Abstract
Staphylococcus caprae is a coagulase-negative staphylococcus commonly associated with animals which can also be a zoonotic human pathogen. To date, there is little data available on S. caprae infections. The aim of this study was to characterize the S. caprae infections identified in two hospitals located, respectively, in rural and urban areas of Catalonia, Spain. In this retrospective, observational study, data were compiled from clinical records of all S. caprae infections diagnosed between January 2010 and December 2023. Over the study period, altogether 31 cases of S. caprae infection were identified, with most (23) of these cases occurring in the second half of the period (2017–2023). The mean age of patients was 58.87 ± 20.65 years, and 58.1% were males. Eight patients had had livestock exposure. The most frequent manifestation of infection was skin and soft subcutaneous tissue infections (10; 32.3%), osteomyelitis (6; 19.4%), and joint prosthetic infections (5; 16.1%). All the strains were susceptible to oxacillin, fluoroquinolones, rifampicin, and trimethoprim–sulfamethoxazole. Twenty-two (71%) of the patients required surgical treatment. Only one patient (3.2%) died, because of aortic prosthetic valve infective endocarditis. Skin and soft tissue infections were the most frequently identified manifestations of S. caprae infection. Over 75% of the cases occurred in the last six years, and 25.8% involved significant exposure to livestock. Ongoing surveillance is necessary to better understand the prevalence and transmission dynamics of this emerging zoonotic pathogen.
1. Introduction
Staphylococcus caprae is an animal-associated coagulase-negative staphylococcus [1] that characteristically colonizes the skin and mammary glands of goats [2]. It was first described by Devriese et al. in 1983 [3], based on a strain isolated from goat’s milk [4], and has also recently been isolated from sheep’s milk samples [5].
In humans, S. caprae may present as a harmless commensal in the nose, skin, and nails [2]. However, it can also become zoonotic and has been isolated from patients who have been in close contact with goats or sheep, such as farm workers or sheep breeders, and individuals bitten by goats [1]. Other risk factors for acquiring the infection are immunosuppression, obesity, open bone fractures, or tissue trauma.
The mechanisms responsible for the development of S. caprae infection remain largely unknown. Watanabe et al. (2018) performed complete genome sequences of three methicillin-resistant S. caprae isolates from humans, revealing that S. caprae is closely related to S. epidermidis and S. capitis at the species level, especially in its ability to form biofilms, which may lead to increased virulence during the development of S. caprae infections [1].
Cases of S. caprae infections have been mainly reported in the following countries: France (n = 48), Spain (n = 16), Canada (n = 12), and USA (n = 11) followed to a lesser extent by Japan, Korea, and other European countries (see Supplementary Table S1, which includes cases of S. caprae infections reported between 1995–2023).
This microorganism has a special predilection for producing osteoarticular infections [6], and further involvement in cases of bacteremia [7], acute otitis externa [8], urinary tract infection, peritonitis [2], and infective endocarditis [9].
In the autonomous community of Catalonia, the goat and sheep industries are significant; nevertheless, to date, little data are available on S. caprae infections in Catalonia. The aim of the present study is to begin to fill this gap by characterizing the 31 cases of S. caprae infections that were diagnosed over a thirteen-year period at two hospitals in Catalonia, one located in a rural setting and the other in an urban one.
2. Material and Methods
2.1. Description of the Study
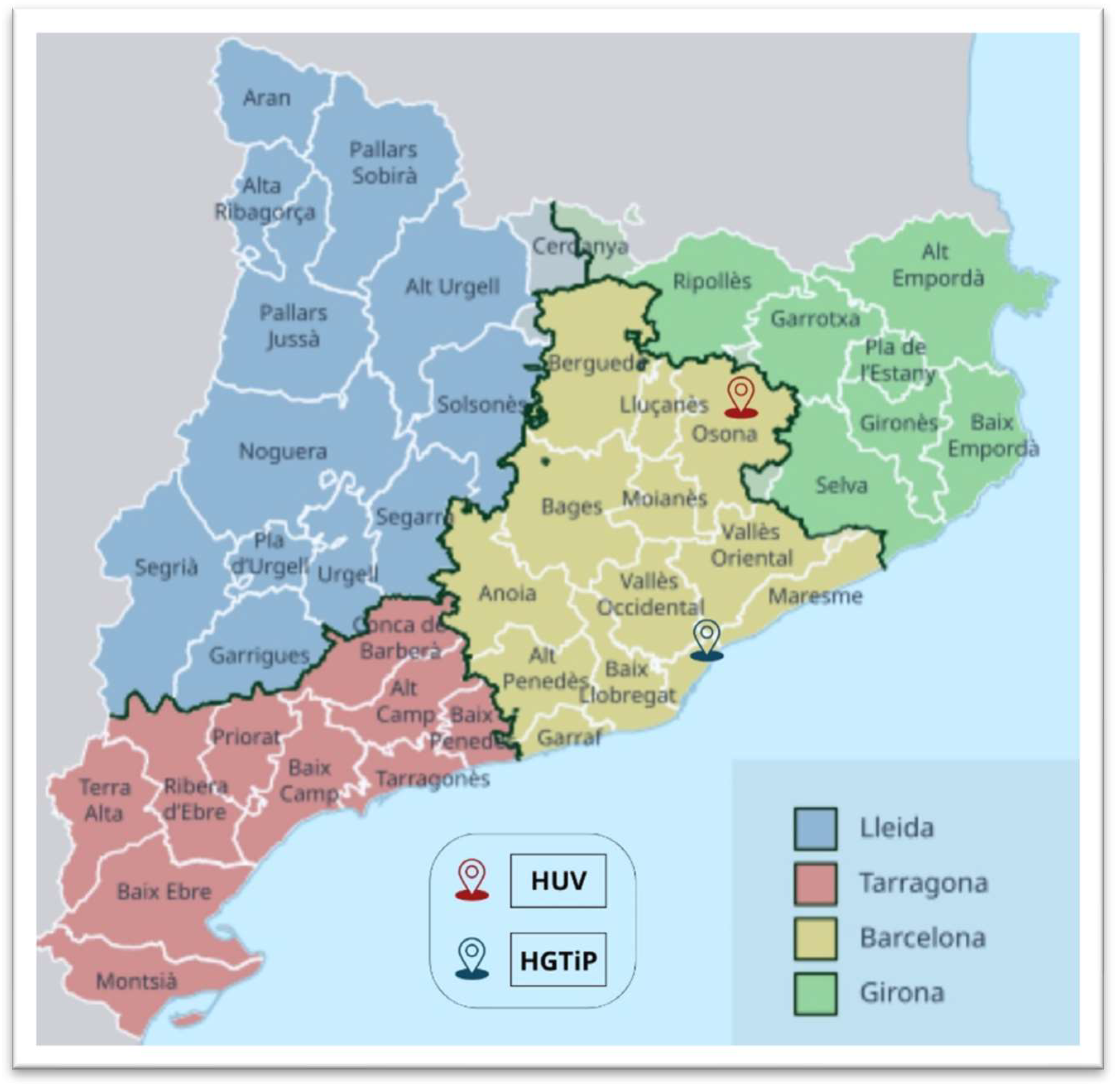
A retrospective, observational study of S. caprae infections was carried out covering the period from January 2010 to December 2023 by examining all the records at the Hospital Germans Trias i Pujol (HGTiP) in Badalona, a city that forms part of the greater Barcelona metropolitan area, and the Hospital Universitari de Vic (HUV), located in the town of Vic, the administrative centre of a rural county. The hospitals serve 800,000 and 156,599 citizens, respectively (Figure 1).
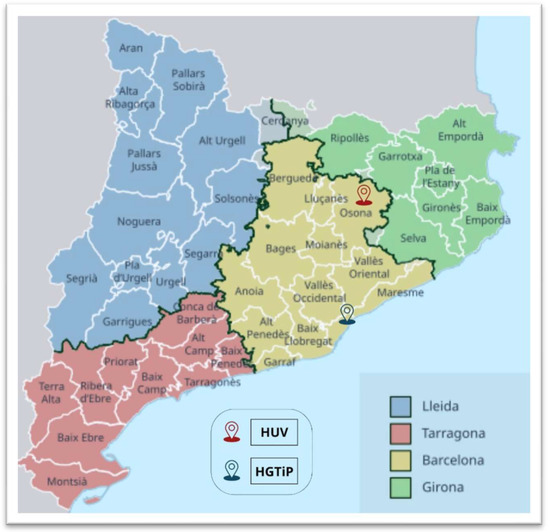
Figure 1.
Map of the administrative subdivisions of Catalonia, Spain, showing the locations of the Hospital Universitari de Vic (HUV) and Hospital Germans Trias i Pujol (HGTiP).
S. caprae infection was defined by the isolation of this microorganism in a sample taken from a normally sterile site (blood, urine, synovial fluid, and bone), purulent discharge and orthopedic devices or catheter in a patient with signs and symptoms of infection.
2.2. Bacterial Identification and Antibiotic Susceptibility Testing
The microorganism was grown on blood agar plates incubated at 37 °C in a 5% CO2 atmosphere for 24–48 h, and bacterial identification was performed using the MALDI-TOF MS Bruker Biotyper (Billerica, MA, USA). The minimal inhibitory concentrations (MICs; μg/mL) of each sample were determined using VITEK®2 (Biomerieux, Marcy-l’Étoile, France), the results were then interpreted in terms of antibiotic susceptibility in accordance with EUCAST clinical breakpoints. These diagnostic techniques have been applied in both hospitals since 2010.
2.3. Statistical Analysis
The descriptive statistics are as follows: Qualitative variables will be presented as absolute frequency and percentage and quantitative variables as mean, standard deviation, median, minimum, and maximum. The inferential statistics are as follows: χ2 (or Fisher’s exact test) was used to analyze the relationship between two variables and Student’s t-test (or a non-parametric Mann–Whitney U test) was used to analyze the relationship between a qualitative and a quantitative variable.
3. Results
A total of 31 cases of S. caprae infection were detected, 16 of them at the HUV and 15 at the HGTiP. Eight cases (25.8%) were detected in the 2010–2016 period and twenty-three (74.2%) in the 2017–2023 period.
The mean of age of the 31 patients was 58.87 ± 20.65 years and a majority were male (18; 58.1%). The mean Charlson Comorbidity Index score was 3.5 ± 3.4. Only one patient (3.2%) was under corticosteroid or other immunosuppressive treatment at the time of infection.
Eight patients had had direct or indirect exposure to livestock, of whom seven (43.8%) lived in the countryside (three had contact with goats, two worked in a slaughterhouse, one patient’s father worked in a slaughterhouse, and another patient’s brother had direct contact with goats), while one patient from an urban area had previously worked as a butcher.
The most frequent manifestation of infection was skin and soft subcutaneous tissue infection (10; 32.3%), osteomyelitis (6; 19.4% → 3 involving toes, 2 the calcaneus, and 1 the tibia), joint prosthesis infection (5; 16.1% → 1 involving hip prosthesis, 1 involving knee prosthesis, and 3 involving osteosynthesis material), bursitis (3; 9.7% → 2 involving elbow and 1 knee), prosthetic valve infective endocarditis (2; 6.5%), urinary tract infection (2; 6.5%), diabetic foot infection (1; 3.2%), tunnelled catheter for haemodialysis infection (1; 3.2%) and otitis media (1; 3.2%).
Most patients required surgical treatment (71%). Only one patient (3.2%) died, due to aortic prosthetic valve infective endocarditis.
Table 1 shows the clinical and epidemiological characteristics of the 31 cases of S. caprae infection.

Table 1.
Clinical and epidemiological characteristics of patients with S. caprae infections at two hospitals in Catalonia.
Table 2 shows the summary of individual characteristics of the 31 patients diagnosed with S. caprae infection as well as the treatment prescribed.

Table 2.
Summary of individual characteristics of the 31 patients diagnosed with S. caprae infections at two hospitals in Catalonia.
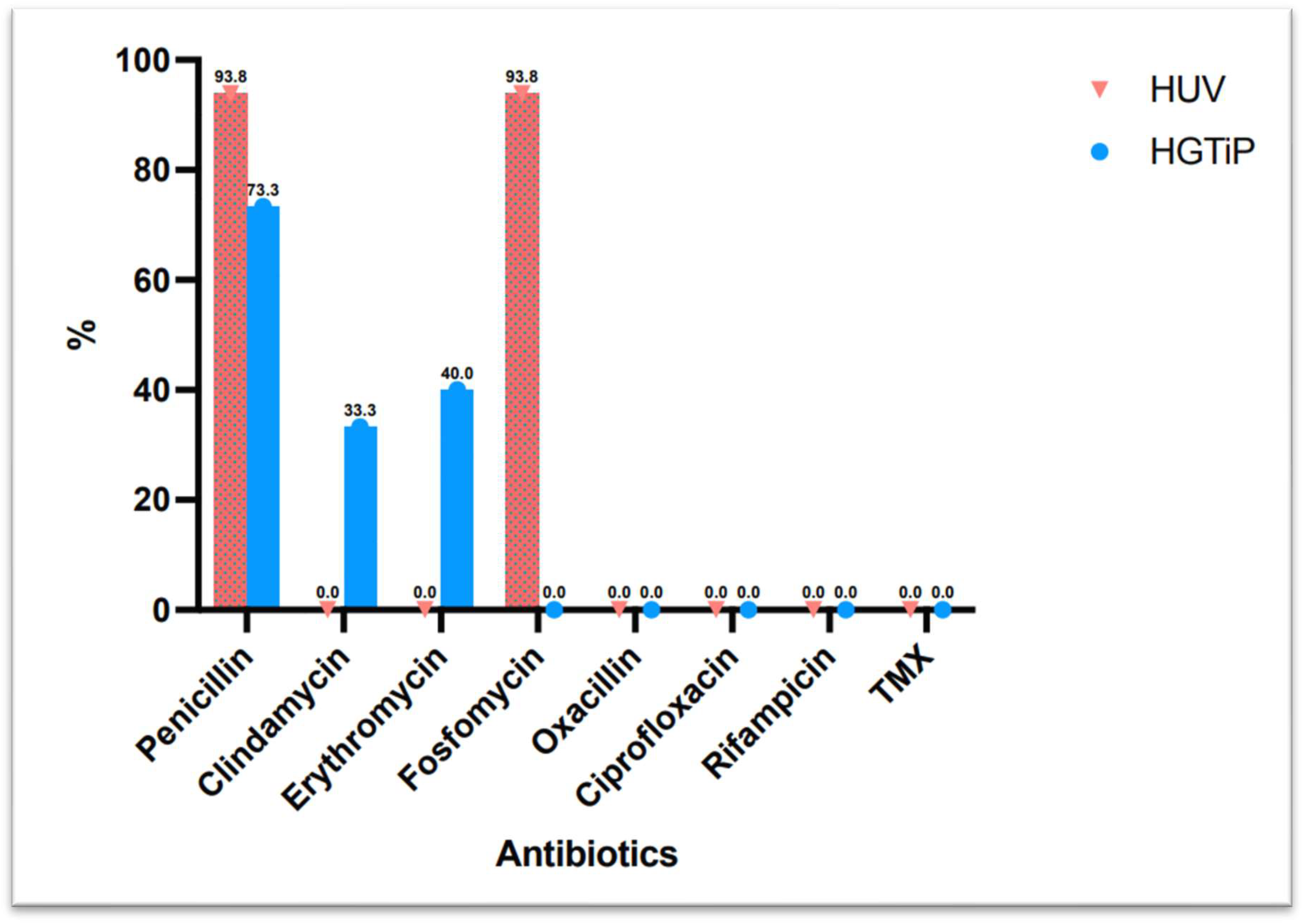
All of the S. caprae strains isolated were susceptible to oxacillin, fluoroquinolones, rifampicin, and trimethoprim–sulfamethoxazole, and 83.9% were susceptible to clindamycin, while only 16.1% were susceptible to penicillin.
Thirteen patients were treated with fluoroquinolones (two of them associated with rifampicin), six patients with amoxicillin/clavulanic acid, two patients with cloxacillin plus rifampicin, and trimethoprim–sulfamethoxazole, clindamycin and linezolid were used for the remaining three patients, respectively. For seven patients, no information was available regarding the antibiotic treatment prescribed.
Figure 2 shows the difference in antibiotic resistance between rural and urban areas.
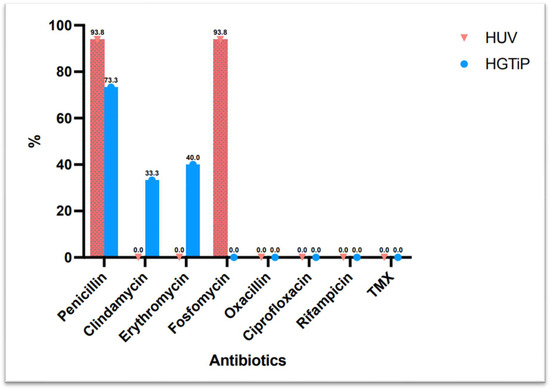
Figure 2.
Difference in antibiotic resistance between isolates from two hospitals in a rural and urban area in Catalonia.
4. Discussion
Although there are almost 70,000 heads of goats registered in Catalonia, goat-farming is more prevalent in several other of the autonomous communities that make up Spain, such as Andalusia and Extremadura [10]. Nonetheless, very little research about S. caprae infections in Spain has been published [8,11,12]. Hence, the present study offers a description of a significant number of cases identified in two hospitals in Catalonia. The distribution of cases between the HUV and the HGTiP was relatively even, with a noticeable increase in the number of infections reported at both sites in the latter half of the study period (2017–2023) compared to the earlier half (2010–2016). This could be influenced by the increase in the number of surgeries in recent years as well as the awareness of surgeons to send representative samples for a correct microbiological diagnosis, suggesting that S. caprae is likely to become a more prevalent casual infectious agent in the near future.
S. caprae infections have been associated with direct or indirect contact with goats or sheep, and indeed in our study we found that seven patients (43.8%) from rural areas had had occupational or environmental exposure and one patient from an urban background had worked as a butcher. This is a higher percentage of cases than that reported in Seng et al. (2014), where 20% of patients in a hospital in south-eastern France were related to close contact with goats or sheep [2], with information about occupational exposure being absent in other reports [11,12,13].
The finding that, overall, 25.8% of patients had had direct or indirect exposure to livestock underscores the zoonotic potential of S. caprae. Such findings highlight the importance of considering occupational and environmental factors in the epidemiology of S. caprae infections, particularly in rural areas where human–animal interactions are more common.
Interestingly, we found that animal contact was predominantly reported among patients from the HUV, which serves a more rural catchment area, supporting the notion of distinct transmission dynamics between rural and urban settings. In urban areas, alternative transmission routes, such as through contaminated food products (i.e., unpasteurised dairy products or contaminated meat) or the environment (mainly in animal work areas, veterinary hospitals, animal clinics, or animal handling facilities, where the bacteria can survive on contaminated surfaces, such as cages, animal handling equipment or clothing, favouring transmission when people come into contact with these contaminated surfaces or utensils), should be considered. This warrants further investigation to elucidate the mechanisms of infection acquisition, particularly in urban populations.
Previous studies have mainly reported infections involving bone and joints such as prosthetic joint infection [2], osteomyelitis [11], septic arthritis [12], or spondylodiscitis [13]. Similarly, in our study more than 70% percent of cases involved skin and soft subcutaneous tissues, bones, bursa, diabetic feet, and prosthetic joints. Interestingly, we had two cases of infective endocarditis, one involving an aortic bioprosthesis and a mitral native valve and the other a pulmonary bioprosthesis, with only three similar cases reported previously. This distribution reflects the organism’s known propensity for causing biofilm-associated infections, particularly in patients with indwelling medical devices [9]. Seng et al. (2014) similarly identified prosthetic joint infections as a common presentation [2], emphasizing the challenges of managing biofilm-forming bacteria in clinical settings.
The antibiotic susceptibility patterns observed in this study, with high susceptibility to oxacillin, fluoroquinolones, rifampicin, and trimethoprim–sulfamethoxazole, are consistent with previously reported data [14]. In contrast to S. epidermidis and S. haemolyticus with high percentages of methicillin-resistant isolates of both species [15], other clinically important coagulase-negative staphylococci are mostly less resistant to oxacillin, such as S. caprae, according previous studies [14,16,17,18,19]. The low susceptibility to penicillin (16.1%) reflects the typical resistance profile of coagulase-negative staphylococci, which often produce beta-lactamase enzymes. These findings highlight the importance of performing susceptibility testing to tailor antibiotic therapy and avoid the use of ineffective treatments. The preference for fluoroquinolones, often combined with rifampicin, as a treatment regimen reflects current best practise for managing biofilm-related infections [14].
The mortality related to S. caprae infections was very low, probably due to the average age of the patients coupled with optimal surgical and antibiotic treatment. Despite the severity of these infections, the study’s low mortality rate, with only one death reported due to prosthetic infective endocarditis, suggests that prompt and aggressive treatment can lead to favourable outcomes. These data are in line with previous studies where only two deaths were reported related to one clinical case of lumbar spondylodiscitis and another clinical case of mastoiditis [20,21].
The present study has several limitations, mainly due to its retrospective design. Therefore, to analyze genomic characteristics of S. caprae such as agrD sequence typing was not possible. The unavailability of some information about which antibiotic treatment was prescribed, the lack of uniformity in the duration of some antibiotic treatment prescribed by different specialists and the relatively short study period make it difficult to draw significant conclusions. Prospective studies with larger cohorts are needed to validate these results and provide more definitive conclusions about the epidemiology and management of S. caprae infection.
5. Conclusions
In conclusion, there are currently few studies describing S. caprae infections in Spain, despite a significant increase in the number of cases in recent years. In the data examined here, most patients from rural areas had had livestock exposure, while this was not the case for patients who lived in an urban setting, showing the importance of information about patients’ occupational field in any investigation of this issue. Skin or soft subcutaneous tissue infections and osteoarticular infections were the most prevalent manifestations of S. caprae infection in our series, and our data showed a low mortality rate. Ongoing surveillance and research are essential to improve our understanding of this emerging pathogen in order to optimize patient care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13010053/s1, Supplementary Table S1. Diagnostic of 99 reported cases published between 1995–2023. References [2,4,6,7,8,9,11,12,13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.D.d.l.R. and E.R.; methodology, N.P.-N., J.S.-P., S.M., M.N. and E.P.; formal analysis, E.P.; investigation, J.D.d.l.R., G.P.-P. and E.R.; writing—original draft preparation, J.D.d.l.R. and E.R.; writing—review and editing, all authors; supervision, L.P.-B., Ó.M. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
This study was carried out following the rules of the Declaration of Helsinki of 1975, which was revised in 2013 and was approved by the Ethics Committee of the HUV (#2022196; 11 April 2022).
Informed Consent Statement
All the participants were asked to provide informed and written consent to participate in this study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The preliminary results of this work were presented in part at the Congreso Nacional de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), Zaragoza, Spain, 30 May–1 June 2024 (e-Póster; presentation code 0757). We would like to thank Analía Treveset and Michael Kennedy-Scanlon for style-checking this manuscript and Silvia Díez de los Ríos González for her help with the design of Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| USA | United States of America |
| SEIMC | Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica |
| S. caprae | Staphylococcus caprae |
| S. epidermidis | Staphylococcus epidermidis |
| S. capitis | Staphylococcus capitis |
| HGTiP | Hospital Germans Trias i Pujol |
| HUV | Hospital Universitari de Vic |
| MALDI-TOF MS | Matrix-assisted laser desorption-ionization time of flight mass spectrometry |
| MICs | minimal inhibitory concentrations |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
References
- Watanabe, S.; Aiba, Y.; Tan, X.E.; Li, F.Y.; Boonsiri, B.; Thitiananpakorn, K.; Cui, B.; Sato’o, Y.; Kiga, K.; Sasahara, T.; et al. Complete genome sequencing of three human clinical isolates of Staphylococcus caprae reveals virulence factors similar to those of S. epidermidis and S. capitis. BMC Genom. 2018, 19, 810. [Google Scholar] [CrossRef]
- Seng, P.; Barbe, M.; Pinelli, P.O.; Gouriet, F.; Drancourt, M.; Minebois, A.; Cellier, N.; Lechiche, C.; Asencio, G.; Lavigne, J.P.; et al. Staphylococcus caprae bone and joint infections: A re-emerging infection? Clin. Microbiol. Infect. 2014, 20, O1052–O1058. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Poutrel, B.; Kilpper-Balz, R.; Schleifer, K.H. Staphylococcus gallinarum and Staphylococcus caprae, two new species from animals. Int. J. Syst. Bacteriol. 1983, 33, 480–486. [Google Scholar] [CrossRef]
- D’Ersu, J.; Aubin, G.G.; Mercier, P.; Nicollet, P.; Bémer, P.; Corvec, S. Characterization of staphylococcus caprae clinical isolates involved in human bone and joint infections, compared with goat mastitis isolates. J. Clin. Microbiol. 2016, 54, 106–113. [Google Scholar] [CrossRef]
- Longheu, C.M.; Azara, E.; Attene, S.; Sanna, S.; Sale, M.; Addis, M.F.; Tola, S. Comparative characterization of human and ovine non- aureus staphylococci isolated in Sardinia (Italy) for antimicrobial susceptibility profiles and resistance genes. Epidemiol. Infect. 2021, 149, e45. [Google Scholar] [CrossRef]
- Gowda, A.; Pensiero, A.L.; Packer, C.D. Staphylococcus caprae: A Skin Commensal with Pathogenic Potential. Cureus 2018, 10, e3485. [Google Scholar] [CrossRef]
- Kini, G.D.; Parris, A.R.; Tang, J.S. A Rare Presentation of Sepsis from Staphylococcus caprae. Open. Microbiol. J. 2009, 3, 67–68. [Google Scholar] [CrossRef]
- Mazur, E.; Żychowski, P.; Juda, M.; Korona-Głowniak, I.; Niedzielska, G.; Malm, A.; Kozioł-Montewka, M. First report of a staphylococcus caprae isolated from middle ear fluid of an infant with recurrent acute otitis media. Ann. Agric. Environ. Med. 2017, 24, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Díez de los Ríos, J.; Hernández-Meneses, M.; Navarro, M.; Montserrat, S.; Perissinotti, A.; Miró, J.M. Staphylococcus caprae: An emerging pathogen related to infective endocarditis. Clin. Microbiol. Infect. 2023, 29, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- SG de Producciones Ganaderas y Cinegéticas. Caracterización del sector ovino y caprino de leche en España. Minist Agric Pesca y Aliment. 2023. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/caracterizacionovinoycaprinolechedatos2023_tcm30-562416.pdf (accessed on 1 December 2024).
- Rodríguez Fernández, L.; Martín Guerra, J.M.; Dueñas Gutiérrez, C.J. Role of Staphylococcus caprae in nosocomial infection. Enferm. Infecc. Microbiol. Clin. 2020, 38, 455–456. [Google Scholar] [CrossRef]
- Rodríguez-Lucas, C.; Iriberri, I.; García-Arenzana, J.M.; Fernández, J. Septic arthritis caused by Staphylococcus caprae following arthroscopic meniscus tear repair in a patient without any foreign device. Enferm. Infecc. Microbiol. Clin. 2019, 37, 421–422. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, Y.; Li, D.; Fei, Q. A rare lumbar pyogenic spondylodiscitis caused by staphylococcus caprae with initial misdiagnosis: Case report and literature review. BMC. Surg. 2020, 20, 200. [Google Scholar] [CrossRef]
- Domashenko, P.; Foukarakis, G.; Kenanidis, E.; Tsiridis, E. A Rare Case of Staphylococcus caprae-Caused Periprosthetic Joint Infection Following Total Hip Arthroplasty: A Literature Review and Antibiotic Treatment Algorithm Suggestion. Cureus 2023, 15, e39471. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Ortega-Peña, S.; Franco-Cendejas, R.; Salazar-Sáenz, B.; Rodríguez-Martínez, S.; Cancino-Díaz, M.E.; Cancino-Díaz, J.C. Prevalence and virulence factors of coagulase negative Staphylococcus causative of prosthetic joint infections in an orthopedic hospital of Mexico. Cir. Cir. 2019, 87, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, P.; Pellizzer, G.; Furlan, F.; Nicolin, R.; Rassu, M.; Sefton, A. Staphylococcus caprae meningitis following intraspinal device infection. J. Med. Microbiol. 2008, 57 Pt 7, 904–906. [Google Scholar] [CrossRef][Green Version]
- Elsner, H.A.; Dahmen, G.P.; Laufs, R.; Mack, D. Intra-articular empyema due to Staphylococcus caprae following arthroscopic cruciate ligament repair. J. Infect. 1998, 37, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Spellerberg, B.; Steidel, K.; Lütticken, R.; Haase, G. Isolation of Staphylococcus caprae from blood cultures of a neonate with congenital heart disease. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 61–62. [Google Scholar] [CrossRef]
- Hilliard, C.A.; El Masri, J.; Goto, M. Staphylococcus caprae bacteraemia and native bone infection complicated by therapeutic failure and elevated MIC: A case report. JMM Case Rep. 2017, 4, e005112. [Google Scholar] [CrossRef]
- Shuttleworth, R.; Behme, R.J.; McNabb, A.; Colby, W.D. Human isolates of Staphylococcus caprae: Association with bone and joint infections. J. Clin. Microbiol. 1997, 35, 2537–2541. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, O.; De Marco, G.; Gavira, N.; Habre, C.; Bartucz, M.; Steiger, C.N.; Dayer, R.; Ceroni, D. Subacute osteomyelitis due to Staphylococcus caprae in a teenager: A case report and review of the literature. World J. Clin. Cases 2023, 11, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Scavelli, K.; Priester, W.B.; Finn, A.P. Numerous White Retinal Lesions Following Cataract Surgery. JAMA Ophthalmol. 2022, 140, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Nakano, S.; Yoshioka, S.; Nakamura, M.; Goto, T.; Hamada, D.; Sairyo, K. A Rare Case of Extremely Severe Heterotopic Ossification after Primary Total Hip Arthroplasty due to Persistent Mild Periprosthetic Joint Infection. Case Rep. Orthop. 2021, 2021, 8849929. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Ben Ali, Z.A.; Charfeddine, S.; Abid, L.; Kammoun, S. Endocardite infectieuse à Staphylococcus caprae compliquée de syndrome coronarien aigu [Staphylococcus caprae infective endocarditis complicated by acute coronary syndrome]. Med. Mal. Infect. 2020, 50, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.J. Puerperal septic shock and necrotizing fasciitis caused by staphylococcus caprae and Escherichia coli. Yeungnam. Univ. J. Med. 2018, 35, 248–252. [Google Scholar] [CrossRef]
- Kwok, T.C.; Poyner, J.; Olson, E.; Henriksen, P.; Koch, O. Staphylococcus caprae native mitral valve infective endocarditis. JMM Case Rep. 2016, 3, e005065. [Google Scholar] [CrossRef] [PubMed]
- Pommepuy, T.; Lons, A.; Benad, K.; Beltrand, E.; Senneville, E.; Migaud, H. Bilateral One-Stage Revision of Infected Total Hip Arthroplasties: Report of Two Cases and Management of Antibiotic Therapy. Case Rep. Orthop. 2016, 2016, 3621749. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.R.; Schwartz, S.G.; Flynn, H.W., Jr. Endophthalmitis following pars plana vitrectomy for vitreous floaters. Clin. Ophthalmol. 2014, 8, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Darrieutort-Laffite, C.; André, V.; Leautez, S.; Tanguy, G.; Cormier, G. Arthrites septiques à Staphylococcus caprae [Staphylococcus caprae arthritis]. Med. Mal. Infect. 2013, 43, 131–132. [Google Scholar] [CrossRef]
- Kato, J.; Mori, T.; Sugita, K.; Murata, M.; Ono, Y.; Yamane, A.; Shimizu, T.; Okamoto, S. Central line-associated bacteremia caused by drug-resistant Staphylococcus caprae after chemotherapy for acute myelogenous leukemia. Int. J. Hematol. 2010, 91, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.L.; Fuss, E.P.; Harrington, S.M.; Cai, M.; Perl, T.M.; Merz, W.G. Methicillin-resistant Staphylococcus caprae in a neonatal intensive care unit. J. Clin. Microbiol. 2005, 43, 363–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blanc, V.; Picaud, J.; Legros, E.; Bes, M.; Etienne, J.; Moatti, D.; Raynaud, M.F. Infection sur prothèse totale de hanche à Staphylococcus caprae. Cas clinique et revue de la littérature [Infection after total hip replacement by Staphylococcus caprae. Case report and review of the literature]. Pathol. Biol. (Paris) 1999, 47, 409–413. [Google Scholar]
- Vandenesch, F.; Eykyn, S.J.; Bes, M.; Meugnier, H.; Fleurette, J.; Etienne, J. Identification and ribotypes of Staphylococcus caprae isolates isolated as human pathogens and from goat milk. J. Clin. Microbiol. 1995, 33, 888–892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).