Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland
Abstract
1. Introduction
2. Materials and Methods
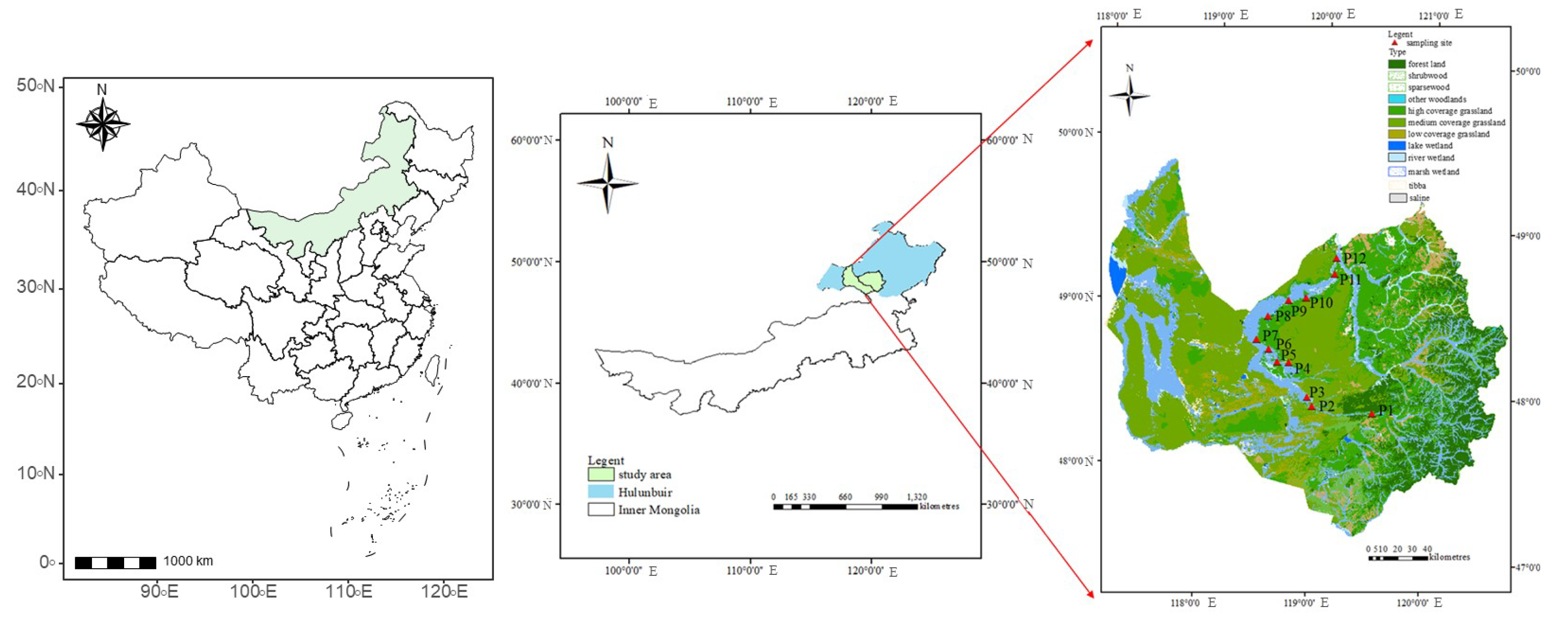
2.1. Study Site Description
2.2. Experimental Design
2.3. Plant and Soil Sampling
2.4. Plant Properties and Soil Physicochemical Properties
2.5. Metagenomic Sequencing of the Soil Samples
2.5.1. DNA Extraction and PCR Amplification
2.5.2. Data Quality Control and De-Host Sequences
2.6. Statistical Analysis Method
3. Results
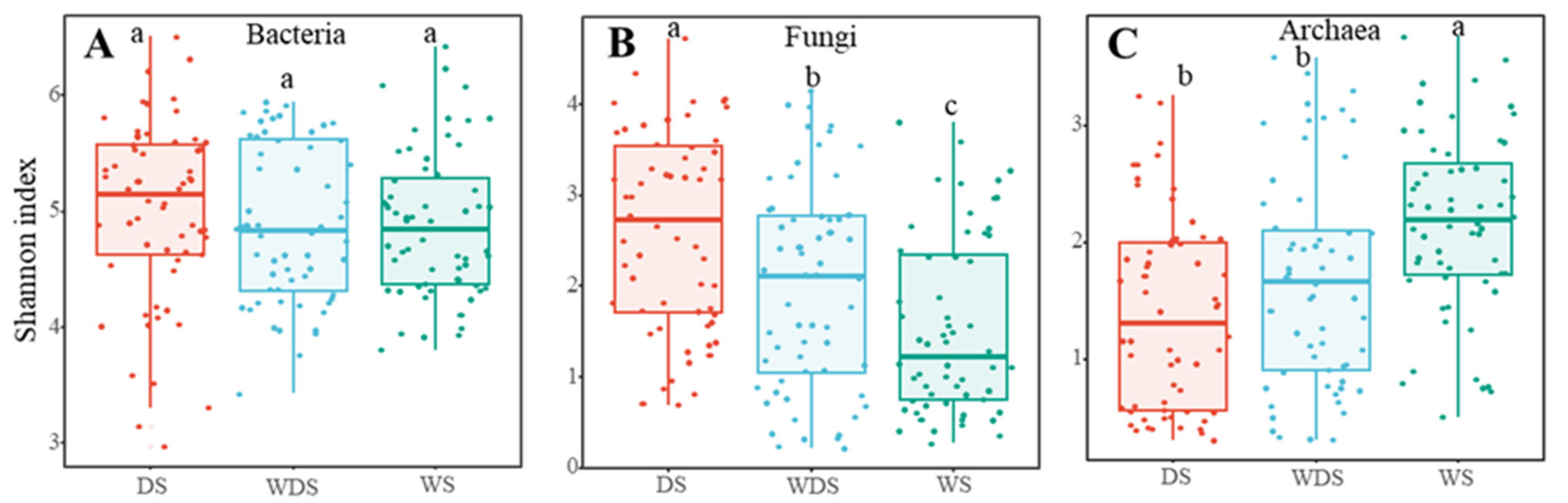
3.1. Soil Microbial Biodiversity and Community Construction
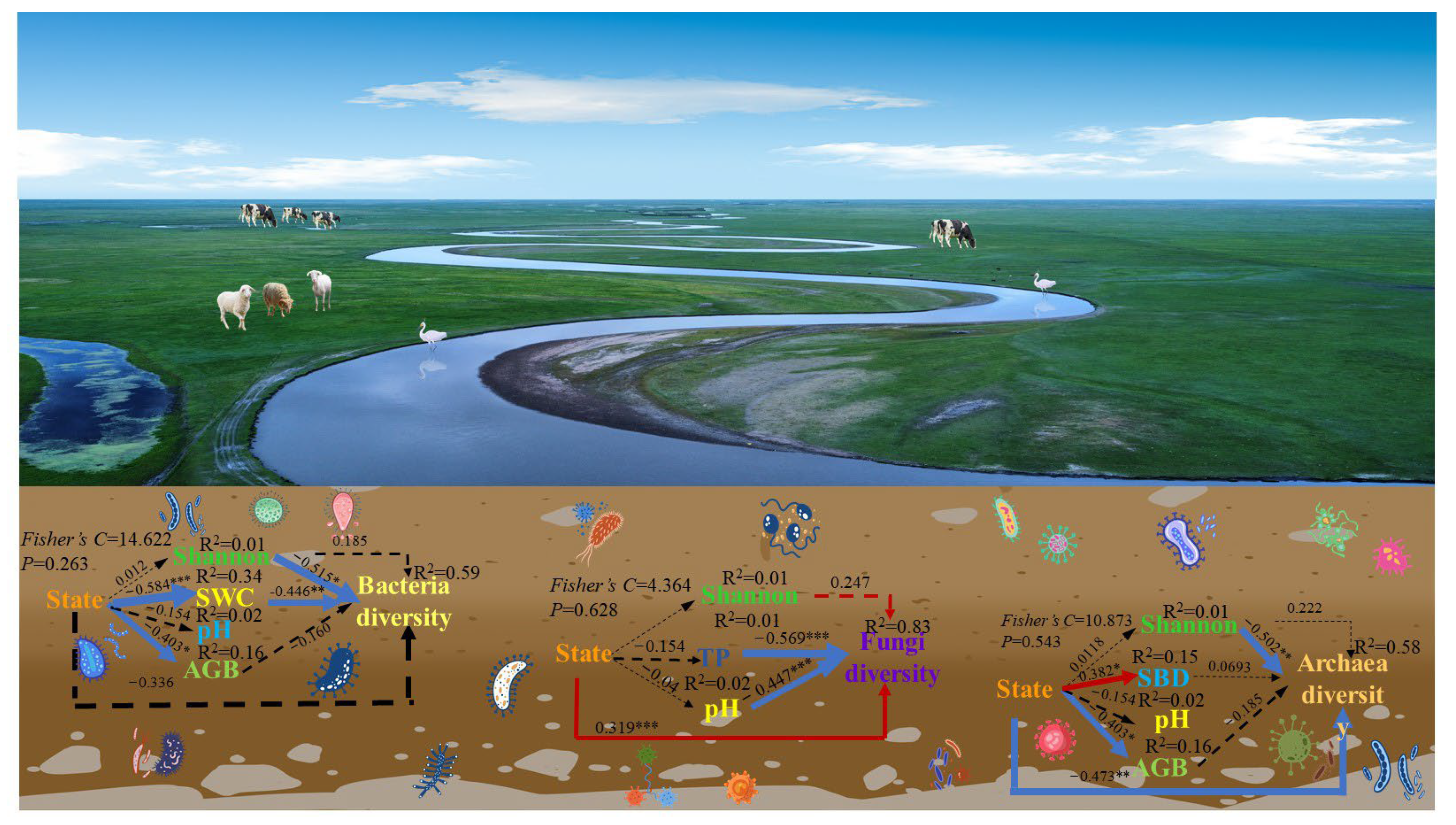
3.2. Key Factors Driving Soil Microbial Communities
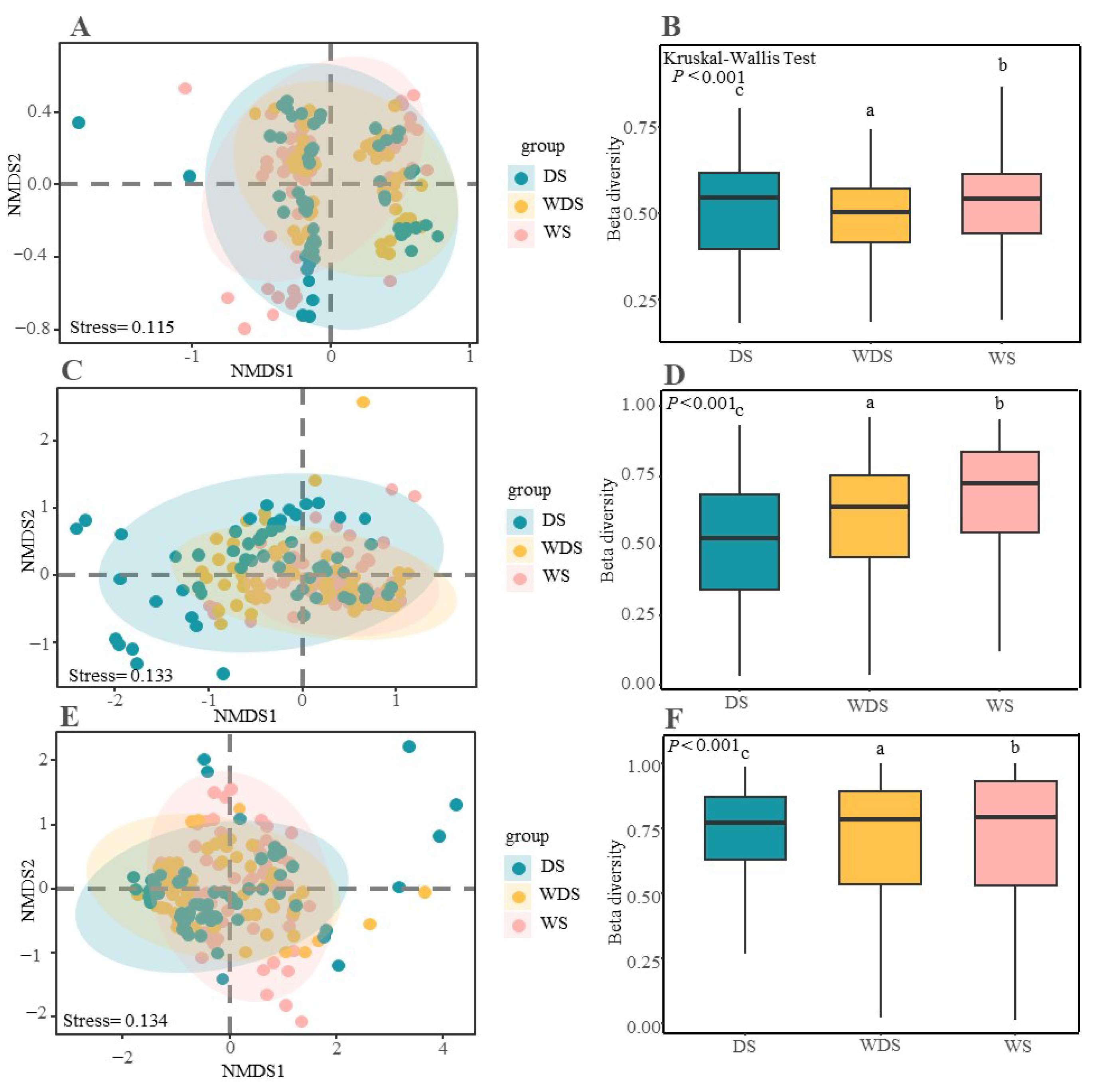
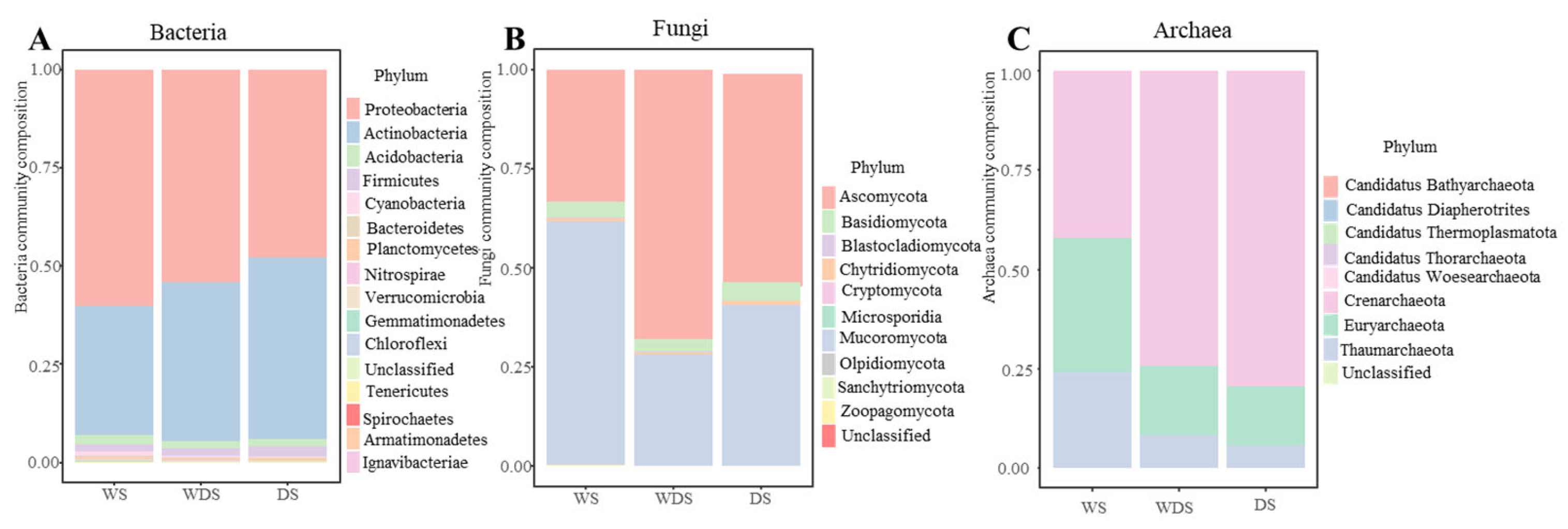
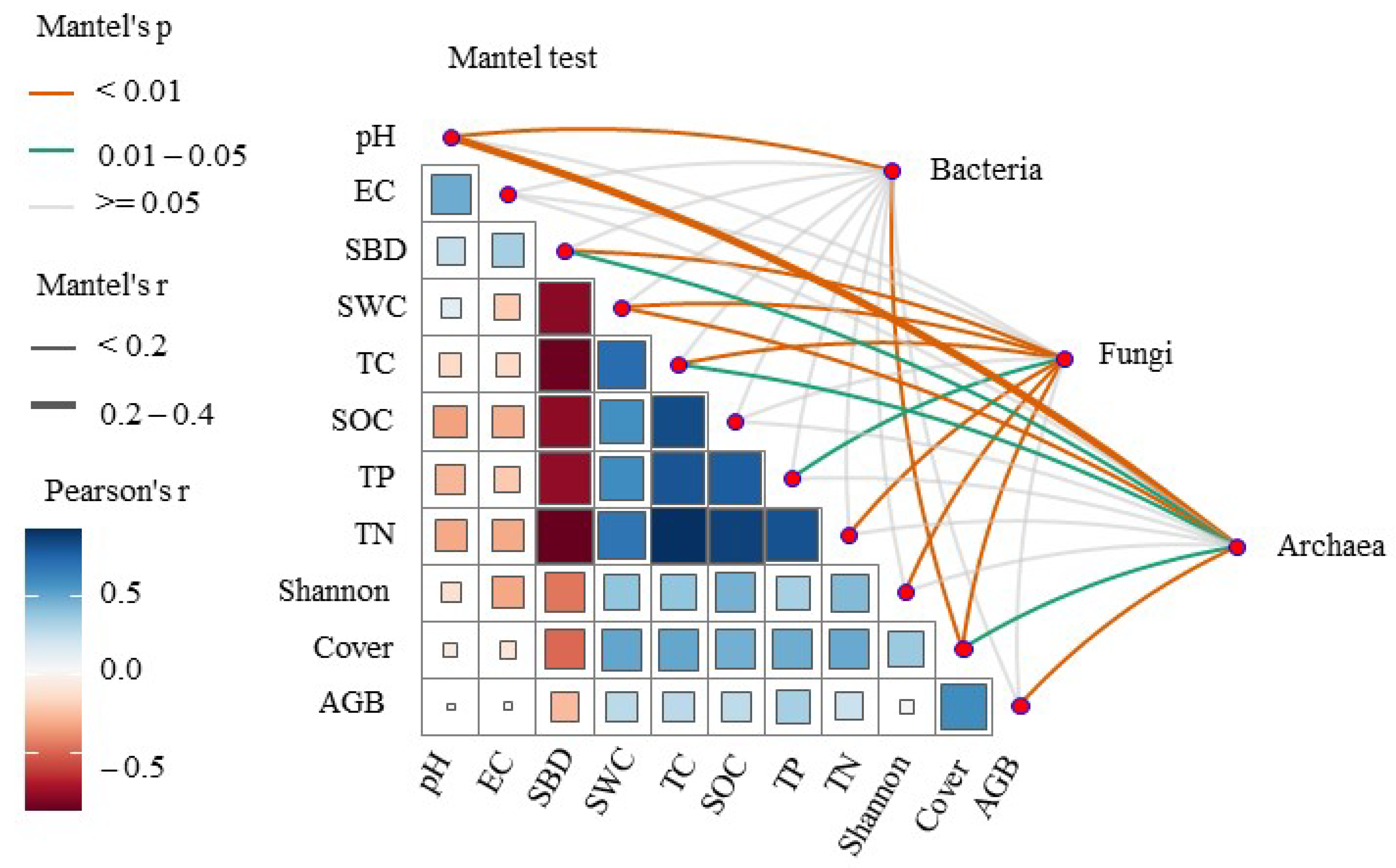
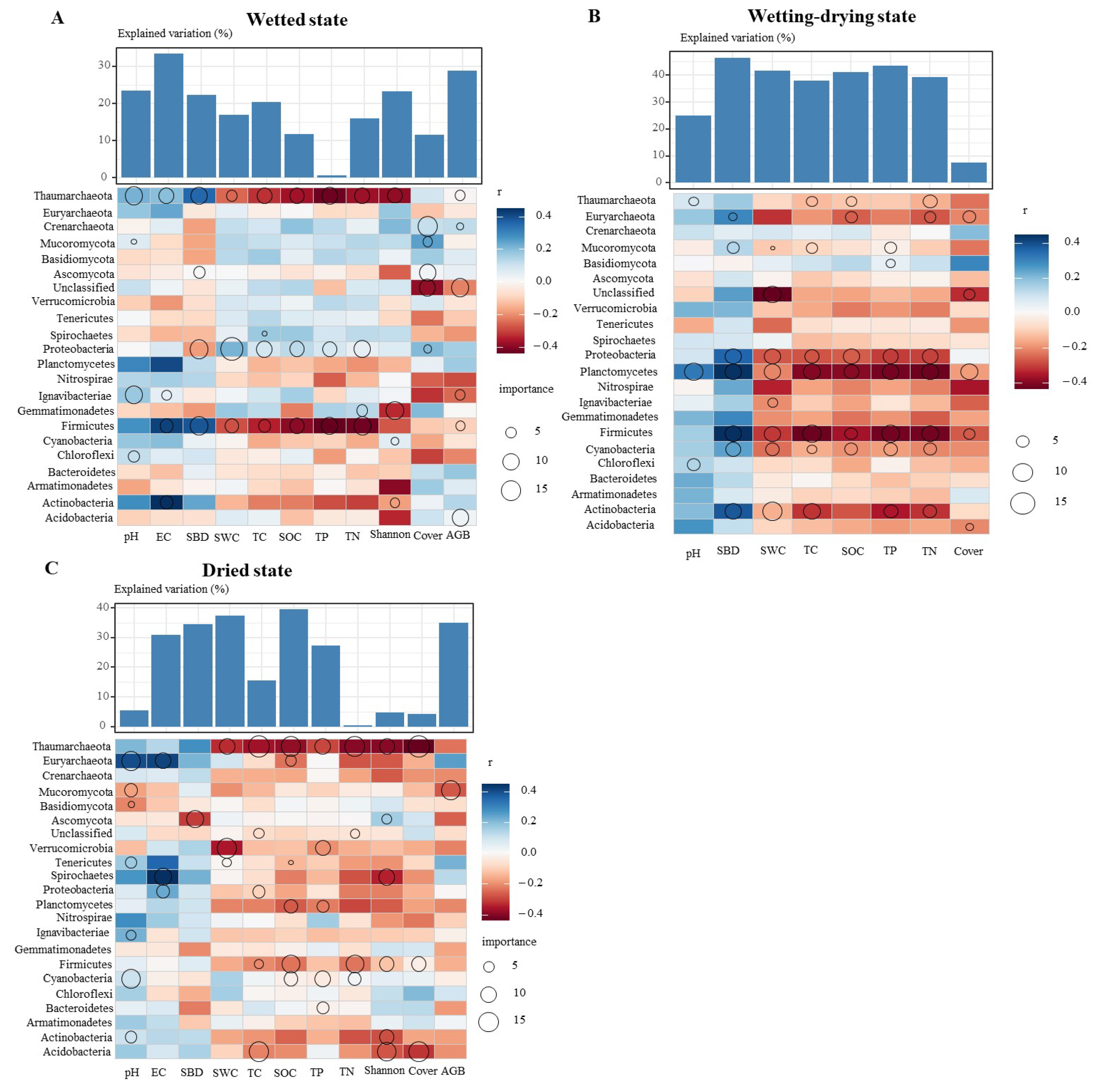
3.3. The Relationships of Soil Microbes with the Plant Community and Soil Environmental Factors
4. Discussions
4.1. The Effects of Different Flooding Conditions on the Microbial Community
4.2. Soil Physicochemical Properties Drive the Microbial Community
4.3. Mechanisms of the Effects of Soil and Plant Properties on Soil Microbes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.; Liang, J.; Zhang, P.; Wang, Q.; Wu, Y.; Ding, Y.; Wang, H.; Fu, C.; Sun, J. Review on strategies of close-to-natural wetland restoration and a brief case plan for a typical wetland in northern China. Chemosphere 2021, 285, 131534. [Google Scholar] [CrossRef]
- Hammer, D.A.; Bastian, R.K. Wetlands ecosystems: Natural water purifiers? In Constructed Wetlands for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2020; pp. 5–19. [Google Scholar]
- Shin, Y.J.; Midgley, G.F.; Archer, E.R.; Arneth, A.; Barnes, D.K.; Chan, L.; Hashimoto, S.; Hoegh-Guldberg, O.; Insarov, G.; Leadley, P. Actions to halt biodiversity loss generally benefit the climate. Glob. Change Biol. 2022, 28, 2846–2874. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Kremenetska, Y.; Wan, S.; Hu, Q.; He, S. Ecological Functions and Environmental Benefits of Reservoir Riparian Zone; SNAU: Sumy, Ukraine, 2021. [Google Scholar]
- Stutter, M.; Baggaley, N.; Wang, C. The utility of spatial data to delineate river riparian functions and management zones: A review. Sci. Total Environ. 2021, 757, 143982. [Google Scholar] [CrossRef] [PubMed]
- Fritz, K.M.; Schofield, K.A.; Alexander, L.C.; McManus, M.G.; Golden, H.E.; Lane, C.R.; Kepner, W.G.; LeDuc, S.D.; DeMeester, J.E.; Pollard, A.I. Physical and chemical connectivity of streams and riparian wetlands to downstream waters: A synthesis. JAWRA J. Am. Water Resour. Assoc. 2018, 54, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.D.; Castillo, M.M.; Capps, K.A.; Allan, J.D.; Castillo, M.M.; Capps, K.A. Energy flow and nutrient cycling in aquatic communities. In Stream Ecology: Structure and Function of Running Waters; Springer: Cham, Switzerland, 2021; pp. 357–381. [Google Scholar]
- Chen, Z.; Arif, M.; Wang, C.; Chen, X.; Li, C. Effects of hydrological regime on foliar decomposition and nutrient release in the riparian zone of the Three Gorges Reservoir, China. Front. Plant Sci. 2021, 12, 661865. [Google Scholar] [CrossRef]
- Cole, L.J.; Stockan, J.; Helliwell, R. Managing riparian buffer strips to optimise ecosystem services: A review. Agric. Ecosyst. Environ. 2020, 296, 106891. [Google Scholar] [CrossRef]
- Lewandowski, J.; Arnon, S.; Banks, E.; Batelaan, O.; Betterle, A.; Broecker, T.; Coll, C.; Drummond, J.D.; Gaona Garcia, J.; Galloway, J. Is the hyporheic zone relevant beyond the scientific community? Water 2019, 11, 2230. [Google Scholar] [CrossRef]
- Hanrahan, B.R.; Tank, J.L.; Dee, M.M.; Trentman, M.T.; Berg, E.M.; McMillan, S.K. Restored floodplains enhance denitrification compared to naturalized floodplains in agricultural streams. Biogeochemistry 2018, 141, 419–437. [Google Scholar] [CrossRef]
- Qian, J.; Liu, J.; Wang, P.; Wang, C.; Hu, J.; Li, K.; Lu, B.; Tian, X.; Guan, W. Effects of riparian land use changes on soil aggregates and organic carbon. Ecol. Eng. 2018, 112, 82–88. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Jia, W.; Huang, P.; Zhu, K.; Gao, X.; Chen, Q.; Chen, J.; Ran, Y.; Chen, S.; Ma, M.; Wu, S. Zonation of bulk and rhizosphere soil bacterial communities and their covariation patterns along the elevation gradient in riparian zones of three Gorges reservoir, China. Environ. Res. 2024, 249, 118383. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Zaimes, G. Mediterranean riparian areas–climate change implications and recommendations. J. Environ. Biol. 2020, 41, 957–965. [Google Scholar] [CrossRef]
- Ye, F.; Ma, M.H.; Wu, S.J.; Jiang, Y.; Zhu, G.B.; Zhang, H.; Wang, Y. Soil properties and distribution in the riparian zone: The effects of fluctuations in water and anthropogenic disturbances. Eur. J. Soil Sci. 2019, 70, 664–673. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Lu, Y.; Chen, L.; Zhang, Z.; Zhang, H.; Wang, X.; Shu, L.; Yang, Q.; Song, Q. When microclimates meet soil microbes: Temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- Zifcakova, L. Factors affecting soil microbial processes. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 439–461. [Google Scholar]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Ye, C.; Butler, O.M.; Chen, C.; Liu, W.; Du, M.; Zhang, Q. Shifts in characteristics of the plant-soil system associated with flooding and revegetation in the riparian zone of Three Gorges Reservoir, China. Geoderma 2020, 361, 114015. [Google Scholar] [CrossRef]
- Zhang, M.; O’Connor, P.J.; Zhang, J.; Ye, X. Linking soil nutrient cycling and microbial community with vegetation cover in riparian zone. Geoderma 2021, 384, 114801. [Google Scholar] [CrossRef]
- Zhao, T.; Bisseling, T.; Yang, Y.; Zhao, M.; Zhang, B.; Han, G. Soil carbon mineralization decreased in desert steppe by light grazing but not fencing management. CATENA 2024, 245, 108321. [Google Scholar] [CrossRef]
- Wang, B.B.; Jia, X.; Huangfu, C.H. Soil water content mediates the spatiotemporal nitrogen uptake by a dominant plant species in a subtropical wetland ecosystem. Plant Soil 2023, 483, 395–410. [Google Scholar] [CrossRef]
- Hallberg, L.; Hallin, S.; Bieroza, M. Catchment controls of denitrification and nitrous oxide production rates in headwater remediated agricultural streams. Sci. Total Environ. 2022, 838, 156513. [Google Scholar] [CrossRef] [PubMed]
- Schad, P. World Reference Base for Soil Resources. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Li, X.; Ding, C.; Bu, H.; Han, L.; Su, D. Effects of submergence frequency on soil C:N:P ecological stoichiometry in riparian zones of Hulunbuir steppe. J. Soils Sediments 2020, 20, 1480–1493. [Google Scholar] [CrossRef]
- Zhang, H.; Zha, T.; Yu, Y.; Zhang, Z.; Zhang, X.; Zhang, H.; Ji, X. Functional vegetation community responses to soil and topographic factors in the Loess Plateau of China. Land Degrad. Dev. 2023, 34, 5355–5372. [Google Scholar] [CrossRef]
- Joshi, R.K.; Garkoti, S.C. Influence of vegetation types on soil physical and chemical properties, microbial biomass and stoichiometry in the central Himalaya. CATENA 2023, 222, 106835. [Google Scholar] [CrossRef]
- Chen, L.; Xu, H.; Sun, J.; Baoyin, T. The short-term impacts of soil disturbance on soil microbial community in a degraded Leymus chinensis steppe, North China. Soil Tillage Res. 2021, 213, 105112. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; de Sousa-Blanco, M.; Marques, J.P.; Queirós, J.; Fernández-Melgar, R.; García-Álvarez, O.; Alves, P.C.; Contreras, M. Impact of vaccination with the Anaplasma phagocytophilum MSP4 chimeric antigen on gene expression in the rabbit host. Res. Vet. Sci. 2024, 178, 105370. [Google Scholar] [CrossRef]
- Zubek, S.; Rożek, K.; Chmolowska, D.; Odriozola, I.; Větrovský, T.; Skubała, K.; Dobler, P.T.; Stefanowicz, A.M.; Stanek, M.; Orzechowska, A.; et al. Dominant herbaceous plants contribute to the spatial heterogeneity of beech and riparian forest soils by influencing fungal and bacterial diversity. Soil Biol. Biochem. 2024, 193, 109405. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, K.; Xie, Y.; Li, X.; Zhang, S.; Liu, W.; Huang, Y.; Cui, L.; Wang, S.; Bao, P. Geographical, climatic, and soil factors control the altitudinal pattern of rhizosphere microbial diversity and its driving effect on root zone soil multifunctionality in mountain ecosystems. Sci. Total Environ. 2023, 904, 166932. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. Unlocking the potential of wetland biomass: Treatment approaches and sustainable resource management for enhanced utilization. Bioresour. Technol. Rep. 2023, 23, 101553. [Google Scholar] [CrossRef]
- Köchy, M.; Hiederer, R.; Freibauer, A. Global distribution of soil organic carbon–Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. Soil 2015, 1, 351–365. [Google Scholar] [CrossRef]
- Evans, S.E.; Allison, S.D.; Hawkes, C.V. Microbes, memory and moisture: Predicting microbial moisture responses and their impact on carbon cycling. Funct. Ecol. 2022, 36, 1430–1441. [Google Scholar] [CrossRef]
- Furtak, K.; Wolińska, A. The impact of extreme weather events as a consequence of climate change on the soil moisture and on the quality of the soil environment and agriculture–A review. Catena 2023, 231, 107378. [Google Scholar] [CrossRef]
- Singh, P.K.; Chudasama, H. Pathways for climate change adaptations in arid and semi-arid regions. J. Clean. Prod. 2021, 284, 124744. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Sun, X.; Wang, T.; Zhang, A.; Han, D.; Wei, G.; Song, W.; Shu, D. Host genotype-specific rhizosphere fungus enhances drought resistance in wheat. Microbiome 2024, 12, 44. [Google Scholar]
- Makhalanyane, T.P.; Valverde, A.; Gunnigle, E.; Frossard, A.; Ramond, J.B.; Cowan, D.A. Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 2015, 39, 203–221. [Google Scholar] [CrossRef]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.-N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Z.; Vosatka, M.; Vymazal, J. Arbuscular mycorrhizal fungi colonization and physiological functions toward wetland plants under different water regimes. Sci. Total Environ. 2020, 716, 137040. [Google Scholar] [CrossRef]
- Fusconi, A.; Mucciarelli, M. How important is arbuscular mycorrhizal colonization in wetland and aquatic habitats? Environ. Exp. Bot. 2018, 155, 128–141. [Google Scholar] [CrossRef]
- Ouyang, J.-X.; He, Y.-D.; Yang, B.; Zhou, J.-Z.; Li, W.; Cao, Y. Elevation, but not phosphorus, shapes arbuscular mycorrhizal fungal colonization of plateau wetland plants: A case study of the Qinghai-Tibet Plateau. Glob. Ecol. Conserv. 2023, 46, e02611. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, C.; Li, J.; Wang, G. Effects of arbuscular mycorrhizal fungi on maize nitrogen uptake strategy under different soil water conditions. Plant Soil 2021, 464, 441–452. [Google Scholar] [CrossRef]
- Xu, T.; Johnson, D.; Bardgett, R.D. Defoliation modifies the response of arbuscular mycorrhizal fungi to drought in temperate grassland. Soil Biol. Biochem. 2024, 192, 109386. [Google Scholar] [CrossRef]
- Spang, A.; Caceres, E.F.; Ettema, T.J. Genomic exploration of the diversity, ecology, and evolution of the archaeal domain of life. Science 2017, 357, eaaf3883. [Google Scholar] [CrossRef]
- Offre, P.; Spang, A.; Schleper, C. Archaea in biogeochemical cycles. Annu. Rev. Microbiol. 2013, 67, 437–457. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, Y.; Zhang, J.; Lu, Y. Environmental filtering drives distinct continental atlases of soil archaea between dryland and wetland agricultural ecosystems. Microbiome 2019, 7, 15. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, H.; Huang, Y.; Dong, B.; Fu, S.; Zhang, C.; Li, X.; Tan, Y.; Zhang, X.; Zhang, X. Driving mechanisms of soil bacterial α and β diversity under long-term nitrogen addition: Subtractive heterogenization based on the environment selection. Geoderma 2024, 445, 116886. [Google Scholar] [CrossRef]
- Ding, N.; Guo, P.; Xi, Y.; Zhang, A.; Lei, X. Low-carbon development in power systems based on carbon emission analysis models: A comprehensive review. Sustain. Energy Technol. Assess. 2024, 65, 103774. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zhai, Q.; Chang, Z.; Li, J. Nitrogen cycling process and application in different prawn culture modes. Rev. Aquac. 2024, 16, 1580–1602. [Google Scholar] [CrossRef]
- Wang, Z.; Li, K.; Yan, F.; Xiang, Q.; Zhao, X.; Ji, L.; Xin, Y.; Sun, J.; Liu, C.; Xu, X. Soil nitrogen content and key functional microorganisms influence the response of wetland anaerobic oxidation of methane to trivalent iron input. Chemosphere 2023, 322, 138183. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2023, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Khan, M.I.; Khattak, M.N.; Zhang, J.; Li, K.; Hassan, I.U. Diversity and distribution of thermophilic microorganisms and their applications in biotechnology. J. Basic Microbiol. 2022, 62, 95–108. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, C.; Singh, J.S. Wetlands: A major natural source responsible for methane emission. In Restoration of Wetland Ecosystem: A Trajectory Towards a Sustainable Environment; Springer: Singapore, 2020; pp. 59–74. [Google Scholar]
- Wang, Y.; Dong, L.; Zhang, M.; Cui, Y.; Bai, X.; Song, B.; Zhang, J.; Yu, X. Dynamic microbial community composition, co-occurrence pattern and assembly in rhizosphere and bulk soils along a coniferous plantation chronosequence. Catena 2023, 223, 106914. [Google Scholar] [CrossRef]
- Xie, L.; Li, W.; Pang, X.; Liu, Q.; Yin, C. Soil properties and root traits are important factors driving rhizosphere soil bacterial and fungal community variations in alpine Rhododendron nitidulum shrub ecosystems along an altitudinal gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Chen, Z.; Jia, Y.; Zhang, D.; Li, J.; Zhang, X. Differential response of soil abundant and rare bacterial subcommunities in the natural restoration process of oil well sites in the Loess Plateau. Appl. Soil Ecol. 2024, 203, 105663. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Zou, L.; Bai, Y.-p.; Huang, J.; Xiao, D.-r.; Yang, G. Soil pH and dissolved organic carbon shape microbial communities in wetlands with two different vegetation types in Changdu area, Tibet. J. Mt. Sci. 2023, 20, 750–764. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jiao, S.; Luo, S.; Ma, B.; Qi, W.; Cao, C.; Zhao, Z.; Du, G.; Ma, X. High soil pH enhances the network interactions among bacterial and archaeal microbiota in alpine grasslands of the Tibetan Plateau. Environ. Microbiol. 2021, 23, 464–477. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Song, W.F.; Chen, R.F.; Zhao, X.Q.; Wen, S.L.; Zheng, Z.S.; Shen, R.F. Biogeographic patterns and co-occurrence networks of diazotrophic and arbuscular mycorrhizal fungal communities in the acidic soil ecosystem of southern China. Appl. Soil Ecol. 2021, 158, 103798. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Chen, Q.; Li, Y.; Guo, D.; Nie, X.; Peng, X. Assessment of heavy metal pollution and the effect on bacterial community in acidic and neutral soils. Ecol. Indic. 2020, 117, 106626. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Zheng, H.; Hu, B.; Dai, X.; Meng, N.; Zhu, J.; Yan, D. Environmental changes drive soil microbial community assembly across arid alpine grasslands on the Qinghai-Tibetan Plateau, China. Catena 2023, 228, 107175. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, K.; Muneer, M.A.; Li, C.; Shi, H.; Tang, Y.; Zhang, J.; Ji, B. Soil moisture and pH differentially drive arbuscular mycorrhizal fungal composition in the riparian zone along an alpine river of Nam Co watershed. Front. Microbiol. 2022, 13, 994918. [Google Scholar] [CrossRef]
- Aguilera, H.; Moreno, L.; Wesseling, J.G.; Jiménez-Hernández, M.E.; Castaño, S. Soil moisture prediction to support management in semiarid wetlands during drying episodes. Catena 2016, 147, 709–724. [Google Scholar] [CrossRef]
- Basyal, B.; Emery, S.M. An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef]
- Du, X.; Bi, Y.; Tian, L.; Li, M.; Yin, K. Enhancing infiltration characteristics of compact soil in open-pit dumps through arbuscular mycorrhizal fungi inoculation in Amorpha fruticosa: Mechanisms and effects. CATENA 2024, 247, 108515. [Google Scholar] [CrossRef]
- Xiao, D.; Deng, L.; Kim, D.G.; Huang, C.; Tian, K. Carbon budgets of wetland ecosystems in China. Glob. Change Biol. 2019, 25, 2061–2076. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Fenn, M.E.; De Vries, W.; Ok, Y.S. Atmospheric Nitrogen Deposition to Global Forests: Status, Impacts and Management Options; Elsevier: Amsterdam, The Netherlands, 2019; Volume 250, pp. 1044–1048. [Google Scholar]
- Bebber, D.P. The gap between atmospheric nitrogen deposition experiments and reality. Sci. Total Environ. 2021, 801, 149774. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Ayoubi, S.; Lorenz, N. Soil microbial communities affected by vegetation, topography and soil properties in a forest ecosystem. Appl. Soil Ecol. 2020, 149, 103514. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Li, Q.; Zhao, C.; Feng, Y. Influence of plants and environmental variables on the diversity of soil microbial communities in the Yellow River Delta Wetland, China. Chemosphere 2021, 274, 129967. [Google Scholar] [CrossRef]
- Wang, L.; Pang, X.; Li, N.; Qi, K.; Huang, J.; Yin, C. Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Huang, L.; Qi, Q.; Liu, L.; Zhao, Y.; Wang, Z.; Zhou, H.; Lv, X.; Mao, Z. Shifts in soil microbial community functional gene structure across a 61-year desert revegetation chronosequence. Geoderma 2019, 347, 126–134. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Roles of plant endosphere microbes in agriculture-a review. J. Plant Growth Regul. 2022, 41, 1411–1428. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, F.; He, L.; Guo, Z.; Wang, N.; Zuo, Y.; Liu, J.; Li, K.; Wang, Y.; Sun, Y. Wetland conversion to cropland alters the microbes along soil profiles and over seasons. Catena 2022, 214, 106282. [Google Scholar] [CrossRef]
- Li, W.; Siddique, M.S.; Liu, M.; Graham, N.; Yu, W. The migration and microbiological degradation of dissolved organic matter in riparian soils. Water Res. 2022, 224, 119080. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, X.; Yan, W.; Zhong, Y.; Shangguan, Z. Changes in soil microbial community structure during long-term secondary succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Hicks, L.C.; Frey, B.; Kjøller, R.; Lukac, M.; Moora, M.; Weedon, J.T.; Rousk, J. Toward a function-first framework to make soil microbial ecology predictive. Ecology 2022, 103, e03594. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, W.; Hou, X.; Li, Y.; Tong, J. How nutrient loads influence microbial-derived carbon accumulation in wetlands: A new insight from microbial metabolic investment strategies. Environ. Res. 2023, 217, 114981. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
| Variables | WS | WDS | DS |
|---|---|---|---|
| TC (g·kg−1) | 48.74 ± 6.126 a | 46.725 ± 6.099 a | 30.383 ± 2.558 b |
| TN (g·kg−1) | 3.395 ± 0.366 a | 3.214 ± 0.381 a | 2.464 ± 0.193 a |
| TP (g·kg−1) | 0.5 ± 0.046 a | 0.572 ± 0.066 a | 0.459 ± 0.039 a |
| SOC(g·kg−1) | 30.788 ± 3.237 a | 32.078 ± 4.37 a | 23.421 ± 2.251 a |
| pH | 8.485 ± 0.17 a | 8.523 ± 0.197 a | 8.319 ± 0.228 a |
| SWC (%) | 48.644 ± 2.313 a | 40.109 ± 2.603 b | 31.965 ± 1.969 c |
| SBD (g·cm−3) | 1.181 ± 0.056 a | 1.252 ± 0.053 a | 1.334 ± 0.027 b |
| Aboveground biomass (g·m−2) | 168 ± 15.29 b | 170 ± 30.48 b | 135 ± 7.97 b |
| Shannon diversity | 230 ± 16.15 b | 221 ± 31.75 b | 286 ± 10.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiqige, B.; Liu, J.; Li, M.; Hu, X.; Guo, W.; Wang, P.; Ding, Y.; Zhi, Q.; Wu, Y.; Guan, X.; et al. Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland. Microorganisms 2025, 13, 154. https://doi.org/10.3390/microorganisms13010154
Qiqige B, Liu J, Li M, Hu X, Guo W, Wang P, Ding Y, Zhi Q, Wu Y, Guan X, et al. Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland. Microorganisms. 2025; 13(1):154. https://doi.org/10.3390/microorganisms13010154
Chicago/Turabian StyleQiqige, Bademu, Jingjing Liu, Ming Li, Xiaosheng Hu, Weiwei Guo, Ping Wang, Yi Ding, Qiuying Zhi, Yuxuan Wu, Xiao Guan, and et al. 2025. "Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland" Microorganisms 13, no. 1: 154. https://doi.org/10.3390/microorganisms13010154
APA StyleQiqige, B., Liu, J., Li, M., Hu, X., Guo, W., Wang, P., Ding, Y., Zhi, Q., Wu, Y., Guan, X., & Li, J. (2025). Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland. Microorganisms, 13(1), 154. https://doi.org/10.3390/microorganisms13010154






