Antibiotic Residues in Muscle Tissues of Lueyang Black-Bone Chickens Under Free-Range Mountainous Conditions and Their Association with Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Animal Resources and Breeding Systems
2.3. Metabolomic Analysis of Intestinal and Muscle Tissues
2.4. The 16S rDNA Analysis of Gut Microbes
2.5. Statistical Analysis
3. Results
3.1. Gut Metabolome Analysis
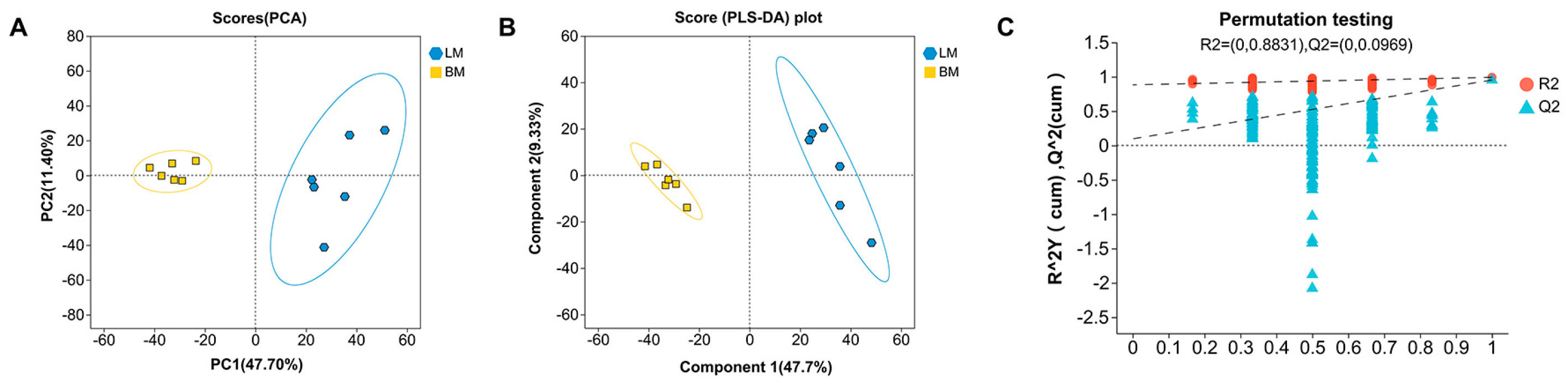
3.1.1. Multivariate Statistical Analysis of Intestinal Metabolites
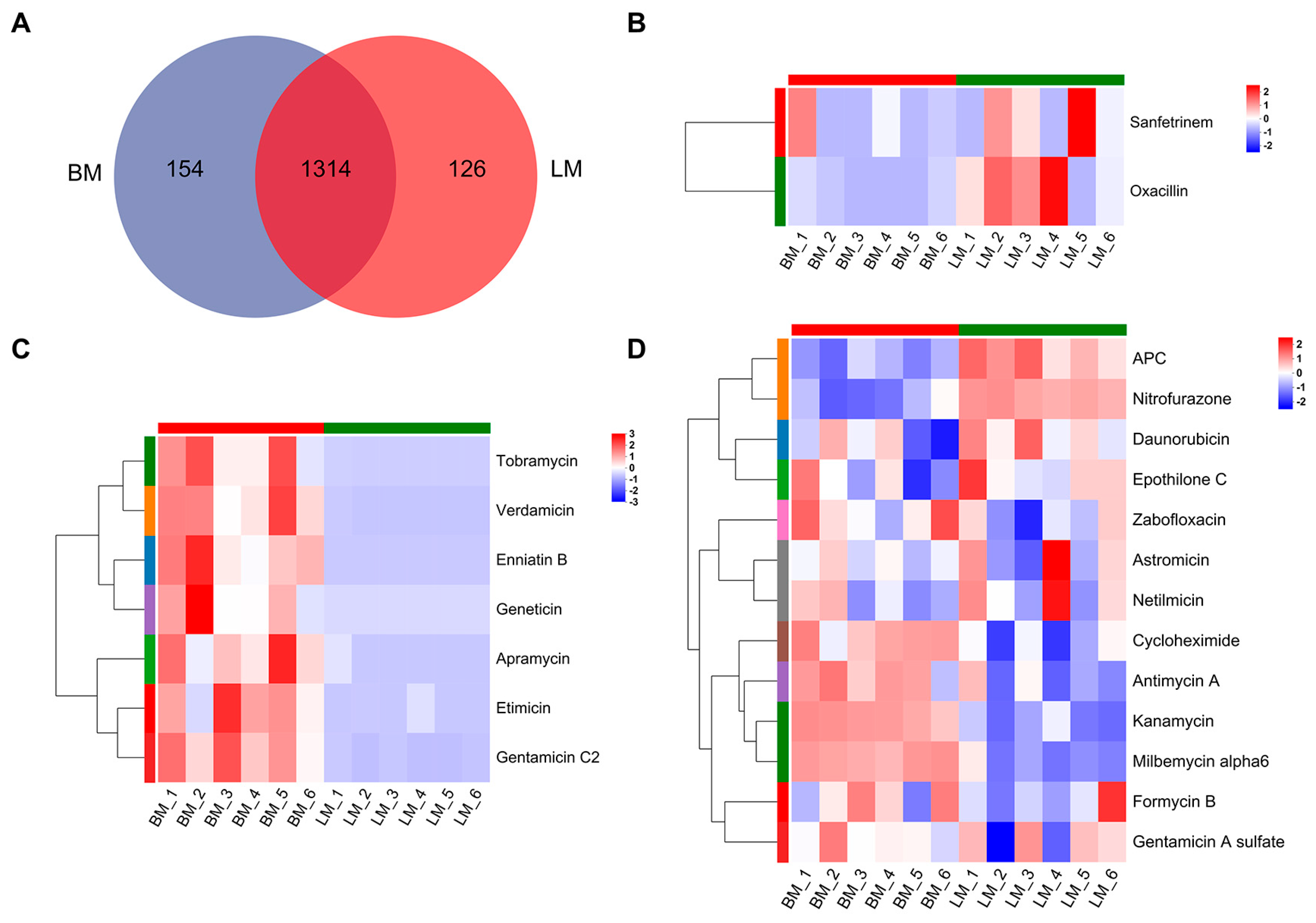
3.1.2. Analysis of Antibiotics in Gut Metabolites
3.2. Muscle Metabolome Analysis
3.2.1. Multivariate Statistical Analysis of Muscle Metabolites
3.2.2. Analysis of Antibiotics in Muscle Metabolites
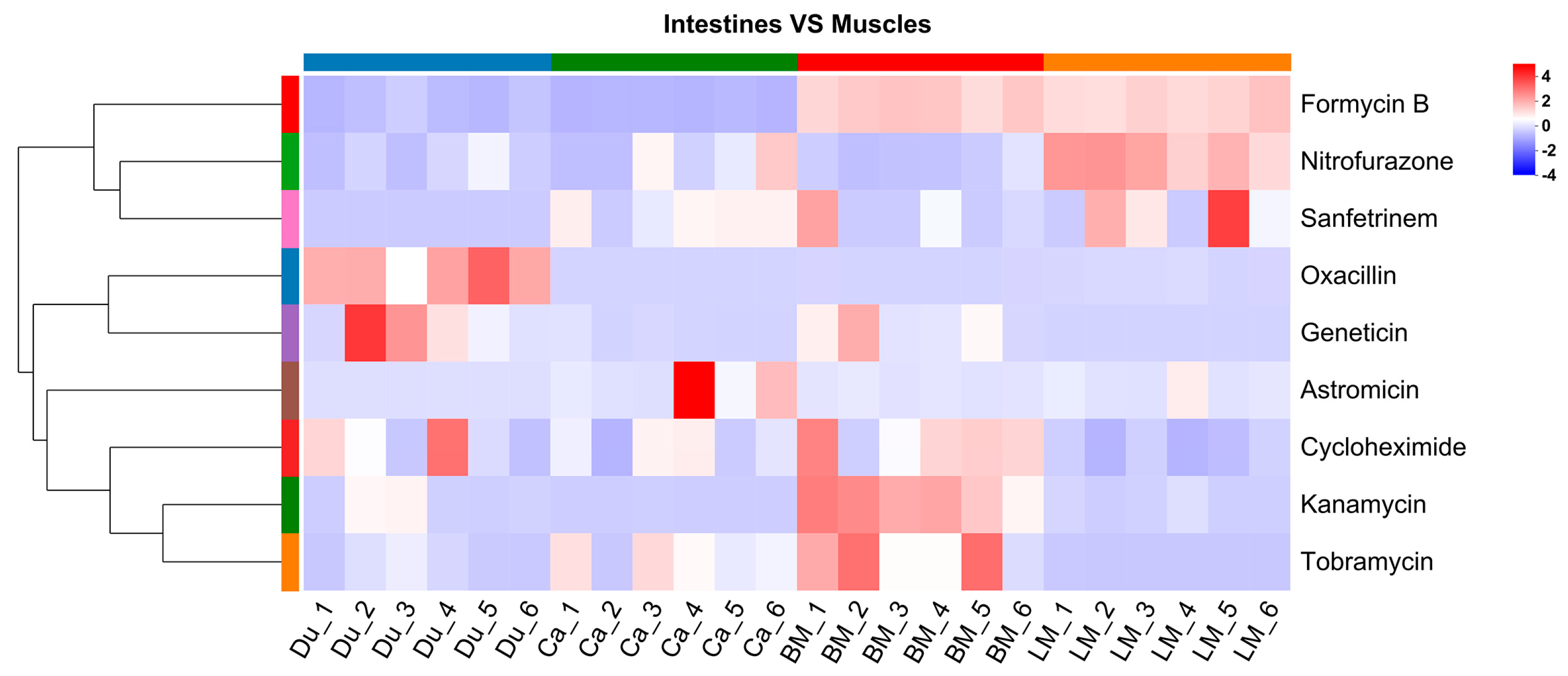
3.3. Analysis of Antibiotics in the Gut and Muscle Tissues
3.4. Gut Microbiota Analysis
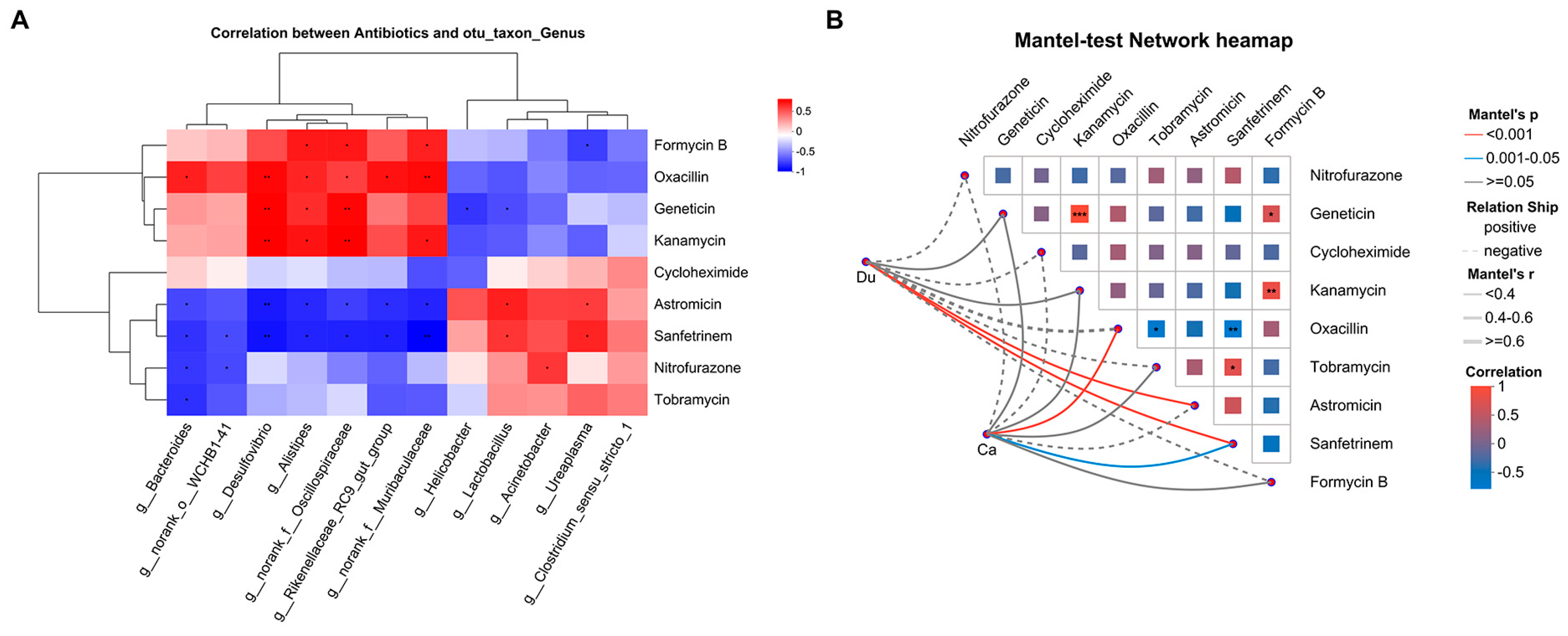
3.5. Association Analysis Between Gut Microbiota and Antibiotics
4. Discussion
4.1. Analysis of Potential Sources of Antibiotic Tissue Residues in Lueyang Black-Bone Chickens
4.2. Antibiotic Tissue Distribution Patterns and Absorption-Transport Characteristics
4.3. Potential Regulatory Role of Intestinal Microbial Differences in Antibiotic Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Low, C.X.; Tan, L.T.; Ab Mutalib, N.S.; Pusparajah, P.; Goh, B.H.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef]
- Broom, L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef] [PubMed]
- Inyinbor, A.A.; Bello, O.S.; Fadiji, A.E.; Inyinbor, H.E. Threats from antibiotics: A serious environmental concern. J. Environ. Chem. Eng. 2018, 6, 784–793. [Google Scholar] [CrossRef]
- Al-Maadheed, S.; Goktepe, I.; Latiff, A.B.A.; Shomar, B. Antibiotics in hospital effluent and domestic wastewater treatment plants in Doha, Qatar. J. Water. Process. Eng. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar–A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin-A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Baloch, Z.; Shah, Z.; Cui, X.; Xia, X. The Intestinal Microbiota: Impacts of Antibiotics Therapy, Colonization Resistance, and Diseases. Int. J. Mol. Sci. 2021, 22, 6597. [Google Scholar] [CrossRef]
- Miyazaki, K.; Sasaki, A.; Mizuuchi, H. Advances in the Evaluation of Gastrointestinal Absorption Considering the Mucus Layer. Pharmaceutics 2023, 15, 2714. [Google Scholar] [CrossRef]
- Nowak, B.; Wanat, M.; Świątko, A.; Mirkowski, K.; Tarkowski, V.; Mrożek, A.; Mazurek, M.; Domanski, J.; Domagała, Z. Exploring the microscopic terrain of the small intestinal epithelium: A comprehensive overview of general architecture and the present understanding of intestinal stem cells. Med. J. Cell Biol. 2023, 11, 87–92. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Liu, B.; Yu, D.; Sun, J.; Ge, L.; Tang, W.; Liu, S. Gut microbiota contributes to the methionine metabolism in host. Front. Microbiol. 2022, 13, 1065668. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Q.; Li, S.; Jiang, Y.; Zhang, C.; Tang, S.; Zhong, R.; Tang, X.; Zhang, S.; Feng, X.; et al. Multi-Omics Uncover Neonatal Cecal Cell Development Potentials. Front. Cell Dev. Biol. 2022, 10, 840298. [Google Scholar] [CrossRef]
- Shao, C.; Song, X.; Wang, L.; Zhang, H.; Liu, Y.; Wang, C.; Chen, S.; Ren, B.; Wen, S.; Xiao, J.; et al. Microbiome Structure and Mucosal Morphology of Jejunum Appendix and Colon of Rats in Health and Dysbiosis. Curr. Microbiol. 2023, 80, 127. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Gong, J.; Fang, Q.; Qi, S.; Li, M.; Han, Y.; Liu, W.; Ge, G. The Chinese herb Styrax triggers pharmacokinetic herb-drug interactions via inhibiting intestinal CYP3A. Front. Pharmacol. 2022, 13, 974578. [Google Scholar] [CrossRef]
- Gompo, T.R.; Sapkota, R.; Subedi, M.; Koirala, P.; Bhatta, D.D. Monitoring of antibiotic residues in chicken meat, Cow and Buffalo milk samples in Nepal. Int. J. Appl. Sci. Biotechnol. 2020, 8, 355–362. [Google Scholar] [CrossRef]
- Ndlovu, K.; Mwanza, M.; Nleya, N.; Ngoma, L. Detection and quantification of antibiotic residues in goat milk in Mahikeng Local Municipality. J. S. Afr. Vet. Assoc. 2024, 95, 121–130. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.X.; Li, Z.Y.; Zheng, Z.Y.; Xiang, Y.; Liu, Y.; Zhao, R.F.; Fang, J. Detection of fluoroquinolone and sulfonamide residues in poultry eggs in Kunming city, southwest China. Poult. Sci. 2022, 101, 101892. [Google Scholar] [CrossRef]
- Mencía-Ares, O.; Argüello, H.; Puente, H.; Gómez-García, M.; Álvarez-Ordóñez, A.; Manzanilla, E.G.; Carvajal, A.; Rubio, P. Effect of antimicrobial use and production system on Campylobacter spp., Staphylococcus spp. and Salmonella spp. resistance in Spanish swine: A cross-sectional study. Zoonoses Public Health 2021, 68, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lan, F.; Li, X.; Yan, W.; Sun, C.; Li, J.; Yang, N.; Wen, C. The Spatial and Temporal Characterization of Gut Microbiota in Broilers. Front. Vet. Sci. 2021, 8, 712226. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Guo, S.; Zhou, Y.; Zhao, J.; Wang, M.; Sang, L.; Chang, B.; Wang, B. Hepatocellular Carcinoma: How the Gut Microbiota Contributes to Pathogenesis, Diagnosis, and Therapy. Front. Microbiol. 2022, 13, 873160. [Google Scholar] [CrossRef]
- Ferraz, M.P. An overview of the relevance of human gut and skin microbiome in disease: The influence on atopic dermatitis. Appl. Sci. 2023, 13, 10540. [Google Scholar] [CrossRef]
- da Silva, J.M.S.; Almeida, A.M.S.; Borsanelli, A.C.; de Athayde, F.R.F.; Nascente, E.P.; Batista, J.M.M.; Gouveia, A.; Stringhini, J.H.; Leandro, N.S.M.; Café, M.B. Intestinal Microbiome Profiles in Broiler Chickens Raised with Different Probiotic Strains. Microorganisms 2024, 12, 1639. [Google Scholar] [CrossRef]
- Kong, M.; Zhao, W.; Wang, C.; Qi, J.; Liu, J.; Zhang, Q. A Well-Established Gut Microbiota Enhances the Efficiency of Nutrient Metabolism and Improves the Growth Performance of Trachinotus ovatus. Int. J. Mol. Sci. 2024, 25, 5525. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing human gut microbiota research by considering gut transit time. Gut 2023, 72, 180–191. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lin, H.L.; Wang, K.L.; Que, G.X.; Cao, T.; Zhu, L.M.; Yang, X.; Yang, X.F. Influence of Gut Microbiota and its Metabolites on Progression of Metabolic Associated Fatty Liver Disease. Chin. Med. Sci. J. 2023, 38, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, S.Y.; Kim, K.M.; Mony, T.J.; Bae, H.J.; Kim, M.S.; Lee, C.H.; Choi, S.E.; Lee, S.H.; Park, S.J. Fermented Sprouts of Codonopsis lanceolata Suppress LPS-Induced Inflammatory Responses by Inhibiting NF-κB Signaling Pathway in RAW 264.7 Macrophages and CD1 Mice. Pharmaceutics 2023, 15, 1793. [Google Scholar] [CrossRef]
- Jarmusch, A.K.; Vrbanac, A.; Momper, J.D.; Ma, J.D.; Alhaja, M.; Liyanage, M.; Knight, R.; Dorrestein, P.C.; Tsunoda, S.M. Enhanced characterization of drug metabolism and the influence of the intestinal microbiome: A pharmacokinetic, microbiome, and untargeted metabolomics study. Clin. Transl. Sci. 2020, 13, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Kissane, R.W.P.; Al-Shammari, A.A.; Egginton, S. The importance of capillary distribution in supporting muscle function, building on Krogh’s seminal ideas. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 254, 110889. [Google Scholar] [CrossRef]
- Harrell, A.W.; Sychterz, C.; Ho, M.Y.; Weber, A.; Valko, K.; Negash, K. Interrogating the relationship between rat in vivo tissue distribution and drug property data for >200 structurally unrelated molecules. Pharmacol. Res. Perspect. 2015, 3, e00173. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, D.; Xu, G.; Li, K.; Wang, Q.; Zhang, Z.; Li, J.; Chen, Y.; Jia, Y.; Qu, L. Effects of different rearing systems on meat production traits and meat fiber microstructure of Beijing-you chicken. Anim. Sci. J. 2015, 86, 729–735. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.C.; Ianiro, G.; Laterza, L.; Ponziani, F.R.; Pulcini, G.; Gasbarrini, A.; Mele, M.C. Diet-induced alterations in gut microbiota composition and function. Best Pract. Res. Clin. Gastroenterol. 2022, 1, 354–373. [Google Scholar]
- dos Santos Lopes, E.; Santaren, K.C.F.; de Souza, L.C.A.; Parente, C.E.T.; Picão, R.C.; de Azevedo Jurelevicius, D.; Seldin, L. Cross-environmental cycling of antimicrobial resistance in agricultural areas fertilized with poultry litter: A one health approach. Environ. Pollut. 2024, 363, 125177. [Google Scholar] [CrossRef] [PubMed]
- Kivits, T.; Broers, H.P.; Beeltje, H.; van Vliet, M.; Griffioen, J. Presence and fate of veterinary antibiotics in age-dated groundwater in areas with intensive livestock farming. Environ. Pollut. 2018, 241, 988–998. [Google Scholar] [CrossRef]
- Hu, H.; Qi, M.; He, P.; Chen, X.; Li, Z.; Cheng, H. Occurrence and risk assessment of quinolones and sulfonamides in freshwater aquaculture ponds in Northeast Zhejiang, China. Sci. Total. Environ. 2024, 953, 176066. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, L.; Wang, Q.; Zeng, H.; Xu, J.; Chen, Z. Antibiotics in aquaculture ponds from Guilin, South of China: Occurrence, distribution, and health risk assessment. Environ. Res. 2022, 204, 112084. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Z.; He, L.Y.; Chen, X.; Chen, J.L.; Yi, X.; He, L.X.; Huang, X.Y.; Chen, Z.Y.; Bai, H.; Zhang, M.; et al. Swine farm groundwater is a hidden hotspot for antibiotic-resistant pathogenic Acinetobacter. ISME Commun. 2023, 3, 34. [Google Scholar] [CrossRef]
- Liu, R.; Yu, H.; Hou, X.; Liu, X.; Bi, E.; Wang, W.; Li, M. Typical Sulfonamide Antibiotics Removal by Biochar-Amended River Coarse Sand during Groundwater Recharge. Int. J. Environ. Res. Public Health 2022, 19, 16957. [Google Scholar] [CrossRef]
- Narayanan, N.; Gupta, S.; Saini, P.; Singh, N. Unraveling fate of sulfonamide antibiotics in sandy loam soil and water of India and environmental risk assessment. Environ. Monit. Assess. 2025, 197, 341. [Google Scholar] [CrossRef]
- Ye-Shan, L.I.; Zhen-Zhen, Y.; Xue-Rong, W.; Meng-Meng, Y.; Zhuo-Yi, Z.; Xiu, Z.; Hong-Na, L.I. Occurrence Characteristics and Research Status of Antibiotic-resistant Bacteria and Antibiotic Resistance Genes in the Air. Chin. J. Agrometeorol. 2024, 45, 472. [Google Scholar]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, S.; Hou, Z.; Lin, H.; Liang, H.; Wang, L.; Luo, Y.; Ren, H. Rainfall facilitates the transmission and proliferation of antibiotic resistance genes from ambient air to soil. Sci. Total. Environ. 2021, 799, 149260. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, T.J.H.; Keizers, P.H.J.; Béen, F.; Meijer, J.; Houtman, C.J.; Al Gharib, I.; Molenaar, D.; Hamers, T.; Lamoree, M.H. Identifying antimicrobials and their metabolites in wastewater and surface water with effect-directed analysis. Chemosphere 2023, 320, 138093. [Google Scholar] [CrossRef]
- Saibi, Y.; Sato, H.; Tachiki, H. Developing in vitro-in vivo correlation of risperidone immediate release tablet. AAPS PharmSciTech 2012, 13, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, M.; Kametani, K.; Ohno, N.; Hiramatsu, K.; Kawasaki, T.; Hasegawa, Y.; Iwasaki, T.; Watanabe, T. The unique physiological features of the broiler pectoralis major muscle as suggested by the three-dimensional ultrastructural study of mitochondria in type IIb muscle fibers. J. Vet. Med. Sci. 2021, 83, 1764–1771. [Google Scholar] [CrossRef]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Cao, Y.; Liu, K.; Shi, H.; Guo, X.; Liu, W.; Hao, R.; Song, H.; Zhao, R. Nanocarriers for the delivery of antibiotics into cells against intracellular bacterial infection. Biomater. Sci. 2023, 11, 432–444. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef]
- Zheng, M.; Mao, P.; Tian, X.; Meng, L. Effects of grazing mixed-grass pastures on growth performance, immune responses, and intestinal microbiota in free-range Beijing-you chickens. Poult. Sci. 2021, 100, 1049–1058. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, B. The Gut-Liver Axis in Health and Disease: The Role of Gut Microbiota-Derived Signals in Liver Injury and Regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, C.; Ma, J.; Wang, Y. Metabolic mediators: Microbial-derived metabolites as key regulators of anti-tumor immunity, immunotherapy, and chemotherapy. Front. Immunol. 2024, 15, 1456030. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Yoshikawa, T. Propionate Promotes Fatty Acid Oxidation through the Up-Regulation of Peroxisome Proliferator-Activated Receptor α in Intestinal Epithelial Cells. J. Nutr. Sci. Vitaminol. 2015, 61, 511–515. [Google Scholar] [CrossRef]
- Yang, R.; Hu, X.; Xie, X.; Chen, H.; Fang, H.; Zhu, L.; Li, Z. Propionic Acid Targets the TLR4/NF-κB Signaling Pathway and Inhibits LPS-Induced Intestinal Barrier Dysfunction: In Vitro and In Vivo Studies. Front. Pharmacol. 2020, 11, 573475. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Leth, M.L.; Pichler, M.J.; Abou Hachem, M. Butyrate-producing colonic clostridia: Picky glycan utilization specialists. Essays Biochem. 2023, 67, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tong, C.; Xiao, D.; Xie, L.; Zhao, R.; Huo, Z.; Tang, Z.; Hao, J.; Zeng, Z.; Xiong, W. Metagenomic Insights into Chicken Gut Antibiotic Resistomes and Microbiomes. Microbiol. Spectr. 2022, 10, e0190721. [Google Scholar] [CrossRef] [PubMed]
- Rainey, P.; Santi, D.V. Metabolism and mechanism of action of formycin B in Leishmania. Proc. Natl. Acad. Sci. USA 1983, 80, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, D.; Judith, A.B. Sodium-dependent nucleoside transport in mouse intestinal epithelial cells. Two transport systems with differing substrate specificities. J. Biol. Chem. 1988, 263, 19419–19423. [Google Scholar] [CrossRef]
- Wojtyś, M.I.; Jaźwiec, R.; Kazazić, S.; Leščić Ašler, I.; Knežević, P.; Aleksić Sabo, V.; Luić, M.; Jagusztyn-Krynicka, E.K.; Bzowska, A. A comprehensive method for determining cellular uptake of purine nucleoside phosphorylase and adenylosuccinate synthetase inhibitors by H. pylori. Appl. Microbiol. Biotechnol. 2021, 105, 7949–7967. [Google Scholar] [CrossRef]
- Santillana, E.; Beceiro, A.; Bou, G.; Romero, A. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc. Natl. Acad. Sci. USA 2007, 104, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.H.; Shrestha, N.K.; Allison, G.M.; Keller, S.C.; Umscheid, C.A. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin. Infect. Dis. 2019, 68, e1–e35. [Google Scholar] [CrossRef] [PubMed]
- Murínová, I.; Švidrnoch, M.; Gucký, T.; Hlaváč, J.; Michálek, P.; Slanař, O.; Šíma, M. Population Pharmacokinetic Analysis Proves Superiority of Continuous Infusion in PK/PD Target Attainment with Oxacillin in Staphylococcal Infections. Antibiotics 2022, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Baudoux, P.; Bles, N.; Lemaire, S.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Van Bambeke, F. Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J. Antimicrob. Chemother. 2007, 59, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Di Modugno, E.; Erbetti, I.; Ferrari, L.; Galassi, G.; Hammond, S.M.; Xerri, L. In vitro activity of the tribactam GV104326 against gram-positive, gram-negative, and anaerobic bacteria. Antimicrob. Agents Chemother. 1994, 38, 2362–2368. [Google Scholar] [CrossRef]
- Iavarone, L.; Bottacini, M.; Pugnaghi, F.; Morandini, C.; Grossi, P. Sanfetrinem and sanfetrinem-cilexetil: Disposition in rat and dog. Xenobiotica 1997, 27, 693–709. [Google Scholar] [CrossRef]
- Ohta, T.; Hasegawa, M. Analysis of the self-defense gene (fmrO) of a fortimicin A (astromicin) producer, Micromonospora olivasterospora: Comparison with other aminoglycoside-resistance-encoding genes. Gene 1993, 127, 63–69. [Google Scholar] [CrossRef]
- Dhondikubeer, R.; Bera, S.; Zhanel, G.G.; Schweizer, F. Antibacterial activity of amphiphilic tobramycin. J. Antibiot. 2012, 65, 495–498. [Google Scholar] [CrossRef]
- Ariza-Mateos, A.; Díaz-Toledano, R.; Block, T.M.; Prieto-Vega, S.; Birk, A.; Gómez, J. Geneticin Stabilizes the Open Conformation of the 5′ Region of Hepatitis C Virus RNA and Inhibits Viral Replication. Antimicrob. Agents Chemother. 2016, 60, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Deb, J. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef]
- Hill, M.; Cunningham, R.N.; Hathout, R.M.; Johnston, C.; Hardy, J.G.; Migaud, M.E. Formulation of Antimicrobial Tobramycin Loaded PLGA Nanoparticles via Complexation with AOT. J. Funct. Biomater. 2019, 10, 26. [Google Scholar] [CrossRef]
- Vila, M.M.; Coelho, S.L.; Chaud, M.V.; Tubino, M.; Oliveira, J.M., Jr.; Balcao, V.M. Development and characterization of a hydrogel containing nitrofurazone for antimicrobial topical applications. Curr. Pharm. Biotechnol. 2014, 15, 182–190. [Google Scholar] [CrossRef]
- Race, P.R.; Lovering, A.L.; Green, R.M.; Ossor, A.; White, S.A.; Searle, P.F.; Wrighton, C.J.; Hyde, E.I. Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone: Reversed binding orientations in different redox states of the enzyme. J. Biol. Chem. 2005, 280, 13256–13264. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Kaplan, E.; Laurieri, N.; Lowe, E.; Sim, E. Activation of nitrofurazone by azoreductases: Multiple activities in one enzyme. Sci. Rep. 2011, 1, 63. [Google Scholar] [CrossRef]
- Gao, Y.-N.; Li, S.-L.; Yang, X.; Wang, J.-Q.; Zheng, N. The protective effects of lactoferrin on aflatoxin M1-induced compromised intestinal integrity. Int. J. Mol. Sci. 2021, 23, 289. [Google Scholar] [CrossRef]
- Zulkifle, N.T.; Abd Khalil, K.; Safian, M.F.; Saat, M.N.; Ariffin, Z.Z. Optimization of nitrofurazone degradation by local Aspergillus tamarii KX610719.1. Asia Pac. J. Mol. Biol. Biotechnol. 2022, 30, 51–61. [Google Scholar] [CrossRef]
- Dündar, H.; Biberoglu, G.; Okur, L.; Tümer, L.; Ezgü, F.S. In vitro translational readthrough by gentamicin and geneticin improves GLA activity in Fabry disease. Mol. Genet. Metab. 2017, 120, S43. [Google Scholar] [CrossRef]
- Hummel, A.S.; Hertel, C.C.; Holzapfel, W.H.; Franz, C.M.A.P. Antibiotic Resistances of Starter and Probiotic Strains of Lactic Acid Bacteria. Appl. Environ. Microbiol. 2007, 73, 730–739. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Kromann, S.; Chen, M.; Shi, L.; Meng, H. Antibiotic Resistance of Lactobacillus spp. and Streptococcus thermophilus Isolated from Chinese Fermented Milk Products. Foodborne Pathog. Dis. 2019, 16, 221–228. [Google Scholar] [CrossRef]
- Sirichoat, A.; Flórez, A.B.; Vázquez, L.; Buppasiri, P.; Panya, M.; Lulitanond, V.; Mayo, B. Antibiotic Susceptibility Profiles of Lactic Acid Bacteria from the Human Vagina and Genetic Basis of Acquired Resistances. Int. J. Mol. Sci. 2020, 21, 2594. [Google Scholar] [CrossRef] [PubMed]
- Efsa Contam, P. Scientific Opinion on nitrofurans and their metabolites in food. EFSA J. 2015, 13, 4140. [Google Scholar] [CrossRef]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Thirapanmethee, K.; Khuntayaporn, P.; Chomnawang, M.T. CRISPR-based gene editing in Acinetobacter baumannii to combat antimicrobial resistance. Pharmaceuticals 2023, 16, 920. [Google Scholar] [CrossRef]
- Iswandana, R.; Putri, K.S.S.; Putri, F.A.; Gunawan, M.; Larasati, S.A. Challenge and development strategy for colon-targeted drug delivery system. Pharm. Sci. Res. 2022, 9, 17–27. [Google Scholar] [CrossRef]
- Li, B.; Lv, R.; Xiao, Y.; Hu, W.; Mai, Y.; Zhang, J.; Lin, L.; Hu, X. A novel nitrite-base aerobic denitrifying bacterium Acinetobacter sp. YT03 and its transcriptome analysis. Front. Microbiol. 2019, 10, 2580. [Google Scholar] [CrossRef]


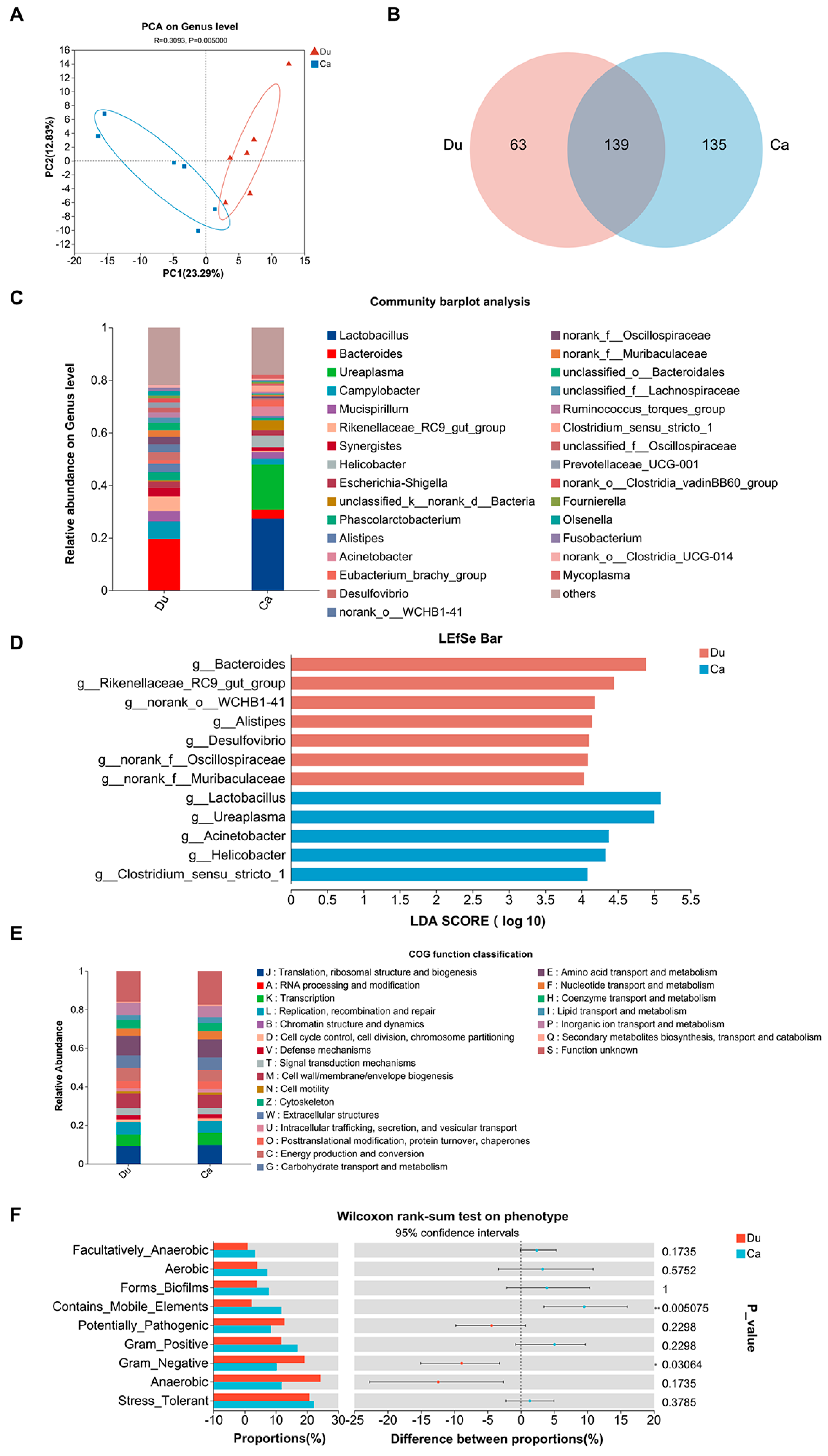
| Groups | Du | Ca | |
|---|---|---|---|
| ASVs | 898 ± 280.90 | 320 ± 141.82 | |
| Good’s coverage | 0.9999 ± 0.0001 | 0.9999 ± 0.0001 | |
| Richness | Sobs | 128.67 ± 12.75 | 114.5 ± 40.68 |
| Chaol | 129.90 ± 12.89 | 116.24 ± 41.42 | |
| Ace | 130.08 ± 12.80 | 116.16 ± 41.01 | |
| Diversity | Shannon | 3.27 ± 0.35 | 2.40 ± 0.90 |
| Simpson | 0.09 ± 0.07 | 0.28 ± 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zeng, S.; Shao, L.; Wang, L.; Zhang, T.; Lu, H.; Zeng, W. Antibiotic Residues in Muscle Tissues of Lueyang Black-Bone Chickens Under Free-Range Mountainous Conditions and Their Association with Gut Microbiota. Microorganisms 2025, 13, 2239. https://doi.org/10.3390/microorganisms13102239
Zhao M, Zeng S, Shao L, Wang L, Zhang T, Lu H, Zeng W. Antibiotic Residues in Muscle Tissues of Lueyang Black-Bone Chickens Under Free-Range Mountainous Conditions and Their Association with Gut Microbiota. Microorganisms. 2025; 13(10):2239. https://doi.org/10.3390/microorganisms13102239
Chicago/Turabian StyleZhao, Mingming, Shuang Zeng, Linqing Shao, Ling Wang, Tao Zhang, Hongzhao Lu, and Wenxian Zeng. 2025. "Antibiotic Residues in Muscle Tissues of Lueyang Black-Bone Chickens Under Free-Range Mountainous Conditions and Their Association with Gut Microbiota" Microorganisms 13, no. 10: 2239. https://doi.org/10.3390/microorganisms13102239
APA StyleZhao, M., Zeng, S., Shao, L., Wang, L., Zhang, T., Lu, H., & Zeng, W. (2025). Antibiotic Residues in Muscle Tissues of Lueyang Black-Bone Chickens Under Free-Range Mountainous Conditions and Their Association with Gut Microbiota. Microorganisms, 13(10), 2239. https://doi.org/10.3390/microorganisms13102239






