The Role and the Regulation of NLRP3 Inflammasome in Irritable Bowel Syndrome: A Narrative Review
Abstract
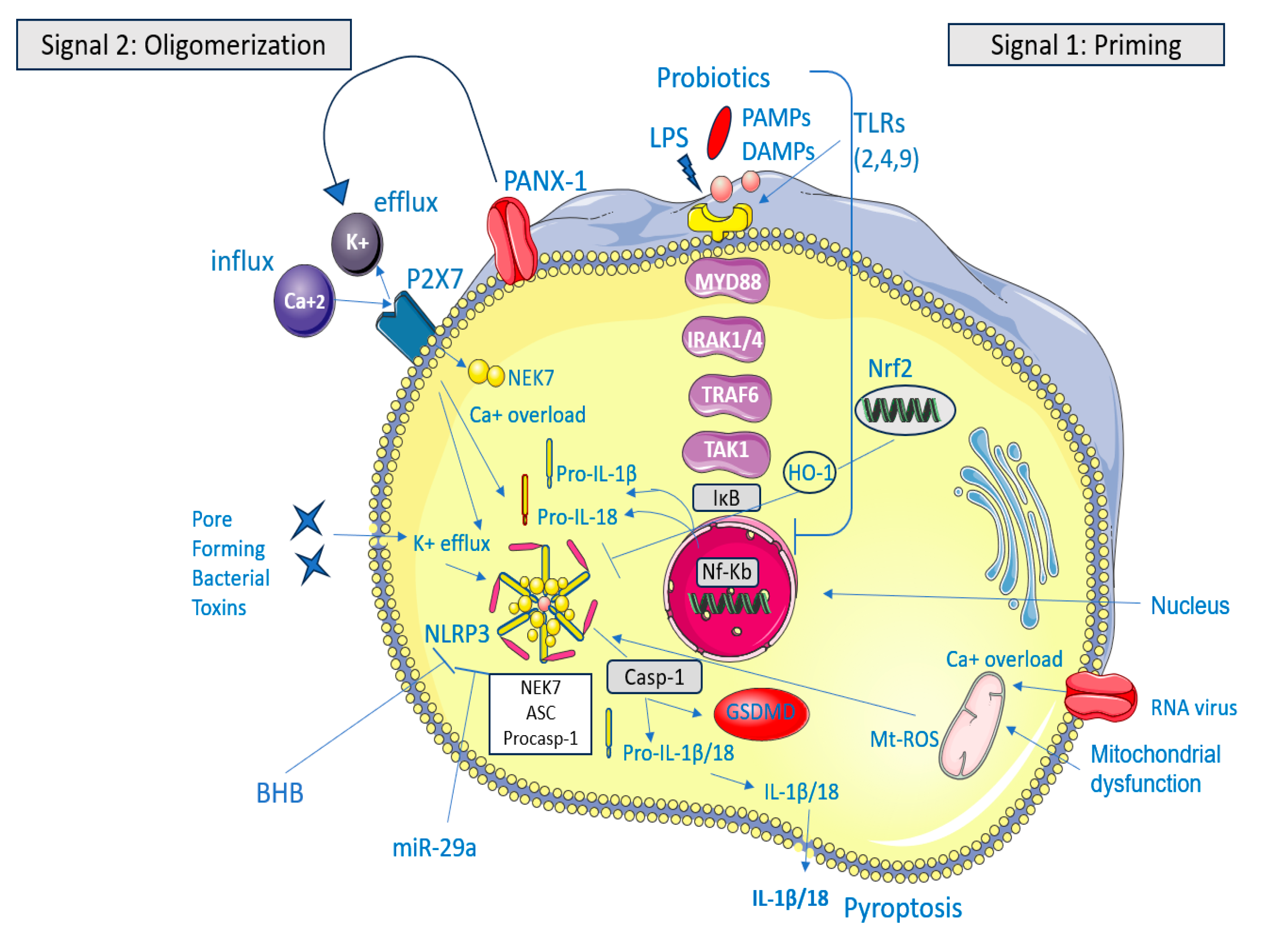
1. Introduction
2. Intestinal Inflammation and IBS
3. Potential Inhibitors of NLRP3 in IBS
3.1. Phytochemicals
3.1.1. Paeoniflorin Through miR-29a
3.1.2. Coptisine Through Nrf2 Signaling Pathway
3.1.3. Chang-Kang-Fang Through TLR4/MyD88/NF-κB
3.2. Synthetic Small Molecules
3.2.1. BAY 11-7082 Through NF-κB Pathway
3.2.2. Tranilast Through ASC Oligomerization
3.3. Organic Compounds
β-Hydroxybutyrate (BHB) Through ASC Oligomerization
3.4. Probiotics
Bifidobacterium longum Through TLR4/MyD88/NF-κB
4. Small Molecules, Phytochemicals, Organic Compounds, and Probiotics: Promising Therapeutic Choices for IBS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| AWR | Abdominal withdrawal reflex |
| ASC | Apoptosis-associated speck-like protein |
| BHB | β-hydroxybutyrate |
| CARD | Caspase recruitment domain |
| CKF | Chang-Kang-Fang |
| COX-2 | Cyclooxygenase-2 |
| COP | Coptisine |
| CRF | Corticotropin-releasing factor |
| DAMPs | Damage-associated molecular patterns |
| DSS | Dextran sulfate sodium |
| ENS | Enteric nervous system |
| GSDMD | Gasdermin D |
| HO-1 | Heme oxygenase-1 |
| IBS-C | Constipation-predominant irritable bowel syndrome |
| IBS-D | Diarrhea-predominant irritable bowel syndrome |
| IECs | Intestinal epithelial cells |
| IκBα | Inhibitor of nuclear factor kappa B |
| IKK | IκB kinase complex |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IRAK | IL-1 Receptor-Associated Kinase |
| LPS | Lipopolysaccharide |
| LRRs | Leucine-rich repeats |
| MAPK | Mitogen-Activated Protein Kinase |
| MCs | Mast cells |
| MDA | Malondialdehyde |
| miRNAs | MicroRNAs |
| MPO | Myeloperoxidase |
| MyD88 | Myeloid Differentiation Factor 88 NC: Normal controls |
| NF-κB | Nuclear factor kappa-B |
| NLR | NOD-like receptor |
| NLRP3 | NLR family pyrin domain-containing protein 3 |
| NMS | Neonatal maternal separation |
| Nrf2 | Transcription factor nuclear factor erythroid 2-related factor 2 |
| PAMPs | Pathogen-associated molecular patterns |
| PBMCs | Peripheral blood mononuclear cells |
| PFK | Pei-Fei-Kang |
| PF | Paeoniflorin |
| PGE2 | Prostaglandin E2 |
| PI-IBS | Post-infectious irritable bowel syndrome |
| PYD | Pyrin domain |
| ROS | Reactive oxygen species |
| RS | Restraint stress TAK1: Transforming Growth Factor-β-activated Kinase 1 |
| TIR | Toll/IL-1 receptor |
| TL | Tranilast |
| TLR4 | Toll-like receptor 4 |
| TNF α | Tumor necrosis factor-alpha |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| TRX | Thioredoxin |
| TXNIP | Thioredoxin-interacting protein |
| ZO-1 | Zonulin protein |
References
- Almansour, O. Prevalence of Irritable Bowel Syndrome (IBS) in the Arab World: A Systematic Review. Cureus 2024, 16, e65421. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Xu, H. Healthcare Costs of Irritable Bowel Syndrome and Irritable Bowel Syndrome Subtypes in the United States. Am. J. Gastroenterol. 2024, 119, 1571–1579. [Google Scholar] [CrossRef]
- Sinagra, E.; Pompei, G.; Tomasello, G.; Cappello, F.; Morreale, G.C.; Amvrosiadis, G.; Rossi, F.; Lo Monte, A.I.; Rizzo, A.G.; Raimondo, D. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J. Gastroenterol. 2016, 22, 2242–2255. [Google Scholar] [CrossRef]
- Burns, G.L.; Talley, N.J.; Keely, S. Immune responses in the irritable bowel syndromes: Time to consider the small intestine. BMC Med. 2022, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.-S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef]
- Nozu, T.; Arie, H.; Miyagishi, S.; Ishioh, M.; Takakusaki, K.; Okumura, T. Tranilast alleviates visceral hypersensitivity and colonic hyperpermeability by suppressing NLRP3 inflammasome activation in irritable bowel syndrome rat models. Int. Immunopharmacol. 2024, 133, 112099. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Lizarraga, J.; Hussein, H.; Boeckxstaens, G.E. Immune activation in irritable bowel syndrome: What is the evidence? Nat. Rev. Immunol. 2022, 22, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Scalavino, V.; Piccinno, E.; Giannelli, G.; Serino, G. Inflammasomes in Intestinal Disease: Mechanisms of Activation and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13058. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Zhang, S.; Hao, Z.; Shen, J. Pomegranate Peel Extract Mitigates Diarrhea-Predominant Irritable Bowel Syndromes via MAPK and NF-κB Pathway Modulation in Rats. Nutrients 2024, 16, 3854. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zheng, Y.; Yu, Q.; Zeng, M.; Bai, L.; Yang, L.; Guo, M.; Jiang, X.; Gan, J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int. J. Mol. Med. 2023, 51, 35. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Hou, X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. Motil. 2016, 22, 181–192. [Google Scholar] [CrossRef]
- O’Malley, D. Immunomodulation of enteric neural function in irritable bowel syndrome. World J. Gastroenterol. 2015, 21, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Shackelford, K. Irritable Bowel Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30521231 (accessed on 8 January 2025).
- Liebregts, T.; Adam, B.; Bredack, C.; Röth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.A.; Moretta, M.; Lim, A.; Grasby, D.J.; Bird, D.; Brierley, S.M.; Liebregts, T.; Adam, B.; Blackshaw, L.A.; Holtmann, G.; et al. Immune derived opioidergic inhibition of viscerosensory afferents is decreased in Irritable Bowel Syndrome patients. Brain. Behav. Immun. 2014, 42, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.A.; Harrington, A.M.; Castro, J.; Liebregts, T.; Adam, B.; Grasby, D.J.; Isaacs, N.J.; Maldeniya, L.; Martin, C.M.; Persson, J.; et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 2013, 62, 1456–1465. [Google Scholar] [CrossRef]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.; Meddings, J.; Collins, S.M. WEL Investigators Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 2004, 20, 1317–1322. [Google Scholar] [CrossRef]
- Shulman, R.J.; Jarrett, M.E.; Cain, K.C.; Broussard, E.K.; Heitkemper, M.M. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J. Gastroenterol. 2014, 49, 1467–1476. [Google Scholar] [CrossRef]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; O’Neill, J.; Carlson, P.; Lamsam, J.; Eckert, D.; Janzow, D.; Burton, D.; et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1262–G1269. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, J.-F.; Kao, D.; Mah, S.J.; Claggett, B.; Saltzman, J.R.; Fedorak, R.N.; Liu, J.J. Breaks in the wall: Increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest. Endosc. 2013, 77, 624–630. [Google Scholar] [CrossRef]
- Bertiaux-Vandaële, N.; Youmba, S.B.; Belmonte, L.; Lecleire, S.; Antonietti, M.; Gourcerol, G.; Leroi, A.-M.; Déchelotte, P.; Ménard, J.-F.; Ducrotté, P.; et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 2011, 106, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Wilcz-Villega, E.M.; McClean, S.; O’Sullivan, M.A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: Implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 1140–1151. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Gao, J.; Li, J.-Y.; Turgeon, D.K.; Owyang, C. Prostaglandin E2, Produced by Mast Cells in Colon Tissues From Patients with Irritable Bowel Syndrome, Contributes to Visceral Hypersensitivity in Mice. Gastroenterology 2020, 158, 2195–2207.e6. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, S.A.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Esposito, E.; Campolo, M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines 2020, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, J.; Zhuo, L.; Zhang, W.; Lv, L.; Zhu, L.; Zhang, J.; Feng, F.; Liu, W.; Han, L.; et al. The TLR4/NF-κB/NLRP3 and Nrf2/HO-1 pathways mediate the neuroprotective effects of alkaloids extracted from Uncaria rhynchophylla in Parkinson’s disease. J. Ethnopharmacol. 2024, 333, 118391. [Google Scholar] [CrossRef]
- Ke, W.; Wang, Y.; Huang, S.; Liu, S.; Zhu, H.; Xie, X.; Yang, H.; Lu, Q.; Gan, J.; He, G.; et al. Paeoniflorin alleviates inflammatory response in IBS-D mouse model via downregulation of the NLRP3 inflammasome pathway with involvement of miR-29a. Heliyon 2022, 8, e12312. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wei, H.; Chen, C.; Jiao, L.; Zhang, J.; Tan, Y.; Zeng, L. Coptisine attenuates post-infectious IBS via Nrf2-dependent inhibition of the NLPR3 inflammasome. Mol. Med. Rep. 2022, 26, 362. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.-Y.; Zhang, J.; Feng, Y.-C. Role of NLRP3 inflammasome in Bifidobacterium longum-regulated visceral hypersensitivity of postinfectious irritable bowel syndrome. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1933–1937. [Google Scholar] [CrossRef]
- Zhou, Q.; Souba, W.W.; Croce, C.M.; Verne, G.N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010, 59, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dothel, G.; Barbaro, M.R.; Di Vito, A.; Ravegnini, G.; Gorini, F.; Monesmith, S.; Coschina, E.; Benuzzi, E.; Fuschi, D.; Palombo, M.; et al. New insights into irritable bowel syndrome pathophysiological mechanisms: Contribution of epigenetics. J. Gastroenterol. 2023, 58, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xiao, X.; Shi, Y.; Wu, Y.; Huang, Y.; Li, D.; Xiong, F.; He, G.; Chai, Y.; Tang, H. Inhibition of miRNA-29a regulates intestinal barrier function in diarrhea-predominant irritable bowel syndrome by upregulating ZO-1 and CLDN1. Exp. Ther. Med. 2020, 20, 155. [Google Scholar] [CrossRef]
- Tastan, B.; Arioz, B.I.; Genc, S. Targeting NLRP3 Inflammasome with Nrf2 Inducers in Central Nervous System Disorders. Front. Immunol. 2022, 13, 865772. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Paeoniflorin Sulfonate. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Paeoniflorin-sulfonate (accessed on 9 January 2025).
- Zeng, L.; Li, K.; Wei, H.; Hu, J.; Jiao, L.; Yu, S.; Xiong, Y. A Novel EphA2 Inhibitor Exerts Beneficial Effects in PI-IBS in Vivo and in Vitro Models via Nrf2 and NF-κB Signaling Pathways. Front. Pharmacol. 2018, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.-M.; Ciobica, A.; Cojocariu, R.; Luca, A.-C.; Gorgan, L. Irritable Bowel Syndrome and Neurological Deficiencies: Is There A Relationship? The Possible Relevance of the Oxidative Stress Status. Medicina 2020, 56, 175. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-R.; Li, C.-Y.; Yazal, T.; Chen, I.-C.; Liu, P.-L.; Chen, Y.-T.; Liu, C.-C.; Lo, J.; Lin, T.-C.; Chang, C.-T.; et al. Protective effects of nordalbergin against LPS-induced endotoxemia through inhibiting MAPK/NF-κB signaling pathway, NLRP3 inflammasome activation, and ROS production. Inflamm. Res. 2024, 73, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, H.; Gao, Y.; Wang, X.; Lyu, L.; Wang, Y. Oral intake of titanium dioxide nanoparticles affect the course and prognosis of ulcerative colitis in mice: Involvement of the ROS-TXNIP-NLRP3 inflammasome pathway. Part. Fibre Toxicol. 2023, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Hu, B.; Yang, L.; Wang, P.; Wang, F.; Meng, X. Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur. J. Pharmacol. 2016, 780, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, S.; Zhang, T.; Zhang, W.; Lu, Q.; Hu, Q.; Shao, H.; Guo, Y.; Luo, Q. Antibacterial activity mechanism of coptisine against Pasteurella multocida. Front. Cell. Infect. Microbiol. 2023, 13, 1207855. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, Z.; Zhang, H.; Lu, Z.; Zhang, Y.Y.Y.; Li, M.; Kang, J.; Yang, Z.; Ma, L.L.L.; Ma, L.L.L.; et al. Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome. Open Life Sci. 2023, 18, 20220568. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Peng, S.; Zhong, J.; Guo, L.; Xu, Y.; Jin, X.; Chu, F. Effects of Chang-Kang-Fang Formula on the Microbiota-Gut-Brain Axis in Rats with Irritable Bowel Syndrome. Front. Pharmacol. 2022, 13, 778032. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; Vaccaro, M.; Bitto, A.; Pallio, G.; Pizzino, G.; Lentini, M.; Arcoraci, V.; Minutoli, L.; Scuruchi, M.; Cutroneo, G.; et al. BAY 11-7082 inhibits the NF-κB and NLRP3 inflammasome pathways and protects against IMQ-induced psoriasis. Clin. Sci. 2017, 131, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Huang, Z.; Wu, H.; Zhou, Y. Tranilast attenuates lipopolysaccharide-induced lung injury via the CXCR4/JAK2/STAT3 signaling pathway. Mol. Med. Rep. 2022, 26, 220. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sonowal, H.; Shukla, K.; Srivastava, S.K.; Ramana, K.V. Aldose reductase mediates NLRP3 inflammasome-initiated innate immune response in hyperglycemia-induced thp1 monocytes and male mice. Endocrinology 2017, 158, 3661–3675. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Hirata, Y.; Shimazaki, S.; Suzuki, S.; Henmi, Y.; Komiyama, H.; Kuwayama, T.; Iwata, H.; Karasawa, T.; Takahashi, M.; Takahashi, H.; et al. β-hydroxybutyrate suppresses NLRP3 inflammasome-mediated placental inflammation and lipopolysaccharide-induced fetal absorption. J. Reprod. Immunol. 2021, 148, 103433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, P.; Kumar, A. Targeted therapy of irritable bowel syndrome with anti-inflammatory cytokines. Clin. J. Gastroenterol. 2022, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics Regulating Inflammation via NLRP3 Inflammasome Modulation: A Potential Therapeutic Approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xiao, Y.; Zhang, X.; Zhu, Z.; Zhang, H.; Wei, J.; Zhao, Z.; Li, J.; Chen, T. Probiotics suppress LL37 generated rosacea-like skin inflammation by modulating the TLR2/MyD88/NF-κB signaling pathway. Food Funct. 2024, 15, 8916–8934. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Grover, M.; Bercik, P.; Corsetti, M.; Ghoshal, U.C.; Ohman, L.; Rajilić-Stojanović, M. Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome. Gastroenterology 2019, 156, 46–58.e7. [Google Scholar] [CrossRef]
- Qiang, R.; Li, Y.; Dai, X.; Lv, W. NLRP3 inflammasome in digestive diseases: From mechanism to therapy. Front. Immunol. 2022, 13, 978190. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef]
- Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.-D. The NLRP3 Inflammasome Protects against Loss of Epithelial Integrity and Mortality during Experimental Colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, C.; Xing, Y.; Xue, G.; Zhang, Q.; Pan, F.; Wu, G.; Hu, Y.; Guo, Q.; Lu, A.; et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 2017, 8, 1896. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhatia, R.; Devi, K.; Rawat, A.; Singh, S.; Bhadada, S.K.; Bishnoi, M.; Sharma, S.S.; Kondepudi, K.K. A synbiotic combination of Bifidobacterium longum Bif10 and Bifidobacterium breve Bif11, isomaltooligosaccharides and finger millet arabinoxylan prevents dextran sodium sulphate induced ulcerative colitis in mice. Int. J. Biol. Macromol. 2023, 231, 123326. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Mustafa, M.A.; Moath Omar, T.; Taher, S.G.; Ubaid, M.; Gilmanova, N.S.; Nasrat Abdulraheem, M.; Saadh, M.J.; Athab, A.H.; Mirzaei, R.; et al. Gut instinct: Harnessing the power of probiotics to tame pathogenic signaling pathways in ulcerative colitis. Front. Med. 2024, 11, 1396789. [Google Scholar] [CrossRef]
- Le Morvan de Sequeira, C.; Kaeber, M.; Cekin, S.E.; Enck, P.; Mack, I. The Effect of Probiotics on Quality of Life, Depression and Anxiety in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3497. [Google Scholar] [CrossRef]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrné, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Vazquez Roque, M.I.; Camilleri, M.; Stephens, D.; Burton, D.D.; Baxter, K.; Thomforde, G.; Zinsmeister, A.R. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005, 17, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Li, M.; Li, Y.-Y.Y.-Q.; Li, L.-X.; Zhai, W.-Z.; Wang, P.; Yang, X.-X.; Gu, X.; Song, L.-J.; Li, Z.; et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018, 8, 2964. [Google Scholar] [CrossRef]
- Wang, X.J.; Carlson, P.; Chedid, V.; Maselli, D.B.; Taylor, A.L.; McKinzie, S.; Camilleri, M. Differential mRNA Expression in Ileal Mucosal Biopsies of Patients with Diarrhea- or Constipation-Predominant Irritable Bowel Syndrome. Clin. Transl. Gastroenterol. 2021, 12, e00329. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Kato, K.; Tsukada, R.; Suzuki, H.; Kaneko, Y.; Kojo, Y.; Sato, H.; Onoue, S. Protective effects of tranilast on experimental colitis in rats. Biomed. Pharmacother. 2017, 90, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kang, C.; Park, S.; Ju, S.; Yoo, J.-W.; Yoon, I.-S.; Yun, H.; Jung, Y. Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation. Pharmaceuticals 2021, 14, 1092. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, L.; Pu, M.; Ye, X.; Lu, Q. Coptisine alleviates colitis through modulating gut microbiota and inhibiting TXNIP/NLRP3 inflammasome. J. Ethnopharmacol. 2024, 335, 118680. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, Y.; Jiang, Q.; Li, S.; Huang, W.; Xiang, L.; Liu, D.; Hu, Y.; Wang, P.; Lu, X.; et al. Coptisine from Coptis chinensis blocks NLRP3 inflammasome activation by inhibiting caspase-1. Pharmacol. Res. 2019, 147, 104348. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Li, M.; Bi, J.; Yang, G.; Li, E. Paeoniflorin protects against dextran sulfate sodium (DSS)-induced colitis in mice through inhibition of inflammation and eosinophil infiltration. Int. Immunopharmacol. 2021, 97, 107667. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lei, J.; Zhao, Z.; Jia, J.; Wang, L. Therapeutic effects of paeoniflorin on irritable bowel syndrome in rats. J. Vet. Sci. 2023, 24, e23. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Liu, W.; Liu, Y.; Zhang, D.; Fan, X.; Zhang, J.; Li, T.; Lu, M. Evaluation of the effectiveness and mechanism of action of the Chang-Kang-Fang formula combined with bifid triple viable capsules on diarrhea-predominant irritable bowel syndrome. Front. Microbiol. 2023, 14, 1160783. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, M.H.; Kim, E.; Cho, J.Y. BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediators Inflamm. 2012, 2012, 416036. [Google Scholar] [CrossRef]
- Guruvaiah, P.; Gupta, R. IκBα kinase inhibitor BAY 11-7082 promotes anti-tumor effect in RAS-driven cancers. J. Transl. Med. 2024, 22, 642. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Basilotta, R.; Lanza, M.; Filippone, A.; Raciti, G.; Puliafito, I.; Colarossi, L.; Esposito, E.; Paterniti, I. NLRP3 Inflammasome Inhibitor BAY-117082 Reduces Oral Squamous Cell Carcinoma Progression. Int. J. Mol. Sci. 2021, 22, 11108. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Scuderi, S.A.; Lanza, M.; Filippone, A.; Mannino, D.; Giuffrida, R.; Colarossi, C.; Mare, M.; Capra, A.P.; De Gaetano, F.; et al. Therapeutic Potential of BAY-117082, a Selective NLRP3 Inflammasome Inhibitor, on Metastatic Evolution in Human Oral Squamous Cell Carcinoma (OSCC). Cancers 2023, 15, 2796. [Google Scholar] [CrossRef] [PubMed]
- Coles, V.E.; Darveau, P.; Zhang, X.; Harvey, H.; Henriksbo, B.D.; Yang, A.; Schertzer, J.D.; Magolan, J.; Burrows, L.L. Exploration of BAY 11-7082 as a Potential Antibiotic. ACS Infect. Dis. 2022, 8, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Sharan, L.; Pal, A.; Babu, S.S.; Kumar, A.; Banerjee, S. Bay 11-7082 mitigates oxidative stress and mitochondrial dysfunction via NLRP3 inhibition in experimental diabetic neuropathy. Life Sci. 2024, 359, 123203. [Google Scholar] [CrossRef] [PubMed]
| Author, Year of Publication | Animal Model | Intervention | NLRP3/Mechanisms of Activity | Outcomes |
|---|---|---|---|---|
| Nozu et al., 2024 [6] | Rats with IBS | Tranilast | Inhibition through ASC oligomerization | Reduction in IL-1β, Inhibition of caspase-1 expression |
| BHB | Inhibition of IL-1β production | |||
| Zhang et al., 2024 [28] | Rats with IBS-D | Chang-Kang-Fang | Inhibition, through TLR4/MyD88/NF-κB | Reduction in IL-1β, IL-6, TNF-α production |
| Ke et al., 2022 [29] | Mice with IBS-D | Paeoniflorin | Inhibition through miR-29a | Reduction in IL-1β, IL-18, TNF-α, MPO production |
| Xiong et al., 2022 [30] | Rats with PI-IBS | Coptisine | Inhibition through Nrf2 signaling | Reduction in IL-1β, IL-18, TNF-α production |
| Scuderi et al., 2020 [27] | Rats with IBS-D | BAY 11-7082 | Inhibition through NF-κB pathway | Reduction in IL-1β, IL-18, TNF-α, MPO, MDA, COX-2. Production Reestablishment of NF-κB/Iκb-α expression |
| Gu et al., 2016 [31] | Mice with PI-IBS | Bifidobacterium longum | Inhibition TLR4/MyD88/NF-κB | Reduction in IL-1β, IL-18 production |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasti, A.; Katsas, K.; Nikolaki, M.D.; Triantafyllou, K. The Role and the Regulation of NLRP3 Inflammasome in Irritable Bowel Syndrome: A Narrative Review. Microorganisms 2025, 13, 171. https://doi.org/10.3390/microorganisms13010171
Kasti A, Katsas K, Nikolaki MD, Triantafyllou K. The Role and the Regulation of NLRP3 Inflammasome in Irritable Bowel Syndrome: A Narrative Review. Microorganisms. 2025; 13(1):171. https://doi.org/10.3390/microorganisms13010171
Chicago/Turabian StyleKasti, Arezina, Konstantinos Katsas, Maroulla D. Nikolaki, and Konstantinos Triantafyllou. 2025. "The Role and the Regulation of NLRP3 Inflammasome in Irritable Bowel Syndrome: A Narrative Review" Microorganisms 13, no. 1: 171. https://doi.org/10.3390/microorganisms13010171
APA StyleKasti, A., Katsas, K., Nikolaki, M. D., & Triantafyllou, K. (2025). The Role and the Regulation of NLRP3 Inflammasome in Irritable Bowel Syndrome: A Narrative Review. Microorganisms, 13(1), 171. https://doi.org/10.3390/microorganisms13010171









