Antiviral Activity of Eugenol Against Largemouth Bass Ranavirus Through Regulation of Autophagy and Apoptosis In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish, Cells and Virus
2.3. Cell Treatment
2.4. Cytotoxicity Assays
2.5. Virus Infection Assay In Vitro
2.6. RNA Isolation and qRT-PCR Analysis
2.7. Western Blotting Analysis
2.8. Annexin V-FITC/PI Apoptosis Assay
2.9. Antiviral Activity of EUG In Vivo
2.10. Statistical Analysis
3. Results
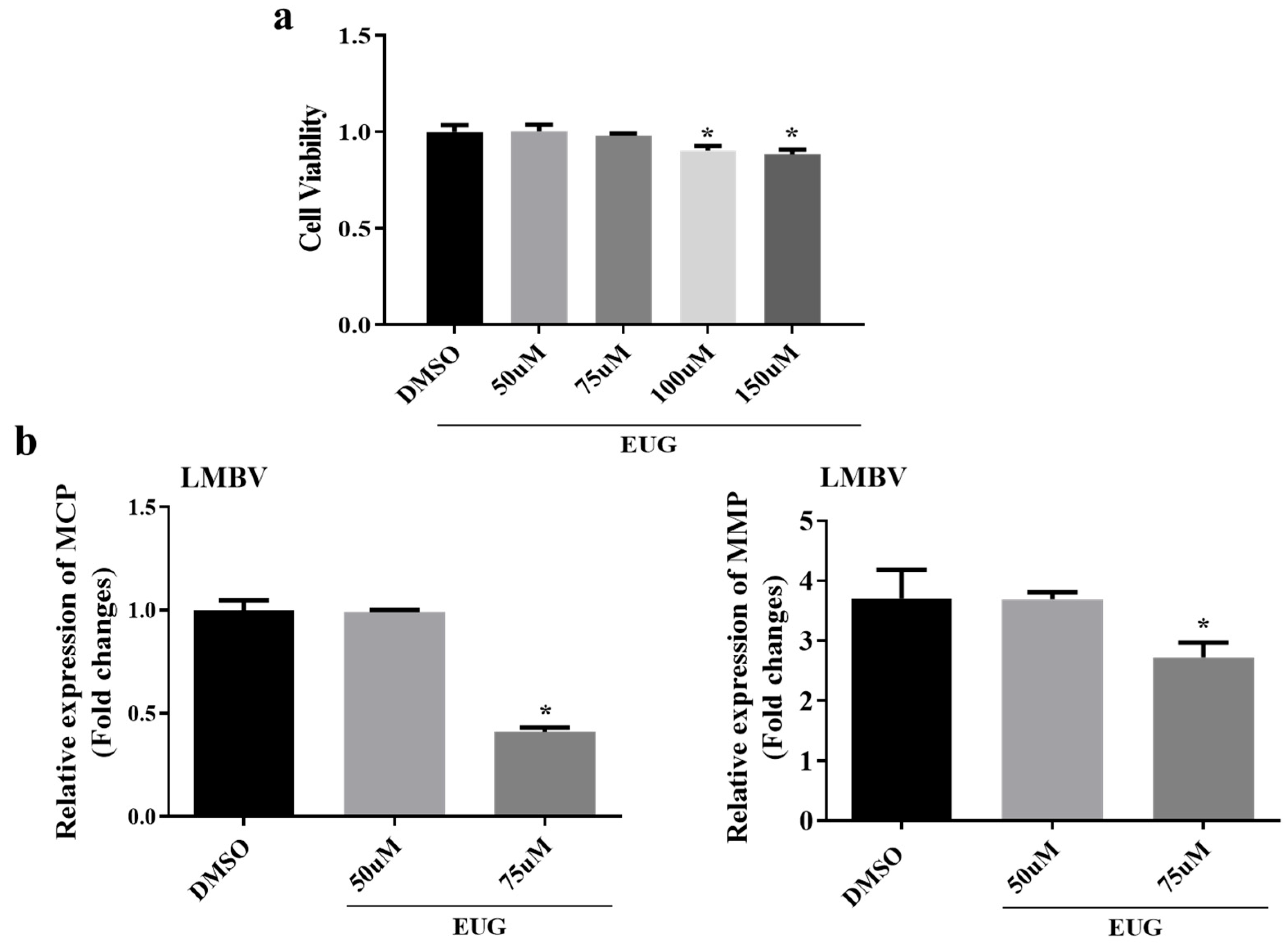
3.1. Effect of EUG in Different Concentrations on LMBV Infection
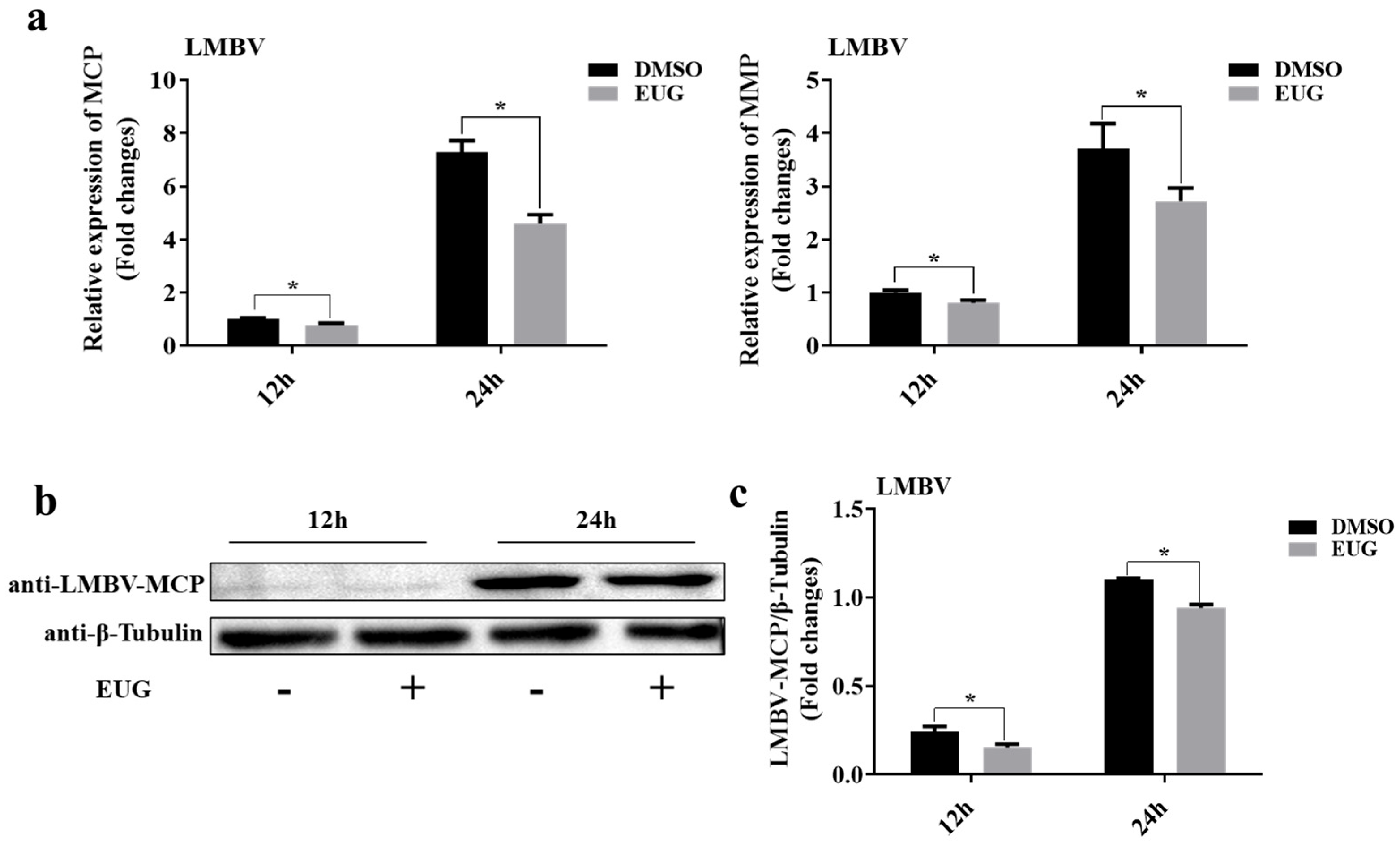
3.2. EUG Inhibits LMBV Infection at Different Time Points
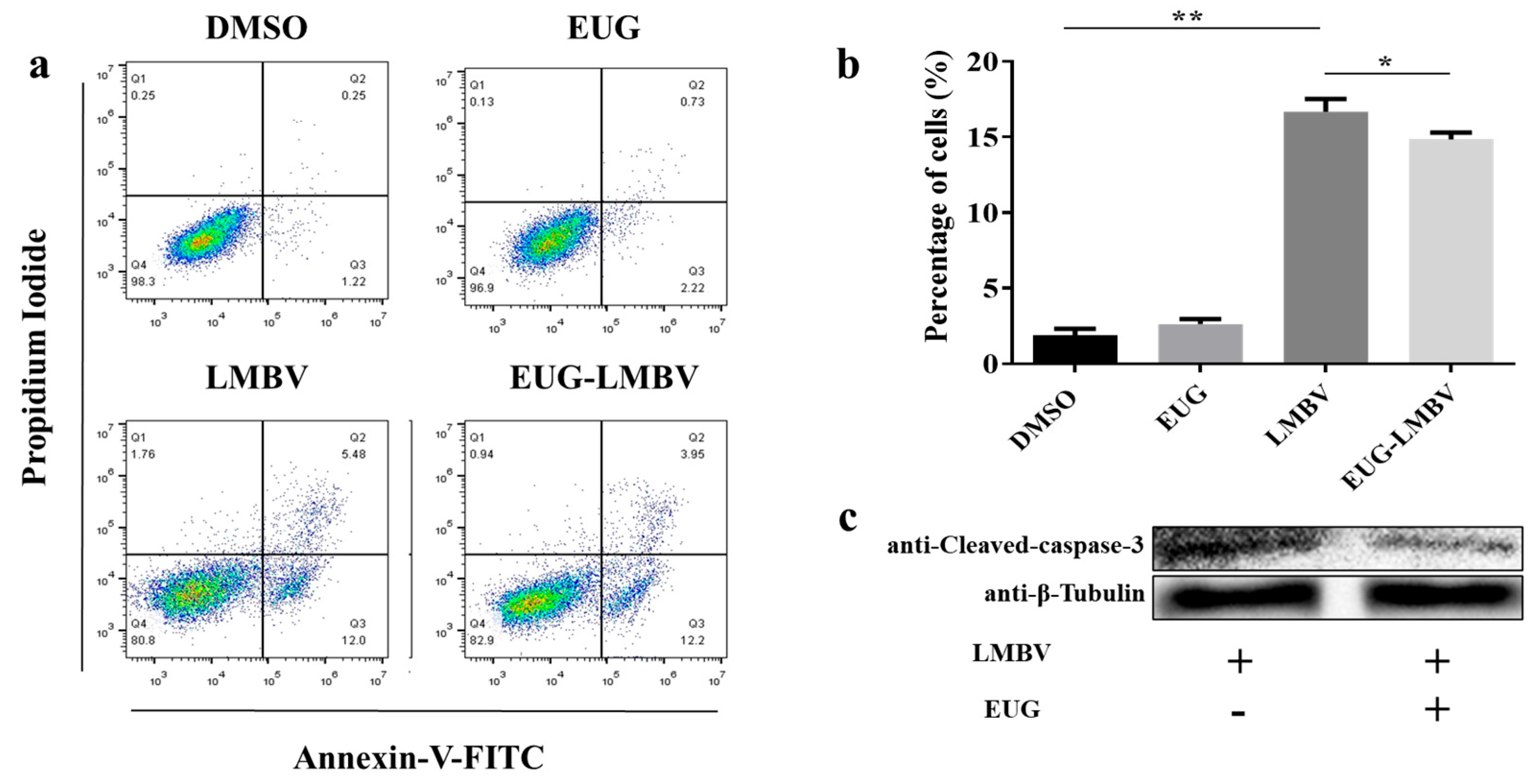
3.3. EUG Inhibits Apoptosis in Cells Infected by LMBV
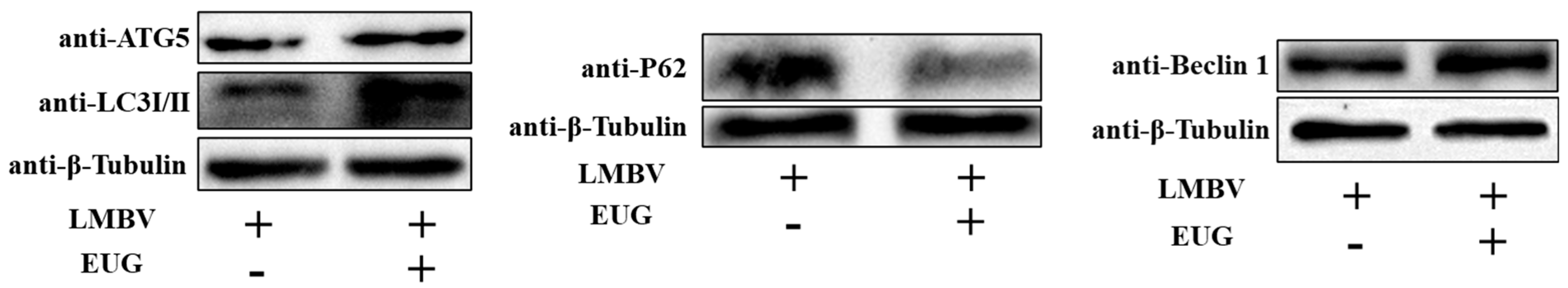
3.4. EUG Promotes Cellular Autophagy in LMBV Infection
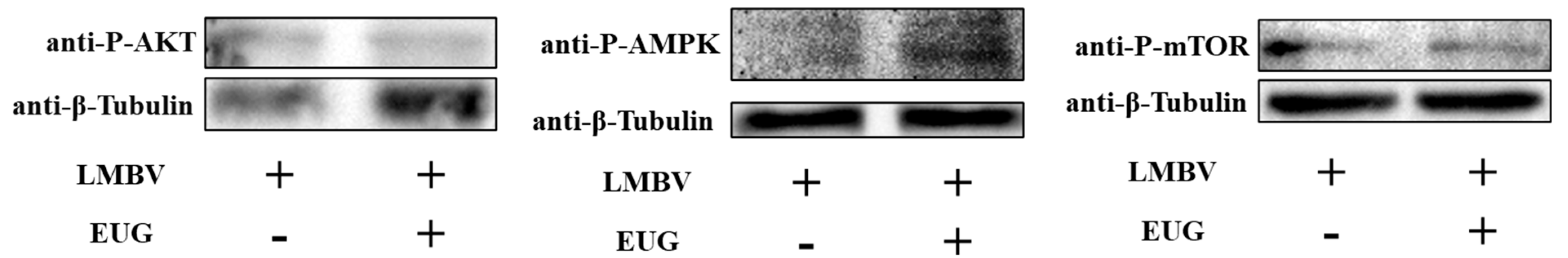
3.5. EUG Regulates AMPK and AKT/mTOR Pathway in LMBV Infection
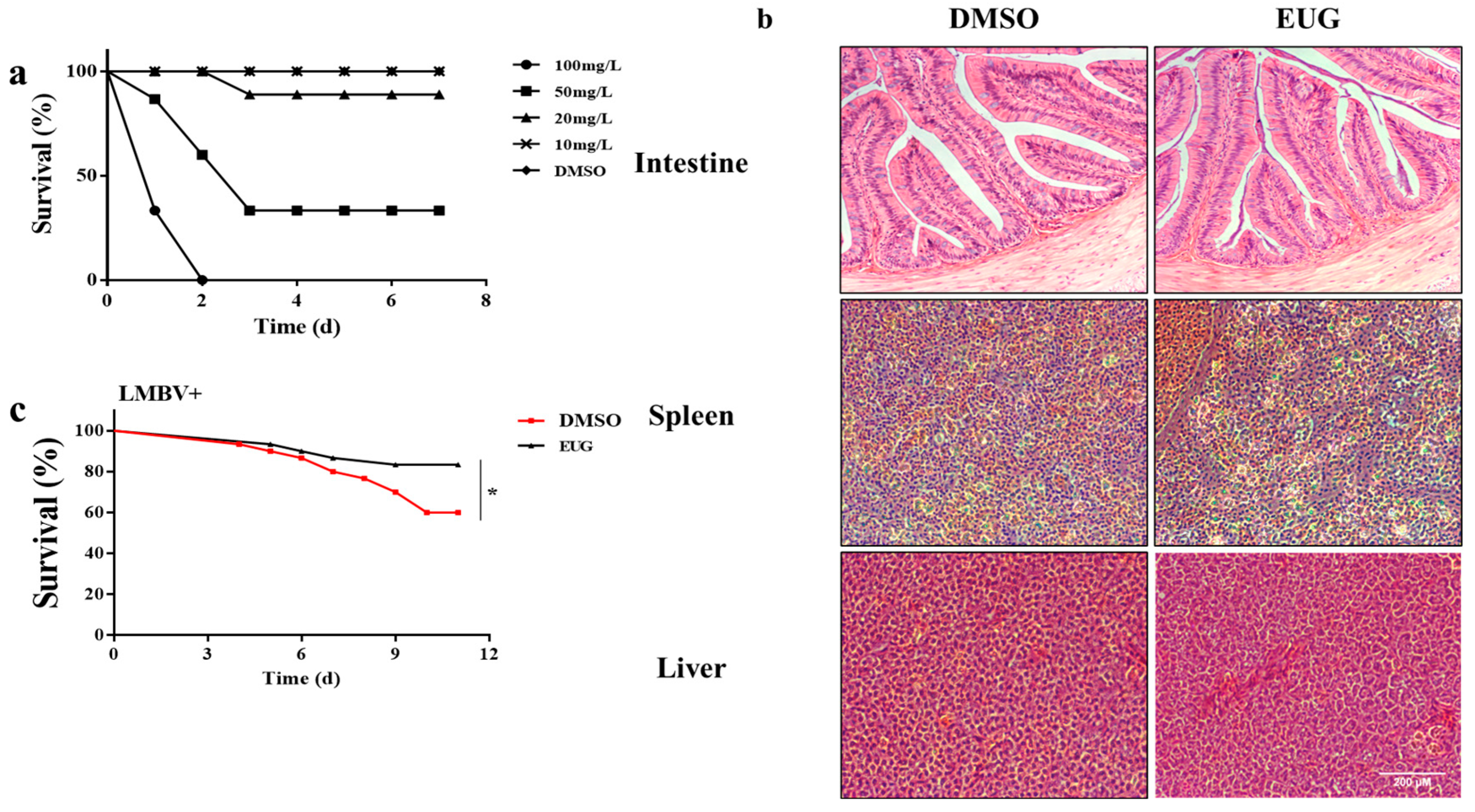
3.6. EUG Reduces Mortality in LMBV-Infected Largemouth Bass
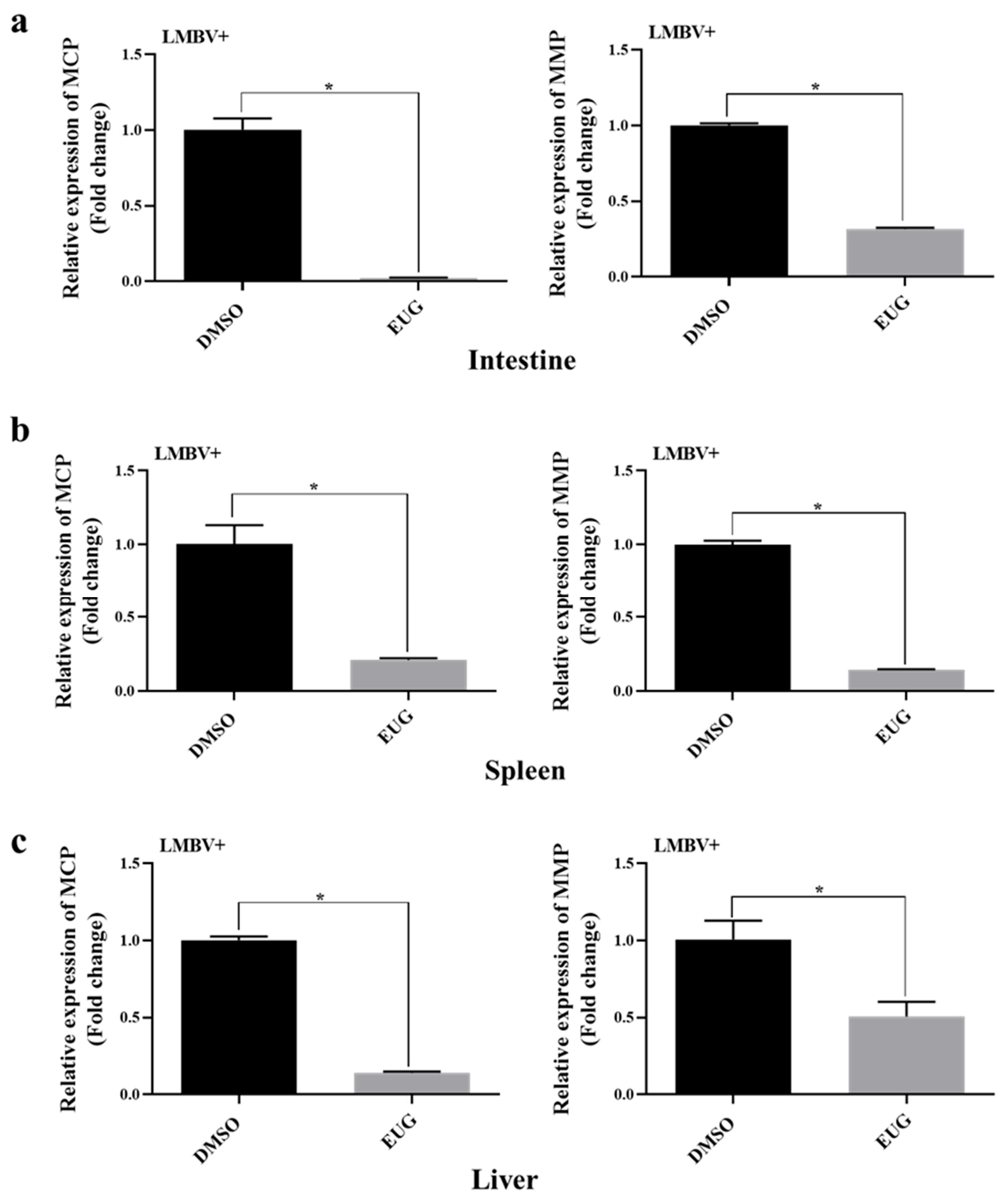
3.7. EUG Inhibits LMBV Infection in Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salazar, V.; Koch, J.D.; Neely, B.C.; Steffen, C.J.; Flores, E.; Steffen, S.F. The effect of largemouth bass virus on bass populations in Kansas impoundments. J. Aquat. Anim. Health. 2022, 34, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhong, Y.; Luo, M.; Zheng, G.; Huang, J.; Wang, G.; Geng, Y.; Qian, X. Largemouth bass ranavirus: Current status and research progression. Aquacult. Rep. 2023, 32, 101706. [Google Scholar] [CrossRef]
- Ma, D.; Deng, G.; Bai, J.; Li, S.; Yu, L.; Quan, Y.; Yang, X.; Jiang, X.; Zhu, Z.; Ye, X. A strain of Siniperca chuatsi rhabdovirus causes high mortality among cultured largemouth bass in South China. J. Aquat. Anim. Health. 2013, 25, 197–204. [Google Scholar] [CrossRef]
- Fogelson, S.B.; Petty, B.D.; Reichley, S.R.; Ware, C.; Bowser, P.R.; Crim, M.J.; Getchell, R.G.; Sams, K.L.; Marquis, H.; Griffin, M.J. Histologic and molecular characterization of Edwardsiella piscicida infection in largemouth bass (Micropterus salmoides). J. Vet. Diagn. Invest. 2016, 28, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G. Ranaviruses (family Iridoviridae): Emerging cold-blooded killers. Arch. Virol. 2002, 147, 447–470. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Zhang, Z.; An, Z.; Zhang, X.; Vakharia, V.N.; Lin, L. Isolation, identification and the pathogenicity characterization of a Santee-Cooper ranavirus and its activation on immune responses in juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2023, 135, 108641. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, C.; Yuan, X.; Huang, L.; Hu, D.; Yu, Z.; Yin, W.; Lin, L.; Pan, X.; Yang, G.; et al. Oral vccination with recombinant pichia pastoris expressing iridovirus major capsid protein elicits protective immunity in largemouth bass (Micropterus salmoides). Front Immunol. 2022, 13, 852300. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Chen, J.; He, X.; He, H.; Qin, Q.; Yang, M. The role of largemouth bass NF-kappa B/p65: Inhibition of LMBV and activator of IL-18 promoter. Fish Shellfish Immunol. 2025, 158, 110120. [Google Scholar] [CrossRef]
- Deng, L.; Feng, Y.; OuYang, P.; Chen, D.; Huang, X.; Guo, H.; Deng, H.; Fang, J.; Lai, W.; Geng, Y. Autophagy induced by largemouth bass virus inhibits virus replication and apoptosis in Epithelioma papulosum cyprini cells. Fish Shellfish Immunol. 2022, 123, 489–495. [Google Scholar] [CrossRef]
- Yang, J.; Xu, W.; Wang, W.; Pan, Z.; Qin, Q.; Huang, X.; Huang, Y. Largemouth bass virus infection induced non-apoptotic cell death in MsF cells. Viruses 2022, 14, 1568. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Huang, Y.; Xu, L.; Qin, Q. Involvement of the PI3K and ERK signaling pathways in largemouth bass virus-induced apoptosis and viral replication. Fish Shellfish Immunol. 2014, 41, 371–379. [Google Scholar] [CrossRef] [PubMed]
- D’, A.F.M.; Oliveira, P.S.; Dutra, F.S.; Fernandes, T.J.; De Pereira, C.M.; De Oliveira, S.Q.; Stefanello, F.M.; Lencina, C.L.; Barschak, A.G. Eugenol derivatives as potential anti-oxidants: Is phenolic hydroxyl necessary to obtain an effect? J. Pharm. Pharmacol. 2014, 66, 733–746. [Google Scholar]
- Amangeldinova, M.; Ersatir, M.; Necip, A.; Yilmaz, M.A.; Cimentepe, M.; Kudrina, N.; Terletskaya, N.V.; Ozturk, C.O.; Yildirim, M. Simultaneous quantitative screening of 53 phytochemicals from Rheum tataricum L. roots: A comparative study of supercritical CO2, subcritical ethanol, and ultrasound-assisted extraction for enhanced antioxidant, antibacterial activities, and molecular docking study. Front. Plant. Sci. 2024, 15, 1513875. [Google Scholar]
- Benencia, F.; Courreges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Chen, J.; Gong, H.; Qin, Q.; Wei, S. In vitro antiviral activity of eugenol on Singapore grouper iridovirus. Fish Shellfish Immunol. 2024, 151, 109748. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhao, X.; Zeng, J.; Wan, Q.; Yang, J.; Li, W.; Chen, X.; Wang, G.; Li, K. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using BiFC technique and mechanism of eugenol on anti-influenza A virus activity. PLoS. One. 2013, 8, e61026. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-induced autophagy and apoptosis in breast cancer cells via PI3K/AKT/FOXO3a pathway inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef]
- Hui, D.; Shisi, H.U.; Zhenduo, Y.; Xiaodan, C. Eugenol attenuates the inflammation of Fusarium-induced keratitis through PI3K/AKT signaling pathway. Int. Eye Sci. 2024, 1194–1199. [Google Scholar] [CrossRef]
- Saleh, D.O.; Baraka, S.M.; Jaleel, G.; Hassan, A.; Ahmed-Farid, O.A. Eugenol alleviates acrylamide-induced rat testicular toxicity by modulating AMPK/p-AKT/mTOR signaling pathway and blood-testis barrier remodeling. Sci. Rep. 2024, 14, 1910. [Google Scholar] [CrossRef]
- Imre, G. Cell death signalling in virus infection. Cell. Signal. 2020, 76, 109772. [Google Scholar] [CrossRef]
- Wei, J.; Zang, S.; Xu, M.; Zheng, Q.; Chen, X.; Qin, Q. TRAF6 is a critical factor in fish immune response to virus infection. Fish Shellfish Immunol. 2017, 60, 6–12. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Cai, J.; Wei, S.; Ouyang, Z.; Qin, Q. Molecular cloning, expression and functional analysis of ISG15 in orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2013, 34, 1094–1102. [Google Scholar] [CrossRef]
- Wang, D.; Yao, H.; Li, Y.H.; Xu, Y.J.; Ma, X.F.; Wang, H.P. Global diversity and genetic landscape of natural populations and hatchery stocks of largemouth bass micropterus salmoides across American and Asian regions. Sci. Rep. 2019, 9, 16697. [Google Scholar] [CrossRef]
- Hyatt, A.D.; Gould, A.R.; Zupanovic, Z.; Cunningham, A.A.; Hengstberger, S.; Whittington, R.J.; Kattenbelt, J.; Coupar, B.E. Comparative studies of piscine and amphibian iridoviruses. Arch. Virol. 2000, 145, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Ting, J.; Wu, M.; Wu, M.; Guo, I.; Chang, C. Complete genome sequence of the grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 2005, 79, 2010–2023. [Google Scholar] [CrossRef]
- Guan, W.; Jian, J.; Niu, B.; Zhang, X.; Yu, J.; Xu, X. Germplasm resources evaluation of cultured largemouth bass (Micropterus salmoides) in China based on whole genome resequencing. Genes 2024, 15, 1307. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Feng, J. Rapid diagnosis of largemouth bass ranavirus in fish samples using the loop-mediated isothermal amplification method. Mol. Cell. Probes. 2020, 52, 101569. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Chinchar, G.D.; Chinchar, V.G. Molecular characterization of a ranavirus isolated from largemouth bass Micropterus salmoides. Dis. Aquat. Organ. 1999, 37, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zilberg, D.; Grizzle, J.M.; Plumb, J.A. Preliminary description of lesions in juvenile largemouth bass injected with largemouth bass virus. Dis. Aquat. Organ. 2000, 39, 143–146. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Xue, M.; Jiang, N.; Li, Y.; Fan, Y.; Zhang, P.; Liu, N.; Xiao, Z.; Zhang, Q.; et al. Oral vaccination of largemouth bass (Micropterus salmoides) against largemouth bass ranavirus (LMBV) using yeast surface display technology. Animals 2023, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; He, H.; Chen, J.; He, X.; Qin, Q.; Yang, M. Largemouth bass Rel exerts antiviral role against fish virus and regulates the expression of interleukin-10. Fish Shellfish Immunol. 2023, 142, 109117. [Google Scholar] [CrossRef]
- Ohlemeyer, S.; Holopainen, R.; Tapiovaara, H.; Bergmann, S.M.; Schutze, H. Major capsid protein gene sequence analysis of the Santee-Cooper ranaviruses DFV, GV6, and LMBV. Dis. Aquat. Organ. 2011, 96, 195–207. [Google Scholar] [CrossRef]
- Kvansakul, M. Viral infection and apoptosis. Viruses 2017, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Micoli, K.J.; Pan, G.; Wu, Y.; Williams, J.P.; Cook, W.J.; McDonald, J.M. Requirement of calmodulin binding by HIV-1 gp160 for enhanced FAS-mediated apoptosis. J. Biol. Chem. 2000, 275, 1233–1240. [Google Scholar] [CrossRef]
- Clarke, P.; Leser, J.S.; Quick, E.D.; Dionne, K.R.; Beckham, J.D.; Tyler, K.L. Death receptor-mediated apoptotic signaling is activated in the brain following infection with West Nile virus in the absence of a peripheral immune response. J. Virol. 2014, 88, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.S.; Sampaio, G.L.; Pereira, C.S.; Campos, G.S.; Sardi, S.I.; Freitas, L.A.; Figueira, C.P.; Paredes, B.D.; Nonaka, C.K.; Azevedo, C.M.; et al. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci. Rep. 2016, 6, 39775. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Chiou, P.P.; Chen, W.J.; Chen, Y.C.; Chen, C.W.; Chiu, I.S.; Chen, S.D.; Cheng, Y.H.; Chang, C.Y. Characterization of apoptosis induced by grouper iridovirus in two newly established cell lines from barramundi, Lates calcarifer (Bloch). J. Fish. Dis. 2008, 31, 825–834. [Google Scholar] [CrossRef]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC. Cancer. 2013, 13, 600. [Google Scholar] [CrossRef]
- Pisano, M.; Pagnan, G.; Loi, M.; Mura, M.E.; Tilocca, M.G.; Palmieri, G.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Ponzoni, M.; et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol. Cancer. 2007, 6, 8. [Google Scholar]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Ahmad, L.; Mostowy, S.; Sancho-Shimizu, V. Autophagy-virus interplay: From cell biology to human disease. Front. Cell. Dev. Biol. 2018, 6, 155. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Nardacci, R.; Amendola, A.; Ciccosanti, F.; Corazzari, M.; Esposito, V.; Vlassi, C.; Taibi, C.; Fimia, G.M.; Del, N.F.; Ippolito, G.; et al. Autophagy plays an important role in the containment of HIV-1 in nonprogressor-infected patients. Autophagy 2014, 10, 1167–1178. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, Y.; Torresilla, C.; Rassart, E.; Barbeau, B. Implication of different HIV-1 genes in the modulation of autophagy. Viruses 2017, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, T.; Hu, B.; Chen, L. Interplay between autophagy and apoptosis in human viral pathogenesis. Virus Res. 2025, 359, 199611. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P.E.; Werneke, S.W.; de la Calle, C.; Guivel-Benhassine, F.; Giodini, A.; Peduto, L.; Levine, B.; Schwartz, O.; Lenschow, D.J.; Albert, M.L. Chikungunya virus-induced autophagy delays caspase-dependent cell death. J. Exp. Med. 2012, 209, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Kleeman, L.K.; Jiang, H.H.; Gordon, G.; Goldman, J.E.; Berry, G.; Herman, B.; Levine, B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998, 72, 8586–8596. [Google Scholar] [CrossRef]
- Yeganeh, B.; Ghavami, S.; Rahim, M.N.; Klonisch, T.; Halayko, A.J.; Coombs, K.M. Autophagy activation is required for influenza A virus-induced apoptosis and replication. Biochim. Biophys. Acta. Mol. Cell. Res. 2018, 1865, 364–378. [Google Scholar] [CrossRef]
- Ariosa, A.R.; Klionsky, D.J. Autophagy core machinery: Overcoming spatial barriers in neurons. J. Mol. Med. 2016, 94, 1217–1227. [Google Scholar] [CrossRef]
- Aita, V.M.; Liang, X.H.; Murty, V.V.; Pincus, D.L.; Yu, W.; Cayanis, E.; Kalachikov, S.; Gilliam, T.C.; Levine, B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 1999, 59, 59–65. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef]
- Nezis, I.P.; Simonsen, A.; Sagona, A.P.; Finley, K.; Gaumer, S.; Contamine, D.; Rusten, T.E.; Stenmark, H.; Brech, A. Ref (2) P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J. Cell. Biol. 2008, 180, 1065–1071. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends. Cell. Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.J.; Ayer, D.E.; Dynlacht, B.D. Foxk proteins repress the initiation of starvation-induced atrophy and autophagy programs. Nat. Cell. Biol. 2014, 16, 1202–1214. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, M.; Marshall, B.; Smith, S.; Covar, J.; Atherton, S. Interplay of autophagy and apoptosis during murine cytomegalovirus infection of RPE cells. Mol. Vis. 2014, 20, 1161–1173. [Google Scholar]
- Li, Q.; Rao, R.; Vazzana, J.; Goedegebuure, P.; Odunsi, K.; Gillanders, W.; Shrikant, P.A. Regulating mammalian target of rapamycin to tune vaccination-induced CD8(+) T cell responses for tumor immunity. J. Immunol. 2012, 188, 3080–3087. [Google Scholar] [CrossRef]
- Dazert, E.; Hall, M.N. mTOR signaling in disease. Curr. Opin. Cell. Biol. 2011, 23, 744–755. [Google Scholar] [CrossRef]
- O’Reilly, K.E.; Rojo, F.; She, Q.B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.I.; Lickteig, R.L.; Estes, R.; Rundell, K.; Walter, G.; Mumby, M.C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 1991, 11, 1988–1995. [Google Scholar]

| Primer | Sequence (5′–3′) | Amplicon Size (bp) |
|---|---|---|
| FHM-β-Actin-RT-F | TACGAGCTGCCTGACGGACA | 195 |
| FHM-β-Actin-RT-R | GGCTGTGATCTCCTTCTGCA | |
| MS-β-Actin-RT-F | CCACCACAGCCGAGAGGGAA | 152 |
| MS-β-Actin-RT-R | TCATGGTGGATGGGGCCAGG | |
| LMBV-MCP-RT-F | CTCGCCACTTATGACAGCCTTGAC | 148 |
| LMBV-MCP-RT-R | AACCCACGGGATAATGCTCTTTGAC | |
| LMBV-MMP-RT-F | GCGTATTTCGCACCCTCTG | 122 |
| LMBV-MMP-RT-R | TAAGCGTCGCCCTTGTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, L.; Ruan, L.; Chen, X.; Song, C.; Wei, S.; Xie, Y. Antiviral Activity of Eugenol Against Largemouth Bass Ranavirus Through Regulation of Autophagy and Apoptosis In Vitro and In Vivo. Microorganisms 2025, 13, 2281. https://doi.org/10.3390/microorganisms13102281
Wang Y, Cao L, Ruan L, Chen X, Song C, Wei S, Xie Y. Antiviral Activity of Eugenol Against Largemouth Bass Ranavirus Through Regulation of Autophagy and Apoptosis In Vitro and In Vivo. Microorganisms. 2025; 13(10):2281. https://doi.org/10.3390/microorganisms13102281
Chicago/Turabian StyleWang, Yewen, Lifang Cao, Leshan Ruan, Xingyu Chen, Chunhui Song, Shina Wei, and Yunchang Xie. 2025. "Antiviral Activity of Eugenol Against Largemouth Bass Ranavirus Through Regulation of Autophagy and Apoptosis In Vitro and In Vivo" Microorganisms 13, no. 10: 2281. https://doi.org/10.3390/microorganisms13102281
APA StyleWang, Y., Cao, L., Ruan, L., Chen, X., Song, C., Wei, S., & Xie, Y. (2025). Antiviral Activity of Eugenol Against Largemouth Bass Ranavirus Through Regulation of Autophagy and Apoptosis In Vitro and In Vivo. Microorganisms, 13(10), 2281. https://doi.org/10.3390/microorganisms13102281






