Abstract
Trypanosoma brucei causes African trypanosomiasis in humans. Infection with T. brucei elicits a potent pro-inflammatory immune response within infected human hosts, and this response is thought to at least be partially due to Toll-like receptor (TLR) activation. In response to stimulation by lipopolysaccharide and other pathogen antigens, TLR4 translocates to lipid rafts, which induces the expression of pro-inflammatory genes. However, cholesterol efflux is acknowledged as anti-inflammatory due to promoting lipid raft disruption. In this study, we wanted to assess the impact of T. brucei “ghosts”, which are non-viable T. brucei essentially devoid of intracellular contents, in stimulating macrophage TLR4 translocation to lipid rafts, and whether promoting cholesterol efflux in macrophages incubated with T. brucei ghosts attenuates TLR4-target gene expression. When cultured macrophages were exposed to T. brucei ghosts, we observed an increase in lipid raft TLR4 protein content, which suggests certain surface molecules of T. brucei serve as ligands for TLR4. However, pretreating macrophages with cholesterol acceptors before T. brucei ghost exposure decreased lipid raft TLR4 protein content and the expression of pro-inflammatory TLR4-target genes. Taken together, these results imply that macrophage cholesterol efflux weakens pro-inflammatory responses which occur from T. brucei infection via increasing macrophage lipid raft disruption.
1. Introduction
Trypanosoma brucei is a eukaryotic pathogen that causes African sleeping sickness in humans and nagana in livestock [1]. African sleeping sickness and nagana are vector-borne diseases because transmission occurs when an infected tsetse fly bites a mammalian host [2]. African trypanosomiasis is considered a neglected tropical disease, as tsetse flies are only thought to inhabit sub-Saharan Africa [3]. If left untreated, African trypanosomiasis is generally fatal [4]. However, conventional treatments for African trypanosomiasis are prone to side effects [5]. Indeed, complications from traditional types of therapeutic interventions for African trypanosomiasis have been reported to be severe enough to becoming life-threatening, and in some cases, even fatal [6,7].
African trypanosomiasis is well-recognized to be endemic to sub-Saharan Africa [8]. While improved screening and increased treatment rates have reduced the number of reported cases for human African trypanosomiasis, this disease is still considered a serious concern in rural sub-Saharan African areas stricken by poverty, since African sleeping sickness often goes under-reported within these regions [3]. African trypanosomiasis is also a significant burden for the veterinary and agricultural industry. It is estimated that between 50 and 55 million cattle are at risk for developing nagana and approximately 3 million cattle die yearly from this disease [9,10]. Animal African trypanosomiasis also drastically afflicts sheep and goats, as approximately 30 million sheep and an estimated 40 million goats are at risk of being infected annually [9,10]. Furthermore, without prompt and effective treatments, African trypanosomiasis in livestock will likely lead to mortality [11,12].
African trypanosomiasis leads to a strong host pro-inflammatory immune response that attempts to clear T. brucei from the body [13]. However, T. brucei is able to escape host immunity by undergoing antigenic variation via variant surface glycoprotein (VSG) switching, allowing T. brucei to persist within infected mammalian hosts [14,15,16]. Therefore, it is not entirely clear what benefit pro-inflammatory immune responses play during T. brucei infection, and some evidence has suggested that this type of host response may be deleterious to infected hosts [17,18]. It is also poorly understood what mechanisms and processes activate the pro-inflammatory immune response from T. brucei infection [19,20]. T. brucei is associated with certain surface molecules [21] that appear capable of acting as ligands for different Toll-like receptors (TLRs). For instance, data suggest that VSG, which is the most abundant surface molecule of bloodstream form T. brucei, may demonstrate the capacity to induce various TLRs [21,22,23,24]. Data have also shown that lipophosphoglycan, which is a minor surface component of T. brucei, serves as a ligand for both TLR2 and TLR4 [25,26]. Thus, TLR activation mediated by T. brucei surface molecules may at least partially explain how T. brucei parasites stimulate inflammation within infected hosts.
Early in T. brucei infection, macrophages in the liver and spleen mediate the humoral clearance of parasites and influence the developing immune response [27,28]. Macrophages contain a high lipid raft content and also exhibit potent pro-inflammatory effects from lipopolysaccharide (LPS) exposure [29]. Moreover, macrophages are known to efficiently efflux cholesterol to apoAI and HDL via the cholesterol efflux transporters ABCA1 and ABCG1, respectively [30]. We and others have shown that ABCA1/apoAI- and ABCG1/HDL-mediated cholesterol efflux decreases pro-inflammatory gene expression in macrophages and other cells that are challenged with the canonical TLR4 ligand LPS or other TLR4 ligands, and these effects are from cholesterol-efflux-reducing lipid raft TLR4 protein content [31,32,33,34,35,36,37]. Regarding TLRs, the TLR which is of central importance to innate immunity is TLR4 [38,39]. Interestingly, for TLR4 to induce pro-inflammatory gene expression, TLR4 first needs to translocate to lipid rafts after being activated by ligands on the cell surface [40]. Furthermore, the removal of cholesterol from cells promotes lipid raft disruption, and so cellular cholesterol efflux has been acknowledged as being anti-inflammatory due to stimulating lipid raft disruption [41]. Macrophages are also well-established to play a critical role in the pro-inflammatory immune response that occurs during T. brucei infection [42,43].
Hence, the purpose of our work was to assess whether apoAI/HDL-mediated cholesterol efflux in macrophages is capable of attenuating the pro-inflammatory response that occurs when these cells are exposed to T. brucei. In this work, we aimed to analyze the capacity of the entire cell surface of T. brucei to act as a ligand for TLR4 within cultured macrophages. In this work, when we utilized non-viable T. brucei “ghosts” and incubated macrophages with these parasite ghosts (PGs), this resulted in increased TLR4 translocation to lipid rafts within the cultured macrophages. However, pretreating cultured macrophages with the cholesterol acceptors apoAI or HDL before challenging these cells with PG decreased TLR4 translocation to lipid rafts. Furthermore, macrophage apoAI/HDL pretreatment also resulted in reducing the pro-inflammatory expression of TLR4-target genes in macrophages incubated with PG. Based on our results, we conclude that apoAI- and HDL-mediated cholesterol efflux is capable of attenuating TLR4-target gene expression in macrophages exposed to T. brucei and that this effect is likely due to promoting macrophage lipid raft disruption which decreases TLR4 translocation to lipid rafts.
2. Materials and Methods
2.1. Cell Culture and Maintenance of Cells
We purchased RAW 264.7 macrophage cells [44] from ATCC (Manassas, VA, USA) and maintained this cell line in tissue culture plates and standard growth medium that contained high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Corning, New York, NY, USA), fetal bovine serum (FBS) (10%; VWR Life Science Seradigm, Radnor, PA, USA), and penicillin-streptomycin (1%; Corning). For this cell line, we replenished the medium approximately biweekly; once cells reached 70–90% confluency, they were either passaged into maintenance tissue culture plates or plated to treatment dishes. Bloodstream form T. brucei brucei Lister 427 strain (BF-427) were cultured within flasks using HMI 9 medium [45] supplemented with 10% heat-inactivated FBS and 1% pencillin-streptomycin. We sub-cultured BF-427 every 2–3 days until cells were ready for their respective treatments. Both types of cells were grown and maintained in an incubator set at 37 °C and 5% CO2.
2.2. Preparation and Characterization of BF-427 Parasite Ghosts (PGs)
We prepared BF-427 PG by adapting methods as previously described [25]. We exposed cultured BF-427 to a working concentration of 1% saponin (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at 4 °C. After saponin treatments, we then either assessed PG via fluorescent microscopy, or collected PG via centrifugation, washed the pelleted PG with phosphate-buffered saline (PBS), repeated centrifugation, and then removed PBS and resuspended PG to utilize for downstream experiments.
We utilized a rat anti-“Tryps” primary antibody to aid in visualizing the PG. To create the anti-Tryps primary antibody, male retired breeder CD1 rats (Charles River, Wilmington, MA, USA) were infected intra-peritoneally (i.p.) with 5 × 105 T. brucei in sterile phosphate-buffered saline (PBS). When parasitemia reached 0.5 × 108, rats were cured with two doses of diminazene [46] (34.1 mg/kg i.p.; Cayman Chemical, Ann Arbor, MI, USA) administered 24 h apart. After 20 days to allow for the development of a mature antibody response, blood from cured rats was collected by cardiac puncture and allowed to clot, and the serum supernatant was stored at −80 °C. Serum proteins were concentrated by ammonium sulfate precipitation, and serum IgMs were affinity-enriched with an IgM Purification Kit (Pierce Biotechnology/Thermo Scientific, Rockford, IL, USA) using the manufacturer’s instructions, except ammonium sulfate precipitate was dialyzed into 20 mM Tris-Cl (pH 7.4), 1.25 M NaCl. The serum IgGs were then enriched by passing the IgM column flow-through (which contained the IgGs and other serum proteins) over a Pierce Melon Gel IgG purification kit (Pierce Biotechnology/Thermo Scientific) using the manufacturer’s instructions to remove serum albumin and other contaminating serum proteins. The resulting affinity-enriched IgG antibody was used then used to visualize the PG.
To stain the PG surface for imaging, we plated prepared PG on poly-L-lysine coated slides and allowed PG to settle for 20 min at room temperature. Residual liquid was removed and then PG were fixed for 1 h in 2% paraformaldehyde at room temperature in a humid chamber. PG were washed 4X in PBS, permeabilized with 0.1% triton-X-100/PBS for 20 min, and then washed again with PBS. We incubated PG in block solution (10% goat serum/0.1% triton-X-100/PBS) for 1 h at room temperature, followed by an overnight, 4 °C incubation with the rat anti-”Tryps” primary antibody (diluted 1:10). Following incubation with this primary antibody, PG were washed 3X in wash solution, incubated in Alexa Fluor 488 goat anti-rat IgG secondary antibody (Invitrogen, Carlsbad, CA, USA), which was diluted 1:200, washed again 3X using wash solution, and then incubated with DAPI (Invitrogen) [47]. Following incubation with DAPI counterstain, PG were washed twice with wash solution, and then mounted in ProLong Gold. We imaged PG with a Leica SP8X confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA) equipped with a 405 nm laser, a tunable white light laser (WLL), HyD detectors, and time gating. To detect DAPI, PG were excited using the 405 nm laser, and emission wavelengths of 410–460 nm were collected by using a HyD detector. For antibody detection, PG were excited using the WLL tuned to 499 nm, and emission wavelengths of 510–550 nm were collected by using the HyD detector with a 0.5 ns time gate. The PG were imaged using a 100X/1.40 N.A. oil immersion lens with 1.5X zoom, and images were collected with using Leica LAS X software (Version 3.5.7.23225, Leica Microsystems, Buffalo Grove, IL, USA) and exported as .TIF image files.
2.3. Macrophage Exposure to PG
We pre-incubated cultured macrophages in serum-free DMEM containing 1% pencillin-streptomycin and 2 mg/mL of fatty acid-free bovine serum albumin (Sigma-Aldrich) [48]. To intially assess TLR4 translocation to lipid rafts in cultured macrophages exposed to PG, we treated cells using these cultured conditions and incubated macrophages with either vehicle only or PG (1:1 PG to macrophage ratio) for 24 h, washed cells with PBS, and then collected the non-raft and lipid raft fractions from macrophages. To promote lipid raft disruption, we first exposed macrophages to either 50 μg/mL apoAI (Academy Bio-Medical Company, Houston, TX, USA), 50 μg/mL HDL (Academy Bio-Medical Company), or vehicle only during serum-free DMEM culturing conditions [49,50]. After 24 h of cholesterol acceptor and vehicle treatments, we removed the medium and washed macrophages with PBS, then challenged cells with PG in serum-free DMEM. After 24 h of PG treatment, we washed cells with PBS and either isolated the non-raft and lipid raft fractions from macrophages or harvested total RNA from the treated cells.
2.4. Isolation of Macrophage Lipid Rafts and Immunoblotting
To isolate non-raft and lipid raft fractions from macrophages, we utilized a detergent-free, gradient ultracentrifugation method [36] successfully used by us previously [33]. Briefly, we spun macrophage lysates at 50,000 rpm maximum speed for 24 h at 4 °C by using an Optima MAX-XP ultracentrifuge (Beckman Coulter, Brea, CA, USA) and an MLS-50 swinging bucket rotor (Beckman Coulter). After ultracentrifugation, we collected 9 equal volume fractions, discarded the fifth fraction, then pooled equal volumes from the 4 remaining lipid-raft fractions and 4 remaining non-raft fractions. We separated proteins from the pooled fractions using SDS-PAGE and then transferred the separated proteins onto PVDF membranes [51]. After incubating PVDF membranes in the blocking buffer, we incubated the membranes overnight with either mouse anti-TLR4 primary antibody (1:1000 dilution, sc-293072; Santa Cruz Biotechnology, Dallas, TX, USA) or rabbit anti-caveolin-1 primary antibody (1:750 dilution; 3267, Cell Signaling Technology, Danvers, MA, USA). After primary antibody incubation and washing steps, we incubated the PVDF membranes with either HRP-conjugated goat anti-mouse IgG secondary antibody (1:10,000 dilution, AP181P; Sigma-Aldrich) or HRP-conjugated goat anti-rabbit IgG secondary antibody (1:10,000 dilution, HAF008; Novus Biologicals, Littleton, CO, USA). To visualize proteins, we utilized an ECL-based detection and imaging ChemiDoc instrument (Analytik Jena US, Upland, CA, USA) [52].
2.5. Macrophage Total RNA Extraction and RT-qPCR
We isolated total RNA from PG-challenged macrophages as previously described [53]. Briefly, we initially lysed macrophages with TRIzol and then extracted total RNA from lysed macrophages by using a Direct-Zol RNA purification kit (Zymo Research, Irvine, CA, USA). We then quantified the purified total RNA using a SpectraMax® QuickDrop™ Micro-Volume Spectrophotometer (Molecular Devices, LLC., San Jose, CA, USA). Using equal mass of RNA per RNA sample, we converted the total RNA into cDNA with Quantabio qScript Ultra SuperMix reagent (Beverly, MA, USA). We used the newly synthesized cDNA for qPCR reactions by utilizing a Quantabio PerfeCTa SYBR Green Fastmix kit along with the following primer pairs to amplify genes of interest and GAPDH housekeeping gene: IL-1β (forward primer, 5′-GAAATGCCACCTTTTGACAGTG-3′; reverse primer, 5′-TGGATGCTCTCATCAGGACAG-3′); IL-6 (forward primer: 5′-TCTATACCACTTCACAAGTCGGA-3′; reverse primer: 5′-GAATTGCCATTGCACAACTCTTT-3′); TNF-α (forward primer: 5′-CAGGCGGTGCCTATGTCTC-3′; reverse primer: 5′-CGATCACCCCGAAGTTCAGTAG-3′); GAPDH internal reference for normalization (forward primer: 5′-CGTGCCGCCTGGAGAAAC-3′; reverse primer: 5′-TGGGAGTTGCTGTTGAAGTCG-3′) [33,54]. The amplified qPCR reactions were quantified with a qTOWER3 G touch qPCR instrument (Analytik Jena US) [55], and gene expression was analyzed by following the delta-delta CT (ΔΔCT) method [56].
2.6. Statistical Analysis
To perform statistical analyses, we utilized SigmaPlot (v14.0) software (Systat Software Inc., San Jose, CA, USA). To determine the appropriateness of conducting Student’s t-test, we initially performed Brown–Forsythe and Shapiro–Wilk tests to assess equal variance and normality, respectively. When the Brown–Forsythe test failed, we performed Welch’s t-test. When the Shapiro–Wilk test failed, we conducted a Mann–Whitney rank-sum test. We set the level of significance for experiments requiring statistical analysis at p < 0.05.
3. Results
3.1. Exposing Cultured Macrophages to T. brucei “Ghosts” Triggers TLR4 Translocation to Lipid Rafts
The host pro-inflammatory immune response against T. brucei is complex [18,57]. For instance, T. brucei is capable of activating TLR9, and data have shown that the intracellular DNA of T. brucei activates this TLR [23,58,59]. Moreover, TLR9 is an endosomal TLR, unlike cell surface TLR4 [60,61]. Therefore, to inhibit the intracellular components of T. brucei stimulating inflammation within cultured macrophages, we generated non-viable BF-427 PG, which results in the removal of intracellular contents from these parasites while still preserving BF-427 surface molecules [25]. Using immunofluorescent staining, we confirmed we were capable of generating PG (Figure 1A,B). We utilized these PGs to treat cultured macrophages to initially assess whether exposing macrophages to PG stimulates TLR4 translocation to lipid rafts. When we used immunoblotting to assess TLR4 protein levels, we observed an increase in TLR4 protein content within the lipid raft fractions of cultured macrophages challenged with PG when compared to control macrophages treated with vehicle only (Figure 1C). Based on this result, we concluded that the cell surface of T. brucei functions as a ligand for TLR4.
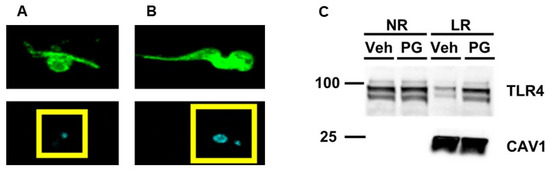
Figure 1.
Enrichment of TLR4 within the lipid raft fractions of cultured macrophages challenged with PG. Immunofluorescence staining of a representative BF-247 PG (A) and viable BF-427 parasite (B) for detecting the cell surface of BF-427 (green in top two panels) and nuclei (blue/cyan in bottom two panels). Yellow boxes enclose nuclear region showing only mitochondrial kinetoplast DNA (kDNA) present in the BF-427 PG (A), while both the nucleus and kDNA were detected in viable BF-427 based on DAPI counterstain. (C) Cultured macrophages were incubated with either vehicle only (Veh) or PG and pooled non-raft (NR) and lipid raft (LR) fractions were collected from macrophages to probe for TLR4 and the lipid raft marker caveolin-1 (CAV1) using immunoblotting. Size markers are in kDa.
3.2. Pretreating Cultured Macrophages with Cholesterol Acceptors Reduces PG-Induced TLR4 Translocation to Lipid Rafts
Cellular lipid raft disruption occurs when cholesterol is removed from cells, and we and others have shown that apoAI- and HDL-mediated cholesterol efflux is a robust method to promote lipid raft disruption [33,35,36]. Thus, we wanted to analyze whether pretreating cultured macrophages with the cholesterol acceptors apoAI or HDL decreases TLR4 translocation to lipid rafts when macrophages later become exposed to PG. In cultured macrophages that were first incubated with either apoAI (Figure 2A) or HDL (Figure 2B) before being challenged with PG, we observed a reduction in TLR4 translocation to lipid rafts when compared to the respective vehicle control pretreated macrophages later incubated with PG. Since apoAI acts as the exclusive cholesterol acceptor for ABCA1, and ABCG1 can participate in cholesterol efflux via interacting with HDL [30], we concluded from these findings that ABC-transporter-dependent cholesterol efflux is able to decrease TLR4 translocation to lipid rafts in macrophages exposed to PG through stimulating lipid raft disruption.
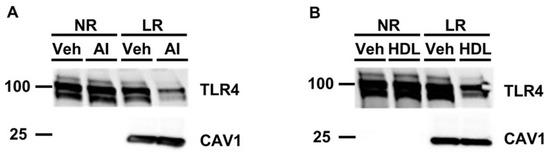
Figure 2.
Exposing cultured macrophages to apoAI or HDL before PG stimulation decreases TLR4 translocation to lipid rafts. (A,B) Cultured macrophages were either pre-incubated with vehicle only (Veh), apoAI (“AI” shown in panel A), or HDL (B) before being challenged with PG. Pooled non-raft (NR) and lipid raft (LR) fractions were collected from treated macrophages to probe for TLR4 and the lipid raft marker caveolin-1 (CAV1) via immunoblotting. Size markers are in kDa.
3.3. ApoAI- and HDL-Mediated Cholesterol Efflux Decreases TLR4-Target Gene Expression in Cultured Macrophages Exposed To PG
Our team and other laboratories have shown that if LPS-mediated TLR4 translocation to lipid rafts becomes inhibited, then this attenuates the expression of pro-inflammatory TLR4-target genes [33,34,35]. Hence, in this study, we wanted to assess if macrophage cholesterol efflux may reduce the expression of TLR4-target genes in these cells when they are exposed to PG. Using RT-qPCR, we measured mRNA expression of the well-established TLR4-target genes IL-1β, IL-6, and TNF-α [62] in macrophages challenged with PG and either pretreated with the cholesterol acceptors apoAI and HDL or pretreated with vehicle only. In these experiments, we observed a significant decrease in the expression of all pro-inflammatory genes within the cultured macrophages pretreated with the cholesterol acceptors when compared to the respective vehicle control pretreatment groups (Figure 3). From these results, we conclude that incubating macrophages with apoAI and HDL attenuates the inflammatory response which normally occurs in these cells upon exposure to T. brucei and that this effect is likely due to ABCA1/apoAI- and ABCG1/HDL-mediated cholesterol efflux promoting lipid raft disruption which reduces macrophage TLR4 translocation to lipid rafts.
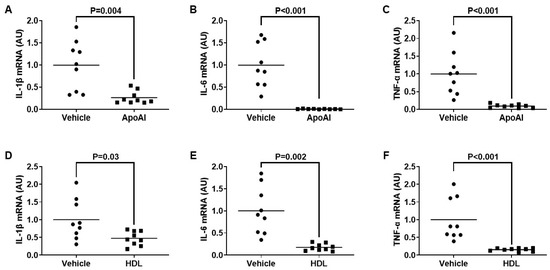
Figure 3.
Pretreating cultured macrophages with cholesterol acceptors attenuates TLR4-target gene expression induced by PG exposure. (A–F) TLR4 target genes IL-1® (A,D), IL-6 (B,E), and TNF-⟨ (C,F) were measured in control macrophages pretreated with vehicle only (A–F), or macrophages pretreated with either apoAI (A–C) or HDL (D–F), before all macrophages were incubated with PG. (A–F) Gene expression measured via qRT-PCR and AU = arbitrary units. Data points are from three independent treatments that include three biological replicates for each respective treatment. Bars are group means.
4. Discussion
In our study, we wanted to assess a possible pro-inflammatory impact that the cell surface of T. brucei may induce in macrophages and if this potential pro-inflammatory response is regulated by TLR4. To capture the entire cell surface landscape of T. brucei, we prepared PG from BF-427 so that parasite viability and the intracellular contents of T. brucei may be controlled when analyzing inflammation in cultured macrophages. When we exposed cultured macrophages to PG, we observed an increase in TLR4 translocation to lipid rafts within these cells, which strongly suggests certain cell surface components of T. brucei serve as a ligand for TLR4. Because TLR4 translocation to lipid rafts is required to robustly trigger a pro-inflammatory immune response [63], we then wanted to directly test whether apoAI- and HDL-mediated cholesterol efflux can confer protection against inflammation within macrophages exposed to PG via reducing TLR4 translocation to lipid rafts, as ABCA1/apoAI- and ABCG1/HDL-mediated cholesterol efflux enhances lipid raft disruption [64]. When macrophages were incubated with either apoAI or HDL before PG exposure, we did observe decreased macrophage TLR4 translocation to lipid rafts, which indicates apoAI- and HDL-mediated cholesterol efflux reduces TLR4 translocation to lipid rafts via promoting lipid raft disruption. Furthermore, when we measured the expression of the TLR4-target genes IL-1β, IL-6, and TNF-α in these same conditions, we detected decreased mRNA expression of all pro-inflammatory TLR4-target genes within the cultured macrophages pretreated with either apoAI or HDL when compared to respective control macrophages pretreated with vehicle only. Taken together, these results suggest ABCA1/ABCG1-dependent cholesterol efflux within macrophages reduces the pro-inflammatory response that occurs when becoming infected with T. brucei. If so, then cholesterol efflux could be considered a potential novel host anti-inflammatory process occurring during T. brucei infection (Figure 4).
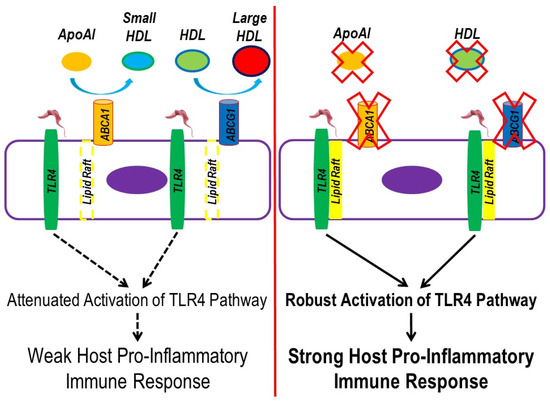
Figure 4.
Working model to propose ABC-transporter mediated cholesterol efflux reduces the pro-inflammatory immune response that initially occurs from T. brucei infection. (Left panel) Functional ABCA1 and ABCG1 efflux cholesterol upon interaction with apoAI and HDL, which promotes lipid raft disruption. During T. brucei infection, ligands on the parasite surface bind to macrophage surface TLR4. However, TLR4 translocation to lipid rafts becomes minimized upon enhanced macrophage ABCA1/apoAI- and ABCG1/HDL-mediated cholesterol efflux. The reduced abundance of lipid rafts in macrophages attenuates activation of the TLR4 signaling pathway, weakening the host pro-inflammatory immune response that normally occurs in T. brucei infection. (Right panel) Impaired ABCA1/apoAI- and ABCG1/HDL-mediated cholesterol efflux in macrophages stabilizes lipid rafts within macrophages, enhancing TLR4 translocation and causing robust activation of the TLR4 signaling pathway in response to T. brucei ligands, which results in a strong host pro-inflammatory immune response.
Though it is well-recognized that infection with T. brucei induces pro-inflammatory immune responses within infected hosts, the processes that govern these responses in hosts are complicated and not entirely well-defined. For instance, while data have shown that the spleen and other cells of the reticuloendothelial system play a large role in modulating the host pro-inflammatory during T. brucei infection [65], emerging evidence has shown that adipocytes exhibit a robust pro-inflammatory immune response in hosts infected with T. brucei [66]. This is intriguing, as adipose tissue has traditionally been thought to be a mostly benign tissue with low hormonal activity, even during times of illness and infection [67,68,69,70,71,72,73]. Other data have observed that the brain and central nervous system, lungs, skin, and reproductive system are intricately related to the immune response that mounts in hosts during T. brucei infection [74,75,76,77,78,79,80,81,82,83,84,85,86]. With so many cell and tissue types being at least indirectly involved with the pro-inflammatory immune response that occurs from T. brucei infection, it is very likely that there are numerous inflammatory and immune factors also present during infection with T. brucei. It is already recognized that an innate immune response occurs during T. brucei infection and that this effect may be TLR-mediated [23,25,26,87]. In this study, we clearly show that non-viable PG are effective in triggering a pro-inflammatory response within cultured macrophages by acting as a ligand for TLR4, which may partially explain how T. brucei activates the innate immune system in infected mammals.
Therapies for human African trypanosomiasis vary based on whether infection has been restricted to the hemolymphatic system, which is known as the first stage of infection, or whether the disease has advanced via parasites crossing the blood–brain barrier to reach the host brain and central nervous system, which is defined as the second meningoencephalitis phase [88]. The second phase is notorious to treat effectively due to certain traditional therapies being extremely toxic to the hosts, sometimes leading to fatality [3,6,88,89]. Although more novel polytherapies have been developed (e.g., nifurtimox-eflornithine combination therapy), the therapeutic regimen of these types of treatments is very complicated, requiring intensive training, labor, and resources for appropriate administration [90,91]. For newer therapies to combat African trypanosomiasis, it is important to note that some treatments may be contraindicated in certain patients, as well as being considered to be cost-prohibitive [88,92,93,94,95,96,97,98,99,100]. Moreover, there are serious potential issues about drug resistance with newer types of treatments for human African trypanosomiasis [95,97,101,102]. Interestingly, the mechanisms of actions for treatments involving African trypanosomiasis are diverse [88], but none appear to directly influence cellular cholesterol efflux and metabolism. While it is not currently known if manipulating cell cholesterol content in hosts infected with T. brucei is either beneficial or harmful to combating disease progression, there is evidence that suggests infectivity with the trypanosomatid parasite Lelishmania donovani depends on host–cell cholesterol metabolism, though these data are convoluted. Indeed, the pathogenesis of L. donovani appears to be enhanced when cells contain high levels of cholesterol, but after disease initiation and especially during the later stages of L. donovani infection, virulence seems to become advanced when host cellular cholesterol becomes depleted [103,104,105,106,107,108,109,110,111]. If cellular cholesterol efflux does impact the pro-inflammatory immune response that occurs within infected hosts during T. brucei infection via inhibiting TLR4-target gene expression through lipid raft disruption, then a similar quandary may exist for host–cell cholesterol metabolism influencing T. brucei pathogenicity. It has been proposed that the initial pro-inflammatory immune response against T. brucei is beneficial, but in the more advanced stages of disease, this response is considered to be deleterious [58,85,86,112,113].
There are limitations in our study. One main limitation is using the RAW 264.7 mouse macrophage cell line in our experiments instead of utilizing primary macrophages. While we have experience working with this macrophage cell line, and RAW 264.7 macrophages contain lipid rafts, robustly express ABCA1 and ABCG1, efflux cholesterol to both apoAI and HDL, and demonstrate a strong pro-inflammatory response to TLR4 ligands such as LPS [44,114,115,116,117], we acknowledge that data generated from immortalized cells may not be comparable to primary cells, as immortalized cell lines sometimes lack similar characteristics and properties as primary cells [118]. Therefore, future studies should be conducted to confirm that the cell surface of T. brucei does serve as a ligand for TLR4 in primary macrophages. Another major limitation of our study is not directly testing the potential pro-inflammatory effects which occur in cultured macrophages exposed to PG that are not mediated by TLR4. A strong strategy to test for this in the future is to utilize TLR4 knockout macrophages derived from TLR4 knockout mice [119,120]. It is certainly possible that the cell surface of T. brucei may also robustly induce the activation of cell surface TLR2 and respective pro-inflammatory genes, which would be a novel finding. In this scenario, future studies should also employ TLR2 knockout macrophages derived from TLR2 knockout mice [119,121] to gauge the pro-inflammatory impact of TLR4 versus TLR2 activation and induction of their respective pro-inflammatory target genes in cultured macrophages exposed to PG. This would enable an assessment of what surface TLR is the main driver in stimulating macrophage inflammation upon exposure to T. brucei surface ligands. Lastly, both apoAI and HDL have been proposed to exhibit anti-inflammatory effects that are independent of their contribution to lipid raft disruption via cholesterol efflux. Therefore, future studies should also be conducted by utilizing ABCA1 and ABCG1 knockout macrophages [122] to directly analyze what possible anti-inflammatory effects occur within cultured macrophages exposed to PG that have been pretreated with the cholesterol acceptors apoAI and HDL, as the ablation of ABCA1/ABCG1 would prevent apoAI/HDL-mediated cholesterol efflux.
5. Conclusions
Our study shows that incubating macrophages with either apoAI or HDL attenuates pro-inflammatory TLR4-target gene expression that is stimulated from T. brucei exposure and that this effect is likely due to ABCA1- and ABCG1-dependent cholesterol efflux. Future studies should focus on which precise surface molecule(s) of T. brucei are responsible for TLR4 activation and whether manipulating apoAI/HDL levels or ABC-transporter expression in animal models infected with T. brucei impacts pro-inflammatory immune responses within these hosts. If modulating cholesterol efflux does alter both pro-inflammatory immune response and parasite load, then possible treatments for African trypanosomiasis may include therapies that can alter apoAI/HDL plasma levels and/or change cellular ABC-transporter expression [44,53,55,123,124,125,126,127], as efficacy for these types of proposed treatments can easily be analyzed within a pre-clinical setting.
Author Contributions
Conceptualization, A.S.; methodology, A.S., R.R.P., T.B., A.P., N.P., J.S., K.P. and K.S.P.; formal analysis, A.S., L.F., J.E.-C., M.M., R.R.P., T.B. and K.S.P.; investigation, A.S., L.F., J.E.-C., M.M., R.R.P., A.P., N.P., J.S. and K.P.; resources, A.S., T.B. and K.S.P.; writing—original draft preparation, A.S., R.R.P., K.S.P. and L.F.; writing—review and editing, A.S.; supervision, A.S., T.B. and K.S.P.; funding acquisition, A.S. and K.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by NIH Eukaryotic Pathogens Innovation Center (EPIC) Centers of Biomedical Research Excellence (COBRE) grants P20GM146584 and P20GM109094-01, as well as USDA-NIFA Hatch Project (project no. SC-1700577; accession no. 1021291). Imaging analyses was conducted at CLIF, and this facility is also supported in part by the NIH EPIC COBRE grant P20GM146584, as well as an NIH SC BioCRAFT COBRE grant 5P30GM131959 and the Clemson University Division of Research. The CLIF Leica SP8X confocal microscope equipment is partially supported by NSF MRI grants 1126407 and 1920095. Jing Echesabal-Chen was partially supported by a CU-FELLOWS R-Initiatives fellowship provided by the Clemson University Division of Research, and Murphy Miller was supported by the Medical Enrichment Through Opportunities in Research (MEnTOR) NIH/NIAID T35 training grant program.
Data Availability Statement
All data that are represented within this study are contained in the manuscript.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dean, S. Basic Biology of Trypanosoma brucei with Reference to the Development of Chemotherapies. Curr. Pharm. Des. 2021, 27, 1650–1670. [Google Scholar] [CrossRef] [PubMed]
- Malvy, D.; Chappuis, F. Sleeping sickness. Clin. Microbiol. Infect. 2011, 17, 986–995. [Google Scholar] [CrossRef]
- Hollingshead, C.M.; Bermudez, R. Human African Trypanosomiasis (Sleeping Sickness). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jacobs, R.T.; Nare, B.; Phillips, M.A. State of the art in African trypanosome drug discovery. Curr. Top. Med. Chem. 2011, 11, 1255–1274. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Ortiz, J.F.; Fabara, S.P.; Eissa-Garces, A.; Reddy, D.; Collins, K.D.; Tirupathi, R. Efficacy and Toxicity of Fexinidazole and Nifurtimox Plus Eflornithine in the Treatment of African Trypanosomiasis: A Systematic Review. Cureus 2021, 13, e16881. [Google Scholar] [CrossRef]
- Brun, R.; Schumacher, R.; Schmid, C.; Kunz, C.; Burri, C. The phenomenon of treatment failures in Human African Trypanosomiasis. Trop. Med. Int. Health 2001, 6, 906–914. [Google Scholar] [CrossRef]
- Bouteille, B.; Oukem, O.; Bisser, S.; Dumas, M. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 2003, 17, 171–181. [Google Scholar] [CrossRef]
- Mulenga, G.M.; Henning, L.; Chilongo, K.; Mubamba, C.; Namangala, B.; Gummow, B. Insights into the Control and Management of Human and Bovine African Trypanosomiasis in Zambia between 2009 and 2019—A Review. Trop. Med. Infect. Dis. 2020, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, A.; Jawo, E.; Amoutchi, A.I.; Koua, H.; Kuye, R.; Dabre, Z.; Bojang, A.; Vieira, R.F.C. Knowledge, Attitude, and Practice of Livestock Owners and Livestock Assistants towards African Trypanosomiasis Control in The Gambia. J. Parasitol. Res. 2022, 2022, 3379804. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; HP, D.E.K.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thevenon, S.; Bossard, G.; Boulange, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. A review on the diagnosis of animal trypanosomoses. Parasit. Vectors 2022, 15, 64. [Google Scholar] [CrossRef]
- Ralston, K.S.; Kabututu, Z.P.; Melehani, J.H.; Oberholzer, M.; Hill, K.L. The Trypanosoma brucei flagellum: Moving parasites in new directions. Annu. Rev. Microbiol. 2009, 63, 335–362. [Google Scholar] [CrossRef] [PubMed]
- Galvao-Castro, B.; Hochmann, A.; Lambert, P.H. The role of the host immune response in the development of tissue lesions associated with African trypanosomiasis in mice. Clin. Exp. Immunol. 1978, 33, 12–24. [Google Scholar] [PubMed]
- Seyfang, A.; Mecke, D.; Duszenko, M. Degradation, recycling, and shedding of Trypanosoma brucei variant surface glycoprotein. J. Protozool. 1990, 37, 546–552. [Google Scholar] [CrossRef]
- Cardoso de Almeida, M.L.; Turner, M.J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature 1983, 302, 349–352. [Google Scholar] [CrossRef]
- Sheader, K.; Vaughan, S.; Minchin, J.; Hughes, K.; Gull, K.; Rudenko, G. Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc. Natl. Acad. Sci. USA 2005, 102, 8716–8721. [Google Scholar] [CrossRef] [PubMed]
- Baral, T.N. Immunobiology of African trypanosomes: Need of alternative interventions. J. Biomed. Biotechnol. 2010, 2010, 389153. [Google Scholar] [CrossRef]
- Onyilagha, C.; Uzonna, J.E. Host Immune Responses and Immune Evasion Strategies in African Trypanosomiasis. Front. Immunol. 2019, 10, 2738. [Google Scholar] [CrossRef]
- Dos-Santos, A.L.; Carvalho-Kelly, L.F.; Dick, C.F.; Meyer-Fernandes, J.R. Innate immunomodulation to trypanosomatid parasite infections. Exp. Parasitol. 2016, 167, 67–75. [Google Scholar] [CrossRef]
- Donelson, J.E.; Hill, K.L.; El-Sayed, N.M. Multiple mechanisms of immune evasion by African trypanosomes. Mol. Biochem. Parasitol. 1998, 91, 51–66. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, M.; Field, M.C. Trypanosoma brucei: Trypanosome-specific endoplasmic reticulum proteins involved in variant surface glycoprotein expression. Exp. Parasitol. 2010, 125, 208–221. [Google Scholar] [CrossRef]
- Hertz, C.J.; Filutowicz, H.; Mansfield, J.M. Resistance to the African trypanosomes is IFN-gamma dependent. J. Immunol. 1998, 161, 6775–6783. [Google Scholar] [CrossRef] [PubMed]
- Drennan, M.B.; Stijlemans, B.; Van den Abbeele, J.; Quesniaux, V.J.; Barkhuizen, M.; Brombacher, F.; De Baetselier, P.; Ryffel, B.; Magez, S. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J. Immunol. 2005, 175, 2501–2509. [Google Scholar] [CrossRef]
- Debierre-Grockiego, F. Glycolipids are potential targets for protozoan parasite diseases. Trends Parasitol. 2010, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, N.; Sang, X.; Feng, Y.; Chen, R.; Chen, Q. Trypanosoma brucei Lipophosphoglycan Induces the Formation of Neutrophil Extracellular Traps and Reactive Oxygen Species Burst via Toll-Like Receptor 2, Toll-Like Receptor 4, and c-Jun N-Terminal Kinase Activation. Front. Microbiol. 2021, 12, 713531. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, N.; Zhang, N.; Yu, L.; Sang, X.; Feng, Y.; Chen, R.; Chen, Q. Trypanosoma brucei Lipophosphoglycan Activates Host Immune Responses via the TLR-mediated p38 MAP Kinase and NF-κB Pathways. Zoonoses 2023, 3, 991. [Google Scholar] [CrossRef]
- Macaskill, J.A.; Holmes, P.H.; Whitelaw, D.D.; McConnell, I.; Jennings, F.W.; Urquhart, G.M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology 1980, 40, 629–635. [Google Scholar]
- Magez, S.; Pinto Torres, J.E.; Obishakin, E.; Radwanska, M. Infections With Extracellular Trypanosomes Require Control by Efficient Innate Immune Mechanisms and Can Result in the Destruction of the Mammalian Humoral Immune System. Front. Immunol. 2020, 11, 382. [Google Scholar] [CrossRef]
- Fang, H.; Pengal, R.A.; Cao, X.; Ganesan, L.P.; Wewers, M.D.; Marsh, C.B.; Tridandapani, S. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J. Immunol. 2004, 173, 360–366. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef]
- Zhu, X.; Owen, J.S.; Wilson, M.D.; Li, H.; Griffiths, G.L.; Thomas, M.J.; Hiltbold, E.M.; Fessler, M.B.; Parks, J.S. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010, 51, 3196–3206. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, J.Y.; Timmins, J.M.; Brown, J.M.; Boudyguina, E.; Mulya, A.; Gebre, A.K.; Willingham, M.C.; Hiltbold, E.M.; Mishra, N.; et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008, 283, 22930–22941. [Google Scholar] [CrossRef] [PubMed]
- Stamatikos, A.; Dronadula, N.; Ng, P.; Palmer, D.; Knight, E.; Wacker, B.K.; Tang, C.; Kim, F.; Dichek, D.A. ABCA1 Overexpression in Endothelial Cells In Vitro Enhances ApoAI-Mediated Cholesterol Efflux and Decreases Inflammation. Hum. Gene Ther. 2019, 30, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Tsuchiya, K.; Tattersall, I.W.; Fotakis, P.; Bochem, A.E.; Molusky, M.M.; Ntonga, V.; Abramowicz, S.; Parks, J.S.; Welch, C.L.; et al. Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Endothelial Cells Accelerates Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1328–1337. [Google Scholar] [CrossRef]
- Cheng, A.M.; Handa, P.; Tateya, S.; Schwartz, J.; Tang, C.; Mitra, P.; Oram, J.F.; Chait, A.; Kim, F. Apolipoprotein A-I attenuates palmitate-mediated NF-kappaB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS ONE 2012, 7, e33917. [Google Scholar] [CrossRef]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Han, C.Y.; Mitra, P.; Averill, M.M.; Tang, C.; Goodspeed, L.; Omer, M.; Subramanian, S.; Wang, S.; Den Hartigh, L.J.; et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ. Res. 2013, 112, 1345–1354. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Bi, X.; Vitali, C.; Cuchel, M. ABCA1 and Inflammation: From Animal Models to Humans. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1551–1553. [Google Scholar] [CrossRef]
- Paulnock, D.M.; Coller, S.P. Analysis of macrophage activation in African trypanosomiasis. J. Leukoc. Biol. 2001, 69, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, S.M.; Singh, R.; Uzonna, J.E. Host Intracellular Signaling Events and Pro-inflammatory Cytokine Production in African Trypanosomiasis. Front. Immunol. 2016, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Echesabal-Chen, J.; Huang, K.; Vojtech, L.; Oladosu, O.; Esobi, I.; Sachdeva, R.; Vyavahare, N.; Jo, H.; Stamatikos, A. Constructing Lipoparticles Capable of Endothelial Cell-Derived Exosome-Mediated Delivery of Anti-miR-33a-5p to Cultured Macrophages. Curr. Issues Mol. Biol. 2023, 45, 5631–5644. [Google Scholar] [CrossRef] [PubMed]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, I.O.; Ugwu, E.N.; Enemuo, O.V.; Obi, C.F.; Iheagwam, C.N.; Ezeokonkwo, R.C.; Onah, D.N. Efficacy of repeated doses of diminazene aceturate (Dinazene((R))) in the treatment of experimental Trypanosoma brucei infection of Albino rats. Iran. J. Vet. Res. 2016, 17, 124–129. [Google Scholar]
- Esobi, I.C.; Barksdale, C.; Heard-Tate, C.; Reigers Powell, R.; Bruce, T.F.; Stamatikos, A. MOVAS Cells: A Versatile Cell Line for Studying Vascular Smooth Muscle Cell Cholesterol Metabolism. Lipids 2021, 56, 413–422. [Google Scholar] [CrossRef]
- Esobi, I.C.; Oladosu, O.; Echesabal-Chen, J.; Powell, R.R.; Bruce, T.; Stamatikos, A. miR-33a Expression Attenuates ABCA1-Dependent Cholesterol Efflux and Promotes Macrophage-Like Cell Transdifferentiation in Cultured Vascular Smooth Muscle Cells. J. Lipids 2023, 2023, 8241899. [Google Scholar] [CrossRef]
- Oladosu, O.; Esobi, I.C.; Powell, R.R.; Bruce, T.; Stamatikos, A. Dissecting the Impact of Vascular Smooth Muscle Cell ABCA1 versus ABCG1 Expression on Cholesterol Efflux and Macrophage-like Cell Transdifferentiation: The Role of SR-BI. J. Cardiovasc. Dev. Dis. 2023, 10, 416. [Google Scholar] [CrossRef]
- Esobi, I.; Olanrewaju, O.; Echesabal-Chen, J.; Stamatikos, A. Utilizing the LoxP-Stop-LoxP System to Control Transgenic ABC-Transporter Expression In Vitro. Biomolecules 2022, 12, 679. [Google Scholar] [CrossRef]
- Oladosu, O.; Chin, E.; Barksdale, C.; Powell, R.R.; Bruce, T.; Stamatikos, A. Inhibition of miR-33a-5p in Macrophage-like Cells In Vitro Promotes apoAI-Mediated Cholesterol Efflux. Pathophysiology 2024, 31, 117–126. [Google Scholar] [CrossRef]
- Huang, K.; Garimella, S.; Clay-Gilmour, A.; Vojtech, L.; Armstrong, B.; Bessonny, M.; Stamatikos, A. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. J. Pers. Med. 2022, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Stamatikos, A.; Knight, E.; Vojtech, L.; Bi, L.; Wacker, B.K.; Tang, C.; Dichek, D.A. Exosome-Mediated Transfer of Anti-miR-33a-5p from Transduced Endothelial Cells Enhances Macrophage and Vascular Smooth Muscle Cell Cholesterol Efflux. Hum. Gene Ther. 2020, 31, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Jo, H.; Echesabal-Chen, J.; Stamatikos, A. Combined LXR and RXR Agonist Therapy Increases ABCA1 Protein Expression and Enhances ApoAI-Mediated Cholesterol Efflux in Cultured Endothelial Cells. Metabolites 2021, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Pitman, M.; Oladosu, O.; Echesabal-Chen, J.; Vojtech, L.; Esobi, I.; Larsen, J.; Jo, H.; Stamatikos, A. The Impact of MiR-33a-5p Inhibition in Pro-Inflammatory Endothelial Cells. Diseases 2023, 11, 88. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Pays, E.; Nolan, D.P. Genetic and immunological basis of human African trypanosomiasis. Curr. Opin. Immunol. 2021, 72, 13–20. [Google Scholar] [CrossRef]
- Stijlemans, B.; Caljon, G.; Van Den Abbeele, J.; Van Ginderachter, J.A.; Magez, S.; De Trez, C. Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Front. Immunol. 2016, 7, 233. [Google Scholar] [CrossRef]
- Yu, L.; Li, Q.; Jiang, N.; Fan, R.; Zhang, N.; Zhang, Y.; Sun, W.; Chen, R.; Feng, Y.; Sang, X.; et al. Toll-like receptor 9 signaling is associated with immune responses to Trypanosoma brucei infection. Int. Immunopharmacol. 2024, 134, 112250. [Google Scholar] [CrossRef]
- Latz, E.; Visintin, A.; Espevik, T.; Golenbock, D.T. Mechanisms of TLR9 activation. J. Endotoxin Res. 2004, 10, 406–412. [Google Scholar] [CrossRef]
- Lee, B.L.; Barton, G.M. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014, 24, 360–369. [Google Scholar] [CrossRef]
- Degirmenci, I.; Ozbayer, C.; Kebapci, M.N.; Kurt, H.; Colak, E.; Gunes, H.V. Common variants of genes encoding TLR4 and TLR4 pathway members TIRAP and IRAK1 are effective on MCP1, IL6, IL1beta, and TNFalpha levels in type 2 diabetes and insulin resistance. Inflamm. Res. 2019, 68, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid rafts as a therapeutic target. J. Lipid Res. 2020, 61, 687–695. [Google Scholar] [CrossRef]
- Ponte-Sucre, A. An Overview of Trypanosoma brucei Infections: An Intense Host-Parasite Interaction. Front. Microbiol. 2016, 7, 2126. [Google Scholar] [CrossRef]
- Machado, H.; Bizarra-Rebelo, T.; Costa-Sequeira, M.; Trindade, S.; Carvalho, T.; Rijo-Ferreira, F.; Rentroia-Pacheco, B.; Serre, K.; Figueiredo, L.M. Trypanosoma brucei triggers a broad immune response in the adipose tissue. PLoS Pathog. 2021, 17, e1009933. [Google Scholar] [CrossRef]
- An, S.M.; Cho, S.H.; Yoon, J.C. Adipose Tissue and Metabolic Health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Adipocytes. Curr. Biol. 2014, 24, R988–R993. [Google Scholar] [CrossRef][Green Version]
- Harvey, I.; Boudreau, A.; Stephens, J.M. Adipose tissue in health and disease. Open Biol. 2020, 10, 200291. [Google Scholar] [CrossRef]
- Nakao, K. Adiposcience and adipotoxicity. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 63. [Google Scholar] [CrossRef][Green Version]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: Dartmouth, MA, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK555602/ (accessed on 16 August 2024).
- Mabille, D.; Dirkx, L.; Thys, S.; Vermeersch, M.; Montenye, D.; Govaerts, M.; Hendrickx, S.; Takac, P.; Van Weyenbergh, J.; Pintelon, I.; et al. Impact of pulmonary African trypanosomes on the immunology and function of the lung. Nat. Commun. 2022, 13, 7083. [Google Scholar] [CrossRef]
- Carvalho, T.; Trindade, S.; Pimenta, S.; Santos, A.B.; Rijo-Ferreira, F.; Figueiredo, L.M. Trypanosoma brucei triggers a marked immune response in male reproductive organs. PLoS Negl. Trop. Dis. 2018, 12, e0006690. [Google Scholar] [CrossRef] [PubMed]
- Capewell, P.; Cren-Travaille, C.; Marchesi, F.; Johnston, P.; Clucas, C.; Benson, R.A.; Gorman, T.A.; Calvo-Alvarez, E.; Crouzols, A.; Jouvion, G.; et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife 2016, 5, e17716. [Google Scholar] [CrossRef]
- Quintana, J.F.; Sinton, M.C.; Chandrasegaran, P.; Lestari, A.N.; Heslop, R.; Cheaib, B.; Ogunsola, J.; Ngoyi, D.M.; Kuispond Swar, N.R.; Cooper, A.; et al. gammadelta T cells control murine skin inflammation and subcutaneous adipose wasting during chronic Trypanosoma brucei infection. Nat. Commun. 2023, 14, 5279. [Google Scholar] [CrossRef] [PubMed]
- Casas-Sanchez, A.; Acosta-Serrano, A. Skin deep. Elife 2016, 5, e21506. [Google Scholar] [CrossRef]
- Reuter, C.; Hauf, L.; Imdahl, F.; Sen, R.; Vafadarnejad, E.; Fey, P.; Finger, T.; Jones, N.G.; Walles, H.; Barquist, L.; et al. Vector-borne Trypanosoma brucei parasites develop in artificial human skin and persist as skin tissue forms. Nat. Commun. 2023, 14, 7660. [Google Scholar] [CrossRef]
- Quintana, J.F.; Chandrasegaran, P.; Sinton, M.C.; Briggs, E.M.; Otto, T.D.; Heslop, R.; Bentley-Abbot, C.; Loney, C.; de Lecea, L.; Mabbott, N.A.; et al. Single cell and spatial transcriptomic analyses reveal microglia-plasma cell crosstalk in the brain during Trypanosoma brucei infection. Nat. Commun. 2022, 13, 5752. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, C.; Xu, Y.Z.; Mumba Ngoyi, D.; Bentivoglio, M. Neural Damage in Experimental Trypanosoma brucei gambiense Infection: The Suprachiasmatic Nucleus. Front. Neuroanat. 2018, 12, 6. [Google Scholar] [CrossRef]
- Laperchia, C.; Palomba, M.; Seke Etet, P.F.; Rodgers, J.; Bradley, B.; Montague, P.; Grassi-Zucconi, G.; Kennedy, P.G.; Bentivoglio, M. Trypanosoma brucei Invasion and T-Cell Infiltration of the Brain Parenchyma in Experimental Sleeping Sickness: Timing and Correlation with Functional Changes. PLoS Negl. Trop. Dis. 2016, 10, e0005242. [Google Scholar] [CrossRef]
- Mogk, S.; Meiwes, A.; Bosselmann, C.M.; Wolburg, H.; Duszenko, M. The lane to the brain: How African trypanosomes invade the CNS. Trends Parasitol. 2014, 30, 470–477. [Google Scholar] [CrossRef]
- Frevert, U.; Movila, A.; Nikolskaia, O.V.; Raper, J.; Mackey, Z.B.; Abdulla, M.; McKerrow, J.; Grab, D.J. Early invasion of brain parenchyma by African trypanosomes. PLoS ONE 2012, 7, e43913. [Google Scholar] [CrossRef]
- Alfituri, O.A.; Quintana, J.F.; MacLeod, A.; Garside, P.; Benson, R.A.; Brewer, J.M.; Mabbott, N.A.; Morrison, L.J.; Capewell, P. To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host. Front. Immunol. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Mabille, D.; Caljon, G. Inflammation following trypanosome infection and persistence in the skin. Curr. Opin. Immunol. 2020, 66, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.N.; Vodnala, S.K.; Masocha, W.; Sun, B.; Kristensson, K.; Rottenberg, M.E. Distinct Toll-like receptor signals regulate cerebral parasite load and interferon alpha/beta and tumor necrosis factor alpha-dependent T-cell infiltration in the brains of Trypanosoma brucei-infected mice. J. Infect. Dis. 2012, 205, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, A.; Tagliazucchi, L.; Lima, C.; Venuti, F.; Malpezzi, G.; Magoulas, G.E.; Santarem, N.; Calogeropoulou, T.; Cordeiro-da-Silva, A.; Costi, M.P. Current Treatments to Control African Trypanosomiasis and One Health Perspective. Microorganisms 2022, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Priotto, G.; Pinoges, L.; Fursa, I.B.; Burke, B.; Nicolay, N.; Grillet, G.; Hewison, C.; Balasegaram, M. Safety and effectiveness of first line eflornithine for Trypanosoma brucei gambiense sleeping sickness in Sudan: Cohort study. BMJ 2008, 336, 705–708. [Google Scholar] [CrossRef]
- Yun, O.; Priotto, G.; Tong, J.; Flevaud, L.; Chappuis, F. NECT is next: Implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e720. [Google Scholar] [CrossRef]
- Jamabo, M.; Mahlalela, M.; Edkins, A.L.; Boshoff, A. Tackling Sleeping Sickness: Current and Promising Therapeutics and Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 12529. [Google Scholar] [CrossRef] [PubMed]
- Papagni, R.; Novara, R.; Minardi, M.L.; Frallonardo, L.; Panico, G.G.; Pallara, E.; Cotugno, S.; Ascoli Bartoli, T.; Guido, G.; De Vita, E.; et al. Human African Trypanosomiasis (sleeping sickness): Current knowledge and future challenges. Front. Trop. Dis. 2023, 4, 1087003. [Google Scholar] [CrossRef]
- Snijders, R.; Fukinsia, A.; Claeys, Y.; Mpanya, A.; Hasker, E.; Meheus, F.; Miaka, E.; Boelaert, M. Cost of a new method of active screening for human African trypanosomiasis in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2020, 14, e0008832. [Google Scholar] [CrossRef] [PubMed]
- Antillon, M.; Huang, C.I.; Crump, R.E.; Brown, P.E.; Snijders, R.; Miaka, E.M.; Keeling, M.J.; Rock, K.S.; Tediosi, F. Cost-effectiveness of sleeping sickness elimination campaigns in five settings of the Democratic Republic of Congo. Nat. Commun. 2022, 13, 1051. [Google Scholar] [CrossRef]
- De Koning, H.P. The Drugs of Sleeping Sickness: Their Mechanisms of Action and Resistance, and a Brief History. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Hasker, E.; Hope, A.; Bottieau, E. Gambiense human African trypanosomiasis: The bumpy road to elimination. Curr. Opin. Infect. Dis. 2022, 35, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Maser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; Akl, E.A.; Villanueva, G.; et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: Substantial changes for clinical practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 1. Anti-infective drugs for regulatory registration. Parasit. Vectors 2023, 16, 82. [Google Scholar] [CrossRef]
- Muraca, G.; Berti, I.R.; Sbaraglini, M.L.; Favaro, W.J.; Duran, N.; Castro, G.R.; Talevi, A. Trypanosomatid-Caused Conditions: State of the Art of Therapeutics and Potential Applications of Lipid-Based Nanocarriers. Front. Chem. 2020, 8, 601151. [Google Scholar] [CrossRef]
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-trypanosomatid drug discovery: Progress and challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. [Google Scholar] [CrossRef]
- Steketee, P.C.; Giordani, F.; Vincent, I.M.; Crouch, K.; Achcar, F.; Dickens, N.J.; Morrison, L.J.; MacLeod, A.; Barrett, M.P. Transcriptional differentiation of Trypanosoma brucei during in vitro acquisition of resistance to acoziborole. PLoS Negl. Trop. Dis. 2021, 15, e0009939. [Google Scholar] [CrossRef]
- Bouazizi-Ben Messaoud, H.; Guichard, M.; Lawton, P.; Delton, I.; Azzouz-Maache, S. Changes in Lipid and Fatty Acid Composition During Intramacrophagic Transformation of Leishmania donovani Complex Promastigotes into Amastigotes. Lipids 2017, 52, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Banerjee, S.; Sen, A.; Banerjee, K.K.; Das, P.; Roy, S. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol. 2005, 175, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Bose, M.; Roy, S.; Bhattacharyya, S.N. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe 2013, 13, 277–288. [Google Scholar] [CrossRef]
- Lal, C.S.; Kumar, A.; Kumar, S.; Pandey, K.; Kumar, N.; Bimal, S.; Sinha, P.K.; Das, P. Hypocholesterolemia and increased triglyceride in pediatric visceral leishmaniasis. Clin. Chim. Acta 2007, 382, 151–153. [Google Scholar] [CrossRef]
- Majumder, S.; Dey, R.; Bhattacharjee, S.; Rub, A.; Gupta, G.; Bhattacharyya Majumdar, S.; Saha, B.; Majumdar, S. Leishmania-induced biphasic ceramide generation in macrophages is crucial for uptake and survival of the parasite. J. Infect. Dis. 2012, 205, 1607–1616. [Google Scholar] [CrossRef]
- Pucadyil, T.J.; Chattopadhyay, A. Cholesterol: A potential therapeutic target in Leishmania infection? Trends Parasitol. 2007, 23, 49–53. [Google Scholar] [CrossRef]
- Roy, K.; Mandloi, S.; Chakrabarti, S.; Roy, S. Cholesterol Corrects Altered Conformation of MHC-II Protein in Leishmania donovani Infected Macrophages: Implication in Therapy. PLoS Negl. Trop. Dis. 2016, 10, e0004710. [Google Scholar] [CrossRef] [PubMed]
- Semini, G.; Paape, D.; Paterou, A.; Schroeder, J.; Barrios-Llerena, M.; Aebischer, T. Changes to cholesterol trafficking in macrophages by Leishmania parasites infection. Microbiologyopen 2017, 6, e00469. [Google Scholar] [CrossRef]
- Winberg, M.E.; Holm, A.; Sarndahl, E.; Vinet, A.F.; Descoteaux, A.; Magnusson, K.E.; Rasmusson, B.; Lerm, M. Leishmania donovani lipophosphoglycan inhibits phagosomal maturation via action on membrane rafts. Microbes Infect. 2009, 11, 215–222. [Google Scholar] [CrossRef]
- Garzon, E.; Holzmuller, P.; Bras-Goncalves, R.; Vincendeau, P.; Cuny, G.; Lemesre, J.L.; Geiger, A. The Trypanosoma brucei gambiense secretome impairs lipopolysaccharide-induced maturation, cytokine production, and allostimulatory capacity of dendritic cells. Infect. Immun. 2013, 81, 3300–3308. [Google Scholar] [CrossRef]
- Stijlemans, B.; Leng, L.; Brys, L.; Sparkes, A.; Vansintjan, L.; Caljon, G.; Raes, G.; Van Den Abbeele, J.; Van Ginderachter, J.A.; Beschin, A.; et al. MIF contributes to Trypanosoma brucei associated immunopathogenicity development. PLoS Pathog. 2014, 10, e1004414. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.; Sundler, R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 2006, 43, 607–612. [Google Scholar] [CrossRef]
- Park, Y.; Pham, T.X.; Lee, J. Lipopolysaccharide represses the expression of ATP-binding cassette transporter G1 and scavenger receptor class B, type I in murine macrophages. Inflamm. Res. 2012, 61, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Feingold, K.R.; Moser, A.H.; Grunfeld, C. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis 1993, 98, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 14, 14.1.1–14.1.14. [Google Scholar] [CrossRef]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef]
- Marquart, T.J.; Allen, R.M.; Ory, D.S.; Baldan, A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 12228–12232. [Google Scholar] [CrossRef] [PubMed]
- Wacker, B.K.; Dronadula, N.; Bi, L.; Stamatikos, A.; Dichek, D.A. Apo A-I (Apolipoprotein A-I) Vascular Gene Therapy Provides Durable Protection Against Atherosclerosis in Hyperlipidemic Rabbits. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Brewer, H.B., Jr.; Schaefer, E.J.; Foldyna, B.; Ghoshhajra, B.B. High-density lipoprotein infusion therapy: A review. J. Clin. Lipidol. 2024, 18, e374–e383. [Google Scholar] [CrossRef]
- Gibson, C.M.; Duffy, D.; Korjian, S.; Bahit, M.C.; Chi, G.; Alexander, J.H.; Lincoff, A.M.; Heise, M.; Tricoci, P.; Deckelbaum, L.I.; et al. Apolipoprotein A1 Infusions and Cardiovascular Outcomes after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J. Apolipoprotein A-I Infusion Therapies for Coronary Disease: Two Outs in the Ninth Inning and Swinging for the Fences. JAMA Cardiol. 2018, 3, 799–801. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).