Dynamic Changes in Microbial Communities in Oil Reservoirs Under a Long-Term Bio-Chemical Flooding Operation
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. RNA Extraction and Sequencing
2.3. Chemical Analysis
2.4. Data Processing and Bioinformatic Analyses
3. Results
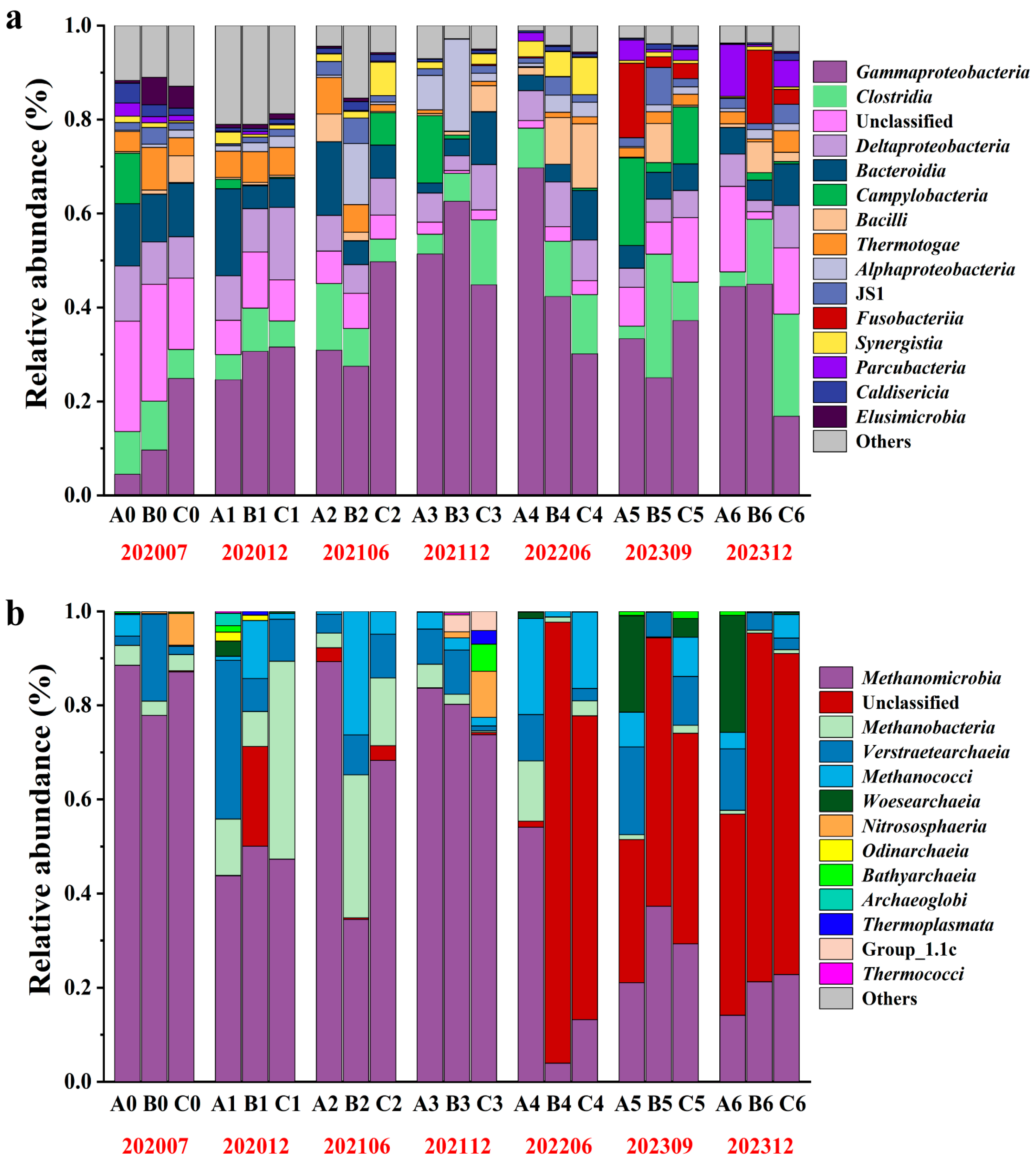
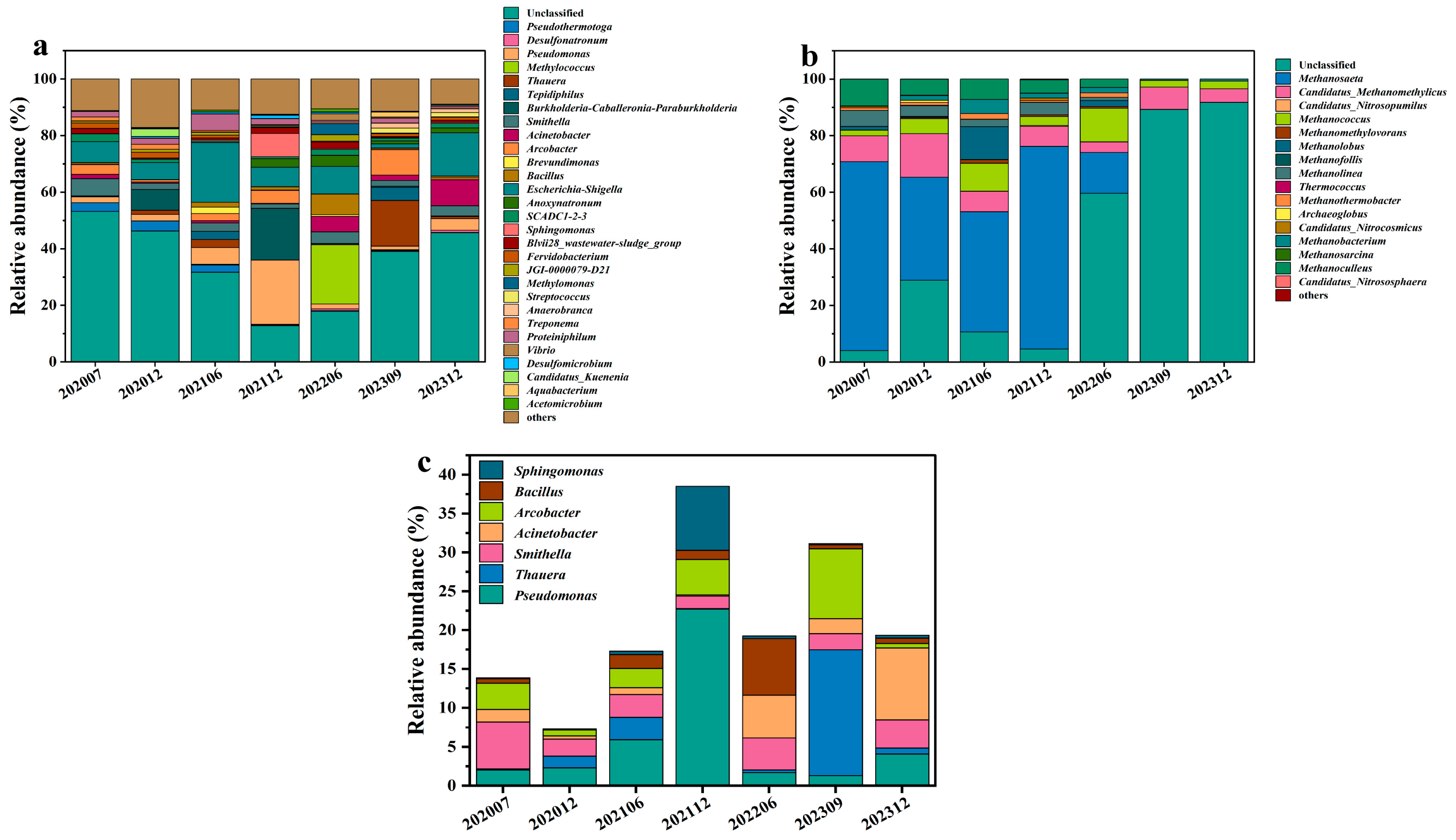
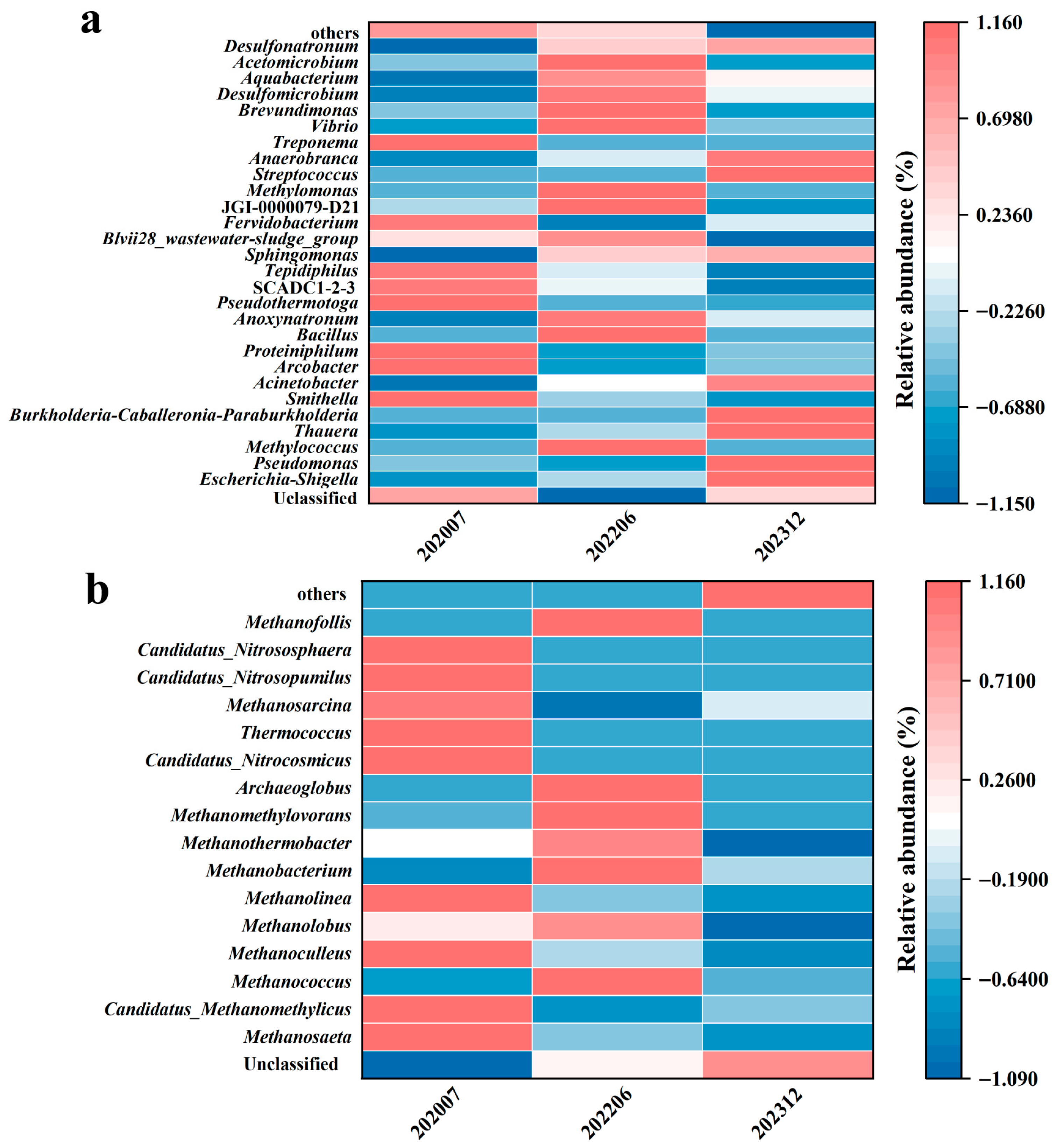
3.1. Characteristics of Microbial Community Composition and Response to Bio-Chemical Flooding
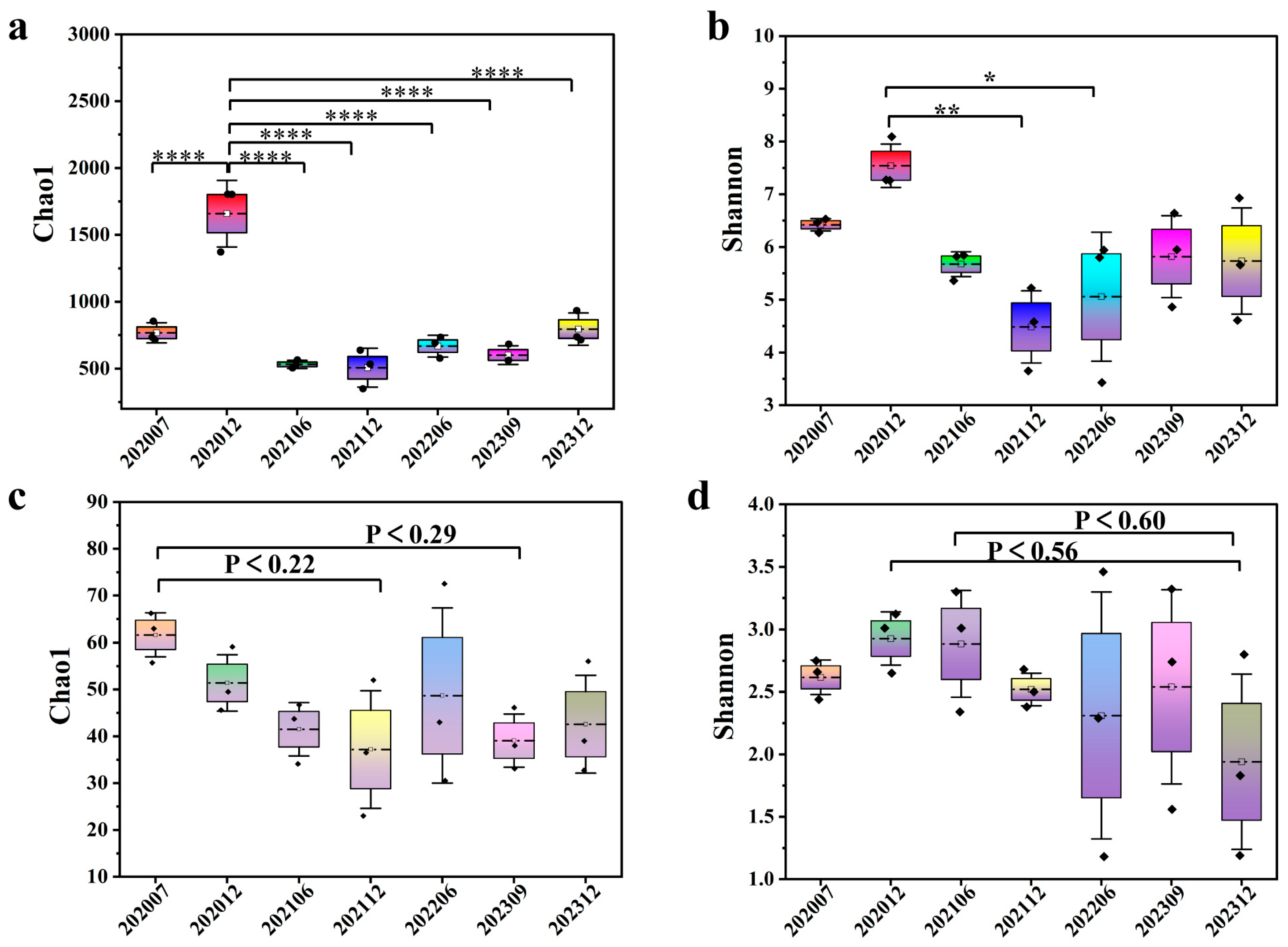
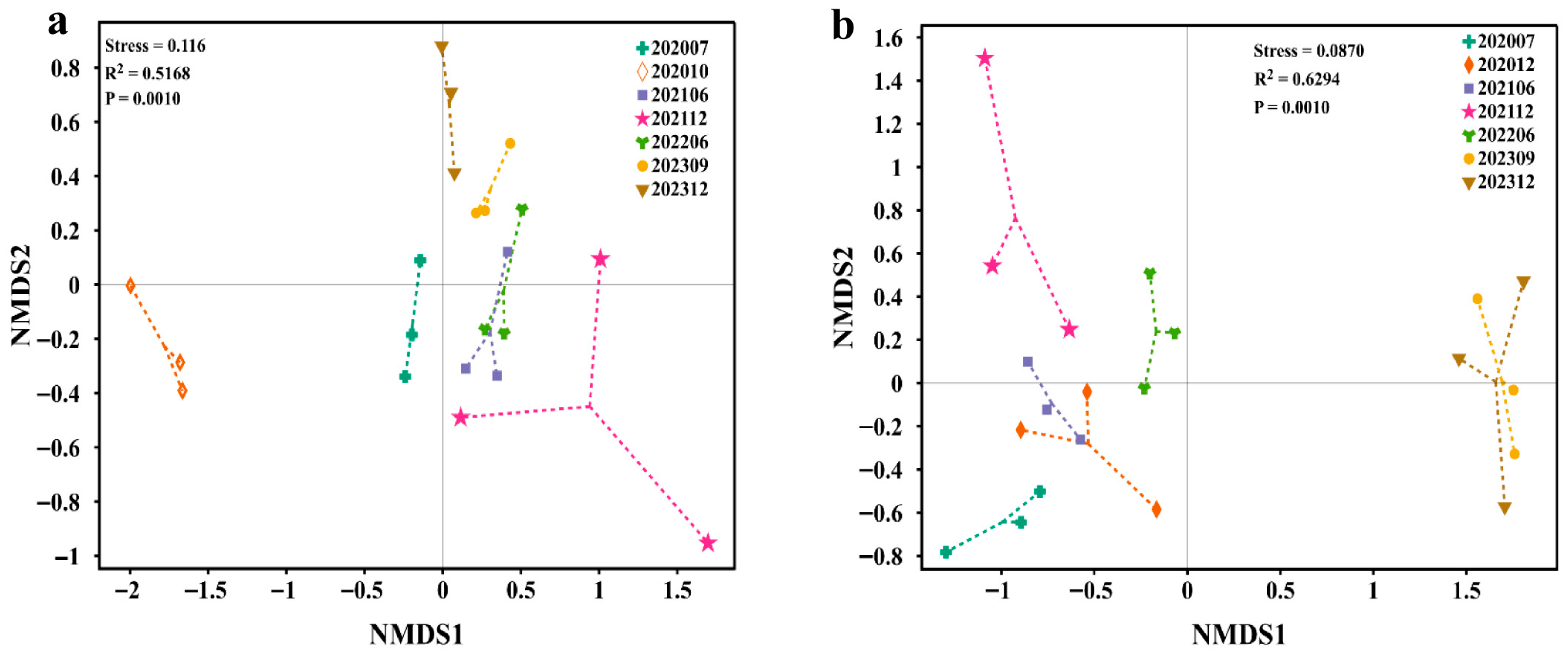
3.2. Microbial Diversity and Dynamic Changes
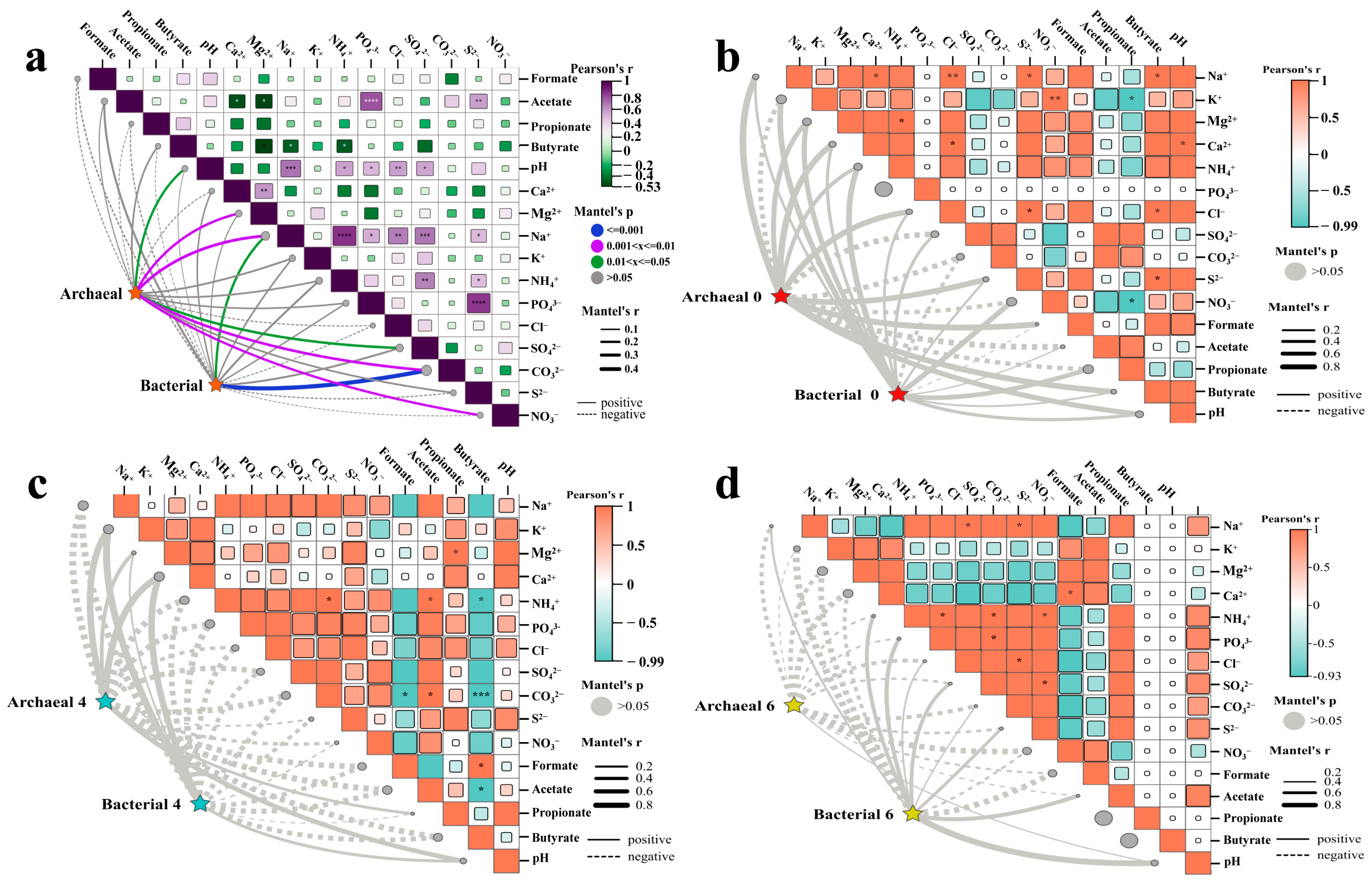
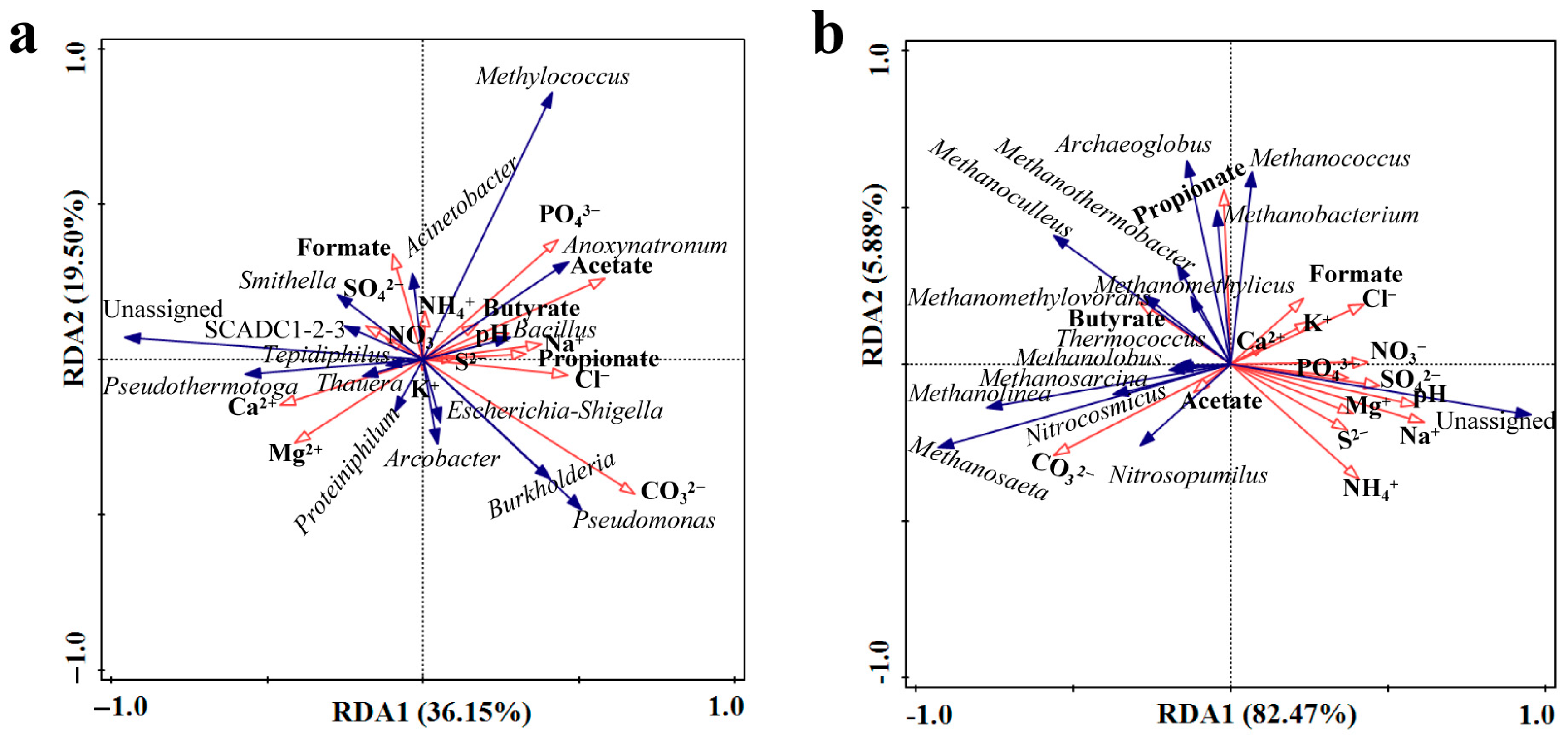
3.3. Influence of Physicochemical Factors on Microbial Community
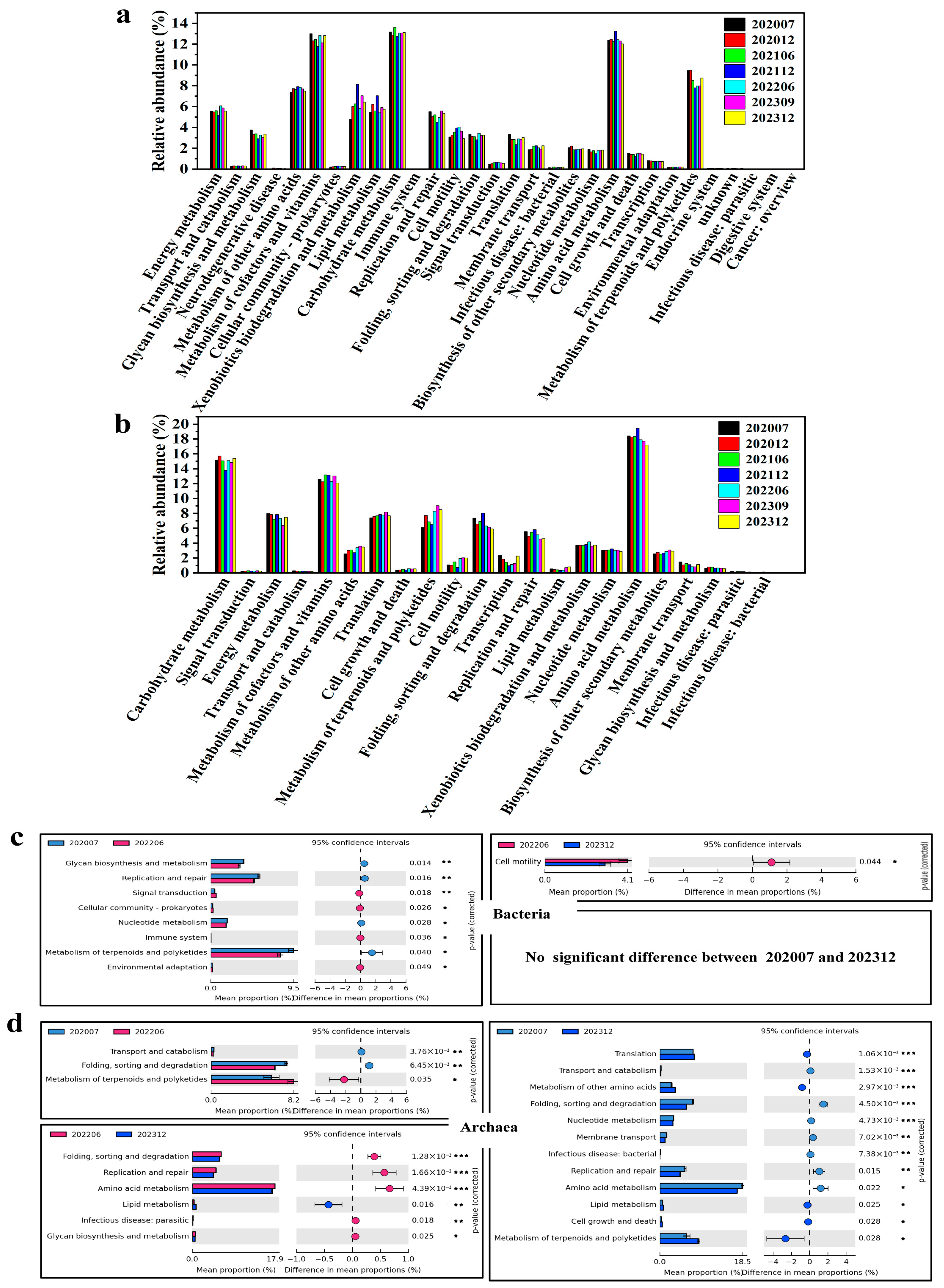
3.4. Metabolic Profiling of Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.X.; Mbadinga, S.M.; Liu, J.F.; Zhou, L.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Microbiota and their affiliation with physiochemical characteristics of different subsurface petroleum reservoirs. Int. Biodeter. Biodegr. 2017, 120, 170–185. [Google Scholar] [CrossRef]
- Lenchi, N.; İnceoğlu, Ö.; Kebbouche-Gana, S.; Gana, M.L.; Llirós, M.; Servais, P.; García-Armisen, T. Diversity of microbial communities in production and injection waters of Algerian oilfields revealed by 16S rRNA gene amplicon 454 pyrosequencing. PLoS ONE 2013, 8, e66588. [Google Scholar] [CrossRef] [PubMed]
- Bastin, E.S.; Greer, F.E.; Merritt, C.A.; Moulton, G. The presence of sulphate reducing bacteria in oil field waters. Science 1926, 63, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Chen, S.C.; Zhao, F.M.; Zhu, W.Y. Whole metagenome of injected and produced fluids reveal the heterogenetic characteristics of the microbial community in a water-flooded oil reservoir. J. Pet. Sci. Eng. 2019, 176, 1198–1207. [Google Scholar] [CrossRef]
- Gao, P.K.; Tian, H.M.; Li, G.Q.; Sun, H.W.; Ma, T. Microbial diversity and abundance in the Xinjiang Luliang long-term water-flooding petroleum reservoir. Microbiologyopen 2015, 4, 332–342. [Google Scholar] [CrossRef]
- Amend, J.P.; Teske, A. Expanding frontiers in deep subsurface microbiology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 131–155. [Google Scholar] [CrossRef]
- Wang, X.B.; Xue, Y.F.; Yuan, S.Q.; Huang, Z.Y.; Ma, Y.H. Influences of microbial community structures and diversity changes by nutrients injection in Shengli oilfield. China. J. Petrol. Sci. Eng. 2015, 133, 421–430. [Google Scholar]
- Wang, G.; Duan, L.S.; Han, F.; Wang, R.; Liu, S.S.; Lei, X.Y.; Jing, J.Z.; Gu, Y.; Lei, Y.C. Research on the change rule of microbial community in the microbial flooding process of Baolige oilfield. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012040. [Google Scholar] [CrossRef]
- Ren, G.L.; Wang, J.L.; Qu, L.N.; Li, W.; Hu, M.; Bian, L.H.; Zhang, Y.T.; Le, J.J.; Dou, X.M.; Chen, X.H.; et al. Compositions and co-occurrence patterns of bacterial communities associated with polymer-and ASP-flooded petroleum reservoir blocks. Front. Microbiol. 2020, 11, 580363. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Lomans, B.P.; Kyrpides, N.C.; Head, I.M.; Tsesmetzis, N. Succession in the petroleum reservoir microbiome through an oil field production lifecycle. ISME J. 2017, 11, 2141–2154. [Google Scholar] [CrossRef]
- Lin, J.H.; Zhang, K.C.; Tao, W.Y.; Wang, D.; Li, S. Geobacillus strains that have potential value in microbial enhanced oil recovery. Appl. Microbiol. Biotechnol. 2019, 103, 8339–8350. [Google Scholar] [CrossRef]
- Yun, Y.; Gui, Z.; Su, T.; Tian, X.; Wang, S.; Chen, Y.; Ma, T. Deep mining decreases the microbial taxonomic and functional diversity of subsurface oil reservoirs. Sci. Total. Environ. 2022, 821, 153564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Wei, H.; Wu, X.; Hou, Z.; Zhang, X.; Yang, E. Research on the Functional Microbe Activation System in a Post-Polymer Flooded Reservoir. Processes 2024, 12, 967. [Google Scholar] [CrossRef]
- Yin, J.; Wei, X.; Hu, F.; Cheng, C.; Song, M.; Zhuang, G.; Ma, A. Alternative stable microbiome state triggered by the introduction of functional microbes in oil reservoirs drives sustainable microbial enhanced oil recovery. Chem. Eng. J. 2023, 475, 146073. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, Y.F.; Zhou, L.; Gang, H.Z.; Liu, J.F.; Sun, G.Z.; Mu, B.Z. Structural diversity of the lipopeptide biosurfactant produced by a newly isolated strain, Geobacillus thermodenitrifcans ME63. ACS Omega 2023, 8, 22150–22158. [Google Scholar] [CrossRef]
- Qin, W.Q.; Fei, D.; Zhou, L.; Guo, Y.J.; An, S.; Gong, O.H.; Mu, B.Z. A new surfactin-C17 produced by Bacillus subtilis TD7 with a low critical micelle concentration and high biological activity. New J. Chem. 2023, 47, 7604–7612. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Q.Q.; Lu, Y.W.; Mbadinga, S.M.; Liu, Y.F.; Li, X.X.; Mu, B.Z. Dominant and active methanogens in the production waters from a high-temperature petroleum reservoir by DNA-and RNA-based analysis. Geomicrobiol. J. 2020, 38, 191–198. [Google Scholar] [CrossRef]
- Shou, L.B.; Hou, Z.W.; Liu, Y.F.; Wang, Y.; Zhou, L.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Wu, X.L.; Mu, B.Z. Composition and transcriptional activity of oil reservoir microorganisms under different gas/liquid ratios. Int. Biodeter. Biodegr. 2023, 181, 105604. [Google Scholar] [CrossRef]
- Sun, G.Z.; Hu, J.; Wang, Z.L.; Li, X.M.; Wang, W.D. Dynamic investigation of microbial activity in microbial enhanced oil recovery (MEOR). Petrol. Sci. Technol. 2018, 36, 1265–1271. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Z.C.; Lu, Y.W.; Ma, L.; Bai, Y.; Li, X.X.; Mbadingan, S.M.; Liu, Y.F.; Yao, X.C.; Qiao, Y.J.; et al. The newly proposed TACK and DPANN archaea detected in the production waters from a high-temperature petroleum reservoir. Int. Biodeter. Biodegr. 2019, 143, 104729. [Google Scholar] [CrossRef]
- Wu, D.; Zou, Y.; Xiao, J.; Mo, L.; Lek, S.; Chen, B.; Fu, Q.Y.; Guo, Z.Q. The spatiotemporal variations of microbial community in relation to water quality in a tropical drinking water reservoir, Southmost China. Front. Microbiol. 2024, 15, 1354784. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Rivals, I.; Personnaz, L.; Taing, L.; Potier, M.C. Enrichment or depletion of a GO category within a class of genes: Which test? Bioinformatics 2006, 23, 401–407. [Google Scholar] [CrossRef]
- Mbadinga, S.M.; Wang, L.Y.; Zhou, L.; Liu, J.F.; Gu, J.D.; Mu, B.Z. Microbial communities involved in anaerobic degradation of alkanes. Int. Biodeter. Biodegr. 2011, 65, 1–13. [Google Scholar] [CrossRef]
- Wang, L.Y.; Duan, R.Y.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Molecular analysis of the microbial community structures in water-flooding petroleum reservoirs with different temperatures. Biogeosciences 2012, 9, 4645–4659. [Google Scholar] [CrossRef]
- Suri, N.; Gassara, F.; Stanislav, P.; Voordouw, G. Microbially enhanced oil recovery by alkylbenzene-oxidizing nitrate-reducing bacteria. Front. Microbiol. 2019, 10, 1243. [Google Scholar] [CrossRef]
- Song, Z.Y.; Zhao, F.M.; Sun, G.Z.; Zhu, W.Y. Long-Term dynamics of microbial communities in a high-permeable petroleum reservoir reveals the spatiotemporal relationship between community and oil recovery. Energy Fuels 2017, 31, 10588–10597. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, J.; Yun, Y.; Zhi, B.; Li, G.Q.; Li, M.; Ma, T. Halomonas plays a central role in the syntrophic community of an alkaline oil reservoir with alkali-surfactant-polymer (ASP) flooding. Sci. Total. Environ. 2020, 747, 141333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, J.; Ji, J.H.; Gao, J.; Liu, Y.F.; Wang, B.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Characteristics of microbiota, core sulfate-reducing taxa and corrosion rates in production water from five petroleum reservoirs in China. Sci. Total. Environ. 2023, 858, 159861. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.K.; Li, G.Q.; Le, J.J.; Liu, X.B.; Liu, F.; Ma, T. Succession of microbial communities and changes of incremental oil in a post-polymer flooded reservoir with nutrient stimulation. Appl. Microbiol. Biot. 2018, 102, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Ishimura, F.; Takeda, T.; Hikuma, M. Isolation of polyacrylamide-degrading microorganisms from soil. Biotechnol. Bioprocess Eng. 2002, 7, 327–330. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Abbasnezhad, H.; Foght, J.M.; Gray, M.R. Adhesion to the hydrocarbon phase increases phenanthrene degradation by Pseudomonas fluorescens LP6a. Biodegradation 2011, 22, 485–496. [Google Scholar] [CrossRef]
- Sugiura, K.; Ishihara, M.; Shimauchi, T.; Harayama, S. Physicochemical properties and biodegradability of crude oil. Environ. Sci. Technol. 1996, 31, 45–51. [Google Scholar] [CrossRef]
- Li, W.; Ma, F.; Zhao, L.J.; Zhang, Z.Z.; Wang, Q. The molecular biology identification of a hydrolyzed polycrylamide (HPAM) degrading bacreria strain H5 and biodegradation product analysis. J. Biotechnol. 2008, 136, 668–669. [Google Scholar] [CrossRef]
- Han, P.; Zheng, L.; Cui, Z.S.; Guo, X.C.; Tian, L. Isolation, identification and diversity analysis of petroleum-degrading bacteria in Shengli Oil Field wetland soil. J. Appl. Ecol. 2009, 20, 1202. [Google Scholar]
- Song, B.; Palleroni, N.J.; Häggblom, M.M. Isolation and characterization of diverse halobenzoate-degrading denitrifying bacteria from soils and sediments. Appl. Environ. Microbiol. 2000, 66, 3446–3453. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified metabolism makes novel Thauera strain highly competitive in low carbon wastewater treatment. Water Res. 2021, 206, 117742. [Google Scholar] [CrossRef]
- Miao, S.J.; Zhou, J.; Qi, G.N.; Liu, J.F.; Yang, S.Z.; Gang, H.Z.; Mu, B.Z. Rheological properties of a new microbial exopolysaccharide produced by Sphingomonas sp. HS and its potential in enhanced oil recovery. Energy Fuels 2022, 36, 1792–1798. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, Y.W.; Wang, D.W.; Zhang, S.L.; Tang, E.G.; Qi, Z.Z.; Xie, S.N.; Wu, J.; Mu, B.Z. Microbial community composition and diversity in production water of a high-temperature offshore oil reservoir assessed by DNA-and RNA-based analyses. Int. Biodeter. Biodegr. 2020, 151, 104970. [Google Scholar] [CrossRef]
- Embree, M.; Nagarajan, H.; Movahedi, N.; Chitsaz, H.; Zengler, K. Single-cell genome and metatranscriptome sequencing reveal metabolic interactions of an alkane-degrading methanogenic community. ISME. J. 2014, 8, 757–767. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, T.; Gao, M.; Gao, P.; Cao, M.; Zhu, X.; Li, G. Characterization of microbial diversity and community in water flooding oil reservoirs in China. World J. Microbiol. Biotechnol. 2012, 28, 3039–3052. [Google Scholar] [CrossRef]
- Hunger, S.; Schmidt, O.; Hilgarth, M.; Horn, M.A.; Kolb, S.; Conrad, R. Competing formate-and carbon dioxide-utilizing prokaryotes in an anoxic methane-emitting fen soil. Appl. Environ. Microbiol. 2011, 77, 3773–3785. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Deng, Y.E.; Zhang, P.; Xue, K.; Liang, Y.T.; Van Nostrand, J.D.; Yang, Y.F.; He, Z.L.; Wu, L.Y.; Stahl, D.A.; et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef]
- Ren, H.Y.; Xiong, S.Z.; Gao, G.J.; Song, Y.T.; Cao, G.Z.; Zhao, L.P.; Zhang, X.Y. Bacteria in the injection water differently impacts the bacterial communities of production wells in high-temperature petroleum reservoirs. Front. Microbiol. 2015, 6, 505. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhao, J.Y.; Tang, Y.Q.; Guo, P.; Yang, Y.; Wu, X.L.; Zhao, F. Species divergence vs. functional convergence characterizes crude oil microbial community assembly. Front. Microbiol. 2016, 7, 1254. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review. Bioresour. Technol. 1995, 51, 1–12. [Google Scholar] [CrossRef]
- Bachoon, D.S.; Araujo, R.; Molina, M.; Hodson, R.E. Microbial community dynamics and evaluation of bioremediation strategies in oil-impacted salt marsh sediment microcosms. J. Ind. Microbiol. Biot. 2001, 27, 72–79. [Google Scholar] [CrossRef]
- Silva, T.R.; Verde, L.C.L.; Neto, E.S.; Oliveira, V.M. Diversity analyses of microbial communities in petroleum samples from Brazilian oil fields. Int. Biodeterior. Biodegrad. 2013, 81, 57–70. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Wang, K.; Hu, Z.X.; Tang, Y.Z. Abundant species diversity and essential functions of bacterial communities associated with dinoflagellates as revealed from metabarcoding sequencing for laboratory-raised clonal cultures. Int. J. Environ. Res. Public Health 2022, 19, 4446. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.Y.; Yan, B.; Wang, H.; Wu, Y.; Wang, X.; Wang, J.Q.; Zhu, Z.H.; Yan, X.X.; Liu, Y.T.; Liu, M.J.; et al. Effects of microplastics on nitrogen and phosphorus cycles and microbial communities in sediments. Environ. Pollut. 2023, 318, 120852. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Gao, P.K.; Zhi, B.; Fu, B.; Gao, G.; Chen, Z.H.; Gao, M.L.; Wu, M.M.; Ma, T. The relative abundance of alkane-degrading bacteria oscillated similarly to a sinusoidal curve in an artificial ecosystem model from oil-well products. Environ. Microbiol. 2018, 20, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, G.-N.; Li, G.-J.; Liu, Y.-F.; Zhou, L.; Ge, Y.-Q.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Dynamic Changes in Microbial Communities in Oil Reservoirs Under a Long-Term Bio-Chemical Flooding Operation. Microorganisms 2025, 13, 2246. https://doi.org/10.3390/microorganisms13102246
Qi G-N, Li G-J, Liu Y-F, Zhou L, Ge Y-Q, Liu J-F, Yang S-Z, Gu J-D, Mu B-Z. Dynamic Changes in Microbial Communities in Oil Reservoirs Under a Long-Term Bio-Chemical Flooding Operation. Microorganisms. 2025; 13(10):2246. https://doi.org/10.3390/microorganisms13102246
Chicago/Turabian StyleQi, Gui-Na, Guo-Jun Li, Yi-Fan Liu, Lei Zhou, Ya-Qing Ge, Jin-Feng Liu, Shi-Zhong Yang, Ji-Dong Gu, and Bo-Zhong Mu. 2025. "Dynamic Changes in Microbial Communities in Oil Reservoirs Under a Long-Term Bio-Chemical Flooding Operation" Microorganisms 13, no. 10: 2246. https://doi.org/10.3390/microorganisms13102246
APA StyleQi, G.-N., Li, G.-J., Liu, Y.-F., Zhou, L., Ge, Y.-Q., Liu, J.-F., Yang, S.-Z., Gu, J.-D., & Mu, B.-Z. (2025). Dynamic Changes in Microbial Communities in Oil Reservoirs Under a Long-Term Bio-Chemical Flooding Operation. Microorganisms, 13(10), 2246. https://doi.org/10.3390/microorganisms13102246







