Risk Factors and Circulating Subtypes of Cryptosporidium spp. and Giardia duodenalis in Hospitalized Children in Mozambique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Population and Inclusion Criterion
2.2. Specimen and Clinical Data Collection
2.3. Microscopic Detection of Cryptosporidium spp. and G. duodenalis
2.4. Genomic DNA Extraction
2.5. Cryptosporidium spp. Identification
2.6. Cryptosporidium hominis and C. parvum Subtyping
2.7. Molecular Typing of G. duodenalis
2.8. Sequencing of ssurRNA and gp60 and bg PCR Amplicons
2.9. Statistical Analysis
3. Results
3.1. Sociodemographic Description of Study Participants
3.2. Prevalence and Risk Factors for Cryptosporidiosis and Giardiasis
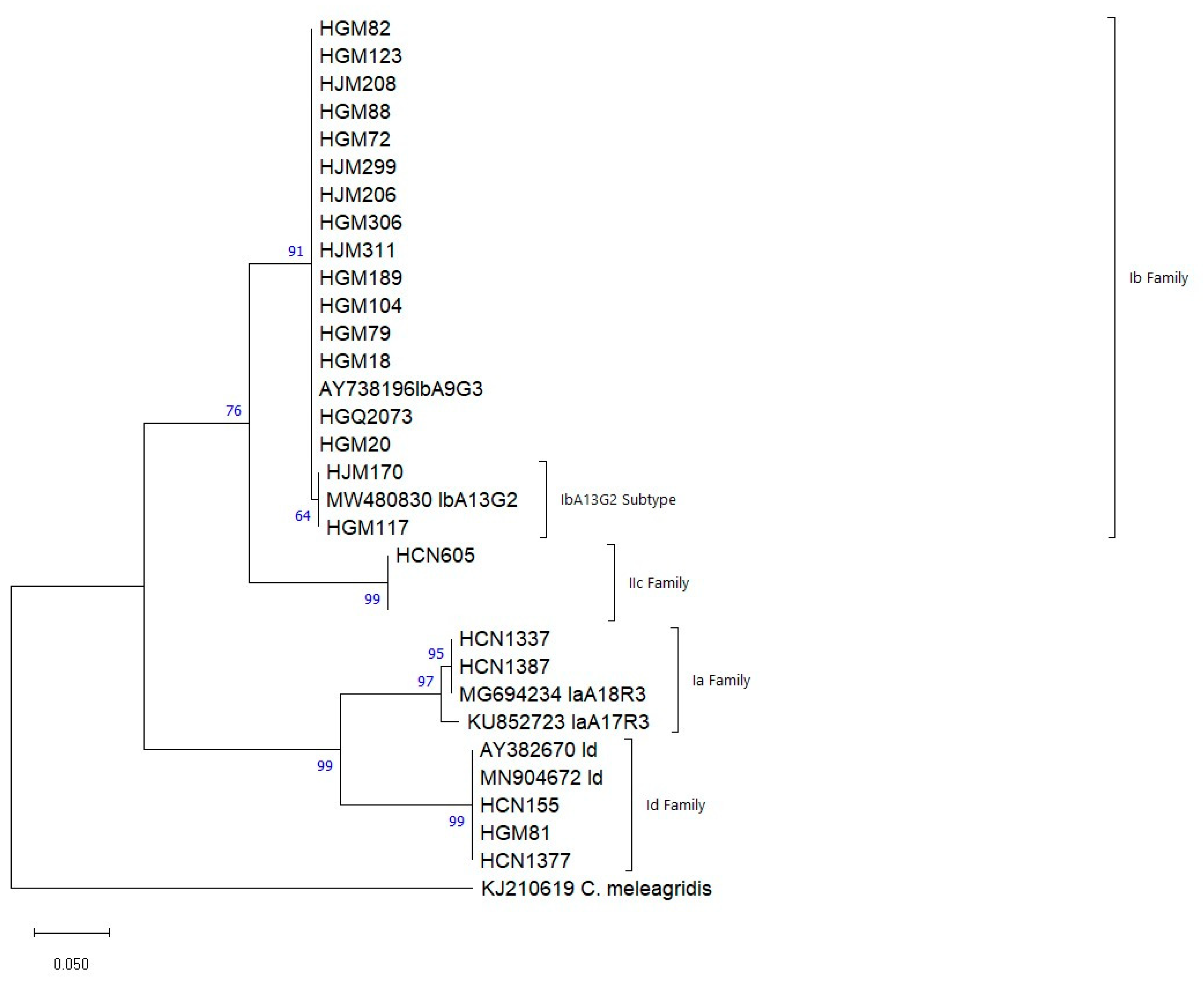
3.3. Genotyping and Subgenotyping of Cryptosporidium spp.
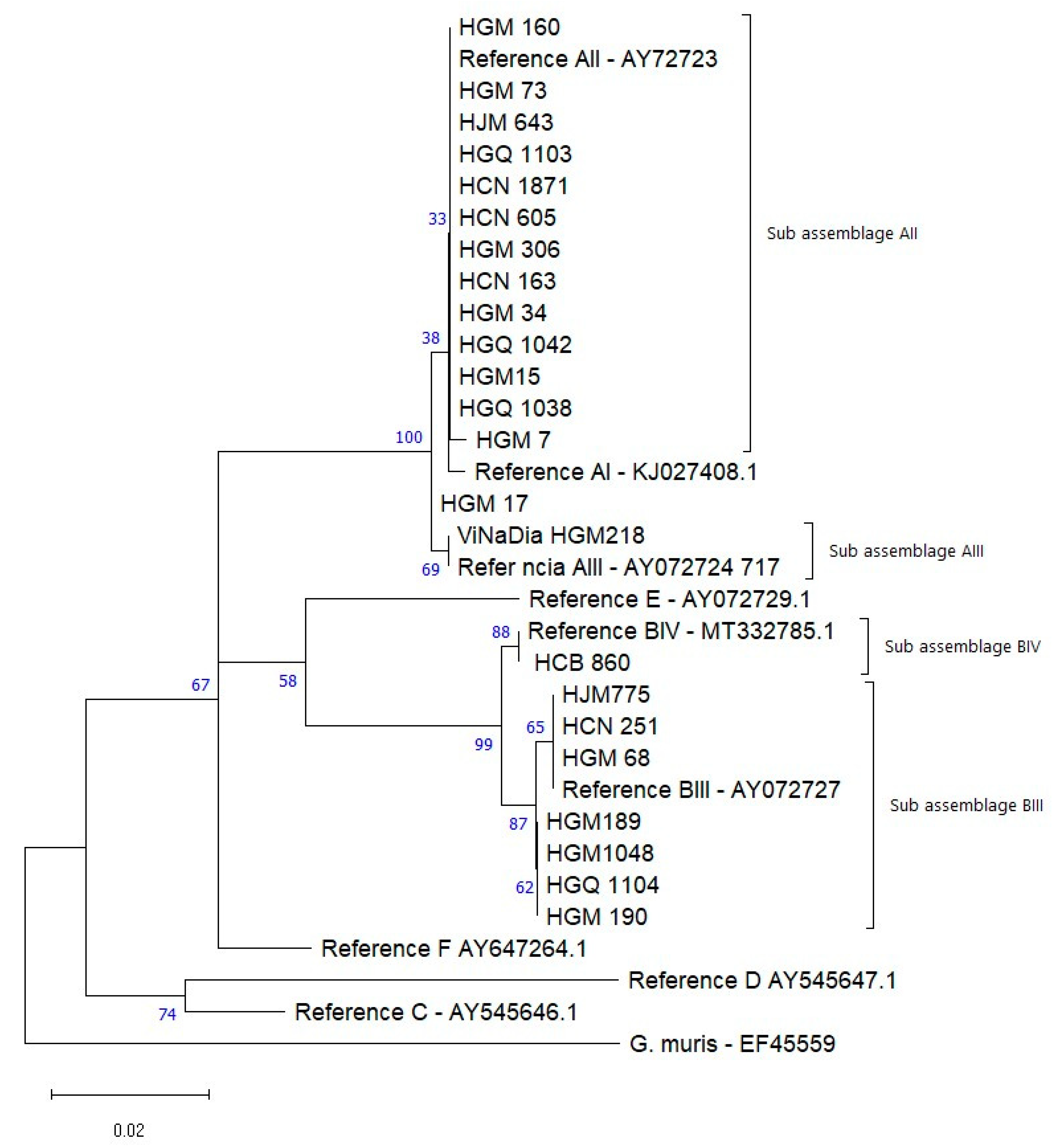
3.4. Genotyping of G. duodenalis
3.5. Local BLAST Alignment and Sequence Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF. UNICEF Data: Monitoring the Situation of Children and Women. Diarrhea. Available online: https://data.unicef.org/topic/child-health/diarrheal-disease/ (accessed on 30 July 2022).
- Shirley, D.-A.T.; Moonah, S.N.; Kotloff, K.L. Burden of disease from cryptosporidiosis. Curr. Opin. Infect. Dis. 2012, 25, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Nhampossa, T.; Mandomando, I.; Acacio, S.; Quintó, L.; Vubil, D.; Ruiz, J.; Nhalungo, D.; Sacoor, C.; Nhabanga, A.; Nhacolo, A.; et al. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0–59 Months Seeking Care at Health Facilities. PLoS ONE 2015, 10, e0119824. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The Burden of Cryptosporidium Diarrheal Disease among Children < 24 Months of Age in Moderate/High Mortality Regions of Sub-Saharan Africa and South Asia, Utilizing Data from the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef]
- Messa, A., Jr.; Köster, P.C.; Garrine, M.; Nhampossa, T.; Massora, S.; Cossa, A.; Bassat, Q.; Kotloff, K.; Levine, M.M.; Alonso, P.L.; et al. Molecular Characterisation of Cryptosporidium spp. in Mozambican Children Younger than 5 Years Enrolled in a Matched Case-Control Study on the Aetiology of Diarrheal Disease. Pathogens 2021, 10, 452. [Google Scholar] [CrossRef]
- Thompson, R.C.A. Towards a better understanding of host specificity and the transmission of Giardia: The impact of molecular epidemiology. In Giardia: The Cosmopolitan Parasite; Olson, B.E., Olson, M.E., Wallis, P.M., Eds.; CAB International: Wallingford, UK, 2002; pp. 55–69. [Google Scholar]
- Bhargava, A.; Cotton, J.A.; Dixon, B.R.; Gedamu, L.; Yates, R.M.; Buret, A.G. Giardia duodenalis Surface Cysteine Proteases Induce Cleavage of the Intestinal Epithelial Cytoskeletal Protein Villin via Myosin Light Chain Kinase. PLoS ONE 2015, 10, e0136102. [Google Scholar] [CrossRef]
- Vivancos, V.; González-Alvarez, I.; Bermejo, M.; Gonzalez-Alvarez, M. Giardiasis: Characteristics, Pathogenesis and New Insights About Treatment. Curr. Top. Med. Chem. 2018, 18, 1287–1303. [Google Scholar] [CrossRef]
- Hanevik, K.; Wensaas, K.-A.; Rortveit, G.; Eide, G.E.; Mørch, K.; Langeland, N. Irritable Bowel Syndrome and Chronic Fatigue 6 Years After Giardia Infection: A Controlled Prospective Cohort Study. Clin. Infect. Dis. 2014, 59, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.N.; Miller, W.A.; Cranfield, M.R.; Ramer, J.; Hassell, J.; Noheri, J.B.; Conrad, P.A.; Gilardi, K.V.K. giardia in mountain gorillas (Gorilla beringei beringei), forest buffalo (Syncerus caffer), and domestic cattle in Volcanoes National Park, Rwanda. J. Wildl. Dis. 2014, 50, 21–30. [Google Scholar] [CrossRef]
- Volotão, A.; Júnior, J.S.; Grassini, C.; Peralta, J.; Fernandes, O. Genotyping of Giardia duodenalis from Southern Brown Howler Monkeys (Alouatta clamitans) from Brazil. Veter. Parasitol. 2008, 158, 133–137. [Google Scholar] [CrossRef]
- Thompson, R.A. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Veter. Parasitol. 2004, 126, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals—A one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Clavero, A.O.; Verdú, M.E.; Pemán, J.; Dario, R.; Gobernado, M. Human intestinal infection due to coccidia in Mozambique: Two cases. Acta Trop. 1999, 72, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Feng, Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017, 8, 14–32. [Google Scholar] [CrossRef]
- Widmer, G.; Lee, Y. Comparison of Single- and Multilocus Genetic Diversity in the Protozoan Parasites Cryptosporidium parvum and C. hominis. Appl. Environ. Microbiol. 2010, 76, 6639–6644. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Fayer, R.; Ryan, U.; Upton, S.J. Cryptosporidium Taxonomy: Recent Advances and Implications for Public Health. Clin. Microbiol. Rev. 2004, 17, 72–97. [Google Scholar] [CrossRef]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Strong, W.B.; Gut, J.; Nelson, R.G. Cloning and Sequence Analysis of a Highly Polymorphic Cryptosporidium parvum Gene Encoding a 60-Kilodalton Glycoprotein and Characterization of Its 15- and 45-Kilodalton Zoite Surface Antigen Products. Infect. Immun. 2000, 68, 4117–4134. [Google Scholar] [CrossRef] [PubMed]
- Monis, P.T.; Andrews, R.H.; Mayrhofer, G.; Ey, P.L. Genetic diversity within the morphological species Giardia intestinalis and its relationship to host origin. Infect. Genet. Evol. 2003, 3, 29–38. [Google Scholar] [CrossRef]
- Squire, S.A.; Ryan, U. Cryptosporidium and Giardia in Africa: Current and future challenges. Parasites Vectors 2017, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.; Beck, R.; Lalle, M.; Marinculic, A.; Pozio, E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int. J. Parasitol. 2008, 38, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S. Estudo do Perfil Epidemiológico Molecular de Giardia duodenalis em Crianças dos 0 aos 59 Meses de Idade No Hospital Central de Nampula e Sua Associação Com o Estado Nutricional, Diarreia e VIH. Ph.D. Thesis, Universidade Nova em Lisboa, Lisbon, Portugal, 2017. Available online: http://hdl.handle.net/10362/27873 (accessed on 20 June 2022).
- Muadica, A.S.; Köster, P.C.; Dashti, A.; Bailo, B.; Hernández-De-Mingo, M.; Balasegaram, S.; Carmena, D. Molecular Diversity of Giardia duodenalis, Cryptosporidium spp., and Blastocystis sp. in Symptomatic and Asymptomatic Schoolchildren in Zambézia Province (Mozambique). Pathogens 2021, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Irisarri-Gutiérrez, M.J.; Mingo, M.H.-D.; de Lucio, A.; Gil, H.; Morales, L.; Seguí, R.; Nacarapa, E.; Muñoz-Antolí, C.; Bornay-Llinares, F.J.; Esteban, J.G.; et al. Association between enteric protozoan parasites and gastrointestinal illness among HIV- and tuberculosis-infected individuals in the Chowke district, southern Mozambique. Acta Trop. 2017, 170, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Messa, A., Jr.; Köster, P.C.; Garrine, M.; Gilchrist, C.; Bartelt, L.A.; Nhampossa, T.; Massora, S.; Kotloff, K.; Levine, M.M.; Alonso, P.L.; et al. Molecular diversity of Giardia duodenalis in children under 5 years from the Manhiça district, Southern Mozambique enrolled in a matched case-control study on the aetiology of diarrhoea. PLoS Negl. Trop. Dis. 2021, 15, e0008987. [Google Scholar] [CrossRef] [PubMed]
- Casmo, V. Giardia intestinalis, Cryptosporidium spp., and Other Intestinal Parasites in Maputo Province, Mozambique. Ph.D. Thesis, Uppsala Universitet, Uppsala, Sweden, 2024; 54p. [Google Scholar]
- Casmo, V.; Lebbad, M.; Maungate, S.; Lindh, J. Occurrence of Cryptosporidium spp. and Cystoisospora belli among adult patients with diarrhoea in Maputo, Mozambique. Heliyon 2018, 4, e00769. [Google Scholar] [CrossRef]
- Cossa-Moiane, I.; Cossa, H.; Bauhofer, A.; Chilaúle, J.; Guimarães, E.; Bero, D.; Cassocera, M.; Bambo, M.; Anapakala, E.; Chissaque, A.; et al. High Frequency of Cryptosporidium hominis Infecting Infants Points to A Potential Anthroponotic Transmission in Maputo, Mozambique. Pathogens 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diarrhoeal Disease. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 12 January 2021).
- World Health Organization. Manual of Basic Tecnhiques for Health Laboratory, 2nd ed.; World Health Organization: Geneva, Switzerland, 2003; Available online: https://apps.who.int/iris/bitstream/handle/10665/42295/9241545305.pdf?sequence=1&isAllowed=y (accessed on 24 February 2022).
- Nhambirre, O.L.; Cossa-Moiane, I.; Bauhofer, A.F.L.; Chissaque, A.; Lobo, M.L.; Matos, O.; de Deus, N. Intestinal Parasites in Children up to 14 Years Old Hospitalized with Diarrhea in Mozambique, 2014–2019. Pathogens 2022, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Bauhofer, A.F.L.; Cossa-Moiane, I.; Marques, S.; Guimarães, E.L.; Munlela, B.; Anapakala, E.; Chilaúle, J.J.; Cassocera, M.; Langa, J.S.; Chissaque, A.; et al. Intestinal protozoan infections among children 0–168 months with diarrhea in Mozambique: June 2014–January 2018. PLoS Negl. Trop. Dis. 2020, 14, e0008195. [Google Scholar] [CrossRef]
- Technologies QS and A. QIAamp® DNA Stool Handbook. For DNA Purification from Stool Samples. 2012. Available online: https://www.qiagen.com/us/resources/resourcedetail?id=a9de0fd4-e405-4bb7-b3a0-a74b336d613e&lang=en (accessed on 15 March 2022).
- Xiao, L.; Morgan, U.M.; Limor, J.; Escalante, A.; Arrowood, M.; Shulaw, W.; Thompson, R.C.A.; Fayer, R.; Lal, A.A. Genetic Diversity within Cryptosporidium parvum and Related Cryptosporidium Species. Appl. Environ. Microbiol. 1999, 65, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Hira, P.R.; Zhou, L.; Al-Ali, F.M.; Al-Shelahi, F.A.; Shweiki, H.M.; Iqbal, J.; Khalid, N.; Xiao, L. Unique Endemicity of Cryptosporidiosis in Children in Kuwait. J. Clin. Microbiol. 2005, 43, 2805–2809. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; De Giacomo, M.; Pozio, E. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction–restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 2002, 32, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Invitrogen by Life Technologies. PureLink® Quick Gel Extraction and PCR Purification Combo Kit. USA. 2011. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/purelink_gel_extraction_pcr_combo_qrc.pdf (accessed on 15 March 2022).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Grau-Pujol, B.; Cuamba, I.; Jairoce, C.; Cossa, A.; Da Silva, J.; Sacoor, C.; Dobaño, C.; Nhabomba, A.; Mejia, R.; Muñoz, J. Molecular Detection of Soil-Transmitted Helminths and Enteric Protozoa Infection in Children and Its Association with Household Water and Sanitation in Manhiça District, Southern Mozambique. Pathogens 2021, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Pereira, F.D.; Martins, M.D. Intestinal parasitic infections in children under five in the Central Hospital of Nampula, Northern Mozambique. J. Infect. Dev. Ctries. 2020, 14, 532–539. [Google Scholar] [CrossRef]
- Muadica, A.S.; Balasegaram, S.; Beebeejaun, K.; Köster, P.C.; Bailo, B.; Hernández-De-Mingo, M.; Dashti, A.; Dacal, E.; Saugar, J.M.; Fuentes, I.; et al. Risk associations for intestinal parasites in symptomatic and asymptomatic schoolchildren in central Mozambique. Clin. Microbiol. Infect. 2020, 27, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Jagai, J.S.; Castronovo, D.A.; Monchak, J.; Naumova, E.N. Seasonality of cryptosporidiosis: A meta-analysis approach. Environ. Res. 2009, 109, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Unicef. The children in Mozambique. In Situation of the Children in Mozambique; Unicef: Pyrmont, NSW, Australia, 2021; Available online: https://www.unicef.org/mozambique/en/children-mozambique (accessed on 15 January 2021).
- Tzipori, S.; Ward, H. Cryptosporidiosis: Biology, pathogenesis and disease. Microbes Infect. 2002, 4, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Maloa, J.M.; Mabjaia, H. Base de Informação Para a Elaboração de Políticas: Moçambique. Available online: https://environmentalmigration.iom.int/sites/g/files/tmzbdl1411/files/documents/base-de-informacao-para-a-elaboracao-de-politicas-migracao-ambiente-e-mudancas-climaticas-mocambique.pdf (accessed on 10 March 2022).
- USAID. Risco Climático em Moçambique: Perfil de Risco do País. Moçambique. 2018. Available online: https://www.climatelinks.org/sites/default/files/asset/document/2018_USAID-ATLAS-Project_Climate-Risk-Profile-Mozambique.pdf (accessed on 8 April 2022).
- Tombang, A.N.; Ambe, N.F.; Bobga, T.P.; Nkfusai, C.N.; Collins, N.M.; Ngwa, S.B.; Diengou, N.H.; Cumber, S.N. Prevalence and risk factors associated with cryptosporidiosis among children within the ages 0–5 years attending the Limbe regional hospital, southwest region, Cameroon. BMC Public Health 2019, 19, 1144. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Messih, I.A.; Wierzba, T.F.; Abu-Elyazeed, R.; Ibrahim, A.F.; Ahmed, S.F.; Kamal, K.; Sanders, J.; Frenck, R. Diarrhea Associated with Cryptosporidium parvum among Young Children of the Nile River Delta in Egypt. J. Trop. Pediatr. 2005, 51, 154–159. [Google Scholar] [CrossRef]
- Nassar, S.A.; Oyekale, T.O.; Oluremi, A.S. Prevalence of Cryptosporidium infection and related risk factors in children in Awo and Iragberi, Nigeria. J. Immunoass. Immunochem. 2016, 38, 2–9. [Google Scholar] [CrossRef]
- Breurec, S.; Vanel, N.; Bata, P.; Chartier, L.; Farra, A.; Favennec, L.; Franck, T.; Giles-Vernick, T.; Gody, J.-C.; Nguyen, L.B.L.; et al. Etiology and Epidemiology of Diarrhea in Hospitalized Children from Low Income Country: A Matched Case-Control Study in Central African Republic. PLoS Negl. Trop. Dis. 2016, 10, e0004283. [Google Scholar] [CrossRef] [PubMed]
- RIDA®QUICK Giardia (Cassettes). Rbiopharm. Available online: https://clinical.r-biopharm.com/products/ridaquick-giardia-cassettes/ (accessed on 8 April 2022).
- Center for Disease Control and Prevention. CDC—DPDx. Diagnostic Procedures—Other Specimens. 2016. Available online: https://www.cdc.gov/dpdx/diagnosticprocedures/stool/specimencoll.html (accessed on 19 February 2022).
- Feleke, B.E.; Beyene, M.B.; Feleke, T.E.; Jember, T.H.; Abera, B. Intestinal parasitic infection among household contacts of primary cases, a comparative cross-sectional study. PLoS ONE 2019, 14, e0221190. [Google Scholar] [CrossRef] [PubMed]
- Tellevik, M.G.; Moyo, S.J.; Blomberg, B.; Hjøllo, T.; Maselle, S.Y.; Langeland, N.; Hanevik, K. Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among Young Children with and without Diarrhea in Dar es Salaam, Tanzania. PLoS Negl. Trop. Dis. 2015, 9, e0004125. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.L.; Augusto, J.; Antunes, F.; Ceita, J.; Xiao, L.; Codices, V.; Matos, O. Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and Other Intestinal Parasites in Young Children in Lobata Province, Democratic Republic of São Tomé and Principe. PLoS ONE 2014, 9, e97708. [Google Scholar] [CrossRef] [PubMed]
- Avendaño Valenzuela, C.; Amaya Martínez, A. Caracterización molecular de los subtipos de la GP60 de Cryptosporidium parvum y Cryptosporidium hominis alrededor del mundo. Rev. MVZ Córdoba 2017, 22, 6339–6354. [Google Scholar] [CrossRef]
- Cama, V.A.; Bern, C.; Roberts, J.; Cabrera, L.; Sterling, C.R.; Ortega, Y.; Gilman, R.H.; Xiao, L. Cryptosporidium Species and Subtypes and Clinical Manifestations in Children, Peru. Emerg. Infect. Dis. 2008, 14, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- WaterAid. Factos e Estatísticas: Moçambique. 2022. Available online: https://www.wateraid.org/mz/quem-somos/factos-e-estatisticas (accessed on 10 December 2023).
- Robertson, L.J.; Johansen, Ø.H.; Kifleyohannes, T.; Efunshile, A.M.; Terefe, G. Cryptosporidium Infections in Africa—How Important Is Zoonotic Transmission? A Review of the Evidence. Front. Veter. Sci. 2020, 7, 575881. [Google Scholar] [CrossRef] [PubMed]
- Mbae, C.; Mulinge, E.; Waruru, A.; Ngugi, B.; Wainaina, J.; Kariuki, S. Genetic Diversity of Cryptosporidium in Children in an Urban Informal Settlement of Nairobi, Kenya. PLoS ONE 2015, 10, e0142055. [Google Scholar] [CrossRef] [PubMed]
- Banda, B.; Siwila, J.; Mukubesa, A.N.; Chitanga, S.; Kaonga, P.; Changula, K.; Simulundu, E.; Saasa, N.; Kelly, P. Cryptosporidiosis is predominantly an urban, anthroponotic infectious disease among Zambian children. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Flecha, M.J.; Benavides, C.M.; Tissiano, G.; Tesfamariam, A.; Cuadros, J.; de Lucio, A.; Bailo, B.; Cano, L.; Fuentes, I.; Carmena, D. Detection and molecular characterisation of Giardia duodenalis, Cryptosporidium spp. and Entamoeba spp. among patients with gastrointestinal symptoms in Gambo Hospital, Oromia Region, southern Ethiopia. Trop. Med. Int. Health 2015, 20, 1213–1222. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.; Sehgal, R.; Malla, N.; Khurana, S. Genetic diversity of Cryptosporidium isolates from patients in North India. Int. J. Infect. Dis. 2013, 17, e601–e605. [Google Scholar] [CrossRef]
- Blanco, M.A.; Montoya, A.; Iborra, A.; Fuentes, I. Identification of Cryptosporidium subtype isolates from HIV-seropositive patients in Equatorial Guinea. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Naguib, D.; El-Gohary, A.H.; Roellig, D.; Mohamed, A.A.; Arafat, N.; Wang, Y.; Feng, Y.; Xiao, L. Molecular characterization of Cryptosporidium spp. and Giardia duodenalis in children in Egypt. Parasites Vectors 2018, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.L.; Mathers, T.C.; Ward, B.J.; Pachebat, J.A.; Swain, M.T.; Robinson, G.; Chalmers, R.M.; Hunter, P.R.; Van Oosterhout, C.; Tyler, K.M. Evolutionary genomics of anthroponosis in Cryptosporidium. Nat. Microbiol. 2019, 4, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Dacal, E.; Saugar, J.M.; de Lucio, A.; Hernández-De-Mingo, M.; Robinson, E.; Köster, P.C.; Aznar-Ruiz-De-Alegría, M.L.; Espasa, M.; Ninda, A.; Gandasegui, J.; et al. Prevalence and molecular characterization of Strongyloides stercoralis, Giardia duodenalis, Cryptosporidium spp., and Blastocystis spp. isolates in school children in Cubal, Western Angola. Parasites Vectors 2018, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Fayer, R. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int. J. Parasitol. 2008, 38, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Di Cristanziano, V.; Santoro, M.; Parisi, F.; Albonico, M.; Shaali, M.; Di Cave, D.; Berrilli, F. Genetic characterization of Giardia duodenalis by sequence analysis in humans and animals in Pemba Island, Tanzania. Parasitol. Int. 2014, 63, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yue, D.; Qi, M.; Wang, R.; Zhao, J.; Li, J.; Shi, K.; Wang, M.; Zhang, L. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Ningxia, northwestern China. BMC Veter. Res. 2014, 10, 292. [Google Scholar] [CrossRef]
- de Lucio, A.; Amor-Aramendía, A.; Bailo, B.; Saugar, J.M.; Anegagrie, M.; Arroyo, A.; López-Quintana, B.; Zewdie, D.; Ayehubizu, Z.; Yizengaw, E.; et al. Prevalence and Genetic Diversity of Giardia duodenalis and Cryptosporidium spp. among School Children in a Rural Area of the Amhara Region, North-West Ethiopia. PLoS ONE 2016, 11, e0159992. [Google Scholar] [CrossRef] [PubMed]
| Cryptosporidium spp. | G. duodenalis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n/N | % | CI95% | p-Value | n/N | % | CI95% | p-Value |
| Gender | 0.427 a | 0.972 a | ||||||
| Male | 63/830 | 7.6 | (6.0; 9.6) | 11/830 | 1.3 | (0.7; 2.3) | ||
| Female | 52/594 | 8.8 | (6.7; 11.3) | 8/594 | 1.3 | (0.7; 2.6) | ||
| Age groups (months) | 0.021 a | 7/682 | 1.0 | (0.5; 2.1) | ||||
| 0–11 | 59/682 | 8.7 | (6.8; 11.0) | 4/196 | 2.0 | (0.8; 5.1) | ||
| 12–23 | 46/481 | 9.6 | (7.3; 12.5) | 0/65 | 0.0 | (0; 00) | ||
| 24–59 | 10/196 | 5.1 | (2.8; 9.1) | 0 | 0.0 | (0; 00) | ||
| Province | 0.038 a | <0.001 b | ||||||
| Maputo | 71/745 | 9.5 | (7.6; 11.9) | 18/745 | 2.4 | (1.5; 3.8) | ||
| Sofala | 8/78 | 10.3 | (5.3; 19.0) | 1/78 | 1.3 | (0.2; 6.9) | ||
| Zambézia | 5/158 | 3.2 | (1.4; 7.2) | 0/158 | 0.0 | (0; 00) | ||
| Nampula | 31/443 | 7.0 | (5.0; 9.8) | |||||
| Source of water | 0.195 b | 0.065 b | ||||||
| Tap water at home | 61/802 | 7.6 | (6.0; 9.7) | 16/802 | 2.0 | (1.2; 3.2) | ||
| Spring water | 37/442 | 6.9 | (6.1; 11.3) | 1/442 | 0.2 | (0.004; 1.3) | ||
| Well water | 10/144 | 6.9 | (3.8; 12.3) | 2/144 | 1.4 | (0.38; 4.9) | ||
| River/Lake/Lagoon | 2/5 | 40.0 | (11.8; 76.9) | 0/5 | 0; 0 | - | ||
| Bottled water | 0/4 | (0; 00) | (0; 00) | 0/4 | 0; 0 | - | ||
| Water Treatment | 0.871 b | 0.040 b | ||||||
| Boiling | 23/243 | 9.5 | (6.4; 13.8) | 4/243 | 1.6 | (0.6; 4.2) | ||
| Chlorination | 20/254 | 7.9 | (5.2; 11.9) | 1/254 | 0.4 | (0.07; 2.2) | ||
| Filtration | 0/11 | - | - | 1/11 | 9.1 | (1.6; 37.7 | ||
| Other | 1/12 | 8.3 | (1.5; 35.4) | 1/12 | 8.3 | (1.5; 35.4) | ||
| Não | 71/881 | 8.1 | (6.4; 10.0) | 12/881 | 1.4 | (0.8; 2.4) | ||
| Types of houses | 0.058 b | 0.002 b | ||||||
| Reed | 8/55 | 14.5 | (7.6; 26.6) | 0/55 | - | (0; 0) | ||
| Mud | 25/417 | 6.0 | (4.1; 8.7) | 0/417 | - | (0; 0) | ||
| Masonry | 74/884 | 8.4 | (6.7; 10.4) | 19/884 | 2.15 | (1.4; 3.3) | ||
| Type of food | 0.716 a | 0.918 b | ||||||
| Breast milk | 25/288 | 8.7 | (6.0; 12.5) | 5/288 | 1.7 | (0.7; 4.0) | ||
| Formula | 7/100 | 7.0 | (3.4; 13.6) | 1/100 | 1.0 | (0.2; 5.5) | ||
| Mixed (breast milk and formula) | 47/537 | 8.8 | (6.7; 11.4) | 6/537 | 1.1 | (0.5; 2.4) | ||
| Other | 33/471 | 7.0 | (5.0; 9.7) | 6/471 | 1.3 | (0.6; 2.8) | ||
| Education level of mother | 0.036 a | 0.895 b | ||||||
| Illiterate | 22/168 | 13.1 | (8.8; 19.0) | 2/168 | 1.2 | (0.3; 4.2) | ||
| Primary | 39/509 | 7.7 | (5.7; 10.3) | 8/509 | 1.6 | (0.8; 3.1) | ||
| Secondary/above | 52/726 | 7.2 | (5.5; 9.3) | 9/726 | 1.2 | (0.7; 2.3) | ||
| Mother’s marital status | 0.058 b | 0.095 b | ||||||
| Married | 63/875 | 7.2 | (5.7; 9.1) | 8/875 | 0.9 | (0.5; 1.8) | ||
| Single | 48/470 | 10.2 | (7.8; 13.3) | 11/470 | 2.3 | (1.3; 4.1) | ||
| Divorce d/widow | 1/45 | 2.2 | (0.4; 11.6) | 0/45 | 0.0 | (0; 00) | ||
| Child caregiver | 0.265 b | 0.591 b | ||||||
| Mother | 104/1257 | 8.3 | (6.9; 9, 9) | 16/1257 | 1.3 | (0.8; 2.1) | ||
| Father | 1/27 | 3.7 | (0.7; 18, 3) | 0/27 | 0; 0 | (0; 00) | ||
| Uncle/aunt | 3/16 | 18.8 | (6.6; 43, 0) | 0/16 | 0; 0 | (0; 00) | ||
| Grandparents | 5/58 | 8.6 | (3.7; 18, 6) | 1/58 | 1.7 | (0.3; 9.1) | ||
| Brothers | 0/5 | (0; 00) | (0; 00) | 0/5 | 0.0 | (0; 00) | ||
| Babysitter | 0/32 | (0; 00) | (0; 00) | |||||
| Agriculture practice | 0.363 a | 1.000 b | ||||||
| Yes | 11/169 | 6.5 | (3.7; 11.3) | 2/169 | 1.2 | (0.3; 4.2) | ||
| No | 96/1119 | 8.6 | (7.1; 10.4) | 13/1119 | 1.2 | (0.7; 2.0) | ||
| Variable | n/N | % | CI95% | p-Value |
|---|---|---|---|---|
| p-value | OR (IC 95%) | 0.427 a | ||
| Age | 0.006 | 0.973 (0.955–0.992) | (6.0; 9.6) | |
| Province | ||||
| Maputo | 0.028 | 2.840 (1.121–7.199) | (6.7; 11.3) | 0.021 a |
| Sofala | 0.035 | 3.499 (1.095–11.185) | (6.8; 11.0) | |
| Zambézia | 0.154 | 2.021 (0.768–5.322 | (7.3; 12.5) | |
| Nampula | 0.070 | Ref. | (2.8; 9.1) | |
| Education level of mother | 0.038 a | |||
| Illiteracy | 0.005 | 2.150 (1.252–3.690) | (7.6; 11.9) | |
| Primary | 0.628 | 1.113 (0.721–1.720) | (5.3; 19.0) | |
| Secondary/above | 0.018 | Ref. | (1.4; 7.2) |
| Gene | Isolate | Family | Subtype | Reference | Extension |
|---|---|---|---|---|---|
| Gp60 | HGM18, HGM20, HGM72, HGM79, HGM82, HGM88, HGM104, HGM123, HGM189, HJM206, HJM208, HJM299, HGM306, HJM311, HGQ2073 | Ib | IbA9G3 | AY738196 | 15–399 |
| HGM117, HJM170 | Ib | IbA13G2 | MW480830 | 18–414 | |
| HCN1337 | Ia | IaA18G2R2 | MG694234.1 | 12–360 | |
| HCN1387 | Ia | IaA17G2R2 | KU852723.1 | 24–363 | |
| HGM81 | Id | IdA20 | JX088404.1 | 24–473 | |
| HCN155 | Id | IdA21 | MN904672.1 | 15–477 | |
| * HCN1377 | Id | IdA17 | AY382670 | 12–478 | |
| HCN605 | IIc | IIcA5G3a | MN904722 | 19–349 |
| . | Cryptosporidium spp. | C. hominis | C. parvum | ||
|---|---|---|---|---|---|
| Variable | Total | Ia | Ib | Id | IIc |
| Gender | n = 23 (%) | n = 2 (%) | n = 17 (%) | n = 3 (%) | n = 1 (%) |
| Male | 14 (60.9) | 0 (0.0) | 11 (64.7) | 2 (66.7) | 1 (100) |
| Female | 9 (39.1) | 2 (100) | 6 (35.3) | 1 (33.3) | 0 (0.0) |
| Age groups (months) | n = 23 (%) | n = 2 (%) | n = 17 (%) | n = 3 (%) | n = 1 (%) |
| 0–11 | 16 (69.4) | 2 (100) | 11 (64.7) | 2 (66.7) | 1 (100) |
| 12–23 | 5 (21.7) | 0 (0.0) | 5 (29.4) | 0 (0.0) | 0 (0.0) |
| 24–59 | 1 (4.3) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| 60–168 | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Province | n = 23 (%) | n = 2 (%) | n = 17 (%) | n = 3 (%) | n = 1 (%) |
| Maputo | 17 (73.9) | 0 (0.0) | 16 (94.1) | 1 (33.3) | 0 (0.0) |
| Zambézia | 1 (4.3) | 0 (100) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Nampula | 5 (21.7) | 2 (100) | 0 (0.0) | 2 (66.7) | 1 (100) |
| Animal contact | n = 23 (%) | n = 2 (%) | n = 17 (%) | n = 3 (%) | n = 1 (%) |
| Yes | 8 (34.8) | 2 (100) | 5 (29.4) | 1 (33.3) | 0 (0.0) |
| No | 15 (65.2) | 0 (0.0) | 12 (70.6) | 2 (66.7) | 1 (100) |
| Diarrhea in the last 7 days | n = 14 (%) | n = 2 (%) | n = 9 (%) | n = 2 (%) | n = 1 (%) |
| Yes | 2 (14.3) | 1 (50.0) | 0 (0–0) | 0 (0.0) | 1 (100) |
| No | 12 (85.7) | 1 (50.0) | 9 (100) | 2 (100) | 0 (0.0) |
| Vomiting | n = 22 (%) | n = 2 (%) | n = 16 (%) | n = 3 (%) | n = 1 (%) |
| Yes | 14 (63.3) | 1 (50.0) | 11 (68.8) | 2 (66.7) | 0 (0.0) |
| No | 8 (36.4) | 1 (50.0) | 5 (31.3) | 1 (33.3) | 1 (100) |
| Fever | n = 22 (%) | n = 2 (%) | n = 16 (%) | n = 3 (%) | n = 1 (%) |
| Yes | 11 (50.0) | 1 (50.0) | 8 (50.0) | 1 (33.3) | 1 (100) |
| No | 11 (50.0) | 1 (50.0) | 8 (50.0) | 2 (66.7) | 0 (0.00) |
| HIV | n = 14 (%) | n = 2 (%) | n = 10 (%) | n = 1 (%) | n = 1 (%) |
| Yes | 1 (7.1) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) |
| No | 13 (92.9) | 2 (100) | 9 (90.0) | 1 (100) | 1 (100) |
| Gene | Isolate | Family | Assemblage | Reference | Extension | ID of the Sequence |
|---|---|---|---|---|---|---|
| β-giardin | HGM160, HGM73, HJM643, HGQ1103, HCN1871, HCN605, HGM306, HCB163, HGM34, HGQ1042, HGM15, HGQ1038, HGM7. | A | AII | AY072723 | 93–603 | MG736240.1 |
| HGM218 | A | AIII | AY072724 | 93–603 | FJ472824.1 | |
| HGM17 | A | Unknown | 93–603 | - | ||
| HJM775, HCN251 HGM68. * HGM189 HGM1048, HGQ1104 e HGM190 | B | BIII | AY072727 | 93–603 | LC508615.1 | |
| HGB860 | B | BIV | MT332785.1 | 93–603 | MK033096.1 |
| G. duodenalis | Assemblage A | Assemblage B | ||||
|---|---|---|---|---|---|---|
| Variables | Total | AII | AIII | BIII | BIV | Not Identified |
| Gender | n = 23 (%) | n = 13 (%) | n = 1 (%) | n = 7 (%) | n = 1 (%) | n = 1 (%) |
| Male | 11 (47.8) | 7 (53.8) | 1 (100) | 2 (28.6) | 0 (0.0) | 1 (100) |
| Female | 12 (52.2) | 6 (46.2) | 0 (0.0) | 5 (71.4) | 1 (100) | 0 (0.0) |
| Age groups (months) | n = 23 (%) | n = 13 (%) | n = 1 (%) | n = 7 (%) | n = 1 (%) | n = 1 (%) |
| 0–11 | 9 (39.1) | 6 (46.2) | 1 (100) | 1 (14.3) | 1 (100) | 0 (0.0) |
| 12–23 | 9 (39.1) | 3 (23.1) | 0 (0.0) | 5 (71.4) | 0 (0.0) | 1 (100) |
| 24–59 | 4 (17.4) | 3 (23.1) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) |
| 60–159 | 1 (4.3) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Province | n = 23 (%) | n = 13 (%) | n = 1 (%) | n = 7 (%) | n = 1 (%) | n = 1 (%) |
| Maputo | 14 (60.9) | 7 (53.8) | 1 (100) | 5 (71.4) | 0 (0.0) | 1 (100) |
| Sofala | 1 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100) | 0 (0.0) |
| Zambézia | 4 (17.4) | 3 (23.1) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) |
| Nampula | 4 (17.4) | 3 (23.1) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) |
| Animal contact | n = 23 (%) | n = 13 (%) | n = 1 (%) | n = 7 (%) | n = 1 (%) | n = 1 (%) |
| Yes | 9 (39.1) | 6 (46.2) | 0 (0.0) | 3 (42.9) | 0 (0.0) | 0 (0.0) |
| No | 14 (60.9) | 7 (53.8) | 1 (100) | 4 (57.1) | 1 (100) | 1 (100) |
| Diarrhea in the past 7 days | n = 16 (%) | n = 11 (%) | n = 1 (%) | n = 4 (%) | n = 0 (%) | n = 0 (%) |
| Yes | 2 (12.5) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 14 (87.5) | 9 (81.8) | 1 (7.1) | 4 (100) | 0 (0.0) | 0 (0.0) |
| Vomiting | n = 21 (%) | n = 12 (%) | n = 1 (%) | n = 8 (%) | n = 1 (%) | n = 1 (%) |
| Yes | 12 (57.1) | 7 (58.3) | 0 (0.0) | 3 (50.0) | 1 (100) | 1 (100) |
| No | 9 (42.9) | 5 (41.7) | 1 (100) | 3 (50.0) | 0 (0.0) | 0 (0.0) |
| Fever | n = 22 (%) | n = 12 (%) | n = 1 (%) | n = 7 (%) | n = 1 (%) | n = 1 (%) |
| Yes | 9 (40.9) | 6 (50.0) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 1 (100) |
| No | 13 (59.1) | 6 (50.0) | 1 (100) | 5 (71.4) | 1 (100) | 0 (0.0) |
| HIV | n = 17 (%) | n = 10 (%) | n = 1 (%) | n = 4 (%) | n = 1 (%) | n = 1 (%) |
| Yes | 1 (5.9) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 16 (94.1) | 9 (90.0) | 1 (100) | 4 (100) | 1 (100) | 1 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhambirre, O.; Lobo, M.L.; Cossa-Moiane, I.; Bauhofer, A.; Deus, N.d.; Matos, O. Risk Factors and Circulating Subtypes of Cryptosporidium spp. and Giardia duodenalis in Hospitalized Children in Mozambique. Microorganisms 2025, 13, 196. https://doi.org/10.3390/microorganisms13010196
Nhambirre O, Lobo ML, Cossa-Moiane I, Bauhofer A, Deus Nd, Matos O. Risk Factors and Circulating Subtypes of Cryptosporidium spp. and Giardia duodenalis in Hospitalized Children in Mozambique. Microorganisms. 2025; 13(1):196. https://doi.org/10.3390/microorganisms13010196
Chicago/Turabian StyleNhambirre, Ofélia, Maria Luísa Lobo, Idalécia Cossa-Moiane, Adilson Bauhofer, Nilsa de Deus, and Olga Matos. 2025. "Risk Factors and Circulating Subtypes of Cryptosporidium spp. and Giardia duodenalis in Hospitalized Children in Mozambique" Microorganisms 13, no. 1: 196. https://doi.org/10.3390/microorganisms13010196
APA StyleNhambirre, O., Lobo, M. L., Cossa-Moiane, I., Bauhofer, A., Deus, N. d., & Matos, O. (2025). Risk Factors and Circulating Subtypes of Cryptosporidium spp. and Giardia duodenalis in Hospitalized Children in Mozambique. Microorganisms, 13(1), 196. https://doi.org/10.3390/microorganisms13010196







