Current Concepts of Local Antibiotic Delivery in Bone and Joint Infections—A Narrative Review of Techniques and Clinical Experiences
Abstract
1. Introduction
2. Modalities of Local Antibiotic Delivery
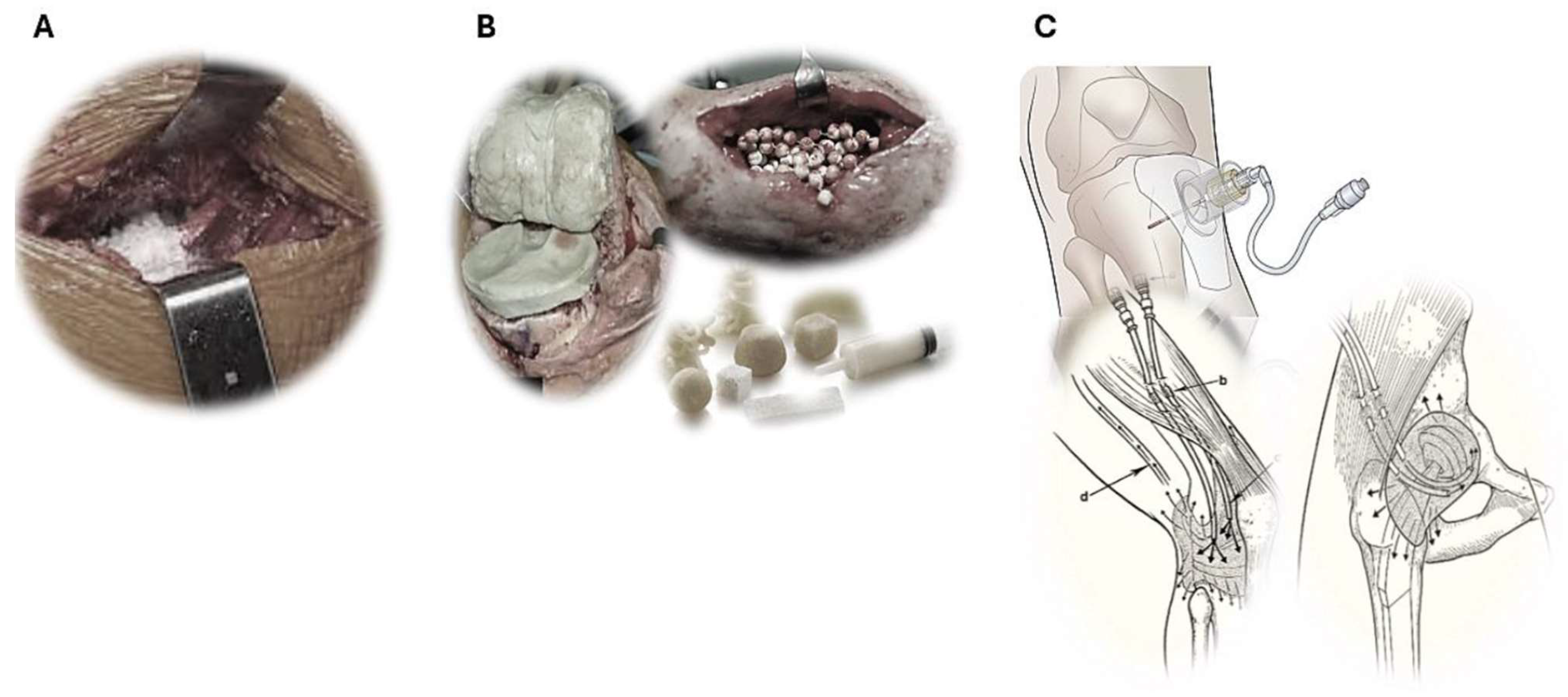
2.1. Local Vancomycin Powder Administration
2.2. Non-Resorbable Biomaterials as Antibiotic Carrier
PMMA-Bone Cement
2.3. Resorbable Antibiotic Carriers
2.3.1. Antibiotic-Loaded Auto- and Allografts
2.3.2. Antibiotic-Loaded Calcium Sulfate
2.3.3. Antibiotic-Loaded Calcium Phosphate/Hydroxyapatite (Alone or as Part of Composite Material)
2.3.4. Bioglass
2.3.5. Hydrogel
2.4. Intraosseous/Intra-Articular Injection or Infusion of Antibiotics
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PJI | Prosthetic Joint Infection |
| FRI | Fracture-Related Infections |
| OM | Osteomyelitis |
| TKA | Total Knee Arthroplasty |
| THA | Total Hip Arthroplasty |
| ALBC | Antibiotic-Loaded Bone Cement |
| PK/PD | Pharmacokinetic/Pharmacodynamic |
| CaSO4 | Calcium sulfate |
| CaP | Calcium phosphate |
| HA | Hydroxyapatite |
| MIC | Minimal Inhibitory Concentration |
| IO | Intraosseous |
| IA | Intra-articular |
| IV | Intravenous |
References
- Nguyen, A.; Lee, P.; Rodriguez, E.K.; Chahal, K.; Freedman, B.R.; Nazarian, A. Addressing the growing burden of musculoskeletal diseases in the ageing US population: Challenges and innovations. Lancet Healthy Longev. 2025, 6, 100707. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Staats, A.; Li, D.; Sullivan, A.C.; Stoodley, P. Biofilm formation in periprosthetic joint infections. Ann. Jt. 2021, 6, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanssen, J.L.J.; van der Linden, H.M.J.; van der Beek, M.T.; van der Wal, R.J.P.; Termaat, M.F.; de Boer, M.G.J.; Scheper, H. Implementation of multidisciplinary team decisions on the management of complex bone and joint infections: An observational study. BMC Musculoskelet. Disord. 2025, 26, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, N.; Rupp, M.; Baertl, S.; Alt, V. The role of multidisciplinary teams in musculoskeletal infection. Bone Jt. Res. 2022, 11, 6–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, S.B.; Pinkney, J.A.; Chen, A.F.; Tande, A.J. Periprosthetic Joint Infection: Current Clinical Challenges. Clin. Infect. Dis. 2023, 77, e34–e45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, J.; Cozzi, F.; Forrest, G.N. Using antibiotics wisely. Curr. Opin. Infect. Dis. 2023, 36, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Ometti, M.; Delmastro, E.; Salini, V. Management of prosthetic joint infections: A guidelines comparison. Musculoskelet. Surg. 2022, 106, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Springorum, H.R.; Baier, C.; Maderbacher, G.; Paulus, A.; Grifka, J.; Goetz, J. Periprosthetic Joint Infections of the Knee-Comparison of Different Treatment Algorithms. J. Clin. Med. 2024, 13, 3718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gatti, M.; Tedeschi, S.; Zamparini, E.; Pea, F.; Viale, P. Pharmacokinetic and pharmacodynamic considerations for optimizing antimicrobial therapy used to treat bone and joint infections: An evidence-based algorithmic approach. Expert Opin. Drug Metab. Toxicol. 2023, 19, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sörgel, F. Penetration of antibacterials into bone: Pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 2009, 48, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Short or Long Antibiotic Regimes in Orthopaedics (Solario): A Randomised Open Label Multi-Centre Clinical Trial. Available online: https://www.expmedndm.ox.ac.uk/research/bone-infection/BoneInfection/STUDIES/SOLARIO (accessed on 15 March 2025).
- Luo, H.; Ren, Y.; Su, Y.; Xue, F.; Hong, Z. Intraoperative vancomycin powder to reduce surgical site infections after posterior spine surgery: A systematic review and meta-analysis. EFORT Open Rev. 2022, 7, 109–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakhsheshian, J.; Dahdaleh, N.S.; Lam, S.K.; Savage, J.W.; Smith, Z.A. The use of vancomycin powder in modern spine surgery: Systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015, 83, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhang, L.; Zhang, D.; Tao, L.; Zhao, Y.; Luo, H. Efficacy and safety of vancomycin for local application in the prevention of surgical site infection after joint arthroplasty: A systematic review and meta-analysis. EFORT Open Rev. 2024, 9, 953–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saka, N.; Yamada, K.; Ono, K.; Iwata, E.; Mihara, T.; Uchiyama, K.; Watanabe, Y.; Matsushita, K. Effect of topical vancomycin powder on surgical site infection prevention in major orthopaedic surgery: A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. J. Hosp. Infect. 2024, 150, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Eckes, S.; Rommens, P.M.; Schmitz, K.; Nickel, D.; Ritz, U. Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dial, B.L.; Lampley, A.J.; Green, C.L.; Hallows, R. Intrawound Vancomycin Powder in Primary Total Hip Arthroplasty Increases Rate of Sterile Wound Complications. Hip Pelvis 2018, 30, 37–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanada, M.; Nishikino, S.; Hotta, K.; Furuhashi, H.; Hoshino, H.; Matsuyama, Y. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, H.W.; Elson, R.A.; Heinert, K. Antibiotic-loaded acrylic cement: Current concepts. Clin. Orthop. Relat. Res. 1984, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Alonso, P.; Rouse, M.S.; Piper, K.E.; Jacofsky, D.J.; Osmon, D.R.; Patel, R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin. Orthop. Relat. Res. 2006, 445, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, J.; Merino, V.; Nácher, A.; Rodrigo, J.L.; Climente, M.; Merino-Sanjuán, M. Antibiotic-loaded Bone Cement as Prophylaxis in Total Joint Replacement. Orthop. Surg. 2017, 9, 331–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neut, D.; Kluin, O.S.; Thompson, J.; van der Mei, H.C.; Busscher, H.J. Gentamicin release from commercially-available gentamicin-loaded PMMA bone cements in a prosthesis-related interfacial gap model and their antibacterial efficacy. BMC Musculoskelet. Disord. 2010, 11, 258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anagnostakos, K.; Kelm, J. Enhancement of antibiotic elution from acrylic bone cement. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 467–475. [Google Scholar] [CrossRef] [PubMed]
- van de Belt, H.; Neut, D.; Uges, D.R.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Tetsworth, K.D. Antibiotic Elution from Cement Spacers and Its Influencing Factors. Antibiotics 2025, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, Y.C.; Hsu, Y.M.; Chang, C.H. Enhancing Drug Release From Antibiotic-loaded Bone Cement Using Porogens. J. Am. Acad. Orthop. Surg. 2016, 24, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Prats-Peinado, L.; Fernández-Fernández, T.; Márquez-Gómez, M.; Matas-Diaz, J.A.; Sánchez-Somolinos, M.; de la Villa-Martínez, S.; Vaquero-Martín, J.; Sanz-Ruiz, P. Do High Doses of Multiple Antibiotics Loaded into Bone Cement Spacers Improve the Success Rate in Staphylococcal Periprosthetic Joint Infection When Rifampicin Cannot Be Employed? Antibiotics 2024, 13, 538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jameson, S.S.; Asaad, A.; Diament, M.; Kasim, A.; Bigirumurame, T.; Baker, P.; Mason, J.; Partington, P.; Reed, M. Antibiotic-loaded bone cement is associated with a lower risk of revision following primary cemented total knee arthroplasty: An analysis of 731,214 cases using National Joint Registry data. Bone Jt. J. 2019, 101-B, 1331–1347. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.W.; Cook, M.J.; O’Neill, T.W.; Board, T.N. Is the use of antibiotic-loaded bone cement associated with a lower risk of revision after primary total hip arthroplasty? Bone Jt. J. 2020, 102-B, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Gil-Gonzalez, S.; Velasco-Regúlez, B.; Cerquides, J.; Hinarejos, P.; Monllau, J.C.; Pelfort, X. Antibiotic-loaded bone cement is associated with a reduction of the risk of revision of total knee arthroplasty: Analysis of the Catalan Arthroplasty Register. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 354–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bendich, I.; Zhang, N.; Barry, J.J.; Ward, D.T.; Whooley, M.A.; Kuo, A.C. Antibiotic-Laden Bone Cement Use and Revision Risk After Primary Total Knee Arthroplasty in U.S. Veterans. J. Bone Jt. Surg. Am. 2020, 102, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- von Hertzberg-Boelch, S.P.; Luedemann, M.; Rudert, M.; Steinert, A.F. PMMA Bone Cement: Antibiotic Elution and Mechanical Properties in the Context of Clinical Use. Biomedicines 2022, 10, 1830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanz-Ruiz, P.; Berberich, C. Infection Risk-Adjusted Antibiotic Prophylaxis Strategies in Arthroplasty: Short Review of Evidence and Experiences of a Tertiary Center in Spain. Orthop. Res. Rev. 2020, 12, 89–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joseph, T.N.; Chen, A.L.; Di Cesare, P.E. Use of antibiotic-impregnated cement in total joint arthroplasty. J. Am. Acad. Orthop. Surg. 2003, 11, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Fragomen, A.T.; Moriarty, T.F.; Morgenstern, M.; Egol, K.A.; Zalavras, C.; Obremskey, W.T.; Raschke, M.; McNally, M.A.; Fracture-Related Infection (FRI) Consensus Group. Evidence-Based Recommendations for Local Antimicrobial Strategies and Dead Space Management in Fracture-Related Infection. J. Orthop. Trauma 2020, 34, 18–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ensing, G.T.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin. Orthop. Relat. Res. 2008, 466, 1492–1498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cara, A.; Ballet, M.; Hemery, C.; Ferry, T.; Laurent, F.; Josse, J. Antibiotics in Bone Cements Used for Prosthesis Fixation: An Efficient Way to Prevent Staphylococcus aureus and Staphylococcus epidermidis Prosthetic Joint Infection. Front. Med. 2021, 7, 576231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cara, A.; Ferry, T.; Laurent, F.; Josse, J. Prophylactic Antibiofilm Activity of Antibiotic-Loaded Bone Cements against Gram-Negative Bacteria. Antibiotics 2022, 11, 137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anagnostakos, K.; Wilmes, P.; Schmitt, E.; Kelm, J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009, 80, 193–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fink, B.; Vogt, S.; Reinsch, M.; Büchner, H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses. Clin. Orthop. Relat. Res. 2011, 469, 3141–3147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anagnostakos, K.; Fink, B. Antibiotic-loaded cement spacers—Lessons learned from the past 20 years. Expert Rev. Med. Devices 2018, 15, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Sprowson, A.P.; Jensen, C.; Chambers, S.; Parsons, N.R.; Aradhyula, N.M.; Carluke, I.; Inman, D.; Reed, M.R. The use of high-dose dual-impregnated antibiotic-laden cement with hemiarthroplasty for the treatment of a fracture of the hip: The Fractured Hip Infection trial. Bone Jt. J. 2016, 98-B, 1534–1541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanz-Ruiz, P.; Matas-Diez, J.A.; Villanueva-Martínez, M.; Santos-Vaquinha Blanco, A.D.; Vaquero, J. Is Dual Antibiotic-Loaded Bone Cement More Effective and Cost-Efficient Than a Single Antibiotic-Loaded Bone Cement to Reduce the Risk of Prosthetic Joint Infection in Aseptic Revision Knee Arthroplasty? J. Arthroplast. 2020, 35, 3724–3729. [Google Scholar] [CrossRef] [PubMed]
- Jenny, J.Y.; Hamon, M.; Klein, S.; Reiter-Schatz, A.; Rondé-Oustau, C.; Boéri, C.; Wisniewski, S.; Gaudias, J. Cement Loaded with High-Dose Gentamicin and Clindamycin Reduces the Risk of Subsequent Infection After One-Stage Hip or Knee Arthroplasty Exchange for Periprosthetic Infection: A Preliminary Study. J. Arthroplast. 2021, 36, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Berberich, C.; Josse, J.; Ruiz, P.S. Patients at a high risk of PJI: Can we reduce the incidence of infection using dual antibiotic-loaded bone cement? Arthroplasty 2022, 4, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anagnostakos, K.; Hitzler, P.; Pape, D.; Kohn, D.; Kelm, J. Persistence of bacterial growth on antibiotic-loaded beads: Is it actually a problem? Acta Orthop. 2008, 79, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Shanks, R.M.Q.; Davis, C.M., III; Craft, D.W.; Wood, T.K.; Hamlin, B.R.; Urish, K.L. Viable bacteria persist on antibiotic spacers following two-stage revision for periprosthetic joint infection. J. Orthop. Res. 2018, 36, 452–458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing) Database. Available online: https://mic.eucast.org/search (accessed on 5 January 2025).
- Masri, B.A.; Duncan, C.P.; Beauchamp, C.P. Long-term elution of antibiotics from bone-cement: An in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J. Arthroplast. 1998, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, J.D.; Fernández-Rodríguez, D.; Goh, G.S.; DeMik, D.E.; Hughes, A.J.; Parvizi, J.; Courtney, P.M.; Purtill, J.J.; Austin, M.S. In Vivo Intra-Articular Antibiotic Concentrations at 24 Hours After TKA Fall Below the Minimum Inhibitory Concentration for Most Bacteria: A Randomized Study of Commercially Available Bone Cement. J. Bone Jt. Surg. Am. 2024, 106, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Wezenberg, D.; Nilsson, M.; Söderquist, B.; Nilsson, L.E.; Schilcher, J. Bone allograft impregnated with tobramycin and vancomycin delivers antibiotics in high concentrations for prophylaxis against bacteria commonly associated with prosthetic joint infections. Microbiol. Spectr. 2024, 12, e0041424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howlin, R.P.; Brayford, M.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015, 59, 111–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahl, P.; Guidi, M.; Benninger, E.; Rönn, K.; Gautier, E.; Buclin, T.; Magnin, J.L.; Livio, F. The levels of vancomycin in the blood and the wound after the local treatment of bone and soft-tissue infection with antibiotic-loaded calcium sulphate as carrier material. Bone Jt. J. 2017, 99-B, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinisch, K.; Schläppi, M.; Meier, C.; Wahl, P. Local antibiotic treatment with calcium sulfate as carrier material improves the outcome of debridement, antibiotics, and implant retention procedures for periprosthetic joint infections after hip arthroplasty—A retrospective study. J. Bone Jt. Infect. 2022, 7, 11–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gramlich, Y.; Johnson, T.; Kemmerer, M.; Walter, G.; Hoffmann, R.; Klug, A. Salvage procedure for chronic periprosthetic knee infection: The application of DAIR results in better remission rates and infection-free survivorship when used with topical degradable calcium-based antibiotics. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, I.K.; Palmer, A.J.R.; Hotchen, A.J.; McNally, M.A.; Young, B.C.; Alvand, A.; Taylor, A.; Kendrick, B.J.L. The use of antibiotic-loaded calcium sulphate beads in debridement, antibiotics, and implant retention (DAIR) for periprosthetic infections: A retrospective comparative cohort on outcome. Acta Orthop. 2024, 95, 707–714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McPherson, E.J.; Crawford, B.M.; Kenny, S.G.; Dipane, M.V.; Salarkia, S.; Stavrakis, A.I.; Chowdhry, M. Point-of-Care Coating of Revision Femoral Stems with Antibiotic-Loaded Calcium Sulfate: Reduction in Infection After 2nd Stage Reimplantation but Not with Aseptic Revisions. Arthroplast. Today 2024, 25, 101302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.; Wu, Y.; Ni, H.; Li, M.; Zhang, C.; Qi, B.; Wei, M.; Wang, T.; Xu, Y. Antibiotic-loaded calcium sulfate in clinical treatment of chronic osteomyelitis: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferguson, J.; Bourget-Murray, J.; Hotchen, A.J.; Stubbs, D.; McNally, M. A comparison of clinical and radiological outcomes between two different biodegradable local antibiotic carriers used in the single-stage surgical management of long bone osteomyelitis. Bone Jt. Res. 2023, 12, 412–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magdaleno, A.; McCauley, R.A. Severe hypercalcemia after joint arthroscopy: Calcium sulfate beads to blame. AACE Clin. Case Rep. 2019, 5, e372–e374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albee, F.H. Studies in bone growth: Triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 1920, 71, 32–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, P.; Bulnheim, U.; Diener, A.; Lüthen, F.; Teller, M.; Klinkenberg, E.D.; Neumann, H.G.; Nebe, B.; Liebold, A.; Steinhoff, G.; et al. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J. Cell. Mol. Med. 2008, 12, 281–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Solberg, B.D.; Gutow, A.P.; Baumgaertner, M.R. Efficacy of gentamycin-impregnated resorbable hydroxyapatite cement in treating osteomyelitis in a rat model. J. Orthop. Trauma 1999, 13, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Lang, Z.G.; Zhang, X.; Guo, Q.; Liang, Y.X.; Yuan, F. Clinical observations of vancomycin-loaded calcium phosphate cement in the 1-stage treatment of chronic osteomyelitis: A randomized trial. Ann. Palliat. Med. 2021, 10, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Dvorzhinskiy, A.; Perino, G.; Chojnowski, R.; van der Meulen, M.C.H.; Bostrom, M.P.G.; Yang, X. Ceramic composite with gentamicin decreases persistent infection and increases bone formation in a rat model of debrided osteomyelitis. J. Bone Jt. Infect. 2021, 6, 283–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stravinskas, M.; Horstmann, P.; Ferguson, J.; Hettwer, W.; Nilsson, M.; Tarasevicius, S.; Petersen, M.M.; McNally, M.A.; Lidgren, L. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: In vitro and clinical release studies. Bone Jt. Res. 2016, 5, 427–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muir, R.; Birnie, C.; Hyder-Wilson, R.; Ferguson, J.; McNally, M.A. Does local implantation of gentamicin impair renal function in patients undergoing surgery for chronic bone infection? Int. J. Res. Orthop. 2021, 7, 438–443. [Google Scholar] [CrossRef]
- McNally, M.A.; Ferguson, J.Y.; Scarborough, M.; Ramsden, A.; Stubbs, D.A.; Atkins, B.L. Mid- to long-term results of single-stage surgery for patients with chronic osteomyelitis using a bioabsorbable gentamicin-loaded ceramic carrier. Bone Jt. J. 2022, 104-B, 1095–1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, X.; Zhao, C.; Gu, Y.; Li, L.; Wang, H.; Huang, W.; Zhou, N.; Wang, D.; Zhu, Y.; Xu, J.; et al. A novel injectable borate bioactive glass cement for local delivery of vancomycin to cure osteomyelitis and regenerate bone. J. Mater. Sci. Mater. Med. 2014, 25, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhao, C.J.; Cui, X.; Gu, Y.F.; Jia, W.T.; Rahaman, M.N.; Wang, Y.; Huang, W.H.; Zhang, C.Q. A novel injectable borate bioactive glass cement as an antibiotic delivery vehicle for treating osteomyelitis. PLoS ONE 2014, 9, e85472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gatti, S.D.; Gaddi, D.; Turati, M.; Leone, G.; Arts, J.J.; Pessina, F.; Carminati, M.; Zatti, G.; De Rosa, L.; Bigoni, M. Clinical outcomes and complications of S53P4 bioactive glass in chronic osteomyelitis and septic non-unions: A retrospective single-center study. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Björkenheim, R.; Jämsen, E.; Eriksson, E.; Uppstu, P.; Aalto-Setälä, L.; Hupa, L.; Eklund, K.K.; Ainola, M.; Lindfors, N.C.; Pajarinen, J. Sintered S53P4 bioactive glass scaffolds have anti-inflammatory properties and stimulate osteogenesis in vitro. Eur. Cells Mater. 2021, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, N.; Geurts, J.; Drago, L.; Arts, J.J.; Juutilainen, V.; Hyvönen, P.; Suda, A.J.; Domenico, A.; Artiaco, S.; Alizadeh, C.; et al. Antibacterial Bioactive Glass, S53P4, for Chronic Bone Infections—A Multinational Study. Adv. Exp. Med. Biol. 2017, 971, 81–92, Erratum in Adv. Exp. Med. Biol. 2017, 971, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Pitarresi, G.; Saiano, F.; Cavallaro, G.; Mandracchia, D.; Palumbo, F.S. A new biodegradable and biocompatible hydrogel with polyaminoacid structure. Int. J. Pharm. 2007, 335, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoccali, C.; Scoccianti, G.; Biagini, R.; Daolio, P.A.; Giardina, F.L.; Campanacci, D.A. Antibacterial hydrogel coating in joint mega-prosthesis: Results of a comparative series. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romanò, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harper, K.D.; Incavo, S.J. Intraosseous Administration of Medications in Total Knee Arthroplasty: An Opportunity for Improved Outcomes and Superior Compliance. JBJS Essent. Surg. Tech. 2024, 14, e22.00071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, S.W.; Zhang, M.; Freeman, J.T.; Mutu-Grigg, J.; Pavlou, P.; Moore, G.A. The Mark Coventry Award: Higher tissue concentrations of vancomycin with low-dose intraosseous regional versus systemic prophylaxis in TKA: A randomized trial. Clin. Orthop. Relat. Res. 2014, 472, 57–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, M.; Wei, Z.; Yang, X.; Xu, Y.; Zhu, W.; Weng, X.; Feng, B. Safety and effectiveness of intraosseous regional prophylactic antibiotics in total knee arthroplasty: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2024, 144, 4233–4245. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.D.; Higuera-Rueda, C.A.; de Beaubien, B.C.; Warner, K.D.; Glassman, A.H.; Parvataneni, H.K.; Piuzzi, N.S. Safety Profile of Seven-Day Intra-articular Antibiotic Irrigation for the Treatment of Chronic Periprosthetic Joint Infection: A Prospective Randomized Phase II Comparative Study. J. Arthroplast. 2024, 39, S229–S235.e1. [Google Scholar] [CrossRef] [PubMed]
- Bruyninckx, S.; Metsemakers, W.J.; Depypere, M.; Henckaerts, L.; van den Hout, E.; Onsea, J.; Ghijselings, S.; Vles, G.F. Local antibiotic delivery via intra-articular catheter infusion for the treatment of periprosthetic joint infection: A systematic review. Arch. Orthop. Trauma Surg. 2024, 144, 5177–5189. [Google Scholar] [CrossRef] [PubMed]
- Lawing, C.R.; Lin, F.C.; Dahners, L.E. Local Injection of Aminoglycosides for Prophylaxis Against Infection in Open Fractures. J. Bone Jt. Surg. Am. 2015, 97, 1844–1851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whiteside, L.A.; Peppers, M.; Nayfeh, T.A.; Roy, M.E. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intra-articular antibiotic infusion. Clin. Orthop. Relat. Res. 2011, 469, 26–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arthur, J.R.; Bingham, J.S.; Clarke, H.D.; Spangehl, M.J.; Young, S.W. Intraosseous Regional Administration of Antibiotic Prophylaxis in Total Knee Arthroplasty. JBJS Essent. Surg. Tech. 2020, 10, e20.00001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, S.W.; Zhang, M.; Freeman, J.T.; Vince, K.G.; Coleman, B. Higher cefazolin concentrations with intraosseous regional prophylaxis in TKA. Clin. Orthop. Relat. Res. 2013, 471, 244–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, S.W.; Zhang, M.; Moore, G.A.; Pitto, R.P.; Clarke, H.D.; Spangehl, M.J. The John N. Insall Award: Higher Tissue Concentrations of Vancomycin Achieved with Intraosseous Regional Prophylaxis in Revision TKA: A Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2018, 476, 66–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Merchan, E.C.; Encinas-Ullan, C.A. Intraosseous Regional Administration of Vancomycin Prophylaxis for Primary and Revision Total Knee Arthroplasty. Arch. Bone Jt. Surg. 2024, 12, 219–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, B.; Wahafu, T.; Li, G.; Zhang, X.; Wang, Y.; Momin, M.; Cao, L. Single-stage treatment of chronically infected total hip arthroplasty with cementless reconstruction: Results in 126 patients with broad inclusion criteria. Bone Jt. J. 2019, 101-B, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Li, G.; Zhang, X.; Wang, Y.; Mu, W.; Cao, L. Effective treatment of single-stage revision using intra-articular antibiotic infusion for culture-negative prosthetic joint infection. Bone Jt. J. 2020, 102-B, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Li, G.; Zhang, X.; Xu, B.; Wang, Y.; Chen, Y.; Cao, L. Effective single-stage revision using intra-articular antibiotic infusion after multiple failed surgery for periprosthetic joint infection : A mean seven years’ follow-up. Bone Jt. J. 2022, 104-B, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, L.A.; Nayfeh, T.A.; LaZear, R.; Roy, M.E. Reinfected revised TKA resolves with an aggressive protocol and antibiotic infusion. Clin. Orthop. Relat. Res. 2012, 470, 236–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spangehl, M.J.; Clarke, H.D.; Moore, G.A.; Zhang, M.; Probst, N.E.; Young, S.W. Higher Tissue Concentrations of Vancomycin Achieved with Low-Dose Intraosseous Injection Versus Intravenous Despite Limited Tourniquet Duration in Primary Total Knee Arthroplasty: A Randomized Trial. J. Arthroplast. 2022, 37, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.D.; Lambert, B.S.; O’Dowd, J.; Sullivan, T.; Incavo, S.J. Clinical outcome evaluation of intraosseous vancomycin in total knee arthroplasty. Arthroplast. Today 2020, 6, 220–223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klasan, A.; Patel, C.K.; Young, S.W. Intraosseous Regional Administration of Vancomycin in Primary Total Knee Arthroplasty Does Not Increase the Risk of Vancomycin-Associated Complications. J. Arthroplast. 2021, 36, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, B.; McEwen, P.; Wilkinson, M.; Hazratwala, K.; Hellman, J.; Kan, H.; McLean, A.; Panwar, Y.; Doma, K.; Grant, A. Intraosseous Regional Prophylactic Antibiotics Decrease the Risk of Prosthetic Joint Infection in Primary TKA: A Multicenter Study. Clin. Orthop. Relat. Res. 2021, 479, 2504–2512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, K.J.; Wininger, A.E.; Sullivan, T.C.; Varghese, B.; Clyburn, T.A.; Incavo, S.J. Superior Clinical Results with Intraosseous Vancomycin in Primary Total Knee Arthroplasty. J. Arthroplast. 2025, 40, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.A.; Wininger, A.E.; Sullivan, T.C.; Brown, T.S.; Clyburn, T.A.; Incavo, S.J.; Park, K.J. The AAHKS Best Podium Presentation Research Award: Intraosseous Vancomycin Reduces the Rate of Periprosthetic Joint Infection Following Aseptic Revision Total Knee Arthroplasty. J. Arthroplast. 2025, 40, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Christopher, Z.K.; Pulicherla, N.; Iturregui, J.M.; Brinkman, J.C.; Spangehl, M.J.; Clarke, H.D.; Bingham, J.S. Low Risk of Periprosthetic Joint Infection After Aseptic Revision Total Knee Arthroplasty with Intraosseous Vancomycin. J. Arthroplast. 2024, 39, S305–S309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Ji, B.; Wulamu, W.; Yushan, N.; Guo, X.; Cao, L. One-stage revision using intra-articular carbapenem infusion effectively treats chronic periprosthetic joint infection caused by Gram-negative organisms. Bone Jt. J. 2023, 105-B, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, L.A.; Roy, M.E. One-stage Revision with Catheter Infusion of Intraarticular Antibiotics Successfully Treats Infected THA. Clin. Orthop. Relat. Res. 2017, 475, 419–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hieda, Y.; Choe, H.; Maruo, A.; Abe, K.; Shimoda, M.; Ike, H.; Kumagai, K.; Kobayashi, N.; Inaba, Y. Clinical outcomes of continuous local antibiotic perfusion in combination with debridement antibiotics and implant retention for periprosthetic hip joint infection. Sci. Rep. 2025, 15, 26017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosugi, K.; Zenke, Y.; Sato, N.; Hamada, D.; Ando, K.; Okada, Y.; Yamanaka, Y.; Sakai, A. Potential of Continuous Local Antibiotic Perfusion Therapy for Fracture-Related Infections. Infect. Dis. Ther. 2022, 11, 1741–1755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abedi, A.O.; Abedi, A.A.; Ferry, T.; Citak, M. Current Applications and the Future of Phage Therapy for Periprosthetic Joint Infections. Antibiotics 2025, 14, 581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Pathogen | Gentamicin or Tobramycin | Clindamycin | Vancomycin |
|---|---|---|---|
| Gram-positive aerobic bacteria | |||
| Coagulase-positive Staphylococci S. aureus (MSSA) | +++ | +++ | +++ |
| S. aureus (MRSA) | ++ | ++ | +++ |
| Coagulase-negative Staphylococci e.g., S. epidermidis | + | + | +++ |
| Streptococci | (+) | ++ | +++ |
| Enterococci | (+) | − | ++ |
| Gram-positive anaerobic bacteria | |||
| C. difficile | − | − | ++ |
| C. acnes | (+) | ++ | ++ |
| Gram-negative aerobic bacteria | |||
| E. coli | ++ | − | − |
| K. pneumoniae | ++ | − | − |
| P. aeruginosa | ++ | − | − |
| Enterobacter, Serratia | ++ | − | − |
| Authors and Study | Number of Patients | Indication and Intended Purpose | Type, Concentration and Duration of Antibiotic Administration | Route of Drug Delivery | Key Findings of Study and Clinical Outcome |
|---|---|---|---|---|---|
| Prophylactic & therapeutic use with endpoints safety & pharmacokinetics | |||||
| Spangehl et al., 2022 [103] | Randomized study with 24 patients | Prophylactic use in primary TKA Patients randomized to receive either vancomycin IV or vancomycin IO | Vancomycin IV (weight-based (15 mg/kg) vs. vancomycin IO (500 mg in 100 mL solution), single-shot | Intraosseous bolus injection via cannula into proximal tibia | Median vancomycin concentrations in tissue were significantly higher (5–15 times) at all time points in the vancomycin IO group |
| Young et al., 2013 [96] | Randomized study with 22 patients | Prophylactic use in primary TKA Patients randomized to receive either cefazolin IV or cefazolin IO | Cefazolin (1 g) IV 10 min before tourniquet inflation. Vs. cefazolin IO (1 g in 200 mL of normal saline), single shot | Intraosseous bolus injection via a tibial cannula after tourniquet inflation | Mean tissue concentration of cefazolin in subcutaneous fat was 186 μg/g in the IO group and 11 μg/g in the IV group. The mean tissue concentration in bone was 130 μg/g in the IO group and 11 μg/g in the IV group. |
| Young et al., 2014 [89] | Randomized study with 30 patients | Prophylactic use in primary TKA Patients randomized to receive either vancomycin IV or vancomycin IO | Vancomycin IV (fixed dose, 1 g) vs. vancomycin IO (250 or 500 mg), single-shot | Intraosseous bolus injection via cannula into proximal tibia | Mean tissue concentration of vancomycin in subcutaneous fat was 14 μg/g in the 250 mg IO group, 44 μg/g in the 500 mg IO group, and 3.2 μg/g in the IV group. Mean concentrations in bone were 16 μg/g in the 250 mg IO group, 38 μg/g in the 500 mg IO group, and 4.0 μg/g in the IV group. |
| Harper et al., 2020 [104] | Retrospective review of 119 TKA patients (100 primary and 19 revision cases) | Prophylactic use in primary and revision TKA | Vancomycin IV vs. vancomycin IO (500 mg in 200 mL saline solution) | Intraosseous injection of 100 mL vancomycin solution via cannula in tibial tubercle region and 100 mL in distal femur | No significant differences in the complication rate or creatinine values were identified between IO and IV groups. |
| Young et al., 2018 [97] | Randomized study with 20 patients | Prophylactic use in revision TKA Patients randomized to receive either vancomycin IV or vancomycin IO | Vancomycin IV (fixed dose, 1 g) vs. vancomycin IO (500 mg), single-shot | Intraosseous bolus injection via cannula into proximal tibia | The mean tissue concentration of vancomycin in fat samples was 3.7 μg/g in the IV group vs. 49.3 μg/g in the IO group; mean tissue concentrations in femoral bone were 6.4 μg/g in the IV group vs. 77.1 μg/g in the IO group. Vancomycin concentrations in the final subcutaneous fat sample taken before closure were 5.3 times higher in the IO group vs. the IV group. |
| Klasan et al., 2021 [105] | Retrospective study of 331 cases receiving IO vancomycin | Prophylactic use in TKA | Vancomycin IO (500 mg), single-shot in addition to weight-based cefazolin | Intraosseous injection via cannula into proximal tibia | IO vancomycin in addition to standard IV cefazolin prophylaxis in TKA is safe without significant adverse effects of vancomycin, such as acute kidney injury, red man syndrome, or neutropenia. The 90-day PJI rate was 0%, and the 1-year PJI rate was 0.2%. |
| Springer et al., 2024 [91] | Randomized multi-center (17 hospitals) study with 76 patients | Treatment use in chronic hip & knee PJI. Patients randomized to 2-stage exchange arthroplasty with either cyclic IA irrigation or with standard ALBC spacer in addition to IV antibiotics | Start with tobramycin IA irrigation using 80 mg in 50 mL of saline (1.600 μg/mL) daily with a 2 h soak followed by 30 min of vacuum to actively drain the intra-articular joint space. The patient then received hourly irrigation using 125 mg of vancomycin IA in 50 mL of saline with a 30 min soak and a 30 min vacuum. Patients received 22 total irrigation cycles per day (approximately 2.750 mg vancomycin/day). Duration: 7 days | Cyclic IA irrigation with instillation and evacuation of antibiotics through a short-term implantable porous titanium spacer | Both detectable vancomycin and tobramycin concentrations were well below established systemic toxicity concentrations, and no case of systemic side effects was observed. Advantage of this therapy modality is that it includes a pump that does not require manual injection of the antibiotics, and the antibiotics are removed through a vacuum system after a soak period, eliminating the concerns for fluid accumulation in the joint. |
| Prophylactic & therapeutic use with endpoints infection control/reinfection | |||||
| Parkinson et al., 2021 [106] | Retrospective multi-center study of 1909 cases | Prophylactic use in primary TKA | Cefazolin IO (1 g) in 324 patients or vancomycin IO (500 mg) in 391 patients, single shot, with or without supplementary IV prophylaxis | Intraosseous injection via cannula into proximal tibia | IO regional antibiotic delivery was associated with a lower risk of infection within 12 months (0.1%) compared with the risk after traditional IV administration (1.4%, relative risk = 0.10; p = 0.03). |
| Yu et al., 2024 [90] | Meta-analysis of 12 studies (7 prospective, 5 retrospective) with 4091 cases | Prophylactic use in primary TKA | Vancomycin IV (weight-based (15 mg/kg) vs. Vancomycin IO (500 mg), single-shot | Intraosseous injection via cannula into proximal tibia | IO vancomycin significantly increased the drug concentration in the periarticular adipose and bone tissue compared to IV vancomycin. Regarding the incidence of postoperative PJI after primary TKA, IO vancomycin was more effective in reducing the occurrence of PJI compared to IV vancomycin (OR: 0.19; 95% CI: 0.06–0.59; p < 0.001). No significant differences were found between the two groups in terms of postoperative pulmonary embolism and vancomycin-related complications. |
| Park et al., 2025 [107] | Retrospective review of 1923 cases | Prophylactic use in primary TKA | Vancomycin IV (weight-based (15 mg/kg) vs. vancomycin IO (500 mg), single-shot | Intraosseous injection via cannula into proximal tibia | IO group had significantly lower incidence of PJI compared to the IV group at 90 days (0.5 vs 1.6%, p = 0.018), 1-year (0.7 vs. 1.8%, p = 0.048), and 2-year (0.9 vs. 2.4%, p = 0.032) follow-up. In addition, there was a lower incidence of nonoperative wound complications requiring oral antibiotics in the IO group, as well as a lower incidence of acute kidney injury |
| McNamara et al., 2025 [108] | Retrospective review of 719 cases | Prophylactic use in aseptic revision TKA | Vancomycin IV (weight-based (15 mg/kg) vs. vancomycin IO (500 mg), single-shot | Intraosseous injection via cannula into proximal tibia | IO cohort with significantly lower PJI incidence compared to the IV cohort at 30 days (0.3 vs. 2.1%, p = 0.03), 90-day (0.9 vs. 3.1%, p = 0.04), and 1-year follow-up (1.6 vs. 4.9%, p = 0.04). There were no reported adverse reactions to vancomycin and no differences in the incidence of acute kidney injury, deep venous thrombosis or pulmonary embolism between the groups. |
| Christopher et al., 2024 [109] | Observational study of 117 cases | Prophylactic use in aseptic revision TKA | Vancomycin IO (500 mg) in conjunction with IV cephalosporins or clindamycin. | Intraosseous injection via cannula into proximal tibia | The rate of PJI was 0% at 3 months postop. Follow-up at 1 year was obtained for 113 of the 117 revision TKAs, and the PJI rate remained 0%. The rate of PJI at the final follow-up of ≥1 year was 0.88%. |
| Ji et al., 2019 [99] | Observational study of 126 cases | Treatment use in hip PJI Single-stage w/o prior patient selection | Vancomycin IA for MDR gram-pos. bacteria Vancomycin + Imipenem IA for polymicrobial organisms with gram-neg. bacteria Fluconazole/Voriconazole IA for fungi Conc. Vancomycin: 500 mg/days Conc. Imipenem: 500 mg/days Conc. Fluconazole/Voriconazole: 100–200 mg/days Mean duration:16–18 days | Catheter-based intra-articular infusion | Total infection-free cases were 89.2% at a mean follow-up time of 58 months. The success rate in patients with multidrug-resistant organisms was 84.2%. |
| Ji et al., 2020 [100] | Observational study of 51 cases | Treatment use in hip and knee culture-negative PJI Single-stage | 500 mg Vancomycin IA + 500 mg Imipinem IA per day, alternately in the morning and afternoon | Catheter-based intra-articular infusion | No additional medical treatment for recurrent infection for 90.2% of cases at a mean of 53.2 months was needed. Impaired kidney function observed in 2 patients. |
| Ji et al., 2022 [101] | Observational study of 78 cases | Treatment use in hip and knee PJI in patients with multiple prior surgical interventions because of infection recurrence | 500 mg Vancomycin IA, 500 mg Imipenem IA, or 100 mg voriconazole IA per day. The antibiotic solution was soaked into the joint for 24 h for a mean of 16 days | Catheter-based intra-articular infusion | The seven-year infection-free survival was 87.6% for all patients. No significant difference in infection-free survival was observed between hip and knee PJIs |
| Li et al., 2023 [110] | Observational study of 32 cases | Treatment use in hip & knee PJI in patients with gram-negative pathogens | 500 mg Imipenem IA for single gram-negative PJI per day 500 mg Vancomycin + 500 mg Imipenem IA for polymicrobial PJI with gram-neg | Catheter-based intra-articular infusion | Of 32 cases, treatment failed to eradicate infection in only three cases (9.4%), at a mean follow-up of 55.1 months. |
| Bruyninckx et al., 2024 [92] | Meta-analysis of 15 articles, encompassing 631 PJIs in 626 patients, all retrospective studies or case series | Treatment use in hip & knee PJI. 79.1% of cases were treated in single-stage revisions with adjuvant IA antibiotic infusion, 12.2% in single-stage revisions with stand-alone IA infusion, 5.7% in DAIR and 3.0% in two-stage revisions | In vast majority of cases vancomycin or gentamicin IA with varying protocols re dosage and duration of administration | Catheter-based intra-articular infusion | Mean duration of IA antibiotic infusion was 19 days (range 3–50). An overall failure rate of approximately 11% was found. In total 117 complications occurred, 71 were non-catheter-related and 46 were catheter-related. The most common catheter-related complications were premature loss of the catheter and elevated blood urea nitrogen and creatinine levels. 17 of the 18 patients had control of infection and achieved durable fixation and a closed wound. 1 case needed re-treatment, but remained then asymptomatic for 28 months post-op. |
| Whiteside et al., 2012 [102] | Observational study of 18 patients | Treatment use in knee PJI after failed one- and two-stage revision | Vancomycin or gentamicin IA. Starting dose of 100 mg vancomcin or 20 mg gentamicin in 3 mL. The concentration and volume were increased daily if the wound remained sealed and quiescent. Dosage was increased to 500 mg vancomycin or 80 mg gentamicin in 8 mL saline. The dose was given every 12 or 24 h for 6 weeks. The injection was alternated between the two catheters to keep them open. The catheters were not flushed. | Hickman double catheter-based infusion into the intraarticular space | |
| Whiteside et al., 2011 [94] | Observational study of 18 patients | Treatment use in knee PJI with MRSA pathogens | Vancomycin IA. 500 mg vancomycin in 10 mL saline solution once or twice daily for 6 weeks; no administration of IV antibiotics after the first 24 h. | Hickman double catheter-based infusion into the intra-articular space | Infection was controlled at last follow-up (42 months) in all but 1 patient with a recurrence of the MRSA pathogens. Re-infection in this case appeared controlled after second intervention until end of follow-up period. |
| Whiteside and Roy, 2017 [111] | Observational study of 30 patients | Treatment use in hip PJI (21 with chronic PJI treated with single-stage exchange, 9 with late acute PJI, treated with DAIR) | Vancomycin, gentamicin IA (in one case). Infusion of drugs was started as soon as the incision was sealed and dry. The dose was increased gradually. Beginning dose was 100 mg for vancomycin in 3 mL water. If tolerated (no wound drainage), daily increase to maintenance dose of 400–500 mg in 5 or 6 mL water. The starting dose for gentamicin was 10 mg in 3 mL saline, and the maintenance dose was 40 mg in 4 mL normal saline. Duration of antibiotic infusion: 6 weeks | Hickman double catheter-based infusion into the intra-articular space | 95% infections in patients with single-stage revision for chronic PJI remained free of infection at a mean follow-up of 63 months- One case grew Candida albicans in the operative cultures and remained free of signs of infection after re-revision followed by infusion of fluconazole. The nine acute PJI cases treated with DAIR and head/liner change all remained free of signs of infection at a mean follow-up of 74 months. No patient had evidence of permanent renal damage. None developed a chronic fistula or had significant drainage from the catheter site. |
| Hieda et al., 2025 [112] | Observational study of 32 cases | Treatment use in hip PJI (all patients treated with DAIR, including 11 chronic PJI cases. In total, 22 patients were treated with DAIR & CLAP, 10 patients with DAIR only. | Gentamicin (amikacine or arbekacin) IA were prepared in saline at 1.2 (or 2 mg/mL, respectively). These solutions were continuously administered at a rate of 2.0 mL/h for 24 h daily using a continuous precision pump through a Salem Sump tube. In two fungal cases micafungin IA was diluted in saline to 50 μg/mL and administered at the same rate and duration. Mean duration of local antibiotic infusion: 15.5 days | Continuous local antibiotic perfusion (CLAP) with a double-lumen tube (antimicrobial administration on one side and negative pressure application on the other). It features multiple holes for suction under continuous negative pressure to prevent blockage. | The implant survival rate after DAIR surgery supplemented with CLAP was 90.9% (20 of 22 cases), and without CLAP was 70.0% (7 of 10 cases). In two cases in the CLAP group, the implants were removed and replaced because of recurrent peri-implant infection. |
| Kosugi et al., 2022 [113] | Restrospective study of 9 cases | Treatment use in fracture-related infections of the lower limb | Gentamicin IO (60 mg in 50 mL NaCl = 1200 μg/mL) 2 mL/h infusion speed Mean duration:17 days | Continuous local antibiotic perfusion (CLAP) | Observational study in refractory FRI with difficult-to-treat pathogens. Implants were preserved until bone union was achieved. Infection was suppressed in all cases (in some cases by repeating this method). No side effects were observed. |
| Lawing et al., 2015 [93] | Restrospective study of 351 cases | Prophylaxis of fracture-related infections (all open fractures) | Gentamicin or Tobramycin IO (intervention group) in addition to IV antibiotics. Control group with only IV antibiotics, Injection of 80 mg of the aminoglycoside, diluted in 40 mL of normal saline (2 mg/mL) by inserting the needle down to the bone and implant after wound closure. In some patients with type-II and III fractures, an additional catheter was placed within the wound and irrigations with a 0.5-mg/mL mixture of aminoglycoside and normal saline were performed every six hours. Duration. 3–5 days | Direct injection into infected dead space (plus catheter-based delivery in some cases of Gustilo type II or III fractures) | The deep and superficial infection rate in the control group was 19.7% (36 of 183 fractures), and 9.5% (16 of 168 fractures) in the intervention group (p = 0.010). When comparing only the deep infections, the infection rate in the control group was 14.2% vs. 6.0% (p = 0.011). After multivariate analysis to adjust for possible confounding factors, the administration of local antibiotics was found to be an independent predictor of lower infection rates in both deep and superficial infections (odds ratio, 2.6) and deep infections only (odds ratio, 3.0). The use of local antibiotics did not have an impact on nonunion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berberich, C.E. Current Concepts of Local Antibiotic Delivery in Bone and Joint Infections—A Narrative Review of Techniques and Clinical Experiences. Microorganisms 2025, 13, 2276. https://doi.org/10.3390/microorganisms13102276
Berberich CE. Current Concepts of Local Antibiotic Delivery in Bone and Joint Infections—A Narrative Review of Techniques and Clinical Experiences. Microorganisms. 2025; 13(10):2276. https://doi.org/10.3390/microorganisms13102276
Chicago/Turabian StyleBerberich, Christof Ernst. 2025. "Current Concepts of Local Antibiotic Delivery in Bone and Joint Infections—A Narrative Review of Techniques and Clinical Experiences" Microorganisms 13, no. 10: 2276. https://doi.org/10.3390/microorganisms13102276
APA StyleBerberich, C. E. (2025). Current Concepts of Local Antibiotic Delivery in Bone and Joint Infections—A Narrative Review of Techniques and Clinical Experiences. Microorganisms, 13(10), 2276. https://doi.org/10.3390/microorganisms13102276






