] [CrossRef]
- Ramadan, Y.N.; Alqifari, S.F.; Alshehri, K.; Alhowiti, A.; Mirghani, H.; Alrasheed, T.; Aljohani, F.; Alghamdi, A.; Hetta, H.F. Microbiome Gut-Brain-Axis: Impact on Brain Development and Mental Health. Mol. Neurobiol. 2025, 62, 10813–10833. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef]
- Peled, J.U.; Hanash, A.M.; Jenq, R.R. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood 2016, 128, 2395–2402. [Google Scholar] [CrossRef]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Perez, L.G.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven inter- leukin-17-producing cells and eosinophils synergize to ac-celerate multiple myeloma progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Artusa, V.; Calabrone, L.; Mortara, L.; Peri, F.; Bruno, A. Microbiota-derived natural products targeting cancer stem cells: Inside the gut pharma factory. Int. J. Mol. Sci. 2023, 24, 4997. [Google Scholar] [CrossRef]
- Tibbs, T.N.; Lopez, L.R.; Arthur, J.C. The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb. Cell 2019, 6, 324–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, M.; Bot, A. The Th17 cell population and the immune homeostasis of the gastrointestinal tract. Int. Rev. Immunol. 2013, 32, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, Y.F.; Van den Brink, M.R.M.; Hazenberg, M.D.; Markey, K.A. The post-hematopoietic cell transplantation microbiome: Relationships with transplant outcome and potential therapeutic targets. Haematologica 2021, 106, 2042–2053. [Google Scholar] [CrossRef]
- Owaga, E.; Hsieh, R.-H.; Mugendi, B.; Masuku, S.; Shih, C.-K.; Chang, J.-S. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2015, 16, 20841–20858. [Google Scholar] [CrossRef]
- Tomkovich, S.; Jobin, C. Microbiota and host immune responses: A love-hate relationship. Immunology 2016, 147, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Staffas, A.; Burgos da Silva, M.; Slingerland, A.E.; Lazrak, A.; Bare, C.J.; Holman, C.D.; Docampo, M.D.; Shono, Y.; Durham, B.; Pickard, A.J.; et al. Nutritional Support from the Intestinal Microbiota Improves Hematopoietic Reconstitution after Bone Marrow Transplantation in Mice. Cell Host Microbe 2018, 23, 447–457.e4. [Google Scholar] [CrossRef] [PubMed]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Singh, V.; Jang, H.; Kim, S.; Ayash, L.; Alavi, A.; Ratanatharathorn, V.; Uberti, J.P.; Deol, A. G-CSF use post peripheral blood stem cell transplant is associated with faster neutrophil engraftment, shorter hospital stay and increased incidence of chronic GVHD. Leuk. Lymphoma 2020, 62, 446–453. [Google Scholar] [CrossRef]
- DeCook, L.J.; Thoma, M.; Huneke, T.; Johnson, N.D.; Wiegand, R.A.; Patnaik, M.M.; Litzow, M.R.; Hogan, W.J.; Porrata, L.F.; Holtan, S.G. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone Marrow Transplant. 2013, 48, 708–714. [Google Scholar] [CrossRef]
- Thoma, M.D.; Huneke, T.J.; DeCook, L.J.; Johnson, N.D.; Wiegand, R.A.; Litzow, M.R.; Hogan, W.J.; Porrata, L.F.; Holtan, S.G. Peripheral Blood Lymphocyte and Monocyte Recovery and Survival in Acute Leukemia Postmyeloablative Allogeneic Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2012, 18, 600–607. [Google Scholar] [CrossRef]
- Varanasi, P.R.; Ogonek, J.; Luther, S.; Dammann, E.; Stadler, M.; Ganser, A.; Borchers, S.; Hambach, L.; Weissinger, E.M. Cytomegalovirus-specific CD8+ T-cells are associated with a reduced incidence of early relapse after allogeneic stem cell transplantation. PLoS ONE 2019, 14, e0213739. [Google Scholar] [CrossRef]
- Ando, T.; Tachibana, T.; Tanaka, M.; Suzuki, T.; Ishiyama, Y.; Koyama, S.; Ogusa, E.; Numata, A.; Matsumoto, K.; Kanamori, H.; et al. Impact of graft sources on immune reconstitution and survival outcomes following allogeneic stem cell transplantation. Blood Adv. 2020, 4, 408–419. [Google Scholar] [CrossRef]
- Li, Y.; Tinoco, R.; Elmén, L.; Segota, I.; Xian, Y.; Fujita, Y.; Sahu, A.; Zarecki, R.; Marie, K.; Feng, Y.; et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5−/− mice. Nat. Commun. 2019, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Q.; Yang, Q.; He, Y.; Zhou, W. Intestinal Microbes and Hematological Malignancies. Cancers 2023, 15, 2284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galloway-Peña, J.; Hanson, B. Tools for Analysis of the Microbiome. Dig. Dis. Sci. 2020, 65, 674–685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elie, C.; Perret, M.; Hage, H.; Sentausa, E.; Hesketh, A.; Louis, K.; Fritah-Lafont, A.; Leissner, P.; Vachon, C.; Rostaing, H.; et al. Comparison of DNA extraction methods for 16S rRNA gene sequencing in the analysis of the human gut microbiome. Sci. Rep. 2023, 13, 10279. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecology 2001, 26, 32–46. [Google Scholar]
- Khan, N.; Lindner, S.; Gomes, A.L.C.; Devlin, S.M.; Shah, G.L.; Sung, A.D.; Sauter, C.S.; Landau, H.J.; Dahi, P.B.; Perales, M.-A.; et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: A multicenter observational study. Blood 2021, 137, 1527–1537. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Kusakabe, S.; Fukushima, K.; Maeda, T.; Motooka, D.; Nakamura, S.; Fujita, J.; Yokota, T.; Shibayama, H.; Oritani, K.; Kanakura, Y. Pre- and post-serial metagenomicanalysis of gut microbiota as a prognostic factor in patients undergoing haematopoietic stem cell transplantation. Br. J. Haematol. 2020, 188, 438–449. [Google Scholar] [CrossRef]
- D’Angelo, C.; Sudakaran, S.; Asimakopoulos, F.; Hematti, P.; El-Gamal, D.; Safdar, N.; Callander, N. Perturbation of the gut microbiome and association with outcomes following autologous stem cell transplantation in patients with multiple myeloma. Leuk. Lymphoma 2023, 64, 87–97. [Google Scholar] [CrossRef]
- Shah, U.A.; Maclachlan, K.H.; Derkach, A. Sustained Minimal Residual Disease Negativity in Multiple Myeloma is Associated with Stool Butyrate and Healthier Plant-based Diets. Clin. Cancer Res. 2022, 28, 5149–5155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pianko, M.J.; Devlin, S.M.; Littmann, E.R.; Chansakul, A.; Mastey, D.; Salcedo, M.; Fontana, E.; Ling, L.; Tavitian, E.; Slingerland, J.B.; et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019, 3, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Schwabkey, Z.I.; Wiesnoski, D.H.; Chang, C.C.; Tsai, W.-B.; Pham, D.; Ahmed, S.S.; Hayase, T.; Turrubiates, M.R.O.; El-Himri, R.K.; Sanchez, C.A.; et al. Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment. Sci. Transl. Med. 2022, 14, eabo3445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laheij, A.M.; Raber-Durlacher, J.E.; Koppelmans, R.G.; Huysmans, M.-C.D.N.J.M.; Potting, C.; van Leeuwen, S.J.M.; Hazenberg, M.D.; Brennan, M.T.; von Bültzingslöwen, I.; Johansson, J.-E.; et al. Microbial changes in relation to oral mucositis in autologous hematopoietic stem cell transplantation recipients. Sci. Rep. 2019, 9, 16929. [Google Scholar] [CrossRef]
- Märtson, A.G.; da Silva Ferreira, A.R.; Veringa, A.; Liu, L.; Wardill, H.R.; Junier, L.A.T.; van der Werf, T.S.; Harmsen, H.J.M.; Sturkenboom, M.G.G.; Span, L.F.; et al. Exposure of anti-infective drugs and the dynamic changes of the gut microbiota during gastrointestinal mucositis in autologous stem cell transplant patients: A pilot study. Ann. Hematol. 2023, 102, 421–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Jurdi, N.; Filali-Mouhim, A.; Salem, I.; Retuerto, M.; Dambrosio, N.M.; Baer, L.; Lazarus, H.M.; Caimi, P.; Cooper, B.; Tomlinson, B.; et al. Gastrointestinal Microbiome and Mycobiome Changes during Autologous Transplantation for Multiple Myeloma: Results of a Prospective Pilot Study. Biol. Blood Marrow Transpl. 2019, 25, 1511–1519. [Google Scholar] [CrossRef]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef]
- Bansal, R.; Park, H.; Taborda, C.C.; Gordillo, C.; Mapara, M.Y.; Assal, A.; Uhlemann, A.-C.; Reshef, R. Antibiotic Exposure, Not Alloreactivity, Is the Major Driver of Microbiome Changes in Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2022, 28, 135–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitts, E.; Grainger, B.; McKenzie, D.; Fiorenza, S. Associations Between the Gut Microbiome and Outcomes in Autologous Stem Cell Transplantation: A Systematic Review. Microorganisms 2025, 13, 2302. https://doi.org/10.3390/microorganisms13102302
Pitts E, Grainger B, McKenzie D, Fiorenza S. Associations Between the Gut Microbiome and Outcomes in Autologous Stem Cell Transplantation: A Systematic Review. Microorganisms. 2025; 13(10):2302. https://doi.org/10.3390/microorganisms13102302
Chicago/Turabian StylePitts, Ema, Brian Grainger, Dean McKenzie, and Salvatore Fiorenza. 2025. "Associations Between the Gut Microbiome and Outcomes in Autologous Stem Cell Transplantation: A Systematic Review" Microorganisms 13, no. 10: 2302. https://doi.org/10.3390/microorganisms13102302
APA StylePitts, E., Grainger, B., McKenzie, D., & Fiorenza, S. (2025). Associations Between the Gut Microbiome and Outcomes in Autologous Stem Cell Transplantation: A Systematic Review. Microorganisms, 13(10), 2302. https://doi.org/10.3390/microorganisms13102302






