Effect of Opaganib on Supplemental Oxygen and Mortality in Patients with Severe SARS-CoV-2 Based upon FIO2 Requirements
Abstract
1. Introduction
2. Methodology
2.1. Study Settings and Trial Design
2.2. Randomization and Intervention
- Whether the patients met three or more high-risk parameters for COVID-19 outcomes at baseline. This was determined by the following eight parameters: age at screening ≥ 60 years; male; HbA1c at screening ≥ 6.5 or on active treatment with insulin or oral hypoglycemics; hypoxemia without commensurate increased work of breathing; known underlying chronic lung disease; known cardiovascular disease or hypertension; BMI ≥ 28.0 kg/m2; known renal disease.
- Whether SoC treatment has established efficacy (yes or no). A standard of care (SoC) treatment with established efficacy was defined by Emergency Use Authorization or full approval granted by either the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), or the Medicines and Healthcare Products Regulatory Agency (MHRA). The proven effective therapies for the purpose of this study were adjusted as new data emerged and were documented and shared with study personnel regularly. The following treatments were included in the list of proven effective therapies at the time the study was being conducted: dexamethasone, remdesivir, COVID-19 convalescent plasma, and baricitinib in combination with remdesivir.
2.3. Primary, Secondary, and Exploratory Outcomes
2.4. Sample Size Calculation
2.5. Statistical Analysis
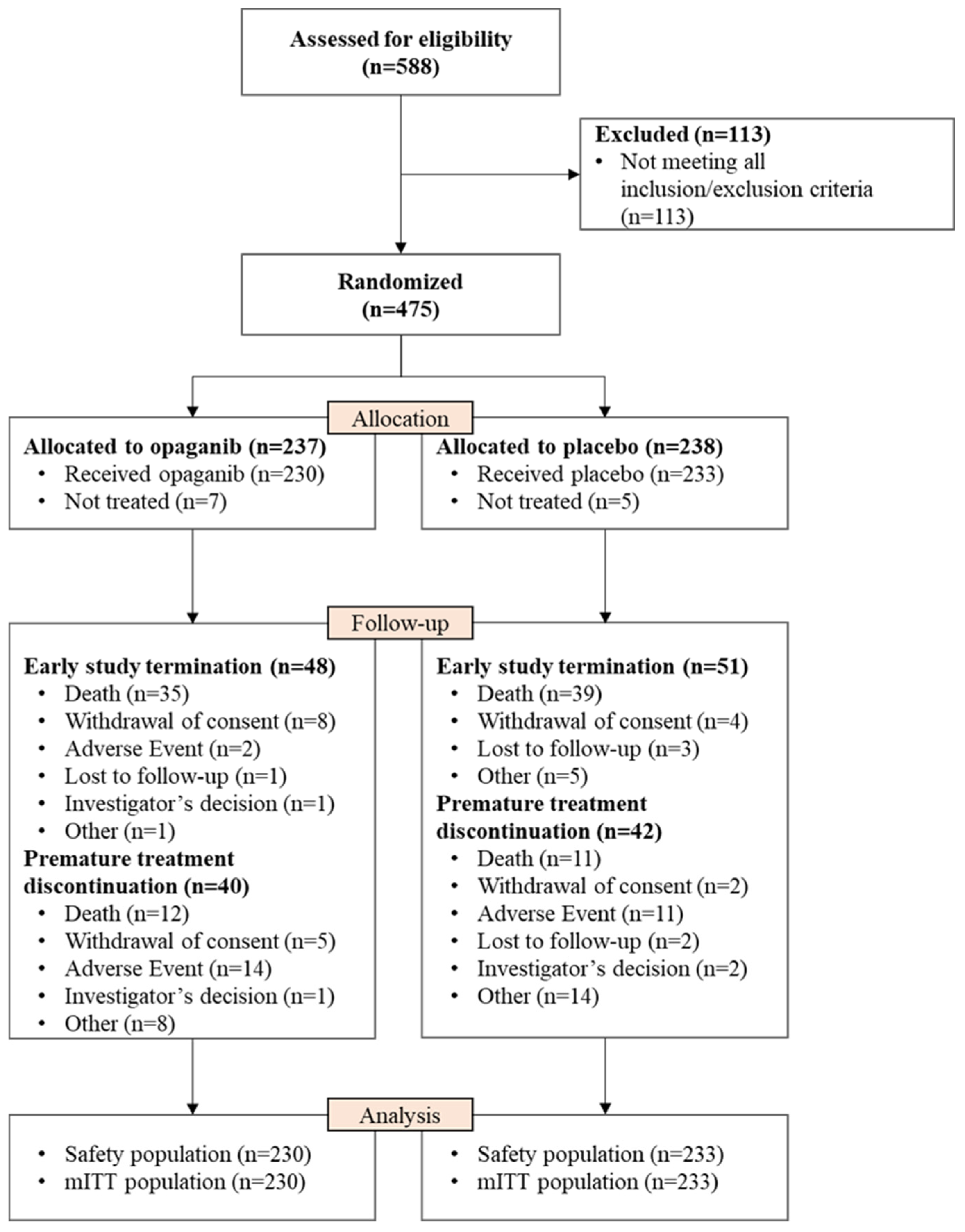
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Primary and Secondary Efficacy Outcomes
3.3. Post-Hoc Efficacy Analysis
3.4. Safety Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO COVID-19 Dashboard; World Health Organization: Geneva, Switzerland, 2020; Updated Daily. Available online: https://covid19.who.int/ (accessed on 15 June 2023).
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Published 26 November 2021. Available online: www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 20 July 2022).
- U.S. Food and Drug Administration. Coronavirus (COVID-19): Drugs. Date Last Updated: 29 December 2023. Available online: www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 5 May 2024).
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for COVID-19—Interim Who Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.P.; Tritsch, S.R.; Kota, K.; Chiang, C.Y.; Dong, L.; Kenny, T.; Brueggemann, E.E.; Ward, M.D.; Cazares, L.H.; Bavari, S. Sphingosine kinase 2 is a chikungunya virus host factor co-localized with the viral replication complex. Emerg. Microbes Infect. 2015, 4, e61. [Google Scholar] [CrossRef] [PubMed]
- Vitner, E.B.; Avraham, R.; Politi, B.; Melamed, S.; Israely, T. Elevation in sphingolipid upon SARS-CoV-2 infection: Possible implications for COVID-19 pathology. Life Sci. Alliance. 2021, 5, e202101168. [Google Scholar] [CrossRef]
- Vitner, E.B.; Achdout, H.; Avraham, R.; Politi, B.; Cherry, L.; Tamir, H.; Yahalom-Ronen, Y.; Paran, N.; Melamed, S.; Erez, N.; et al. Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus. J. Biol. Chem. 2021, 296, 100470. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Maines, L.W.; Keller, S.N.; Katz Ben-Yair, V.; Fathi, R.; Plasse, T.F.; Levitt, M.L. Recent progress in the development of opaganib for the treatment of COVID-19. Drug Des. Devel. Ther. 2022, 16, 2199–2211. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Berdyshev, E.V.; Bronova, I.A.; Liu, Y.; Tiruppathi, C.; Komarova, Y.; Benevolenskaya, E.V.; Suryadevara, V.; Ha, A.W.; Harijith, A.; et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax 2019, 74, 579–591. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Skolnick, A.W.; Rafiq, A.M.; Beegle, S.H.; Suszanski, J.; Koehne, G.; Barnett-Griness, O.; Bibliowicz, A.; Fathi, R.; Anderson, P.; et al. Opaganib in Coronavirus Disease 2019 pneumonia: Results of a Randomized, Rlacebo-Controlled Phase 2a Trial. Open Forum. Infect. Dis. 2022, 9, ofac232. [Google Scholar] [CrossRef] [PubMed]
- RedHill Biopharma Limited. Opaganib, a Sphingosine Kinase-2 (SK2) Inhibitor in COVID-19 Pneumonia. clinicaltrials.gov; 2021. Date last updated: 20 July 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04467840 (accessed on 14 June 2023).
- Assouline, O.; Ben-Chetrit, E.; Helviz, Y.; Kurd, R.; Leone, M.; Einav, S. Experimental and Compassionate Drug Use During the First Wave of the COVID-19 Pandemic: A Retrospective Single-Center Study. Adv. Ther. 2021, 38, 5165–5177. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Information on COVID-19 Treatment, Prevention and Research. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 15 June 2023).
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Diaz, G.; Gottlieb, R.L.; Lobo, S.M.; Robinson, P.; Hunter, B.D.; Cavalcante, A.W.; Overcash, J.S.; Hanania, N.A.; Skarbnik, A.; et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial. Intensive. Care Med. 2021, 47, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. Date Last Updated: 22 December 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 (accessed on 18 April 2022).
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef]

| No. (%) | |||
|---|---|---|---|
| Parameter | Opaganib (N = 230) | Placebo (N = 233) | Overall (N = 463) |
| Age (years) | 55.7 (28–80) | 57.3 (26–80) | 56.5 (26–80) |
| Sex | |||
| Male | 145 (63.0) | 158 (67.8) | 303 (65.4) |
| Female | 85 (37.0) | 75 (32.2) | 160 (34.6) |
| Ethnicity | |||
| Hispanic or Latino | 68 (29.6) | 65 (27.9) | 133 (28.7) |
| Not Hispanic or Latino | 158 (68.7) | 162 (69.5) | 320 (69.1) |
| Unknown | 4 (1.7) | 6 (2.6) | 10 (2.2) |
| Race | |||
| White | 192 (83.5) | 198 (85.0) | 390 (84.2) |
| American Indian or Alaska Native | 5 (2.2) | 4 (1.7) | 9 (1.9) |
| Asian | 1 (0.4) | 0 | 1 (0.2) |
| Black or African American | 10 (4.3) | 10. (4.3) | 20 (4.3) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 |
| White/Black or African American | 1 (0.4) | 2 (0.9) | 3 (0.6) |
| White/American Indian or Alaska Native | 0 | 1(0.4) | 1 (0.2) |
| Other | 21 (9.1) | 18 (7.7) | 38 (8.4 |
| Smoking status | |||
| Never | 169 (73.5) | 169 (72.5) | 338 (73.0) |
| Former | 35 (15.2) | 32 (13.7) | 67 (14.5) |
| Current | 11 (4.8) | 15 (6.4) | 26 (5.6) |
| Missing | 15 (6.5) | 17 (7.3) | 32 (6.9) |
| HbA1c at screening | |||
| <6.5 and not on active treatment with insulin or oral hypoglycemics | 147 (63.9) | 153 (65.7) | 300 (64.8) |
| ≥6.5 or on active treatment with insulin or oral hypoglycemics | 83 (36.1) | 79 (33.9) | 162 (35.0) |
| Missing | 0 | 1 (0.4) | 1 (0.2) |
| BMI at baseline (Kg/m2), n = | 223 | 228 | 451 |
| Mean (SD) | 31.02 (5.676) | 30.47 (5.158) | 30.74 (5.421) |
| Median (min, max) | 30.39 (20.1, 54.8) | 29.73 (19.6, 49.4) | 29.99 (19.6, 54.8) |
| Supplemental oxygen at baseline | |||
| Yes | 229 (99.6) | 233 (100) | 462 (99.8) |
| No | 1 (0.4) | 0 | 1 (0.2) |
| Oxygen type (for oxygen requirement—yes) * | |||
| Intubation/mechanical ventilation | 1 (0.4) | 2 (0.9) | 3 (0.6) |
| Low flow nasal cannulas | 3 (1.3) | 1 (0.4) | 4 (0.9) |
| Non-invasive ventilation/HFNC/mask with reservoir/mask without reservoir | 225 (98.3) | 230 (98.7) | 455 (98.5) |
| type of non-invasive ventilation/HFNC/mask with reservoir/mask without reservoir | |||
| BIPAP | 5 (2.2) | 7 (3.0) | 12 (2.6) |
| CPAP | 64 (27.9) | 61 (26.2) | 125 (27.1) |
| HFNC | 82 (35.8) | 88 (37.8) | 170 (36.8) |
| Face mask (without reservoir) | 9 (3.9) | 11 (4.7) | 20 (4.3) |
| Non-rebreather (reservoir) mask = | 65 (28.4) | 63 (27.0) | 128 (27.7) |
| Not on non-invasive ventilation/HFNC/mask with reservoir/mask without reservoir | 4 (1.7) | 3 (1.3) | 7 (1.5) |
| Oxygen flow at baseline, L/min, n = , mean (SD) † | 222 24.6 (19.59) | 219 24.9 (19.83) | 441 24.8 (19.69) |
| Oxygen in the gas mix (%), n = , mean (SD) | 222 65.5 (18.31) | 222 64.1 (19.00) | 444 64.8 (18.65) |
| Temperature at baseline (Celsius), n = mean (SD) | 226 36.79 (0.641) | 232 36.78 (0.586) | 458 36.78 (0.613) |
| Respiratory rate at baseline (breaths/minute), n = , mean (SD) | 198 21.9 (4.86) | 205 21.7 (4.78) | 403 21.8 (4.81) |
| Oxygen saturation at baseline (%), n = , mean (SD) ‡ | 229 93.2 (5.03) | 233 93.0 (5.04) | 462 93.1 (5.03) |
| Systolic blood pressure at baseline (mmHg), n = , mean (SD) | 229 127.0 (15.04) | 233 127.9 (15.12) | 462 127.5 (15.07) |
| Diastolic blood pressure at baseline (mmHg), n = , mean (SD) | 229 75.7 (10.32) | 233 75.9 (10.51) | 462 75.8 (10.40) |
| Pulse rate at baseline (beats/min), n = , mean (SD) | 229 78.6 (12.70) | 233 78.6 (13.08) | 462 78.6 (12.88) |
| Efficacious SoC concomitant medication § | |||
| Glucocorticoids | 217 (94.3) | 219 (94.0) | |
| Remdesivir | 43 (18.7) | 37 (15.9) | |
| Hyperimmune plasma COVID-19 | 3 (1.3) | 5 (2.1) | |
| COVID-19 vaccine | 0 | 1 (0.4) | |
| No. (%) | |||
|---|---|---|---|
| Parameter | Opaganib (N = 230) | Placebo (N = 233) | Opaganib vs. Placebo |
| Patients no longer receiving supplemental Oxygen (“Success” *), n (%) | 139 (60.43) | 132 (56.65) | |
| Difference † | +3.78 | ||
| 95% CI for the difference † | −5.19, 12.75 | ||
| p-value † | 0.391 | ||
| “Failure” ‡ | 91 (39.57) | 101 (43.35) | |
| Due to the need for supplemental oxygen at day 14, n (%) | 70 (30.43) | 75 (32.19) | |
| Due to death up to day 14, n (%) | 18 (7.83) | 21 (9.01) | |
| Due to lost to follow-up by day 14, n (%) | 3 (1.30) | 4 (1.72) | |
| Due to missing status at day 42 with prior success, n (%) | 0 | 1 (0.43) | |
| Parameter | Opaganib (N = 218) | Placebo (N = 219) |
|---|---|---|
| The time to two consecutive negative swabs for SARS-CoV-2 by RT-PCR, at least 24 h apart, up to 14 days | ||
| Number of events | 93 (42.7) | 79 (36.1) |
| Number of censored observations | 125 (57.3) | 140 (63.9) |
| Reasons for censoring | ||
| No post-baseline results available | 13 | 8 |
| Less than two results and discharged by day 5 | 7 | 7 |
| Less than two results and not discharged by day 5 | 21 | 24 |
| At least two results that are not two sequential negatives | 84 | 101 |
| Log-rank test statistic * | 12.58 | |
| p-value * | 0.043 | |
| Hazard ratio (HR) and 95% CI † | 1.34 (0.99–1.82) | |
| Kaplan–Meier median estimate and 95% CI ‡ | 10.00 (8.00–NA) | NA (10.00–NA) |
| Cumulative incidence {%} ‡ | ||
| Day 7 | 42.09 | 33.25 |
| 95% CI | 35.19, 49.75 | 26.89, 40.65 |
| Day 14 | 54.57 | 47.69 |
| 95% CI | 46.87, 62.63 | 39.70, 56.41 |
| No. (%) | |||
|---|---|---|---|
| Parameter | Opaganib | Placebo | Outcome |
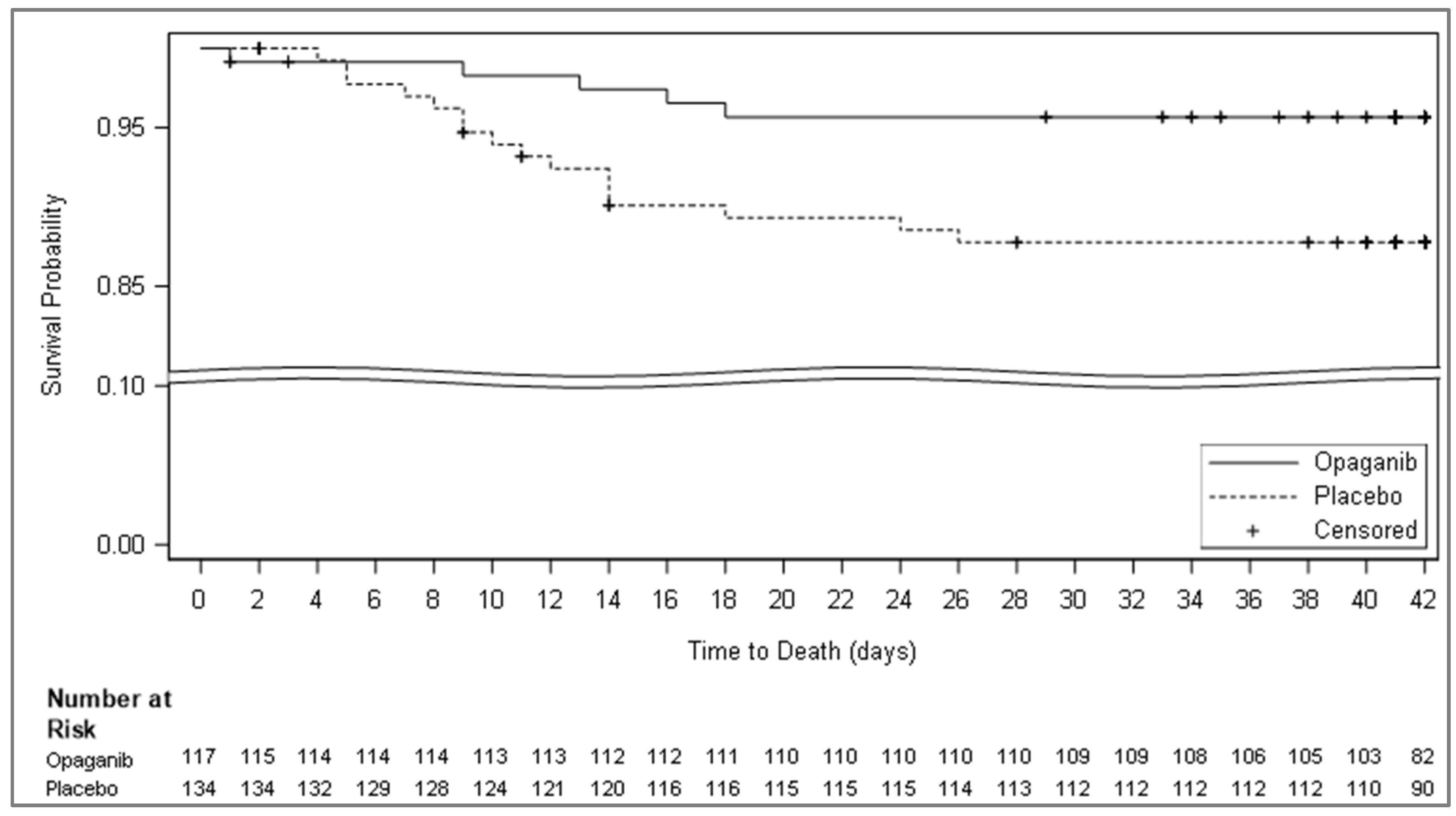
| Mortality due to any cause at day 28 * | 2 (4.65) | 10 (21.28) | |
| Difference (Opaganib% − Placebo%) | −16.63 | ||
| Percentage change (Opaganib%/placebo% × 100) | −81.1 | ||
| 95% CI | 0.00, 10.95 | 9.58, 32.98 | −29.91, −3.34 |
| p-value | 0.024 | ||
| Mortality due to any cause at day 42 | 3 (6.98) | 11 (23.40) | |
| Percentage change (Opaganib%/placebo% × 100) | −16.43 | ||
| 95% CI | 0.00, 14.59 | 11.30, 35.51 | −30.73, −2.1 |
| p-value | 0.034 | ||
| Parameter | Opaganib (N = 230) | Placebo (N = 233) |
|---|---|---|
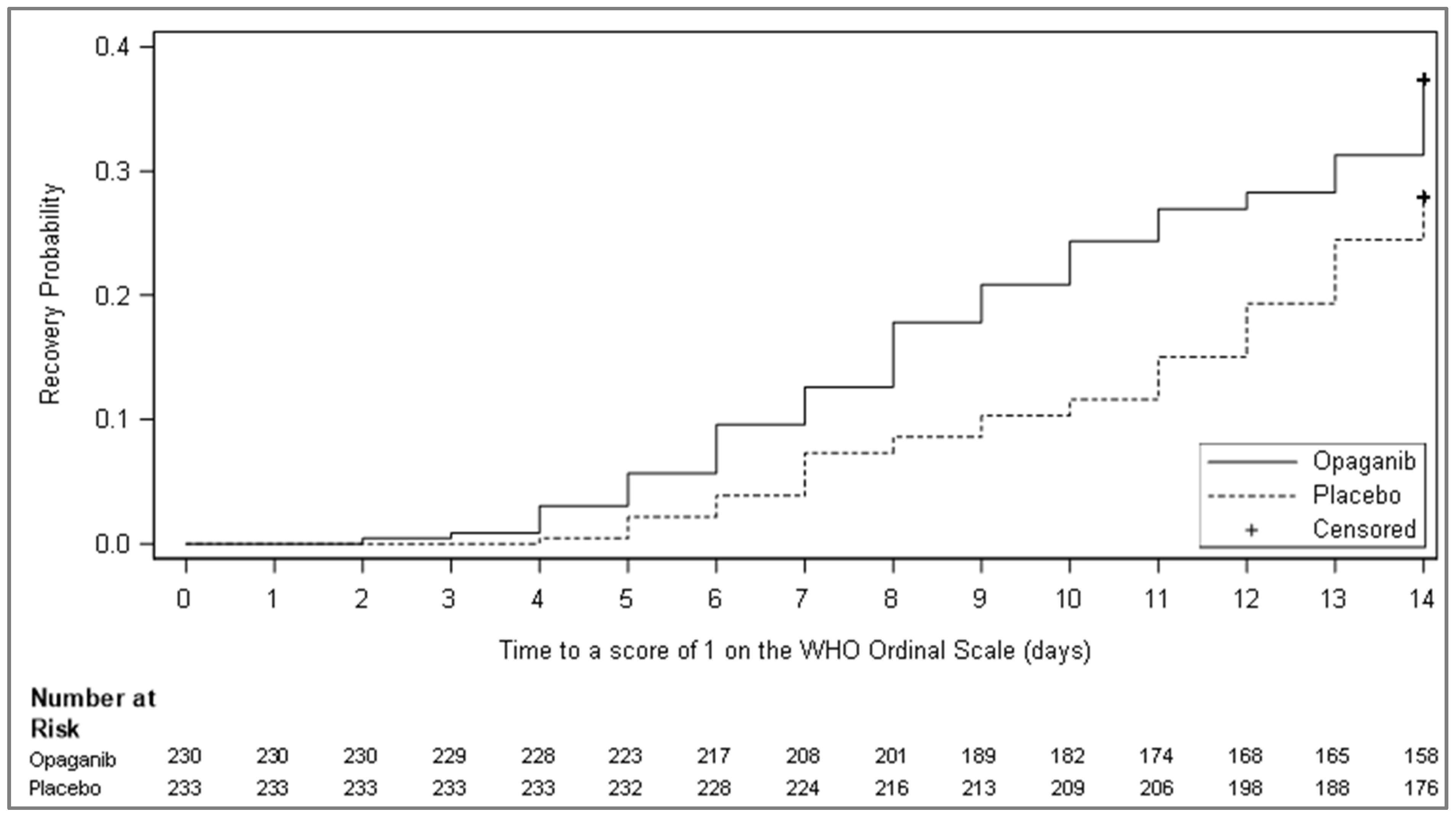
| Time to recovery as defined by improvement to a score of 1 or less on the WHO Ordinal Scale for Clinical Improvement, up to 14 days | ||
| Number of events (%) | 86 (37.4) | 65 (27.9) |
| Number of censored observations (%) | 144 (62.6) | 168 (72.1) |
| Log-rank test statistic 1 | 14.89 | |
| p-value 1 | 0.013 | |
| Hazard ratio (HR) and 95% CI 2 | 1.49 (1.08–2.05) | |
| Kaplan–Meier median estimate and 95% CI 3 | NA (NA–NA) | NA (NA–NA) |
| Cumulative incidence, % 3 | ||
| Day 7 | 12.61 | 7.30 |
| Day 14 | 37.39 | 27.90 |
| No. (%) | |||
|---|---|---|---|
| Opaganib (N = 117) | Placebo (N = 134) | Opaganib vs. Placebo | |
| Primary Outcomes | |||
| Patients no longer receiving supplemental oxygen (“Success”) * | 90 (76.9) | 85 (63.4) | |
| Difference † | 13.49 | ||
| 95% CI for the difference † | 2.32, 24.66 | ||
| p-value † | 0.033 | ||
| “Failure” | 27 (23.08) | 49 (36.57) | |
| Due to the need for supplemental oxygen at day 14 | 24 (20.51) | 32 (23.88) | |
| Due to death up to day 14 | 3 (2.56) | 13 (9.70) | |
| Due to lost to follow-up by day 14 | 0 | 3 (2.24) | |
| Due to missing status at day 42 with prior success | 0 | 1 (0.75) | |
| Secondary Outcomes | |||
| Patients with an improvement of 2 or more on the WHO Ordinal Scale compared to baseline by day 14 ‡ | 93 (79.49) | 88 (65.67) | |
| Difference | 13.82 | ||
| Percentage change (Opaganib%/Placebo% × 100−1) | 21 | ||
| p-value | 0.023 | ||
| Time to a score of ≤3 on the WHO Ordinal Scale | |||
| Number of events | 93 (79.5) | 88 (65.7) | |
| Kaplan–Meier median (days) | 8.00 | 10.00 | |
| 95% CI | 7.00–9.00 | 9.00–12.00 | |
| p-value | 0.010 | ||
| Time to low oxygen flow via nasal cannula (from high flow nasal cannula or CPAP/BiPAP) | |||
| Number of events | 102 (87.2) | 106 (79.1) | |
| Kaplan–Meier median (days) | 4.00 | 5.00 | |
| 95% CI | 3.00–5.00 | 4.00–6.00 | |
| p-value | 0.028 | ||
| Time to discharge by day 42 | |||
| Number of Events (%) | 110 (94.0) | 109 (81.3) | |
| Kaplan–Meier median (days) | 10.00 | 14.00 | |
| 95% CI | 9.00–13.00 | 11.00–14.00 | |
| p-value § | 0.004 | ||
| Patients requiring intubation and mechanical ventilation by day 42 ll | 8 (6.84) | 24 (17.91) | |
| Difference | −11.07 | ||
| Percentage change (Opaganib%/Placebo% × 100−1) | −61.8 | ||
| 95% CI | 2.26, 11.41 | 11.42, 24.40 | −19.01, −3.13 |
| Intubation without death | 2 (1.71) | 3 (2.24) | |
| Intubation with death | 4 (3.42) | 13 (9.70) | |
| Death without intubation | 1 (0.85) | 3 (2.24) | |
| Early termination/missing data (alive without intubation) | 1 (0.85) | 5 (3.73) | |
| p-value | 0.012 | ||
| Mortality due to any cause at day 42 (“Failure”) ** | 7 (5.98) | 21 (15.67) | |
| Difference | −9.69 | ||
| Percentage change (Opaganib%/Placebo% × 100−1) | −61.8 | ||
| 95% CI | 1.69, 10.28 | 9.52, 21.83 | −17.20, −2.18 |
| p-value | 0.019 | ||
| Low FIO2 | High FIO2 | ||||||
|---|---|---|---|---|---|---|---|
| Marker | N | Median | Q1, Q3 | N | Median | Q1, Q3 | p-Value * |
| Lymphocytes (109/L) | 249 | 0.990 | 0.71, 1.38 | 178 | 0.780 | 0.50, 1.20 | <0.0001 |
| C reactive protein (mg/L) | 240 | 60.800 | 21.40, 153.40 | 186 | 102.800 | 38.67, 200.30 | 0.0005 |
| Ferritin (µg/L) | 228 | 666.950 | 370.17, 1297.50 | 167 | 1000.000 | 500.60, 2000.00 | 0.0008 |
| D-dimer (µg/mL) | 240 | 0.450 | 0.18, 1.06 | 178 | 0.806 | 0.31, 1.79 | 0.0004 |
| Lactate dehydrogenase (IU/L) | 230 | 361.150 | 290.60, 522.00 | 183 | 469.230 | 344.00, 644.00 | <0.0001 |
| Mortality at 42-Day-Rate (%) (Kaplan–Meier Analysis) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | # Subjects with bl. Data | Cut-Point for Low High Groups (Median) | High Marker Group | Low Marker Group | High–Low Difference | CI | Two-Sided p-Value |
| Age | 463 | 57.000 | 26.2% | 7.0% | 19.2% | [12.5%, 25.8%] | <0.0001 |
| Oxygen in gas mix at baseline (%) | 463 | 60.000 | 27.5% | 8.5% | 18.9% | [11.6%, 26.2%] | <0.0001 |
| # of risk factors | 463 | 3.000 | 25.5% | 8.7% | 16.8% | [9.9%, 23.7%] | <0.0001 |
| Lactate dehydrogenase (IU/L) | 431 | 405.000 | 24.5% | 8.6% | 16.0% | [9.0%, 22.9%] | <0.0001 |
| Lymphocytes (109/L) | 457 | 0.900 | 9.1% | 23.7% | −14.6% | [−21.4%, −7.8%] | <0.0001 |
| D-dimer (µg/mL) | 436 | 0.578 | 21.5% | 11.0% | 10.6% | [3.6%, 17.5%] | 0.0029 |
| C reactive protein (mg/L) | 445 | 82.800 | 21.9% | 11.5% | 10.4% | [3.4%, 17.4%] | 0.0036 |
| Ferritin (µ/L) | 411 | 758.000 | 21.5% | 11.6% | 9.9% | [2.6%, 17.1%] | 0.0075 |
| Oxygen saturation at baseline (%) | 463 | 94.000 | 11.8% | 20.1% | −8.3% | [−15.0%, −1.6%] | 0.0152 |
| Pulse rate at baseline (beats/min) | 463 | 79.000 | 19.6% | 13.7% | 6.0% | [−0.9%, 12.9%] | 0.0903 |
| BMI (CRF) at baseline (kg/m2) | 463 | 29.988 | 18.9% | 14.1% | 4.8% | [−2.1%, 11.7%] | 0.1754 |
| Systolic blood pressure at baseline (mmHg) | 463 | 129.000 | 18.9% | 14.2% | 4.7% | [−2.1%, 11.6%] | 0.1770 |
| Weight at baseline (kg) | 463 | 87.100 | 17.3% | 15.3% | 2.0% | [−4.9%, 8.9%] | 0.5693 |
| Oxygen flow at baseline (L/min) | 463 | 15.000 | 17.9% | 16.1% | 1.8% | [−5.5%, 9.0%] | 0.6274 |
| Time from the onset of symptoms to randomization (Days) | 455 | 11.000 | 17.0% | 15.8% | 1.3% | [−5.7%, 8.3%] | 0.7200 |
| Temperature at baseline (C) | 463 | 36.700 | 17.3% | 16.1% | 1.2% | [−5.8%, 8.1%] | 0.7409 |
| Opaganib | Placebo | ||||||
|---|---|---|---|---|---|---|---|
| Biomarker | N | Median | Q1, Q3 | N | Median | Q1, Q3 | p-Value * |
| Lymphocytes (109/L) | 116 | 0.910 | 0.69, 1.36 | 133 | 1.010 | 0.72, 1.41 | 0.2534 |
| C Reactive Protein (mg/L) | 113 | 67.100 | 26.77, 173.00 | 127 | 45.000 | 18.60, 145.60 | 0.1910 |
| Ferritin (µg/L) | 104 | 727.600 | 381.65, 1383.45 | 124 | 592.300 | 368.02, 1201.20 | 0.3025 |
| D-Dimer (µg/mL) | 111 | 0.499 | 0.17, 1.17 | 129 | 0.380 | 0.18, 1.04 | 0.6619 |
| Lactate Dehydrogenase (IU/L) | 106 | 359.400 | 300.00, 507.00 | 124 | 368.750 | 287.55, 536.34 | 0.5522 |
| No. (%) | ||
|---|---|---|
| Event | Opaganib * (N = 230) | Placebo * (N = 233) |
| Any TEAEs | 155 (67.4) | 147 (63.1) |
| Serious TEAEs | 52 (22.6) | 52 (22.3) |
| Grade 1 TEAEs mild | 115 (50.0) | 109 (46.8) |
| Grade 2 TEAEs moderate | 58 (25.2) | 67 (28.8) |
| Grade 3 TEAEs severe | 32 (13.9) | 29 (12.4) |
| Grade 4 TEAEs life-threatening | 17 (7.4) | 16 (6.9) |
| Grade 5 TEAEs death | 36 (15.7) | 40 (17.2) |
| Treatment-related TEAEs | 39 (17.0) | 29 (12.4) |
| Treatment-related TESAEs | 1 (0.4) † | 0 |
| TEAEs resulting in dose reduced | 2 (0.9) | 3 (1.3) |
| TEAEs resulting in drug withdrawn | 26 (11.3) | 26 (11.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuenschwander, F.C.; Barnett-Griness, O.; Piconi, S.; Maor, Y.; Sprinz, E.; Assy, N.; Khmelnitskiy, O.; Lomakin, N.V.; Goloshchekin, B.M.; Nahorecka, E.; et al. Effect of Opaganib on Supplemental Oxygen and Mortality in Patients with Severe SARS-CoV-2 Based upon FIO2 Requirements. Microorganisms 2024, 12, 1767. https://doi.org/10.3390/microorganisms12091767
Neuenschwander FC, Barnett-Griness O, Piconi S, Maor Y, Sprinz E, Assy N, Khmelnitskiy O, Lomakin NV, Goloshchekin BM, Nahorecka E, et al. Effect of Opaganib on Supplemental Oxygen and Mortality in Patients with Severe SARS-CoV-2 Based upon FIO2 Requirements. Microorganisms. 2024; 12(9):1767. https://doi.org/10.3390/microorganisms12091767
Chicago/Turabian StyleNeuenschwander, Fernando Carvalho, Ofra Barnett-Griness, Stefania Piconi, Yasmin Maor, Eduardo Sprinz, Nimer Assy, Oleg Khmelnitskiy, Nikita V. Lomakin, Boris Mikhailovich Goloshchekin, Ewelina Nahorecka, and et al. 2024. "Effect of Opaganib on Supplemental Oxygen and Mortality in Patients with Severe SARS-CoV-2 Based upon FIO2 Requirements" Microorganisms 12, no. 9: 1767. https://doi.org/10.3390/microorganisms12091767
APA StyleNeuenschwander, F. C., Barnett-Griness, O., Piconi, S., Maor, Y., Sprinz, E., Assy, N., Khmelnitskiy, O., Lomakin, N. V., Goloshchekin, B. M., Nahorecka, E., Joaquim Westheimer Calvacante, A., Ivanova, A., Vladimirovich Zhuravel, S., Yurevna Trufanova, G., Bonora, S., Saffoury, A., Mayo, A., Shvarts, Y. G., Rizzardini, G., ... Levitt, M. L. (2024). Effect of Opaganib on Supplemental Oxygen and Mortality in Patients with Severe SARS-CoV-2 Based upon FIO2 Requirements. Microorganisms, 12(9), 1767. https://doi.org/10.3390/microorganisms12091767










