Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Infection of Mice with B. burgdorferi
2.2. DNA Extraction/PCR
2.3. Immunohistochemistry (IHC)
2.4. Combined Fluorescent In Situ Hybridization (FISH) and IHC
2.5. Atomic Force Microscopy (AFM)
3. Results
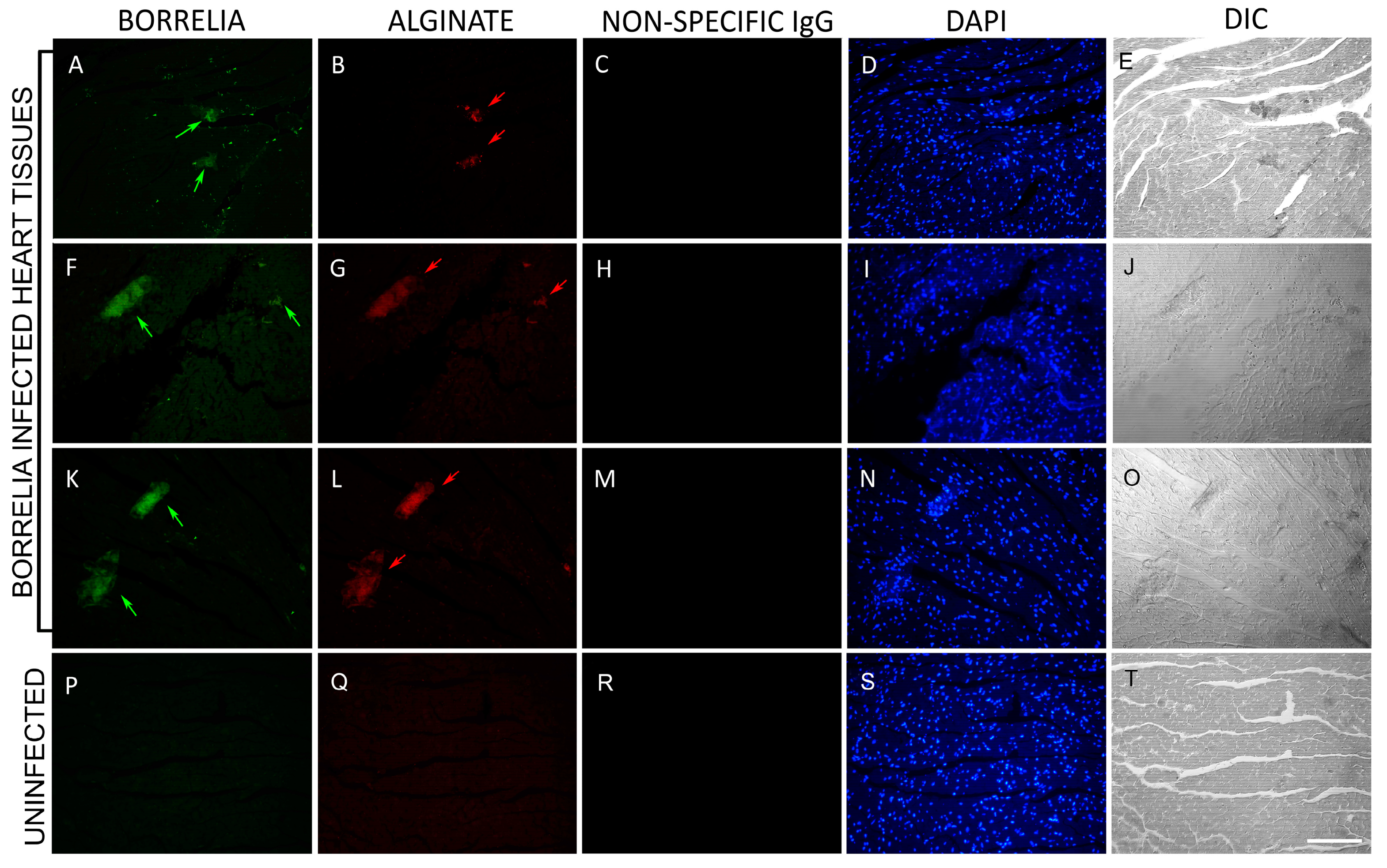
3.1. B. burgdorferi Spirochete and Biofilm Presence in Mouse Heart Tissues
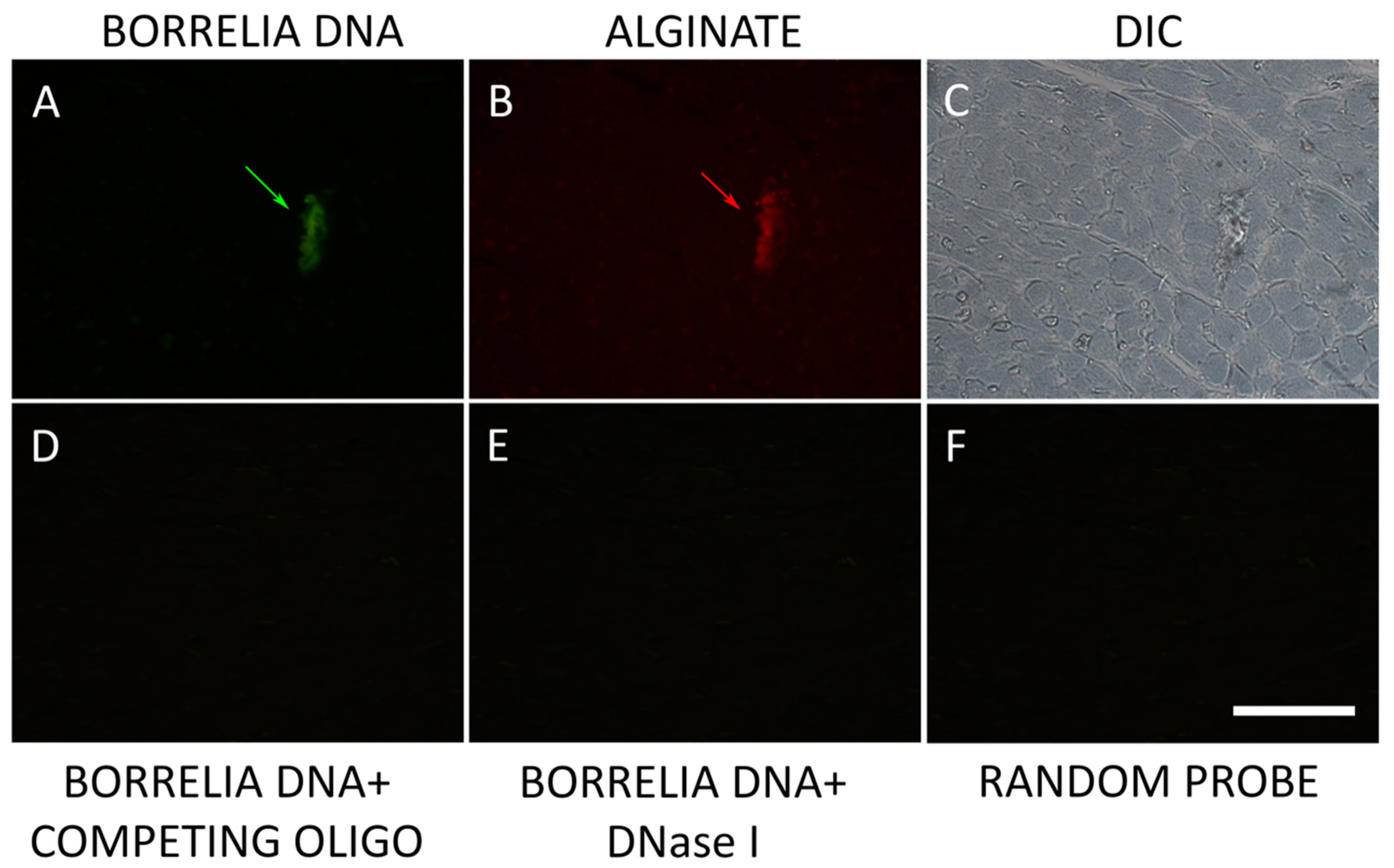
3.2. Combined FISH and IHC Confirmed the In Vivo Existence of B. burgdorferi Biofilm in Infected Mouse Heart Tissues
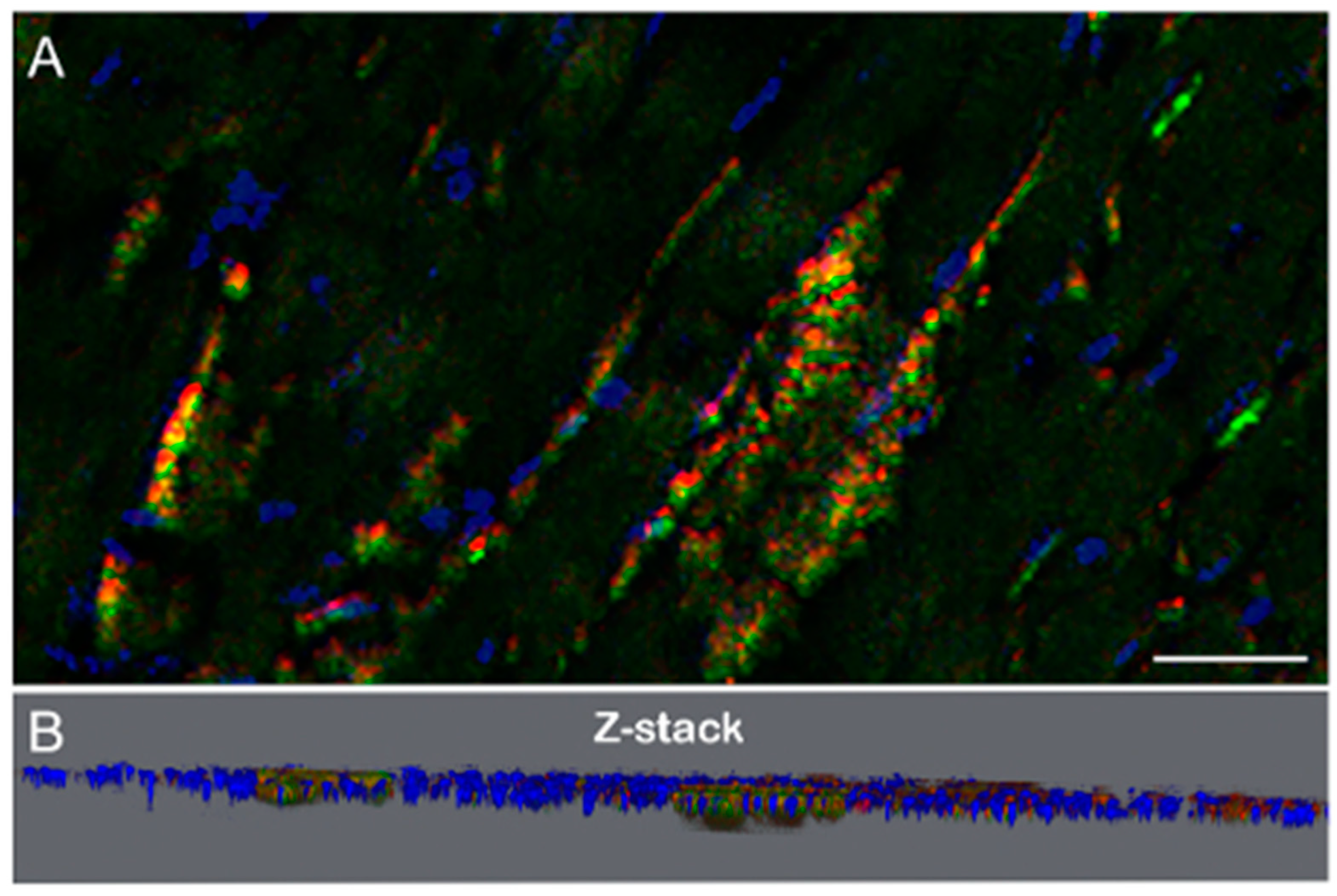
3.3. Three-Dimensional (3D) Microscopic Analysis of B. burgdorferi Biofilm in Infected Mouse Heart Tissues
3.4. Ultra-Structural Analysis of the B. burgdorferi and Alginate Positive Aggregates in Mouse Heart Tissues by Atomic Force Microscopy (AFM)
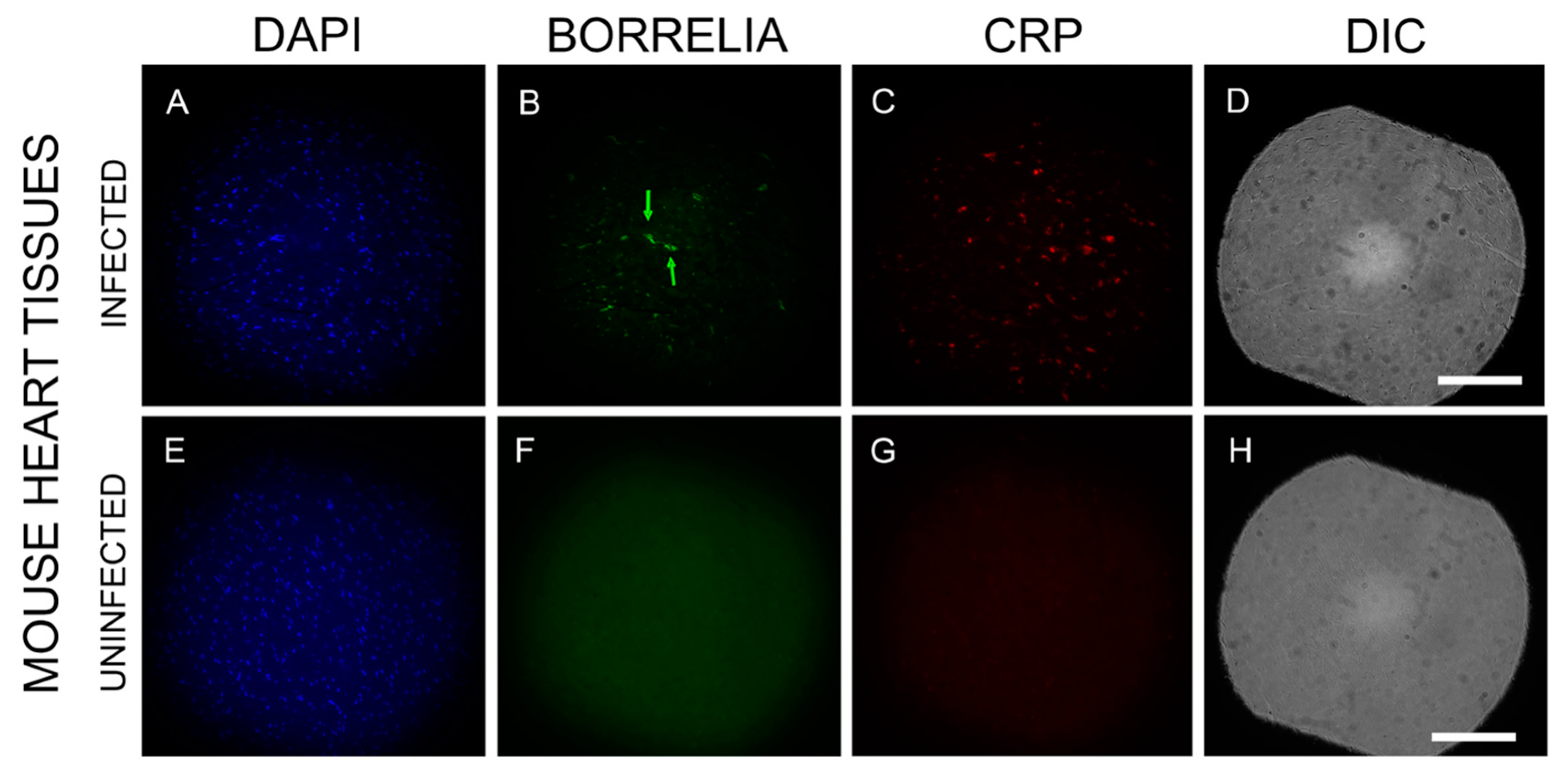
3.5. B. burgdorferi Biofilm Effect on Host Inflammatory Response in Mouse Heart Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perronne, C. Lyme and associated tick-borne diseases: Global challenges in the context of a public health threat. Front. Cell. Infect. Microbiol. 2014, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Garcia, B.; Hu, L.T.; Jewett, M.W.; Kraiczy, P.; Norris, S.J.; Skare, J. Lyme Disease Pathogenesis. Curr. Issues. Mol. Biol. 2021, 42, 473–518. [Google Scholar]
- Liegner, K.B.; Shapiro, J.R.; Ramsay, D.; Halperin, A.J.; Hogrefe, W.; Kong, L. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting Borrelia burgdorferi infection. J. Am. Acad. Dermatol. 1993, 28, 312–314. [Google Scholar] [CrossRef]
- Stricker, R.B.; Johnson, L. Lyme disease: The next decade. Infect. Drug Resist. 2011, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, E.; Imai, D.; Feng, S.; Barthold, S.W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE 2014, 9, e86907. [Google Scholar] [CrossRef]
- Cabello, F.C.; Embers, M.E.; Newman, S.A.; Godfrey, H.P. Borreliella burgdorferi antimicrobial-tolerant persistence in Lyme disease and posttreatment Lyme disease syndromes. mBio 2022, 13, e0344021. [Google Scholar] [CrossRef]
- Brorson, O.; Brorson, S.H. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 1997, 25, 240–246. [Google Scholar] [CrossRef]
- Brorson, O.; Brorson, S.H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 1998, 26, 144–150. [Google Scholar] [CrossRef]
- Brorson, O.; Brorson, S.H. A rapid method for generating cystic forms of Borrelia burgdorferi, and their reversal to mobile spirochetes. APMIS 1998, 106, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. A life cycle for Borrelia spirochetes? Med. Hypotheses. 2006, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. Plaques of Alzheimer’s disease originate from cysts of Borrelia burgdorferi, the Lyme disease spirochete. Med. Hypotheses. 2006, 67, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.S.; Pham, T.V.; et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS ONE 2012, 7, e48277. [Google Scholar] [CrossRef]
- Brady, R.A.; Leid, J.G.; Camper, A.K.; Costerton, J.W.; Shirtliff, M.E. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated host immune response to a biofilm infection. Infect. Immun. 2006, 74, 3415–3426. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Helaine, S.; Holden, D.W. Heterogeneity of intracellular replication of bacterial pathogens. Curr. Opin. Microbiol. 2013, 16, 184–191. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.O.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Høiby, N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Staphylococcus aureus endocarditis: A consequence of medical progress. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef]
- Hoyle, B.D.; Costerton, J.W. Bacterial resistance to antibiotics: The role of biofilms. Prog. Drug Res. 1991, 37, 91–105. [Google Scholar]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.S.; et al. Evidence of in vivo existence of Borrelia biofilm in borrelial lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Kasliwala, R.S.; Ismail, H.; Torres, J.P.; Oldakowski, M.; Markland, S.; Gaur, G.; Melillo, A.; Eisendle, K.; Liegner, K.B.; et al. The long-term persistence of Borrelia burgdorferi antigens and DNA in the tissues of a patient with Lyme disease. Antibiotics 2019, 8, 183. [Google Scholar] [CrossRef]
- Sapi, E.; Kaur, N.; Anyanwu, S.; Luecke, D.F.; Datar, A.; Patel, S.; Rossi, M.; Stricker, R.B. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 2011, 4, 97–113. [Google Scholar] [PubMed]
- Kostić, T.; Momčilović, S.; Perišić, Z.D.; Apostolović, S.R.; Cvetković, J.; Jovanović, A.; Barać, A.; Šalinger-Martinović, S.; Tasić-Otašević, S. Manifestations of Lyme carditis. Int. J. Cardiol. 2017, 232, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Baranchuk, A. Systematic approach to the diagnosis and treatment of Lyme carditis and high-degree atrioventricular block. Healthcare 2018, 6, 119. [Google Scholar] [CrossRef]
- Radesich, C.; Del Mestre, E.; Medo, K.; Vitrella, G.; Manca, P.; Chiatto, M.; Castrichini, M.; Sinagra, G. Lyme Carditis: From pathophysiology to clinical management. Pathogens 2022, 11, 582. [Google Scholar] [CrossRef]
- Burmolle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homoe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—a matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Barthold, S.W.; Beck, D.S.; Hansen, G.M.; Terwilliger, G.A.; Moody, K.D. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 1990, 162, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W.; Persing, D.H.; Armstrong, A.L.; Peeples, R.A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 1991, 139, 263–273. [Google Scholar]
- Armstrong, A.L.; Barthold, S.W.; Persing, D.H.; Beck, D.S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 1992, 47, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W.; de Souza, M.S.; Janotka, J.L.; Smith, A.L.; Persing, D.H. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 1993, 143, 959–971. [Google Scholar] [PubMed]
- Torres, J.P.; Senejani, A.G.; Gaur, G.; Oldakowski, M.; Murali, K.; Sapi, E. Ex vivo murine skin model for B. burgdorferi biofilm. Antibiotics. 2020, 9, 528. [Google Scholar] [CrossRef]
- Morrison, T.B.; Ma, Y.; Weis, J.H.; Weis, J.J. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 1999, 37, 987–992. [Google Scholar] [CrossRef]
- Timmaraju, V.A.; Theophilus, P.A.; Balasubramanian, K.; Shakih, S.; Luecke, D.F.; Sapi, E. Biofilm formation by Borrelia burgdorferi sensu lato. FEMS Microbiol. Lett. 2015, 362, fnv120. [Google Scholar] [CrossRef][Green Version]
- Barthold, S.W.; Hodzic, E.; Tunev, S.; Feng, S. Antibody-mediated disease remission in the mouse model of lyme borreliosis. Infect. Immun. 2006, 74, 4817–4825. [Google Scholar] [CrossRef]
- Nikolić, A.; Boljević, D.; Bojić, M.; Veljković, S.; Vuković, D.; Paglietti, B.; Micić, J.; Rubino, S. Lyme endocarditis as an emerging infectious disease: A review of the literature. Front. Microbiol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in Rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Li, X.; McHugh, G.A.; Damle, N.; Sikand, V.K.; Glickstein, L.; Steere, A.C. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or lyme arthritis. Arthritis Rheum. 2011, 63, 2238–2247. [Google Scholar] [CrossRef]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [CrossRef]
- Bispo, P.J.; Haas, W.; Gilmore, M.S. Biofilms in infections of the eye. Pathogens 2015, 4, 111–136. [Google Scholar] [CrossRef]
- González, M.J.; Robino, L.; Iribarnegaray, V.; Zunino, P.; Scavone, P. Effect of different antibiotics on biofilm produced by uropathogenic Escherichia coli isolated from children with urinary tract infection. Pathog. Dis. 2017, 75, ftx053. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Szymczyk, P.; Ziółkowski, G.; Karuga-Kuzniewska, E.; Smutnicka, D.; Bil-Lula, I.; Bartoszewicz, M.; Mahabady, S.; Sedghizadeh, P.P. Bad to the bone: On in vitro and ex vivo microbial biofilm ability to directly destroy colonized bone surfaces without participation of host immunity or osteoclastogenesis. PLoS ONE 2017, 12, e0169565. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.L.F.; López, J.J.G.; Goyenechea, A.; Fraile, J.; de Górgolas, M. Endocarditis caused by Staphylococcus aureus: A reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine 2009, 88, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Marks, L.R.; Pettigrew, M.M.; Hakansson, A.P. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front. Cell Infect. Microbiol. 2015, 4, 194. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P.Ø. Biofilms and host response—Helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef]
- Liegner, K.B. Lyme disease and the use of C-reactive protein as a marker of inflammation. Clin. Infect. Dis. 2016, 63, 1399–1405. [Google Scholar]
- Udge, A.; Lanza, F.C. Reactive protein and its role in carditis. J. Clin. Med. Res. 2016, 8, 345–352. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.M.; Horowitz, R.; Johnson, R.; Prestiano, R.A.; Klein, B.I. The systemic oral health connection: Biofilms. Medicine 2022, 101, e30517. [Google Scholar] [CrossRef] [PubMed]
- Kasliwala, R. Effect of Borrelia Biofilm on the Host Inflammatory Markers in Infected Human Tissues. Master’s Thesis, University of New Haven, West Haven, CT, USA, 2017. [Google Scholar]
- Soloski, M.J.; Crowder, L.A.; Lahey, L.J.; Wagner, C.A.; Robinson, W.H.; Aucott, J.N. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS ONE 2014, 9, e93243. [Google Scholar] [CrossRef] [PubMed]
- Raveche, E.S.; Schutzer, S.E.; Fernandes, H.; Bateman, H.; McCarthy, B.A.; Nickell, S.P.; Cunningham, M.W. Evidence of Borrelia autoimmunity-induced component of Lyme carditis and arthritis. J. Clin. Microbiol. 2005, 43, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, D.; Bai, Y.; Hodzic, E.; Narayan, K.; Barthold, S.W.; Pachner, A.R. Cardiac involvement in non-human primates infected with the Lyme disease spirochete Borrelia burgdorferi. Lab. Investig. 2004, 84, 1439–1450. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thippani, S.; Patel, N.J.; Jathan, J.; Filush, K.; Socarras, K.M.; DiLorenzo, J.; Balasubramanian, K.; Gupta, K.; Ortiz Aleman, G.; Pandya, J.M.; et al. Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues. Microorganisms 2024, 12, 1766. https://doi.org/10.3390/microorganisms12091766
Thippani S, Patel NJ, Jathan J, Filush K, Socarras KM, DiLorenzo J, Balasubramanian K, Gupta K, Ortiz Aleman G, Pandya JM, et al. Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues. Microorganisms. 2024; 12(9):1766. https://doi.org/10.3390/microorganisms12091766
Chicago/Turabian StyleThippani, Sahaja, Niraj Jatin Patel, Jasmine Jathan, Kate Filush, Kayla M. Socarras, Jessica DiLorenzo, Kunthavai Balasubramanian, Khusali Gupta, Geneve Ortiz Aleman, Jay M. Pandya, and et al. 2024. "Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues" Microorganisms 12, no. 9: 1766. https://doi.org/10.3390/microorganisms12091766
APA StyleThippani, S., Patel, N. J., Jathan, J., Filush, K., Socarras, K. M., DiLorenzo, J., Balasubramanian, K., Gupta, K., Ortiz Aleman, G., Pandya, J. M., Kavitapu, V. V., Zeng, D., Miller, J. C., & Sapi, E. (2024). Evidence for the Presence of Borrelia burgdorferi Biofilm in Infected Mouse Heart Tissues. Microorganisms, 12(9), 1766. https://doi.org/10.3390/microorganisms12091766






