Abstract
Blooms of the dinoflagellate Karenia selliformis in Chile, often associated with massive fish kills, have been noted alongside other species from the Kareniaceae family, such as Karenia spp. and Karlodinium spp. However, the potential allelopathy impact of Chilean K. selliformis on other phytoplankton species remains unexplored. Here, we assessed the allelopathic effects of cell-free exudates from a Chilean K. selliformis strain on six phytoplankton strains representing diverse microalgal groups. The findings of these experiments offer valuable insights into the varied responses of both non-toxic and toxic microalgae to allelochemicals produced by a toxic microalga, showcasing the intricate and multifaceted nature of allelopathic interactions in microalgal communities. The study revealed species-dependent effects, with variable response in cell growth, photosynthetic efficiency (i.e., Fv/Fm), and intracellular reactive oxygen species (ROS) production. While certain strains exhibited significant growth inhibition in response to the allelochemicals, others demonstrated no apparent effect on cell proliferation, indicating varying sensitivity to specific allelochemicals or potentially distinct detoxification mechanisms. Similarly, the diverse effects on Fv/Fm highlight the complexity of allelopathic interactions, with some species showing reduced efficiency without alterations in intracellular ROS production, while others displayed increased ROS production alongside impaired photosynthesis.
1. Introduction
Harmful algal blooms (HABs) are natural events characterized by the proliferation of certain phytoplankton species, which sometimes lead to water discoloration [1]. During HAB events, numerous metabolites produced by the blooming microalgae are released into the water [1,2]. Studies on the effect of these bioactive compounds have been traditionally focused on their toxic effect on humans or animals, either directly or through the trophic web, causing a range of health issues from mild to severe, and in the most severe cases, death [3,4]. However, there is limited understanding of the impact of microalgal secondary metabolites on plankton assemblages.
One of the main open questions in phytoplankton ecology is the reason behind the formation of blooms by certain microalgae. In general, species that can effectively outcompete with others for nutrients are expected to dominate phytoplankton assemblages. However, HABs often consist of dinoflagellates, which, due to their slower growth and nutrient uptake rates [5], are considered less competitive compared to other phytoplankton groups like diatoms [6]. It has been proposed that the main ecological function of producing “toxic” secondary compounds by some HAB species is to hinder the growth of competing phytoplankton species and/or to decrease cell losses by deterring their grazers [7].
Allelopathy is defined as the direct or indirect interactions facilitated by the release of secondary metabolites (i.e., allelochemicals) by some organisms, including plants, algae, bacteria, and fungi, which exhibit an inhibitory effect on the germination, growth, survival, and reproduction of other organisms [8,9]. Growing observational and experimental evidence indicates that allelopathic interactions play a crucial role in mediating phytoplankton species succession and the proliferation of HAB species. By inhibiting the growth and competitiveness of specific organisms, allelochemicals can create favorable conditions for the proliferation of species that are resistant to those compounds. This selective suppression of competing species through allelopathic interactions has the potential to drive shifts in community structure [10,11,12,13]. Common microalgal allelochemical-induced effects include inhibition of growth and photosynthesis inhibition, as well as the death of the target cells [14,15]. However, the mechanisms triggering microalgae allelopathy are not yet well understood [16]. Examples include the inhibitory impact of metabolites released by the raphidophytes Heterosigma akashiwo Hada ex Hara & Chihara and Chattonella antiqua (Hada) Ono on the growth of the diatom Skeletonema costatum (Greville) Cleve [17], and the oxidative effects induced by Karenia mikimotoi (Miyake & Kominami ex Oda) Gert Hansen & Moestrup on the diatom Thalassiosira pseudonana Hasle & Heimdal, leading to decreased photosynthesis efficiency and nutrient uptake [15]. Blooms of the toxic dinoflagellate Karenia selliformis A.J.Haywood, K.A. Steidinger & L. MacKenzie are among the most harmful recorded HAB events due to their production of biotoxins, ichthyotoxins, and allelochemicals that have adverse effects on other phytoplankton species, trophic food webs, and wildlife populations such as birds, mammals, and fish [18]. In Chile, these blooms often coincide with other species from the family Kareniaceae (i.e., Karenia spp. and Karlodinium spp.), making it challenging to isolate the specific environmental effects of K. selliformis toxins. However, recent characterization of two K. selliformis strains isolated during a large bloom of Kareniaceae species in southern Chilean Patagonia in 2018 revealed intriguing findings [19]. Notably, the absence of gymnodimines (GYMs), typically associated with HAB events of Kareniaceae species in different coastal regions, was observed. Instead, there was a high presence of potentially harmful polyunsaturated fatty acids (PUFAs) and the identification of two compounds with similar mass transition to brevenal, a brevetoxin-related non-toxic compound [20]. In vitro assays demonstrated the high cytotoxicity of the two strains of K. selliformis on the rainbow trout gill RTgill-W1 cell line, suggesting significant potential for fish mortality. However, the potential allelopathic effect of these secondary metabolites remains to be investigated.
In this study, we assessed the allelopathic impact of one of the two Chilean K. selliformis strains isolated during the 2018 Kareniaceae bloom on various phytoplankton species. The bioassays involved exposing the cell-free exudate of K. selliformis (strain CREAN-KS02 isolated from the Aysén region at 43° S) to the target microalgae Phaeodactylum tricornutum Bohlin UCN-B018-1, Thalassiossira pseudonana UCN-B011-2, Rhodomonas salina (Wisłouch) D.R.A. Hill & R. Wetherbee CS-174, and Dunaliella tertiolecta Butcher UTEX-999, as well as two strains of H. akashiwo isolated from Chilean Patagonia (CREAN HA-01) and New Zealand (CCMP302). Effects on cell density, photosynthetic efficiency, production of reactive oxygen species (ROS), and morphology of the exposed cells were evaluated.
2. Materials and Methods
2.1. Microalgae Culture
The K. selliformis (strain CREAN-KS02), isolated from the Chilean Aysen region [19], was obtained from the Collection of Harmful Algae hosted at the Center for Harmful Algae Studies at the Chilean Institute for Fishery Development (CREAN/IFOP; Puerto Montt, Chile) and subsequently maintained at the Laboratory of Marine Biotoxins of the University of Concepción (LBTx-UdeC; Concepción, Chile). The strains of the six microalgal species used as targets were obtained from different sources. The chlorophyte D. tertiolecta (UTEX-999; CCM-UDEC-75) and the cryptophyte R. salina (CS-174/CCM-UDEC-153), isolated from the Oslofjord (Norway) and Milford (CT, USA), respectively, were provided by the Laboratory of Phycology of the University of Concepción (FICOLAB-UdeC). The diatoms P. tricornutum (strain UCN-B018-1) and T. pseudonana (strain UCN-B011-2), with unknown geographical origins, were donated by Dr. Alvarez-Vergara from the Department of Aquaculture at the Northern Catholic University (Antofagasta, Chile). Additionally, two strains of the raphidophyte H. akashiwo, strain CCMP302 from New Zealand and strain CREAN-HA01 from the Chilean Los Lagos region, were utilized. All strains were cultured in L1 medium (supplemented with sodium silicate for diatoms [21], prepared with seawater from the coastal area off central Chile (36°31′ S, 73°08′ W), 0.2 µm filtered, and autoclaved, with salinity previously adjusted to 32 PSU (the salinity at which blooms of K. selliformis CREAN-KS02 have been observed in the field [22]). Cultures were maintained in medium L1 at 17 °C, under irradiance of 100 μmol photon m−2 s−1 and a 16 h:8 h light:dark photoperiod (except for K. selliformis and H. akashiwo strains, which were grown at 140 μmol photon m−2 s−1).
Growth curves of K. selliformis CREAN-KS02 were determined from 500 mL cultures started at 1000 cells mL−1, inoculated from stock cultures in the exponential phase. Daily cell densities were determined using Sedgewick Rafter chambers [23]. Growth curves of the target phytoplankton species were derived from 50 mL cultures initiated at 20,000 cells mL−1 from stock cultures at the exponential phase, with daily cell counts obtained with Neubauer chambers [23]. For both K. selliformis and target phytoplankton, cell growth rates (i.e., divisions per day) were determined during the exponential growth phase [24] according to the following equation:
where µ−1 is the growth rate, n1 is the initial cell concentration, n2 is the final cell concentration, t1 is the initial time, and t2 is the final time.
2.2. Experimental Setup
Exudates from K. selliformis CREAN-KS02 were collected in triplicate from cultures in the exponential growth phase (9000–11,000 cells mL−1) by gentle vacuum filtration (<300 mm Hg) through a 0.47 µm glass fiber filter (Merck, Millipore, Dublin, Ireland). To prevent the potential absorption of secondary metabolites by plastic materials [25], the obtained cell-free exudates were gathered into glass tubes at room temperature and promptly used to avoid degradation of potential allelopathic compounds. Inoculations were carried out in triplicates using 100 mL glass Erlenmeyer flasks, where 20 mL of fresh cell-free exudate was added to cultures of the various target phytoplankton species (final concentration was ~20,000 cells mL−1) and topped with L1 media to reach a final volume of 50 mL. The control cultures of the target phytoplankton species (also in triplicate) received only fresh L1 medium without the addition of exudate.
2.3. Allelopathic Effect on Phytoplankton Growth
The maximal growth rates of the target phytoplankton species were determined based on cell counts obtained at 0 h, 12 h, 24 h, and 48 h, following the methodology previously outlined. Additionally, microphotographs were taken 12 h post-exposure using a Nikon Eclipse Ti E200 epifluorescence microscope (Tokyo, Japan) with a Euromex MOD DC.6000I camera (Duiven, The Netherlands). Additionally, microphotographs were taken 12 h post-exposure using the Eclipse Ti E200 and the Euromex MOD DC.6000I.
2.4. Photosynthetic Efficiency (Fv/Fm)
Samples (2 mL) of the target phytoplankton strains from all replicates were collected on day 0 immediately after inoculation and acclimated to darkness for 15 min before assessing their photochemical performance (every 15 min for 105 min) using pulse amplitude modulation fluorometry (PAM) with a portable AquaPen-C AP-C 100 (Photon Systems Instruments, Drassov, Czech Republic). Then, the photosynthetic efficiency (Fv/Fm; [26]) was estimated using the following formula:
where F0 is the initial fluorescence intensity, Fm is the maximum intensity under saturated light conditions (0.5 s at 1500 mmol photon m−2 s−1), and Fv is 1/4 of (Fm − F0).
2.5. Reactive Oxygen Species (ROS)
The relative levels of reactive oxygen species (ROS) were determined using 2′,7′dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen, San Diego, CA, USA), which, in the presence of ROS, is converted into the highly fluorescent compound 2′,7′dichlorofluorescein (DCF). This method has previously been used to measure intracellular ROS production in dinoflagellates [5,27]. In this process, duplicate samples (190 µL) taken from each replicate at 0 h were inoculated with 10 µL of H2DCFDA and the emitted fluorescence was measured every 30 min for 12 h using a Synergy H1 microplate reader (BioTek, Winooski, VT, USA), with excitation of 485/20 nm and emission at 528/20 nm. DCF fluorescence data were expressed as absolute fluorescent units, and background fluorescence 0 h was subtracted from each measurement.
2.6. Statistical Analysis
Growth, photosynthetic activity, and ROS production in the six phytoplankton strains exposed to K. selliformis CREAN-KS02 cell-free exudate were compared to controls (i.e., cultures not exposed to exudate) by t tests using the t.test function in basic package of R software (https://cran.r-project.org/ (accessed on 20 March 2024)). Prior to analysis, the within-group normality in each experimental treatment and controls was checked using the Shapiro–Wilk test (function shapiro_test in R basic package), while the homogeneity of variance was checked with Levene’s test (leveneTest function in ‘car’ package).
3. Results
3.1. Allelopathic Effect of K. selliformis on Phytoplankton Growth
K. selliformis CREAN-KS02 cultures were typically grown to reach cell densities of 35,000 cells mL−1, with the exponential phase lasting from day 5 to 13 (Figure 1). Cell-free exudate was collected from cells during the mid-exponential phase on day 9. When exposed to K. selliformis cell-free exudate, D. tertiolecta UTEX-999 did not show any negative effects, as its cell concentrations remained consistent with those of the control throughout the experiment (Figure 2A,B). On the other hand, R. salina CS-174 experienced a drastic lethal effect upon exposure to K. selliformis CREAN-KS02, with 100% mortality observed shortly (12 h) after inoculation (Figure 2C,D). Contrasting results were observed for the two tested diatoms: while P. tricornutum UCN-B018-1 did not exhibit any adverse effect (Figure 2E,F), a significant inhibitory effect was observed in T. pseudonana UCN-B011-2 12–24 h post-inoculation (Figure 2G,H). H. akashiwo CREAN-HA01 from Chilean Patagonia displayed considerable susceptibility to K. selliformis cell-free exudate (~50% mortality) after 48 h of exposure (Figure 2I,J). However, this effect was less pronounced compared to the response of H. akashiwo CCPM302 from New Zealand, which exhibited a substantial decrease in cell concentration compared to the control cultures (>90%) 12 h after inoculation (Figure 2K,L). Additionally, abnormal cell morphology was observed by optical microscopy (Figure 3).
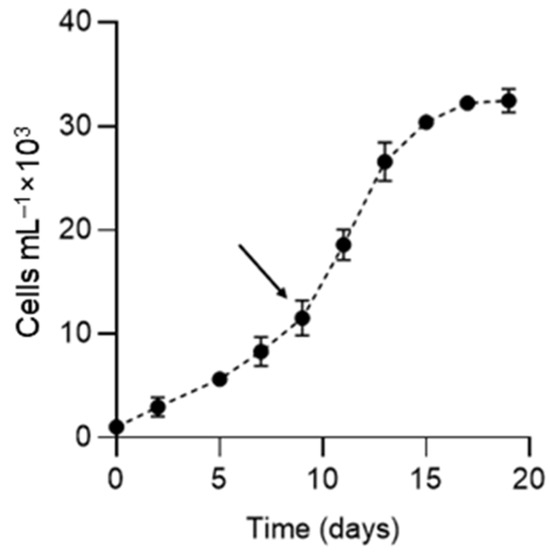
Figure 1.
Growth curve of Karenia selliformis CREAN-KS02. The arrow shows the time at which the cell-free exudate was obtained. Data are presented as means ± SD (n = 3).
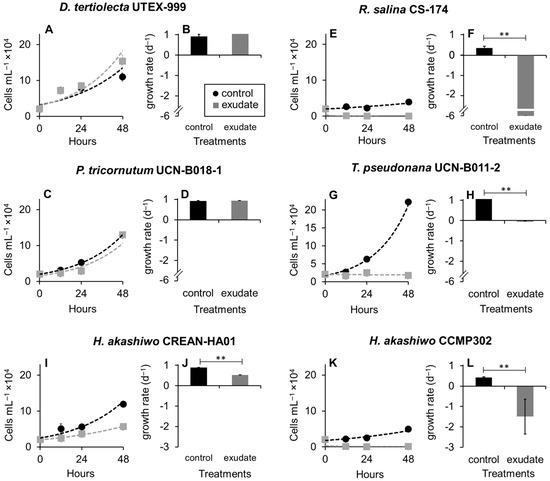
Figure 2.
Effect of Karenia selliformis CREAN-KS02 cell-free exudate on the cell concentration (A,C,E,G,I,L) and growth rates (B,D,F,H,J,L) of the six target phytoplankton species over 48 h of exposure: (A,B) Dunaliella tertiolecta UTEX-999, (C,D) Rhodomonas salina CS-174, (E,F) Phaeodactylum tricornutum UCN-B018-1, (G,H) Thalassiosira pseudonana UCN-B011-2, (I,J) Heterosigma akashiwo CREAN-HA01, (K,L) H. akashiwo CCMP302. The data are shown as means ± SD (n = 3), ** p > 0.01.
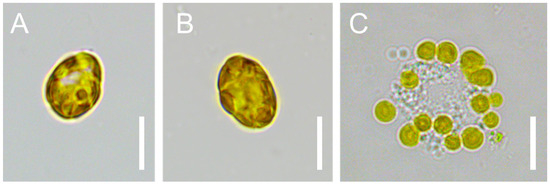
Figure 3.
Cells of Heterosigma akashiwo CCPM302 in control (A,B) and exposed (C) to Karenia sellliformis CREAN-HA01 cell-free exudate. Scale bars = 10 µm.
3.2. Photosynthetic Efficiency (Fv/Fm)
Under control conditions, the quantum yields (Fv/Fm) for the six phytoplankton strains were between 0.6 and 0.7 (Figure 4). The addition of K. selliformis CREAN-KS02 cell-free exudate affected the Fv/Fm values of D. tertiolecta UTEX-999 (Figure 4A), while sustained decrease in this parameter was observed in R. salina CS-174 (Figure 4B), P. tricornutum UCN-B018-1 (Figure 4C), and T. pseudonana UCN-B011-2 (Figure 4D) within the initial 120 min of exposure. Conversely, no significant effect was observed on the Fv/Fm values of H. akashiwo CCMP302 (Figure 4E) and H. akashiwo CREAN-HA01 (Figure 4F) in comparison to control cultures.
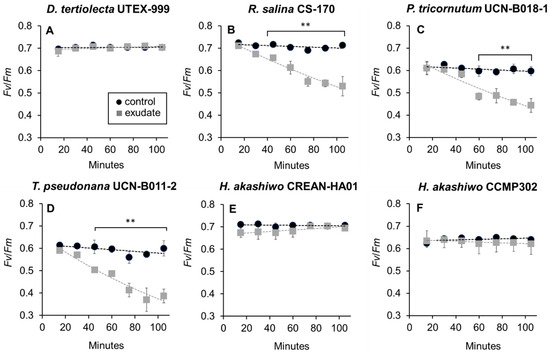
Figure 4.
Photosystem II maximum quantum performance (Fv/Fm) of the phytoplankton species exposed to Karenia selliformis CREAN-KS02 cell-free exudate compared to control cultures: (A) Dunaliella tertiolecta UTEX-999, (B) Rhodomonas salina CS-174, (C) Phaeodactylum tricornutum UCN-B018-1, (D) Thalassiosira pseudonana UCN-B011-2, (E) Heterosigma akashiwo CREAN-HA01, (F) H. akashiwo CCMP302. The data are shown as means ± SD (n = 3), ** p < 0.01.
3.3. ROS Production
The intracellular levels of ROS were assessed using the fluorescent DCF probe after 6 h and 12 h of exposure to K. selliformis CREAN-KS02 cell-free exudates (Figure 5). In general, DCF fluorescence was distributed in the whole area of the microalgal cells under a fluorescence microscopen. Following 6 h and 12 h of exposure, a significant increase in DCF fluorescence was detected in D. tertiolecta UTEX-999 (Figure 5A), T. pseudonana UCN-B011-2 (Figure 5D), and H. akashiwo CREAN-HA01 (Figure 5E). Conversely, there were no significant differences observed in DCF fluorescence levels in R. salina CS-174 (Figure 5B), P. tricornutum UCN-B018-1 (Figure 5C), or H. akashiwo CCMP302 (Figure 5F) compared to the control cultures.
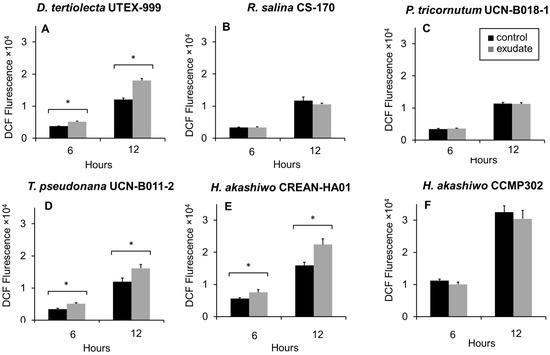
Figure 5.
Fluorescence of 2′,7′dichlorofluorescein (DCF) associated with intracellular reactive oxygen species (ROS) production in the target phytoplankton strains after 6 h and 12 h of exposure to Karenia selliformis CREAN-KS02 cell-free exudate: (A) Dunaliella tertiolecta UTEX-999, (B) Rhodomonas salina CS-174, (C) Phaeodactylum tricornutum UCN-B018-1, (D) Thalassiosira pseudonana UCN-B011-2, (E) Heterosigma akashiwo CREAN-HA01, (F) H. akashiwo CCMP302. The data are shown as means ± SD (n = 3), * p < 0.05.
4. Discussion
Blooms of K. selliformis in Chile have often coincided with other species of the family Kareniaceae [28,29,30]). While the toxin profile of Chilean K. selliformis strains has been only partially characterized [19], their high cytotoxicity has been confirmed through in vitro bioassays based on the RTgill-W1 cell line [30,31]. However, the potential allelopathic effect of Chilean K. selliformis is yet to be evaluated. Plankton allelopathy is typically studied in laboratory-controlled experiments using target microalgal species (e.g., [12,32,33]. For that, it is crucial to carefully select the target organism and employ a range of methods (proxies) to elucidate the inhibition mechanisms fully or partially. In this study, we conducted the first assessment of the effects of exudates from a Chilean K. selliformis strain (CREAN-KS02) on cell growth, photosynthetic efficiency (i.e., Fv/Fm), and intracellular ROS production of six phytoplankton microalgal strains from various taxonomic groups. Our findings demonstrate that the allelopathic effect of Chilean K. selliformis CREAN-KS02 was not only species-dependent but also exhibited variability among strains within the same target phytoplankton species.
In this study, all target phytoplankton species exhibited adverse effects from the cell-free exudates derived from K. selliformis CREAN-KS02, although high variability was observed in both the strength and nature of the allelopathic impact (Figure 3, Figure 4 and Figure 5, Table 1). The allelochemical compounds originating from microalgae encompass a wide array of metabolites with diverse chemical properties (e.g., PUFAS, ROS, and phycotoxins) that can influence phytoplankton species through various mechanisms, including cell membrane damage by lytic activity, inhibition of photosynthesis and enzymatic activity, increased oxidative stress, apoptosis, and effects on cell mobility [34,35,36,37]. Such a diversity in toxic mechanisms is expected to cause diverse effects on target phytoplankton species from different microalgal groups.

Table 1.
Summary of allelopathic effects of K. selliformis CREAN-KS02 cell-free extract on growth, photosynthetic efficiency (Fv/Fm) and ROS production of the six tested target phytoplankton strains. Positive (+) and negative (−) signs indicate significant (p < 0.05) and insignificant differences from controls, respectively.
Cell growth inhibition was observed in R. salina CS-174, T. pseudonana UCN-B011-2, and the two H. akashiwo strains (CREAN-HA01 and CCMP302). Among the impacted strains, T. pseudonana UCN-B011-2 and R. salina CS-174 displayed a significant reduction in growth (Figure 3B and Figure 3C, respectively), with R. salina CS-174 experiencing a more severe decline, reaching zero cell densities just 12 h post-exposure. Additionally, both strains exhibited decreased Fv/Fm values (Figure 4B and Figure 4C, respectively), while T. pseudonana UCN-B011-2 showed increased intracellular ROS production (Figure 5C). This prominent susceptibility to allelopathic compounds aligns with previous studies using strains of these two species as target organisms [14,15,38,39,40], with R. salina strains being frequently used as model species in lithic bioassays for the screening of allelopathic activities in microalgae [11,32].
The growth of the two Heterosigma akashiwo strains was negatively affected by K. selliformis CREAN-KS02 cell-free exudate (Figure 4D,E). However, strain CCMP302 (from New Zealand) exhibited a substantially higher mortality rate of 90% compared to strain CREA-HA01, which displayed a 50% reduction in growth compared to control replicates. While the photosynthetic efficiency (Fv/Fm) remained unaffected in both strains, only CREA-HA01 showed an increased intracellular ROS production upon exposure to K. selliformis CREAN-KS02 cell-free exudate. It is noteworthy that the two tested H. akashiwo strains originated from distinct geographical regions, with the Chilean strain CREAN-HA01, isolated from the same location where K. selliformis blooms have also been reported, demonstrating greater resistance to the allopathic effect of strain K. selliformis CREAN-KS02. This observation may suggest a sympatric adaptation of the Chilean H. akashiwo strain to the allelopathic compounds produced by K. selliformis. This underscores the importance of gaining a deeper understanding of the specific impacts of toxic microalgal exudates on various local phytoplankton species, which could have ecological implications for aquatic ecosystems.
Despite D. tertiolecta UTEX-999 and P. tricornutum UCN-B018-1 not exhibiting growth inhibition when exposed to K. selliformis CREAN-KS02 cell-free exudates (Figure 3A and Figure 3C, respectively), both strains were affected to some extent, with D. tertiolecta experiencing a decrease in photosynthetic efficiency (Figure 4A) and P. tricornutum showing an increase in intracellular ROS production (Figure 5C). These findings differ from previous allelopathy studies using D. tertiolecta as the target species, which reported negative impacts of polyunsaturated aldehydes (PUAs) on cell growth and cell membrane integrity [41]. Similarly, previous research has indicated that okadaic acid (OA) can impair growth and photosynthetic electron transport, leading to increased intracellular ROS production in D. tertiolecta [42]. Moreover, inhibitory effects on cell division and chloroplast damage have been reported in P. tricornutum when co-cultured with Alexandrium tamarense [43], while domoic acid (DA) has been shown to increase intracellular ROS production in this species [44].
Photosynthetic efficiency is a widely used indicator of microalgal cell health in both field populations and cultures [45]. Here, Fv/Fm values in all control replicates fell between 0.65 and 0.7, a range typically associated with photosynthetically healthy cultures [46], indicating that the six microalgal strains were in a photosynthetically efficient state prior to exudate exposure. Allelopathic compounds have the potential to disrupt photosynthetic machinery by affecting processes like chlorophyll synthesis or electron transport chain activity, resulting in decreased efficiency in converting light into chemical energy [47]. These compounds can trigger stress responses in microalgae, leading to reduced photosynthetic rates as cells allocate resources to cope with the stressor instead of investing in growth and reproduction [48]. Decreased photosynthetic efficiency can thus be used as a proxy for assessing the allelopathic effect of compounds on microalgae, with changes in efficiency reflecting cellular stress and physiological impairment [12,33]. For instance, reports have shown that the diatom T. pseudonana experienced allelopathically effects through inhibition of its photosynthetic apparatus when exposed to exudates from the dinoflagellates Alexandrium pacificum [14] and Karenia brevis [15]. This highlights that incorporating methods like pulse amplitude fluorometry (PAM) in studies of allelopathic effects is a valuable and easily implementable methodological strategy to include in this type of ecological toxicity study [12].
Allelopathic compounds can trigger oxidative stress in microalgae by disrupting the cellular redox balance, resulting in an increased production of intracellular ROS (e.g., O2−, H2O2) as a defense mechanism [48,49]. This spike in intracellular ROS levels can subsequently trigger apoptosis pathways [50,51] and/or damage the photosynthetic apparatus [47,48]. The continued increase in ROS production observed up to 12 h of exposure suggests a lasting impact of allelopathic compounds on the target species, a phenomenon reported for other microalgal species exposed to HAB-producing ichthyotoxic microalgae, such as species of the raphidophytes genera Heterosigma and Chatonella [52,53,54,55].
The absence of increased intracellular ROS production in R. salina CS-174 and the two H. akashiwo strains CREA-HA01 and CCMP302, despite exhibiting high cell mortality when exposed to K. selliformis cell-free exudate, indicates a complex interplay of factors influencing cellular responses to allelopathic compounds, with several explanations accounting for this observation. One possibility is that exposure to allelochemicals may trigger rapid extensive cell death, resulting in the cessation of metabolic activity or cell lysis before a significant accumulation of ROS occurs [56]. Alternatively, the primary damage in R. salina could be lytic, as described by other researchers [32].
While it is typically expected that decreased photosynthetic efficiency would be correlated with increased ROS production [5], the findings from this study revealed some unique patterns. Among the tested strains, only T. pseudonana UCN-B011-2 displayed both decreased Fv/Fm and increased intracellular ROS production (Figure 3D and Figure 4D; Table 1). The elevated intracellular ROS production observed in P. tricornutum UNC-B018-1 suggests that this strain might have experienced oxidative stress early in the experiment, resulting in ROS generation either as a defense mechanism or due to cellular damage induced by allelochemicals. In contrast, D. tertiolecta UTEX-999 exhibited decreased Fv/Fm levels without a concomitant increase in intracellular ROS production. This discrepancy could be explained by the timing of measurements, since Fv/Fm was measured at the onset of the experiment (0 h), likely capturing the immediate impact of allelopathic compounds on photosynthetic processes. Meanwhile, intracellular ROS production was evaluated at later times (6 h and 12 h), allowing for the detection of ROS-related responses that may have emerged after the initial assessment of photosynthetic efficiency.
The findings of this study indicate that Chilean K. selliformis populations produce allelochemicals, with the allelopathic effect being specific to each target species. Interestingly, the two H. akashiwo strains exhibited contrasting responses, with the strain isolated from the same geographic region as K. selliformis demonstrating greater resistance to its allelopathic effect. The observed discrepancies, such as changes in Fv/Fm or intracellular ROS production without concomitant alterations in cell growth, underscore the intricate nature of allelopathic interactions and the varied cellular responses to allelochemicals. These differences could signify varying sensitivities to specific allelochemicals or variability in the activation of defense mechanisms across microalgal species. Moreover, the timing of allelochemical exposure and cellular responses may play a role in the observed variability, emphasizing the need for further studies to unravel the mechanisms driving species-specific responses to allelopathy in microalgae.
5. Conclusions
Our results underscore the significance of ongoing research on K. selliformis toxicity and its ecological effects on marine ecosystems in Chile, particularly in understanding the impact of its allelopathic effects on phytoplankton ecology and harmful algal bloom dynamics in the Chilean fjords. Documented allelopathic interactions shed light on how allelochemicals can affect various microalgal species, influencing factors such as cell growth, photosynthetic efficiency, and intracellular ROS production. Future investigations should delve deeper into the specific allelochemical mechanisms at play and their effects on different target species. Furthermore, exploring the adaptability of local microalgal populations, such as the Chilean H. akashiwo strain, to allelopathic compounds produced by K. selliformis could offer insights into local ecological dynamics and potential adaptations. By further exploring these allelopathic effects, researchers can enhance their understanding of how these interactions shape phytoplankton communities and potentially impact marine ecosystems in the Chilean fjords.
Author Contributions
Conceptualization, A.A.-V. and V.A.-A.; methodology, A.A.-V., J.J.G.-R., V.A.-A., S.J.-T. and A.R.-L.; formal analysis, A.A.-V. and C.A.-d.-S.; investigation, A.A.-V., V.A.-A., J.J.G.-R. and S.J.-T.; data curation, C.A.-d.-S.; writing—original draft, V.A.-A., S.J.-T., A.A.-V., C.A.-d.-S. and J.J.G.-R.; writing—review and editing, C.A.-d.-S., A.A.-V., J.J.G.-R., J.I.M. and V.A.-A.; supervision, A.A.-V.; funding acquisition, A.A.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project FONDECYT REGULAR 1200845 (ANID, Chile) and COPAS-COASTAL ANID FB210021 (Chile).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to extend their warm gratitude to their colleagues from the UdeC Biotoxin Laboratory (LBTx-UdeC), the Center for Harmful Algae Studies at the Chilean Institute for Fishery Development (CREAN/IFOP), and the Phycology Laboratory of the University of Concepción (FICOLAB-UdeC). Special thanks are due to Gonzalo Alvarez-Vergara from the Department of Aquaculture at the Northern Catholic University for generously providing the diatom strains used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine Harmful Algal Blooms, Human Health and Wellbeing: Challenges and Opportunities in the 21st Century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in Understanding Harmful Algal Blooms: Paradigm Shifts and New Technologies for Research, Monitoring, and Management. Ann. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of Harmful Algal Blooms on the Aquaculture Industry: Chile as a Case Study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Montes, R.M.; Rojas, X.; Artacho, P.; Tello, A.; Quiñones, R.A. Quantifying Harmful Algal Bloom Thresholds for Farmed Salmon in Southern Chile. Harmful Algae 2018, 77, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Gallardo Rodríguez, J.J.; Sánchez Mirón, A.; Cerón García, M.D.C.; Belarbi, E.H.; García Camacho, F.; Chisti, Y.; Molina Grima, E. Macronutrients Requirements of the Dinoflagellate Protoceratium reticulatum. Harmful Algae 2009, 8, 239–246. [Google Scholar] [CrossRef]
- Hakanen, P.; Suikkanen, S.; Kremp, A. Allelopathic Activity of the Toxic Dinoflagellate Alexandrium ostenfeldii: Intra-Population Variability and Response of Co-Occurring Dinoflagellates. Harmful Algae 2014, 39, 287–294. [Google Scholar] [CrossRef]
- Granéli, E.; Weberg, M.; Salomon, P.S. Harmful Algal Blooms of Allelopathic Microalgal Species: The Role of Eutrophication. Harmful Algae 2008, 8, 94–102. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the Role of Allelochemicals in Plant Defence; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 82. [Google Scholar] [CrossRef]
- Wu, N.; Tong, M.; Gou, S.; Zeng, W.; Xu, Z.; Jiang, T. Hemolytic Activity in Relation to the Photosynthetic System in Chattonella marina and Chattonella ovata. Mar. Drugs 2021, 19, 336. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Zumaya-Higuera, M.G.; López-Cortés, D.J.; Leyva-Valencia, I.; Quijano-Scheggia, S.I.; Hernández-Guerrero, C.J. Allelopathic Effects of Margalefidinium polykrikoides and Gymnodinium impudicum in the Growth of Gymnodinium catenatum. Harmful Algae 2020, 96, 101846. [Google Scholar] [CrossRef] [PubMed]
- Fistarol, G.O.; Legrand, C.; Selander, E.; Hummert, C.; Stolte, W.; Granéli, E. Allelopathy in Alexandrium spp.: Effect on a Natural Plankton Community and on Algal Monocultures. Aquat. Microb. Ecol. 2004, 35, 45–56. [Google Scholar] [CrossRef]
- Long, M.; Tallec, K.; Soudant, P.; Lambert, C.; Le Grand, F.; Sarthou, G.; Jolley, D.; Hégaret, H. A Rapid Quantitative Fluorescence-Based Bioassay to Study Allelochemical Interactions from Alexandrium Minutum. Environ. Pollut. 2018, 242, 1598–1605. [Google Scholar] [CrossRef]
- Wang, R.; Wu, J.; Zhou, S.; Cao, R.; Chan, L.L. A Preliminary Study on the Allelopathy and Toxicity of the Dinoflagellate Karlodinium veneficum. Mar. Pollut. Bull. 2020, 158, 111400. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.T.; Xu, R.X.; Gao, Y.; Li, H.Y.; Liu, J.S.; Yang, W.D. Allelopathy of Alexandrium pacificum on Thalassiosira pseudonana in Laboratory Cultures. Ecotoxicol. Env. Saf. 2021, 215, 112123. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, X.; Ye, M.; Li, H.; Liu, J.; Yang, W. Allelopathy and Underlying Mechanism of Karenia mikimotoi on the Diatom Thalassiosira pseudonana under Laboratory Condition. Algal Res. 2021, 54, 102229. [Google Scholar] [CrossRef]
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A Review of Allelopathy on Microalgae. Microbiology 2019, 165, 587–592. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Uchida, T.; Yuichi, K. Effect of Culture Filtrate of Raphydophytes Heterosigma akashiwo and Chatonella antiqua on the Growth of Diatom Skeletonema costatum. Bull. Fish. Environ. Inland Sea 2000, 2, 57–66. [Google Scholar]
- Orlova, T.Y.; Aleksanin, A.I.; Lepskaya, E.V.; Efimova, K.V.; Selina, M.S.; Morozova, T.V.; Stonik, I.V.; Kachur, V.A.; Karpenko, A.A.; Vinnikov, K.A.; et al. A Massive Bloom of Karenia Species (Dinophyceae) off the Kamchatka Coast, Russia, in the Fall of 2020. Harmful Algae 2022, 120, 102337. [Google Scholar] [CrossRef]
- Mardones, J.I.; Norambuena, L.; Paredes, J.; Fuenzalida, G.; Dorantes-Aranda, J.J.; Chang, K.J.L.; Guzmán, L.; Krock, B.; Hallegraeff, G. Unraveling the Karenia selliformis Complex with the Description of a Non-Gymnodimine Producing Patagonian Phylotype. Harmful Algae 2020, 98, 101892. [Google Scholar] [CrossRef] [PubMed]
- Bourdelais, A.J.; Campbell, S.; Jacocks, H.; Naar, J.; Wright, J.L.C.; Carsi, J.; Baden, D.G. Brevenal Is a Natural Inhibitor of Brevetoxin Action in Sodium Channel Receptor Binding Assays. Cell. Mol. Neurobiol. 2004, 24, 553–563. [Google Scholar] [CrossRef]
- Andersen, R.A. (Ed.) Algal Culturing Techniques, 1st ed.; Elsevier/Academic Press: Burlington, MA, USA, 2005; pp. 21–34. [Google Scholar]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the Environmental Processes Responsible for the World’s Largest Farmed Fish-Killing Harmful Algal Bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef]
- Karlson, B.; Cusack, C.; Bresnan, E. Introduction to Methods for Quantitative Phytoplankton Analysis. In IOC Manuals and Guides No. 55; UNESCO: Paris, France, 2010; pp. 5–12. [Google Scholar] [CrossRef]
- Guillard, R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: London, UK, 1973. [Google Scholar]
- Tillmann, U.; Alpermann, T.; John, U.; Cembella, A. Allelochemical Interactions and Short-Term Effects of the Dinoflagellate Alexandrium on Selected Photoautotrophic and Heterotrophic Protists. Harmful Algae 2008, 7, 52–64. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Astuya, A.; Rivera, A.; Vega-Drake, K.; Aburto, C.; Cruzat, F.; Ulloa, V.; Caprile, T.; Gallardo-Rodríguez, J.J. Study of the Ichthyotoxic Microalga Heterosigma akashiwo by Transcriptional Activation of Sublethal Marker Hsp70b in Transwell Co-Culture Assays. PLoS ONE 2018, 13, e0201438. [Google Scholar] [CrossRef] [PubMed]
- Clement, A.; Seguel, M.; Arzul, G.; Guzman, L.; Alarcón, C. Widespread Outbreak of a Haemolytic, Ichthyotoxic Gymnodinium sp. in Chile. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J.S., Lewis, R.J., Eds.; International Oceanographic Commission: Paris, France, 2001; pp. 66–69. [Google Scholar]
- Uribe, J.C.; Ruiz, M. Gymnodinium Brown Tide in the Magellanic Fjords, Southern Chile. Mar. Pollut. Bull. 2001, 36, 155–164. [Google Scholar] [CrossRef]
- Mardones, J.I. Screening of Chilean Fish-Killing Microalgae Using a Gill Cell-Based Assay. Lat. Am. J. Aquat. Res. 2020, 48, 329–335. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Chen, Z.M.; Li, D.W.; Li, A.F.; Ji, Y.; Li, H.Y.; Yang, W.D. Toxicity and Potential Underlying Mechanism of Karenia selliformis to the Fish Oryzias melastigma. Aquat. Toxicol. 2023, 262, 106643. [Google Scholar] [CrossRef]
- Ma, H.; Krock, B.; Tillmann, U.; Cembella, A. Preliminary Characterization of Extracellular Allelochemicals of the Toxic Marine Dinoflagellate Alexandrium tamarense Using a Rhodomonas salina Bioassay. Mar. Drugs 2009, 7, 497–522. [Google Scholar] [CrossRef]
- Long, M.; Peltekis, A.; González-Fernández, C.; Hégaret, H.; Bailleul, B. Allelochemicals of Alexandrium minutum: Kinetics of Membrane Disruption and Photosynthesis Inhibition in a Co-Occurring Diatom. Harmful Algae 2021, 103, 101997. [Google Scholar] [CrossRef]
- Legrand, C.; Rengefors, K.; Fistarol, G.O.; Granéli, E. Allelopathy in Phytoplankton—Biochemical, Ecological and Evolutionary Aspects. Phycologia 2003, 42, 406–419. [Google Scholar] [CrossRef]
- Prince, E.K.; Myers, T.L.; Kubanek, J. Effects of Harmful Algal Blooms on Competitors: Allelopathic Mechanisms of The Red Tide Dinoflagellate “Karenia brevis”. Limnol. Ocean. 2008, 53, 531–541. [Google Scholar] [CrossRef]
- Fernández-Herrera, L.J.; Band-Schmidt, C.J.; Zenteno-Savín, T.; Leyva-Valencia, I.; Hernández-Guerrero, C.J.; Muñoz-Ochoa, M. Cell Death and Metabolic Stress in Gymnodinium catenatum Induced by Allelopathy. Toxins 2021, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Astuya-Villalón, A.; López, B.; Avello, V.; Rivera, A.; Aballay-González, A.; Ulloa, V.; Aguilera-Belmonte, A.; Gallardo-Rodriguez, J.J. In Vitro Evaluation of the Potential Allelopathic and Ichthyotoxic Effect of the Raphidophyte Heterosigma akashiwo and the Dinoflagellate Alexandrium catenella. Mar. Environ. Res. 2023, 183, 105800. [Google Scholar] [CrossRef] [PubMed]
- Uronen, P.; Lehtinen, S.; Legrand, C.; Kuuppo, P.; Tamminen, T. Haemolytic Activity and Allelopathy of the Haptophyte Prymnesium parvum in Nutrient-Limited and Balanced Growth Conditions. Mar. Ecol. Prog. Ser. 2005, 299, 137–148. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, B.; Tang, Y.; Xu, N. Allelopathic Effects of Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton: The Significance of Nutritional Ecology. J. Oceanol. Limnol. 2021, 39, 903–917. [Google Scholar] [CrossRef]
- Poulin, R.X.; Hogan, S.; Poulson-Ellestad, K.L.; Brown, E.; Fernández, F.M.; Kubanek, J. Karenia brevis Allelopathy Compromises the Lipidome, Membrane Integrity, and Photosynthesis of Competitors. Sci. Rep. 2018, 8, 9572. [Google Scholar] [CrossRef]
- Ribalet, F.; Berges, J.A.; Ianora, A.; Casotti, R. Growth Inhibition of Cultured Marine Phytoplankton by Toxic Algal-Derived Polyunsaturated Aldehydes. Aquat. Toxicol. 2007, 85, 219–227. [Google Scholar] [CrossRef]
- Perreault, F.; Matias, M.S.; Oukarroum, A.; Matias, W.G.; Popovic, R. Okadaic Acid Inhibits Cell Growth and Photosynthetic Electron Transport in the Alga Dunaliella tertiolecta. Sci. Total Environ. 2012, 414, 198–204. [Google Scholar] [CrossRef]
- Zheng, J.W.; Li, D.W.; Lu, Y.; Chen, J.; Liang, J.J.; Zhang, L.; Yang, W.D.; Liu, J.S.; Lu, S.H.; Li, H.Y. Molecular Exploration of Algal Interaction between the Diatom Phaeodactylum tricornutum and the Dinoflagellate Alexandrium tamarense. Algal Res. 2016, 17, 132–141. [Google Scholar] [CrossRef]
- Cabrera, J.; Puntarulo, S.; González, P.M. Domoic Acid Oxidative Effects on the Microalgae Phaeodactylum tricornutum. Life 2023, 13, 676. [Google Scholar] [CrossRef]
- Bermejo, E.; Filali, R.; Taidi, B. Microalgae Culture Quality Indicators: A Review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Contreras-Gómez, A.; Molina-Grima, E. An Optimisation Approach for Culturing Shear-Sensitive Dinoflagellate Microalgae in Bench-Scale Bubble Column Photobioreactors. Bioresour. Technol. 2015, 197, 375–382. [Google Scholar] [CrossRef]
- Qian, H.; Xu, X.; Chen, W.; Jiang, H.; Jin, Y.; Liu, W.; Fu, Z. Allelochemical Stress Causes Oxidative Damage and Inhibition of Photosynthesis in Chlorella vulgaris. Chemosphere 2009, 75, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zhu, Q.Q.; Chen, C.Y.; Xie, B.; Tang, B.G.; Fan, M.H.; Hu, Q.-J.; Liao, Z.; Yan, X.-J. The Growth Inhibitory Effects and Non-Targeted Metabolomic Profiling of Microcystis aeruginosa Treated by Scenedesmus sp. Chemosphere 2023, 338, 139446. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Ross, T.; Pyecroft, S.; Hallegraeff, G. Superoxide Production by Marine Microalgae: II. Towards Understanding Ecological Consequences and Possible Functions. Mar. Biol. 2005, 147, 541–549. [Google Scholar] [CrossRef]
- Jiménez, C.; Capasso, J.M.; Edelstein, C.L.; Rivard, C.J.; Lucia, S.; Breusegem, S.; Berl, T.; Segovia, M. Different Ways to Die: Cell Death Modes of the Unicellular Chlorophyte Dunaliella viridis Exposed to Various Environmental Stresses Are Mediated by the Caspase-like Activity DEVDase. J. Exp. Bot. 2009, 60, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, S.; Hu, J.; Effiong, K.; Ge, Z.; Tang, T.; Xiao, X. Programmed Cell Death Process in Freshwater Microcystis aeruginosa and Marine Phaeocystis globosa Induced by a Plant Derived Allelochemical. Sci. Total Environ. 2022, 838, 156055. [Google Scholar] [CrossRef]
- Twiner, M.J.; Trick, C.G. Possible Physiological Mechanisms for Production of Hydrogen Peroxide by the Ichthyotoxic Flagellate Heterosigma akashiwo. J. Plankton Res. 2000, 22, 1961–1975. [Google Scholar] [CrossRef]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G.M. Fish Gill Damage by the Dinoflagellate Alexandrium catenella from Chilean Fjords: Synergistic Action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Cho, K.; Ueno, M.; Liang, Y.; Kim, D.; Oda, T. Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms. Antioxidants 2022, 11, 206. [Google Scholar] [CrossRef]
- Kim, D.; Watanabe, M.; Nakayasu, Y.; Kohata, K. Production of Superoxide Anion and Hydrogen Peroxide Associated with Cell Growth of Chattonella antiqua. Aquat. Microb. Ecol. 2004, 35, 57–64. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, N.; Xu, H.; Tan, L.; Wang, J. Allelochemicals of Alexandrium tamarense and Its Algicidal Mechanism for Prorocentrum donghaiense and Heterosigma akashiwo. Chemosphere 2024, 357, 141953. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).