Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis
Abstract
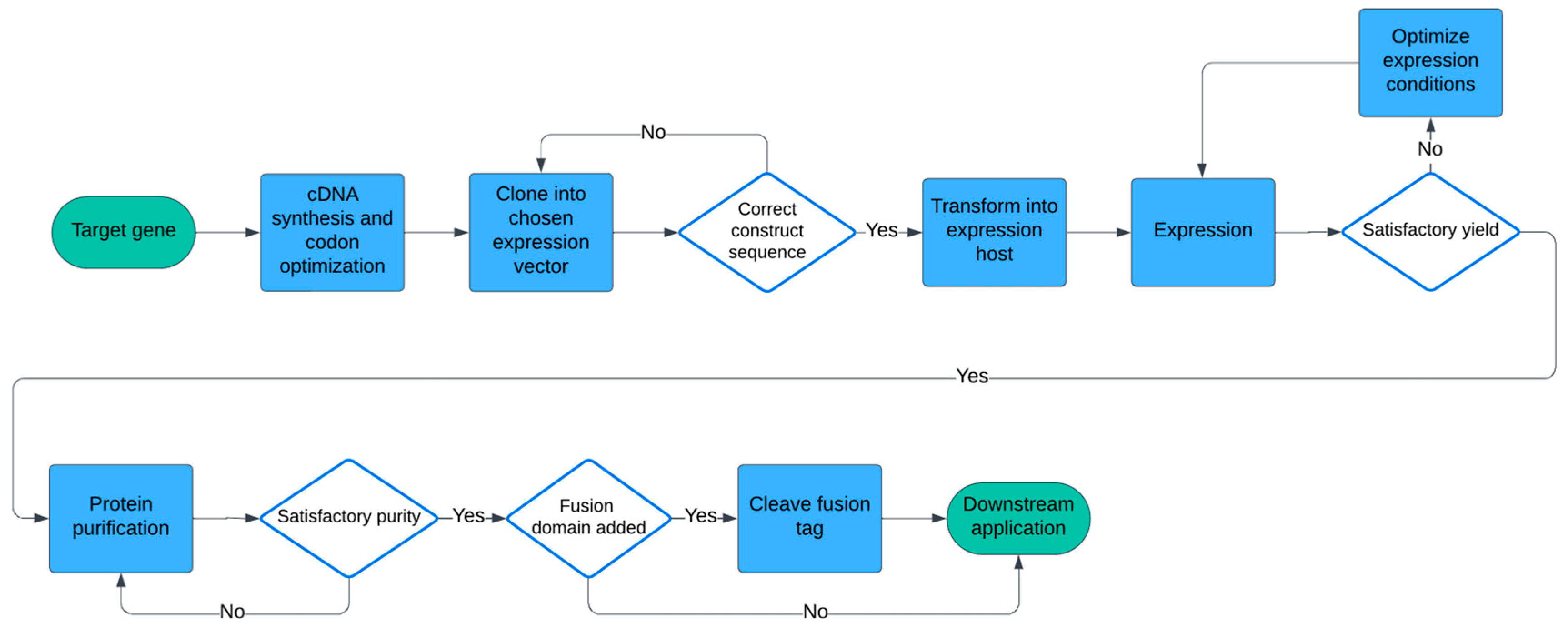
1. Introduction
2. Materials and Methods
3. Prokaryotic Expression Systems
3.1. E. coli Expression Systems
T7 System
| Plasmid Vector | E. coli Host Strain | Protein | Expression Conditions | Yield | Application | Results | Reference, Year | |
|---|---|---|---|---|---|---|---|---|
| Diagnostic | Vaccine | |||||||
| pET29 | BL21 (DE3) pLysS | S-B10a-6xHis | 37 °C, 3 h | − | + | − | Strong immunoreactivity with human sera from both chronic and acute infections. | 1998, [49] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-GRA630–231-6xHis 6xHis-P35b26–170-6xHis 6xHis-SAG230–170-6xHis | LB, 30 °C, 8 h LB, 30 °C, 8 h LB, 37 °C, 3 h | 60–80 mg/L induced bacterial culture | + | − | Both r-GRA6 and r-p35 antigens detected antibodies more frequently (p < 0.01) from acute (93.9 and 87.9%) rather than chronic (60.6 and 53.0%) infections. The r-SAG2 gave a similar sensitivity in both groups of patients (93.9 and 95.5%) Both r-GRA6 and r-p35 antigens detected antibodies more frequently (p < 0.01) from acute (93.9 and 87.9%) rather than chronic (60.6 and 53.0%) infections. The r-SAG2 gave a similar sensitivity in both groups of patients (93.9 and 95.5%) GRA6 and p35 detected antibodies more frequently in acute infections (93.9% and 87.9%) compared to chronic infections (60.6% and 53.0%). SAG2 showed similar sensitivity in both acute and chronic cases (93.9% and 95.5%). | 2005, [50] |
| pET32a(+) | BL21 (DE3) | TRX-(Hisx6)-GRA2 | LB, 37 °C, 3 h | 12 mg/L induced bacterial culture | + | − | Specificity of IgG ELISA was 96.4%. Sensitivity was 95.8% to 100% for acute infection sera and 65.7% to 71.4% for chronic infection sera. | 2007, [51] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-MAG130–222-6xHis | LB, 37 °C, 16 h | 90 mg/L induced bacterial culture | + | − | Can distinguish between acute (97.3% sensitivity) and chronic (7.5% sensitivity) phases of toxoplasmosis. | 2007, [52] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-MIC125–182-6xHis 6xHis-MIC1183–456-6xHis 6xHis-MIC125–456-6xHis | LB, 37 °C | 16–24 mg/L induced bacterial culture | + | − | The three recombinant MIC1 proteins showed similar antigenicity for acute toxoplasmosis sera, but chronic infection sera had significantly lower sensitivity. | 2008, [53] |
| pET-28b(+) | Rosetta (DE3) | 6xHis-GRA718–236-6xHis | LB, 37 °C, 5 h | − | + | − | Western blot results indicated strong recognition of GRA7 by acute sera, weak detection by chronic sera, and no specific bands in negative sera. | 2009, [54] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-ROP185–396-6xHis 6xHis-GRA224–185-6xHis | LB, 37 °C, 16 h | 16 mg/L 28 mg/L induced bacterial culture | + | − | GRA2 and ROP1 showed higher sensitivity in acute infection sera (100% and 94.6%) than in chronic infection sera (22.5% and 15.5%). | 2009, [55] |
| pUET1 | BL21 (DE3) pLysS | 6xHis-GRA526–120-6xHis | LB, 30 °C, 8 h | 15 mg/L induced bacterial culture | + | − | Anti-GRA5 IgG antibodies were found in 70.9% of seropositive samples, similar to TLA-ELISA results. | 2010, [56] |
| pET-28b(+) | Rosetta (DE3) | 6xHis-S-GRA823–169-6xHis | LB, 30 °C, 5 h | 68 mg/L induced bacterial culture | + | − | IgM GRA8-ELISA had 97.1% specificity and 60.6% sensitivity. | 2011, [57] |
| pET-32c | BL21 (DE3) | Trx-(Hisx6)-SAG1309–318-SAG2109–118-SAG3347–356-6xHis | LB, 37 °C, 4 h | − | + | − | IgG ELISA showed 94.4% sensitivity and 100% specificity. IgM ELISA showed 96.9% sensitivity and 100% specificity | 2012, [58] |
| pUET1 | Rosetta (DE3) pLacI | 6xHis-MIC125–182-MAG130–222-6xHis | LB, 37 °C, 16 h | 43 mg/L induced bacterial culture | + | − | The IgG MIC1-MAG1-ELISA showed 90.8% sensitivity, similar to TLA (91.8%) and higher than MIC1, MAG1, or their mixture. | 2012, [59] |
| pUET1 | - | 6xHis-MIC125–182-MAG130–222-SAG149–198-6xHis | − | 20 mg/L induced bacterial culture | + | − | The MIC1-MAG1-SAG1-ELISA had 100% specificity and nearly 100% sensitivity, outperforming assays with just MIC1-MAG1 protein. | 2012, [60] |
| pET-30a(+) | Rosetta (DE3) | 6xHis-PDIc-6xHis | 25 °C, 8 h | − | − | + | Nasal immunization with PDI induced a protective immune response in mice, increasing the 30-day survival rate by about 31% compared to the control group. | 2013, [61] |
| pET-32a | BL21 (DE3) pLysS | 6xHis-GRA4-6xHis | LB, 6–8 h | − | − | + | Subcutaneous immunization induced high IgG levels, which declined after one week, and elevated IFN-γ, interleukin (IL) 10, and IL-4. However, it did not confer protection against T. gondii in mice. | 2013, [62] |
| pET-30 Ek/LIC | Rosetta (DE3) pLacI | 6xHis-SAG2-GRA1-ROP185–396-6xHis 6xHis-SAG2-GRA1-ROP185–250-6xHis | LB, 30 °C, 18 h | 31 mg/L 33 mg/L induced bacterial culture | + | − | IgG ELISA using SAG2-GRA1-ROP185–396—100%, sensitivity. IgG ELISA using SAG2-GRA1-ROP185–250—88.4% sensitivity. | 2015, [63] |
| pUET1 | Rosetta (DE3) pLacI | 6xHis-P3526–170-MAG130–222-6xHis 6xHis-MIC125–182-ROP1113–295-6xHis 6xHis-MAG130–222-ROP1113–295-6xHis | LB, 37 °C, 16 h | 43 mg/L 25 mg/L 36 mg/L induced bacterial culture | + | − | The reactivity of IgG ELISA for acute toxoplasmosis sera was 100% for P35-MAG1, 77.3% for MIC1-ROP1, and 86.4% for MAG1-ROP1, significantly higher than for chronic infection sera (26.2%, 36.1%, and 32.8%, respectively). IgM ELISA using P35-MAG1, MIC1-ROP1, and MAG1-ROP1 had a sensitivity of 81.8%, 72.7%, and 59.1%, respectively | 2015, [64] |
| pET-30a(+) | BL21 (DE3) | 6xHis-ADFd-6xHis | LB, 25 °C, 12 h | − | − | + | Intranasal immunization increased secretory IgA, IgG titers, splenocyte proliferation, and IL-2 and IFN-γ secretion. It improved survival by 36.36% and reduced liver and brain tachyzoite loads by 67.77% and 51.01%. | 2016, [65] |
| pET-28α | BL21 (DE3) | 6xHis-ROP18-6xHis | LB, 30 °C, 6 h | − | − | + | Intranasal immunizations with ROP18 in nanospheres induced higher levels of IgA and IgG2a as compared to groups inoculated intranasally with ROP18 alone or subcutaneously injected with ROP18 in montanide adjuvant. | 2017, [66] |
| pET-28α | BL21 (DE3) | 6xHis-SAG1-6xHis | LB, 30 °C | − | − | + | Intranasal immunization with SAG1 in nanospheres induced higher specific IgA and IgG2a responses compared to controls. | 2018, [67] |
| pET28a | BL21 | 6xHis-CDPK3e-6xHis | LB, 30 °C, 6 h | − | − | + | Intramuscular immunization induced spleen cell proliferation, IFN-γ release, and high IgG titers. It partially protected against acute toxoplasmosis, reducing brain cysts by 46.5%. | 2019, [68] |
| pET28a | BL21 (DE3) | 6xHis-GRA2-6xHis 6xHis-GRA7-6xHis 6xHis-TPIf-6xHis | LB, 37 °C, 4 h | − | + | − | The sensitivities of GRA2, GRA7, TPI, and their mixture were 85%, 83.3%, 88.3%, and 96.7%, respectively, with specificities of 85%, 90%, 100%, and 100%. GRA2 and GRA7 showed cross-reactivity. | 2019, [69] |
| pET-30 Ek/LIC | Rosetta (DE3) pLacI | 6xHis -SAG2-GRA1-ROP1-AMA167–287-6xHis 6xHis-AMA167–287-SAG2-GRA1-ROP1-6xHis 6xHis-AMA1287–569-SAG2-GRA1-ROP1-6xHis 6xHis-AMA167–569-SAG2-GRA1-ROP1-6xHis | TB, 23 °C, 18 h | 11–23 mg/L induced bacterial culture | + | − | All chimeric proteins demonstrated 100% sensitivity and specificity in IgG ELISA. Avidity results were comparable to commercial assays. | 2019, [70] |
| pET-30 Ek/LIC | Rosetta (DE3) pLysS | 6xHis-AMA167–287-6xHis 6xHis-AMA1287–569-6xHis 6xHis-AMA167–569-6xHis | LB, 30 °C, 4 h | 33 mg/L 31 mg/L 15 mg/L induced bacterial culture | + | − | The full-length AMA1 antigen outperforms its fragments in diagnostic assays. High reactivity with anti-T. gondii IgG (99.4%) and IgM (80.0%) antibodies. | 2020, [71] |
| pET-28a | BL21 (DE3) | 6xHis-MIC330–180-ROP885–185-SAG185–235-6xHis | LB, 37 °C, 6 h | − | − | + | Mice immunized with MIC3-ROP8-SAG1 showed stronger humoral and Th1 responses. Co-immunization with Freund and calcium phosphate nanoparticles impaired responses. Survival time increased by 15 days. | 2021, [72] |
| pET-28a (+) | BL21 (DE3) | 6xHis-ROP18377–546—MIC4302–471–SAG1130–299-6xHis | LB, 37 °C, 6 h | − | − | + | Vaccinated mice, particularly with ROP18-MIC4-SAG1-Freund, showed high levels of total IgG, IgG2a, and IFN-γ. Survival time increased by 15 days after the challenge. | 2023, [73] |
3.2. Protein Solubility
4. Yeast Expression Systems
5. Leishmania Tarentolae
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global Status of Toxoplasma gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar] [PubMed]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The Life-Cycle of Toxoplasma gondii Reviewed Using Animations. Parasit. Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From Animals to Humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Deganich, M.; Boudreaux, C.; Benmerzouga, I. Toxoplasmosis Infection during Pregnancy. Trop. Med. Infect. Dis. 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, Diagnosis and Prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B.S.; Juhas, M.; Zhang, Y. Toxoplasma gondii Secretory Proteins and Their Role in Invasion and Pathogenesis. Microbiol. Res. 2019, 227, 126293. [Google Scholar] [CrossRef]
- Holec-Gasior, L. Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef]
- Kotresha, D.; Noordin, R. Recombinant Proteins in the Diagnosis of Toxoplasmosis. APMIS 2010, 118, 529–542. [Google Scholar] [CrossRef]
- Buxton, D.; Thomson, K.M.; Maley, S.; Wright, S.; Bos, H.J. Experimental Challenge of Sheep 18 Months after Vaccination with a Live (S48) Toxoplasma gondii Vaccine. Vet. Rec. 1993, 133, 310–312. [Google Scholar] [CrossRef]
- Buxton, D. Toxoplasmosis: The First Commercial Vaccine. Parasitol. Today 1993, 9, 335–337. [Google Scholar] [CrossRef]
- Karakavuk, T.; Gül, C.; Karakavuk, M.; Gül, A.; Erkunt Alak, S.; Can, H.; Ün, C.; Döşkaya, M.; Gürüz, A.Y.; Değirmenci Döşkaya, A. Biotechnological Based Recombinant Protein Vaccines Developed Against Toxoplasmosis. Turk. Parazitolojii Derg. 2022, 46, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.J.; Fathollahi, A.; Arab-Mazar, Z.; Kohansal, K.; Fathollahi, M.; Spotin, A.; Bashiri, H.; Bozorgomid, A. Toxoplasma gondii Vaccine Candidates: A Concise Review. Ir. J. Med. Sci. 2023, 192, 231–261. [Google Scholar] [CrossRef]
- Kuriakose, A.; Chirmule, N.; Nair, P. Immunogenicity of Biotherapeutics: Causes and Association with Posttranslational Modifications. J. Immunol. Res. 2016, 2016, 1298473. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P.S. Identification of Conformational B-Cell Epitopes in an Antigen from Its Primary Sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of Bacterial Expression Systems for Heterologous Protein Production: From Molecular and Biochemical Fundamentals to Commercial Systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Chen, R. Bacterial Expression Systems for Recombinant Protein Production: E. coli and Beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Samuelson, J.C. Recent Developments in Difficult Protein Expression: A Guide to E. coli Strains, Promoters, and Relevant Host Mutations. Methods Mol. Biol. 2011, 705, 195–209. [Google Scholar] [CrossRef]
- Sweet, C.R. Expression of Recombinant Proteins From Lac Promoters. In E. coli Plasmid Vectors: Methods and Applications; Casali, N., Preston, A., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 277–288. ISBN 978-1-59259-409-2. [Google Scholar]
- de Boer, H.A.; Comstock, L.J.; Vasser, M. The Tac Promoter: A Functional Hybrid Derived from the Trp and Lac Promoters. Proc. Natl. Acad. Sci. USA 1983, 80, 21–25. [Google Scholar] [CrossRef]
- Hehl, A.B.; Lekutis, C.; Grigg, M.E.; Bradley, P.J.; Dubremetz, J.F.; Ortega-Barria, E.; Boothroyd, J.C. Toxoplasma gondii Homologue of Plasmodium Apical Membrane Antigen 1 Is Involved in Invasion of Host Cells. Infect. Immun. 2000, 68, 7078–7086. [Google Scholar] [CrossRef]
- Dando, C.; Schroeder, E.R.; Hunsaker, L.A.; Deck, L.M.; Royer, R.E.; Zhou, X.; Parmley, S.F.; Vander Jagt, D.L. The Kinetic Properties and Sensitivities to Inhibitors of Lactate Dehydrogenases (LDH1 and LDH2) from Toxoplasma gondii: Comparisons with PLDH from Plasmodium falciparum. Mol. Biochem. Parasitol. 2001, 118, 23–32. [Google Scholar] [CrossRef]
- Reiff, S.B.; Vaishnava, S.; Striepen, B. The HU Protein Is Important for Apicoplast Genome Maintenance and Inheritance in Toxoplasma gondii. Eukaryot. Cell 2012, 11, 905–915. [Google Scholar] [CrossRef]
- Heintzelman, M.B.; Schwartzman, J.D. Characterization of Myosin-A and Myosin-C: Two Class XIV Unconventional Myosins from Toxoplasma gondii. Cell Motil. Cytoskeleton 1999, 44, 58–67. [Google Scholar] [CrossRef]
- Dunn, J.D.; Ravindran, S.; Kim, S.-K.; Boothroyd, J.C. The Toxoplasma gondii Dense Granule Protein GRA7 Is Phosphorylated upon Invasion and Forms an Unexpected Association with the Rhoptry Proteins ROP2 and ROP4. Infect. Immun. 2008, 76, 5853–5861. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, F.L.; Nickdel, M.B.; McLeod, R.; Lyons, R.E.; Lyons, K.; Dubremetz, J.F.; Grigg, M.E.; Samuel, B.U.; Roberts, C.W. Toxoplasma gondii Dense Granule Protein 3 (GRA3) Is a Type I Transmembrane Protein That Possesses a Cytoplasmic Dilysine (KKXX) Endoplasmic Reticulum (ER) Retrieval Motif. Parasitology 2005, 131, 169–179. [Google Scholar] [CrossRef]
- Hehl, A.; Krieger, T.; Boothroyd, J.C. Identification and Characterization of SRS1, a Toxoplasma gondii Surface Antigen Upstream of and Related to SAG1. Mol. Biochem. Parasitol. 1997, 89, 271–282. [Google Scholar] [CrossRef]
- Lekutis, C.; Ferguson, D.J.; Boothroyd, J.C. Toxoplasma gondii: Identification of a Developmentally Regulated Family of Genes Related to SAG2. Exp. Parasitol. 2000, 96, 89–96. [Google Scholar] [CrossRef]
- Manger, I.D.; Hehl, A.B.; Boothroyd, J.C. The Surface of Toxoplasma Tachyzoites Is Dominated by a Family of Glycosylphosphatidylinositol-Anchored Antigens Related to SAG1. Infect. Immun. 1998, 66, 2237–2244. [Google Scholar] [CrossRef]
- Kimple, M.E.; Brill, A.L.; Pasker, R.L. Overview of Affinity Tags for Protein Purification. Curr. Protoc. Protein Sci. 2013, 73, 9.9.1–9.9.23. [Google Scholar] [CrossRef]
- Pina, A.S.; Batalha, Í.L.; Dias, A.M.G.C.; Roque, A.C.A. Affinity Tags in Protein Purification and Peptide Enrichment: An Overview. Methods Mol. Biol. 2021, 2178, 107–132. [Google Scholar] [CrossRef]
- Tenter, A.M.; Johnson, A.M. Recognition of Recombinant Toxoplasma gondii Antigens by Human Sera in an ELISA. Parasitol. Res. 1991, 77, 197–203. [Google Scholar] [CrossRef]
- Parmley, S.F.; Sgarlato, G.D.; Mark, J.; Prince, J.B.; Remington, J.S. Expression, Characterization, and Serologic Reactivity of Recombinant Surface Antigen P22 of Toxoplasma gondii. J. Clin. Microbiol. 1992, 30, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Redlich, A.; Müller, W.A. Serodiagnosis of Acute Toxoplasmosis Using a Recombinant Form of the Dense Granule Antigen GRA6 in an Enzyme-Linked Immunosorbent Assay. Parasitol. Res. 1998, 84, 700–706. [Google Scholar] [CrossRef]
- Lecordier, L.; Fourmaux, M.P.; Mercier, C.; Dehecq, E.; Masy, E.; Cesbron-Delauw, M.F. Enzyme-Linked Immunosorbent Assays Using the Recombinant Dense Granule Antigens GRA6 and GRA1 of Toxoplasma gondii for Detection of Immunoglobulin G Antibodies. Clin. Diagn. Lab. Immunol. 2000, 7, 607–611. [Google Scholar] [CrossRef]
- Wang, H.-L.; Zhang, T.-E.; Yin, L.-T.; Pang, M.; Guan, L.; Liu, H.-L.; Zhang, J.-H.; Meng, X.-L.; Bai, J.-Z.; Zheng, G.-P.; et al. Partial Protective Effect of Intranasal Immunization with Recombinant Toxoplasma gondii Rhoptry Protein 17 against Toxoplasmosis in Mice. PLoS ONE 2014, 9, e108377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, C.-D.; Chang, G.-N.; Chao, D. Protective Immunity against Toxoplasma gondii in Mice Induced by a Chimeric Protein RSAG1/2. Parasitol. Res. 2004, 92, 58–64. [Google Scholar] [CrossRef]
- Elisa, B.; Andrea, S.; Luca, B.; Wilma, B.; Nicola, G. Chimeric Antigens of Toxoplasma gondii: Toward Standardization of Toxoplasmosis Serodiagnosis Using Recombinant Products. J. Clin. Microbiol. 2006, 44, 2133–2140. [Google Scholar] [CrossRef]
- Song, K.J.; Yang, Z.; Chong, C.-K.; Kim, J.-S.; Lee, K.C.; Kim, T.-S.; Nam, H.-W. A Rapid Diagnostic Test for Toxoplasmosis Using Recombinant Antigenic N-Terminal Half of SAG1 Linked with Intrinsically Unstructured Domain of Gra2 Protein. Korean J. Parasitol. 2013, 51, 503–510. [Google Scholar] [CrossRef]
- Yang, Z.; Ahn, H.-J.; Nam, H.-W. High Expression of Water-Soluble Recombinant Antigenic Domains of Toxoplasma gondii Secretory Organelles. Korean J. Parasitol. 2014, 52, 367–376. [Google Scholar] [CrossRef]
- Arab-Mazar, Z.; Fallahi, S.; Koochaki, A.; Haghighi, A.; Seyyed Tabaei, S.J. Immunodiagnosis and Molecular Validation of Toxoplasma gondii-Recombinant Dense Granular (GRA) 7 Protein for the Detection of Toxoplasmosis in Patients with Cancer. Microbiol. Res. 2016, 183, 53–59. [Google Scholar] [CrossRef]
- Arab-Mazar, Z.; Javadi Mamaghani, A.; Fallahi, S.; Rajaeian, S.; Koochaki, A.; Seyyed Tabaei, S.J.; Rezaee, H. Immunodiagnosis and Molecular Validation of Toxoplasma gondii-Recombinant Dense Granular (GRA) 5 Protein for the Detection of Toxoplasmosis in Hemodialysis Patients. Semin. Dial. 2021, 34, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Shimoda, N.; Nakamura, S.; Fox, B.A.; Bzik, D.J.; Ushio-Watanabe, N.; Nishikawa, Y. Protective Efficacy of Recombinant Toxoplasma gondii Dense Granule Protein 15 against Toxoplasmosis in C57BL/6 Mice. Vaccine 2024, 42, 2299–2309. [Google Scholar] [CrossRef]
- Carson, M.; Johnson, D.H.; McDonald, H.; Brouillette, C.; Delucas, L.J. His-Tag Impact on Structure. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Tran, K.C.; Arnold, J.J.; Teng, M.N. Purification and Characterization of Recombinant Human Respiratory Syncytial Virus Nonstructural Protein NS1. Protein Expr. Purif. 2008, 57, 261–270. [Google Scholar] [CrossRef]
- Yap, W.B.; Tey, B.T.; Ng, M.Y.T.; Ong, S.T.; Tan, W.S. N-Terminally His-Tagged Hepatitis B Core Antigens: Construction, Expression, Purification and Antigenicity. J. Virol. Methods 2009, 160, 125–131. [Google Scholar] [CrossRef]
- Goel, A.; Colcher, D.; Koo, J.-S.; Booth, B.J.M.; Pavlinkova, G.; Batra, S.K. Relative Position of the Hexahistidine Tag Effects Binding Properties of a Tumor-Associated Single-Chain Fv Construct. Biochim. Biophys. Acta-Gen. Subj. 2000, 1523, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Legler, P.M.; Mease, R.M.; Duncan, E.H.; Bergmann-Leitner, E.S.; Angov, E. Histidine Affinity Tags Affect MSP1(42) Structural Stability and Immunodominance in Mice. Biotechnol. J. 2012, 7, 133–147. [Google Scholar] [CrossRef]
- Nockemann, S.; Dlugonska, H.; Henrich, B.; Kitzerow, A.; Däubener, W. Expression, Characterization and Serological Reactivity of a 41 KDa Excreted–Secreted Antigen (ESA) from Toxoplasma gondii. Mol. Biochem. Parasitol. 1998, 97, 109–121. [Google Scholar] [CrossRef]
- Hiszczyńska-Sawicka, E.; Kur, J.; Pietkiewicz, H.; Holec-Gasior, L.; G1sior, A.; Myjak, P. Efficient Production of the Toxoplasma gondii GRA6, P35 and SAG2 Recombinant Antigens and Their Applications in the Serodiagnosis of Toxoplasmosis. Acta Parasitol. 2005, 50, 249–254. [Google Scholar]
- Golkar, M.; Rafati, S.; Abdel-Latif, M.S.; Brenier-Pinchart, M.-P.; Fricker-Hidalgo, H.; Sima, B.K.; Babaie, J.; Pelloux, H.; Cesbron-Delauw, M.-F.; Mercier, C. The Dense Granule Protein GRA2, a New Marker for the Serodiagnosis of Acute Toxoplasma Infection: Comparison of Sera Collected in Both France and Iran from Pregnant Women. Diagn. Microbiol. Infect. Dis. 2007, 58, 419–426. [Google Scholar] [CrossRef]
- Holec, L.; Hiszczyńska-Sawicka, E.; Gasior, A.; Brillowska-Dabrowska, A.; Kur, J. Use of MAG1 Recombinant Antigen for Diagnosis of Toxoplasma gondii Infection in Humans. Clin. Vaccine Immunol. 2007, 14, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Holec, L.; Gasior, A.; Brillowska-Dabrowska, A.; Kur, J. Toxoplasma gondii: Enzyme-Linked Immunosorbent Assay Using Different Fragments of Recombinant Microneme Protein 1 (MIC1) for Detection of Immunoglobulin G Antibodies. Exp. Parasitol. 2008, 119, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sadeghiani, G.; Zare, M.; Babaie, J.; Shokrgozar, M.-A.; Azadmanesh, K.; Fard-Esfahani, P.; Golkar, M. Heterologous Production of Dense Granule GRA7 Antigen of Toxoplasma gondii in Escherichia coli. Southeast Asian J. Trop. Med. Public Health 2009, 40, 692–700. [Google Scholar] [PubMed]
- Holec-Gasior, L.; Kur, J.; Hiszczyńska-Sawicka, E. GRA2 and ROP1 Recombinant Antigens as Potential Markers for Detection of Toxoplasma gondii-Specific Immunoglobulin G in Humans with Acute Toxoplasmosis. Clin. Vaccine Immunol. 2009, 16, 510–514. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Kur, J. Toxoplasma gondii: Recombinant GRA5 Antigen for Detection of Immunoglobulin G Antibodies Using Enzyme-Linked Immunosorbent Assay. Exp. Parasitol. 2010, 124, 272–278. [Google Scholar] [CrossRef]
- Babaie, J.; Miri, M.; Sadeghiani, G.; Zare, M.; Khalili, G.; Golkar, M. Expression and Single-Step Purification of GRA8 Antigen of Toxoplasma gondii in Escherichia coli. Avicenna J. Med. Biotechnol. 2011, 3, 67–77. [Google Scholar]
- Jianfang, D.; Min, J.; Yanyun, W.; Lili, Q.; Rujun, G.; Jin, S. Evaluation of a Recombinant Multiepitope Peptide for Serodiagnosis of Toxoplasma gondii Infection. Clin. Vaccine Immunol. 2012, 19, 338–342. [Google Scholar] [CrossRef]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D.; Lautenbach, D.; Kur, J. A New MIC1-MAG1 Recombinant Chimeric Antigen Can Be Used Instead of the Toxoplasma gondii Lysate Antigen in Serodiagnosis of Human Toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 57–63. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Ferra, B.; Drapala, D. MIC1-MAG1-SAG1 Chimeric Protein, a Most Effective Antigen for Detection of Human Toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 1977–1979. [Google Scholar] [CrossRef][Green Version]
- Wang, H.-L.; Li, Y.-Q.; Yin, L.-T.; Meng, X.-L.; Guo, M.; Zhang, J.-H.; Liu, H.-L.; Liu, J.-J.; Yin, G.-R. Toxoplasma gondii Protein Disulfide Isomerase (TgPDI) Is a Novel Vaccine Candidate against Toxoplasmosis. PLoS ONE 2013, 8, e70884. [Google Scholar] [CrossRef]
- Ram, H.; Rao, J.R.; Tewari, A.K.; Banerjee, P.S.; Sharma, A.K. Molecular Cloning, Sequencing, and Biological Characterization of GRA4 Gene of Toxoplasma gondii. Parasitol. Res. 2013, 112, 2487–2494. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. A New Toxoplasma gondii Chimeric Antigen Containing Fragments of SAG2, GRA1, and ROP1 Proteins—Impact of Immunodominant Sequences Size on Its Diagnostic Usefulness. Parasitol. Res. 2015, 114, 3291–3299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drapała, D.; Holec-Gasior, L.; Kur, J. New Recombinant Chimeric Antigens, P35-MAG1, MIC1-ROP1, and MAG1-ROP1, for the Serodiagnosis of Human Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2015, 82, 34–39. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, L.; Li, Y.; Yuan, F.; Zhang, X.; Ma, J.; Liu, H.; Wang, Y.; Zheng, K.; Cao, J. Intranasal Immunization with Recombinant Toxoplasma gondii Actin Depolymerizing Factor Confers Protective Efficacy against Toxoplasmosis in Mice. BMC Immunol. 2016, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Nabi, H.; Rashid, I.; Ahmad, N.; Durrani, A.; Akbar, H.; Islam, S.; Bajwa, A.A.; Shehzad, W.; Ashraf, K.; Imran, N. Induction of Specific Humoral Immune Response in Mice Immunized with ROP18 Nanospheres from Toxoplasma gondii. Parasitol. Res. 2017, 116, 359–370. [Google Scholar] [CrossRef]
- Naeem, H.; Sana, M.; Islam, S.; Khan, M.; Riaz, F.; Zafar, Z.; Akbar, H.; Shehzad, W.; Rashid, I. Induction of Th1 Type-Oriented Humoral Response through Intranasal Immunization of Mice with SAG1-Toxoplasma gondii Polymeric Nanospheres. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; An, R.; Chen, Y.; Chen, T.; Wen, H.; Yan, Q.; Shen, J.; Chen, L.; Du, J. Vaccination with Recombinant Toxoplasma gondii CDPK3 Induces Protective Immunity against Experimental Toxoplasmosis. Acta Trop. 2019, 199, 105148. [Google Scholar] [CrossRef]
- Luo, J.; Wan, J.; Tang, Z.; Shen, S. Identification of Novel Antigens for Serum IgG Diagnosis of Human Toxoplasmosis. Exp. Parasitol. 2019, 204, 107722. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K.; Grąźlewska, W.; Lautenbach, D. The First Study on the Usefulness of Recombinant Tetravalent Chimeric Proteins Containing Fragments of SAG2, GRA1, ROP1 and AMA1 Antigens in the Detection of Specific Anti-Toxoplasma gondii Antibodies in Mouse and Human Sera. PLoS ONE 2019, 14, e0217866. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K. Toxoplasma gondii Recombinant Antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies. Pathogens 2020, 9, 43. [Google Scholar] [CrossRef]
- Dodangeh, S.; Fasihi-Ramandi, M.; Daryani, A.; Valadan, R.; Asgarian-Omran, H.; Hosseininejad, Z.; Nayeri Chegeni, T.; Pagheh, A.S.; Javidnia, J.; Sarvi, S. Protective Efficacy by a Novel Multi-Epitope Vaccine, Including MIC3, ROP8, and SAG1, against Acute Toxoplasma gondii Infection in BALB/c Mice. Microb. Pathog. 2021, 153, 104764. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Fasihi-Ramandi, M.; Valadan, R.; Asgarian-Omran, H.; Ajami, A.; Khalilian, A.; Hosseininejad, Z.; Dodangeh, S.; Javidnia, J.; et al. Enhancement of Immune Responses by Vaccine Potential of Three Antigens, Including ROP18, MIC4, and SAG1 against Acute Toxoplasmosis in Mice. Exp. Parasitol. 2023, 244, 108427. [Google Scholar] [CrossRef]
- Qing, G.; Ma, L.-C.; Khorchid, A.; Swapna, G.V.T.; Mal, T.K.; Takayama, M.M.; Xia, B.; Phadtare, S.; Ke, H.; Acton, T.; et al. Cold-Shock Induced High-Yield Protein Production in Escherichia coli. Nat. Biotechnol. 2004, 22, 877–882. [Google Scholar] [CrossRef]
- Francis, D.M.; Page, R. Strategies to Optimize Protein Expression in E. coli. Curr. Protoc. Protein Sci. 2010, 5, 5.24.1–5.24.29. [Google Scholar] [CrossRef]
- Sonaimuthu, P.; Fong, M.Y.; Kalyanasundaram, R.; Mahmud, R.; Lau, Y.L. Sero-Diagnostic Evaluation of Toxoplasma gondii Recombinant Rhoptry Antigen 8 Expressed in E. coli. Parasit. Vectors 2014, 7, 297. [Google Scholar] [CrossRef][Green Version]
- Sonaimuthu, P.; Ching, X.T.; Fong, M.Y.; Kalyanasundaram, R.; Lau, Y.L. Induction of Protective Immunity against Toxoplasmosis in BALB/c Mice Vaccinated with Toxoplasma gondii Rhoptry-1. Front. Microbiol. 2016, 7, 808. [Google Scholar] [CrossRef]
- Atroshenko, D.L.; Sergeev, E.P.; Golovina, D.I.; Pometun, A.A. Additivities for Soluble Recombinant Protein Expression in Cytoplasm of Escherichia coli. Fermentation 2024, 10, 120. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of Protein Fold Stability on Immunogenicity and Its Implications for Vaccine Design. Expert Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Nozach, H.; Fruchart-Gaillard, C.; Fenaille, F.; Beau, F.; Ramos, O.H.P.; Douzi, B.; Saez, N.J.; Moutiez, M.; Servent, D.; Gondry, M.; et al. High Throughput Screening Identifies Disulfide Isomerase DsbC as a Very Efficient Partner for Recombinant Expression of Small Disulfide-Rich Proteins in E. coli. Microb. Cell Fact. 2013, 12, 37. [Google Scholar] [CrossRef]
- Baneyx, F.; Mujacic, M. Recombinant Protein Folding and Misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, A.; Kaur, J. Strategies for Optimization of Heterologous Protein Expression in E. coli: Roadblocks and Reinforcements. Int. J. Biol. Macromol. 2018, 106, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Hatahet, F.; Salo, K.E.H.; Enlund, E.; Zhang, C.; Ruddock, L.W. Pre-Expression of a Sulfhydryl Oxidase Significantly Increases the Yields of Eukaryotic Disulfide Bond Containing Proteins Expressed in the Cytoplasm of E. coli. Microb. Cell Fact. 2011, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Stern, D.; Seeber, F. Expression of in Vivo Biotinylated Recombinant Antigens SAG1 and SAG2A from Toxoplasma gondii for Improved Seroepidemiological Bead-Based Multiplex Assays. BMC Biotechnol. 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Grigg, M.E.; Boothroyd, J.C.; Garcia, K.C. Structure of the Immunodominant Surface Antigen from the Toxoplasma gondii SRS Superfamily. Nat. Struct. Biol. 2002, 9, 606–611. [Google Scholar] [CrossRef]
- Karyolaimos, A.; de Gier, J.-W. Strategies to Enhance Periplasmic Recombinant Protein Production Yields in Escherichia coli. Front. Bioeng. Biotechnol. 2021, 9, 797334. [Google Scholar] [CrossRef]
- Allahyari, M.; Mohabati, R.; Babaie, J.; Amiri, S.; Siavashani, Z.J.; Zare, M.; Sadeghiani, G.; Golkar, M. Production of In-Vitro Refolded and Highly Antigenic SAG1 for Development of a Sensitive and Specific Toxoplasma IgG ELISA. J. Immunol. Methods 2015, 416, 157–166. [Google Scholar] [CrossRef]
- Jacquet, A.; Daminet, V.; Haumont, M.; Garcia, L.; Chaudoir, S.; Bollen, A.; Biemans, R. Expression of a Recombinant Toxoplasma gondii ROP2 Fragment as a Fusion Protein in Bacteria Circumvents Insolubility and Proteolytic Degradation. Protein Expr. Purif. 1999, 17, 392–400. [Google Scholar] [CrossRef]
- Graille, M.; Stura, E.A.; Bossus, M.; Muller, B.H.; Letourneur, O.; Battail-Poirot, N.; Sibaï, G.; Gauthier, M.; Rolland, D.; Le Du, M.-H.; et al. Crystal Structure of the Complex between the Monomeric Form of Toxoplasma gondii Surface Antigen 1 (SAG1) and a Monoclonal Antibody That Mimics the Human Immune Response. J. Mol. Biol. 2005, 354, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, A.; Saadatnia, G.; Golkar, M.; Babaie, J.; Noordin, R. Production of Refolded Toxoplasma gondii Recombinant SAG1-Related Sequence 3 (SRS3) and Its Use for Serodiagnosis of Human Toxoplasmosis. Protein Expr. Purif. 2017, 133, 66–74. [Google Scholar] [CrossRef]
- Nigro, M.; Martin, V.; Kaufer, F.; Carral, L.; Angel, S.O.; Pszenny, V. High Level of Expression of the Toxoplasma gondii-Recombinant Rop2 Protein in Escherichia coli as a Soluble Form for Optimal Use in Diagnosis. Mol. Biotechnol. 2001, 18, 269–273. [Google Scholar] [CrossRef]
- Petsch, D.; Anspach, F.B. Endotoxin Removal from Protein Solutions. J. Biotechnol. 2000, 76, 97–119. [Google Scholar] [CrossRef]
- Daly, R.; Hearn, M.T.W. Expression of Heterologous Proteins in Pichia pastoris: A Useful Experimental Tool in Protein Engineering and Production. J. Mol. Recognit. 2005, 18, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, T.; Xu, C.; Li, J.; Liu, G.; Yin, K.; Cui, Y.; Wei, Q.; Huang, B.; Sun, H. Protective Immune Response against Toxoplasma gondii Elicited by a Novel Yeast-Based Vaccine with Microneme Protein 16. Vaccine 2018, 36, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Darby, R.A.J.; Cartwright, S.P.; Dilworth, M.V.; Bill, R.M. Which Yeast Species Shall I Choose? Saccharomyces cerevisiae versus Pichia pastoris (Review). Methods Mol. Biol. 2012, 866, 11–23. [Google Scholar] [CrossRef]
- Legastelois, I.; Buffin, S.; Peubez, I.; Mignon, C.; Sodoyer, R.; Werle, B. Non-Conventional Expression Systems for the Production of Vaccine Proteins and Immunotherapeutic Molecules. Hum. Vaccin. Immunother. 2017, 13, 947–961. [Google Scholar] [CrossRef]
- Chahed Bel-Ochi, N.; Bouratbine, A.; Mousli, M. Design and Characterization of a Recombinant Colorimetric SAG1–Alkaline Phosphatase Conjugate to Detect Specific Antibody Responses against Toxoplasma gondii. J. Immunol. Methods 2013, 394, 107–114. [Google Scholar] [CrossRef]
- Odenthal-Schnittler, M.; Tomavo, S.; Becker, D.; Dubremetz, J.F.; Schwarz, R.T. Evidence for N-Linked Glycosylation in Toxoplasma gondii. Biochem. J. 1993, 291 Pt 3, 713–721. [Google Scholar] [CrossRef]
- Biemans, R.; Grégoire, D.; Haumont, M.; Bosseloir, A.; Garcia, L.; Jacquet, A.; Dubeaux, C.; Bollen, A. The Conformation of Purified Toxoplasma gondii SAG1 Antigen, Secreted from Engineered Pichia pastoris, Is Adequate for Serorecognition and Cell Proliferation. J. Biotechnol. 1998, 66, 137–146. [Google Scholar] [CrossRef]
- Letourneur, O.; Gervasi, G.; Gaïa, S.; Pagès, J.; Watelet, B.; Jolivet, M. Characterization of Toxoplasma gondii Surface Antigen 1 (SAG1) Secreted from Pichia pastoris: Evidence of Hyper O-Glycosylation. Biotechnol. Appl. Biochem. 2001, 33, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Lau, Y.L.; Zulqarnain, M. Characterization of Secreted Recombinant Toxoplasma gondii Surface Antigen 2 (SAG2) Heterologously Expressed by the Yeast Pichia pastoris. Biotechnol. Lett. 2008, 30, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-L.; Fong, M.-Y.; Idris, M.M.; Ching, X.-T. Cloning and Expression of Toxoplasma gondii Dense Granule Antigen 2 (GRA2) Gene by Pichia pastoris. Southeast Asian J. Trop. Med. Public Health 2012, 43, 10–16. [Google Scholar] [PubMed]
- Lau, Y.L.; Hasan, M.T.; Thiruvengadam, G.; Idris, M.M.; Init, I. Cloning and Expression of Toxoplasma gondii Dense Granular Protein 4 (GRA4) in Pichia pastoris. Trop. Biomed. 2010, 27, 525–533. [Google Scholar]
- Lau, Y.L.; Thiruvengadam, G.; Lee, W.W.; Fong, M.Y. Immunogenic Characterization of the Chimeric Surface Antigen 1 and 2 (SAG1/2) of Toxoplasma gondii Expressed in the Yeast Pichia pastoris. Parasitol. Res. 2011, 109, 871–878. [Google Scholar] [CrossRef]
- Chang, P.Y.; Fong, M.Y.; Nissapatorn, V.; Lau, Y.L. Evaluation of Pichia pastoris-Expressed Recombinant Rhoptry Protein 2 of Toxoplasma gondii for Its Application in Diagnosis of Toxoplasmosis. Am. J. Trop. Med. Hyg. 2011, 85, 485–489. [Google Scholar] [CrossRef]
- Lau, Y.L.; Fong, M.Y. Toxoplasma gondii: Serological Characterization and Immunogenicity of Recombinant Surface Antigen 2 (SAG2) Expressed in the Yeast Pichia pastoris. Exp. Parasitol. 2008, 119, 373–378. [Google Scholar] [CrossRef]
- Thiruvengadam, G.; Init, I.; Fong, M.Y.; Lau, Y.L. Optimization of the Expression of Surface Antigen SAG1/2 of Toxoplasma gondii in the Yeast Pichia pastoris. Trop. Biomed. 2011, 28, 506–513. [Google Scholar]
- Ling, L.Y.; Ithoi, I.; Yik, F.M. Optimization for High-Level Expression in Pichia pastoris and Purification of Truncated and Full Length Recombinant SAG2 of Toxoplasma gondii for Diagnostic Use. Southeast Asian J. Trop. Med. Public Health 2010, 41, 507–513. [Google Scholar]
- Fournier, J. A Review of Glycan Analysis Requirements. Biopharm Int. 2015, 28, 32–37. [Google Scholar]
- Werner, R.G.; Kopp, K.; Schlueter, M. Glycosylation of Therapeutic Proteins in Different Production Systems. Acta Paediatr. 2007, 96, 17–22. [Google Scholar] [CrossRef]
- Zhang, P.; Woen, S.; Wang, T.; Liau, B.; Zhao, S.; Chen, C.; Yang, Y.; Song, Z.; Wormald, M.R.; Yu, C.; et al. Challenges of Glycosylation Analysis and Control: An Integrated Approach to Producing Optimal and Consistent Therapeutic Drugs. Drug Discov. Today 2016, 21, 740–765. [Google Scholar] [CrossRef]
- Shen, W.; Xue, Y.; Liu, Y.; Kong, C.; Wang, X.; Huang, M.; Cai, M.; Zhou, X.; Zhang, Y.; Zhou, M. A Novel Methanol-Free Pichia pastoris System for Recombinant Protein Expression. Microb. Cell Fact. 2016, 15, 178. [Google Scholar] [CrossRef]
- Cos, O.; Ramón, R.; Montesinos, J.L.; Valero, F. Operational Strategies, Monitoring and Control of Heterologous Protein Production in the Methylotrophic Yeast Pichia pastoris under Different Promoters: A Review. Microb. Cell Fact. 2006, 5, 17. [Google Scholar] [CrossRef]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous Protein Production Using the Pichia pastoris Expression System. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Karbalaei, M.; Soleimanpour, S.; Mosavat, A.; Rezaee, S.A.; Ghazvini, K.; Farsiani, H. Practical Methods for Expression of Recombinant Protein in the Pichia pastoris System. Curr. Protoc. 2021, 1, e155. [Google Scholar] [CrossRef]
- Zhou, H.; Gu, Q.; Zhao, Q.; Zhang, J.; Cong, H.; Li, Y.; He, S. Toxoplasma gondii: Expression and Characterization of a Recombinant Protein Containing SAG1 and GRA2 in Pichia pastoris. Parasitol. Res. 2007, 100, 829–835. [Google Scholar] [CrossRef]
- Fernández-Robledo, J.A.; Vasta, G.R. Production of Recombinant Proteins from Protozoan Parasites. Trends Parasitol. 2010, 26, 244–254. [Google Scholar] [CrossRef][Green Version]
- Niimi, T. Recombinant Protein Production in the Eukaryotic Protozoan Parasite Leishmania tarentolae: A Review. Methods Mol. Biol. 2012, 824, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Taheri, T.; Seyed, N.; Mizbani, A.; Rafati, S. Leishmania-Based Expression Systems. Appl. Microbiol. Biotechnol. 2016, 100, 7377–7385. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, C.; Sitz, M.; Wolf, M.; Pohl, H.-D. Development of a Defined Medium for Heterologous Expression in Leishmania tarentolae. J. Basic Microbiol. 2008, 48, 488–495. [Google Scholar] [CrossRef]
- Kalef, D.A. Leishmania mexicana Recombinant Filamentous Acid Phosphatase as Carrier for Toxoplasma gondii Surface Antigen 1 Expression in Leishmania tarentolae. J. Parasit. Dis. 2021, 45, 1135–1144. [Google Scholar] [CrossRef]
- Majidiani, H.; Dalimi, A.; Ghaffarifar, F.; Pirestani, M. Multi-Epitope Vaccine Expressed in Leishmania tarentolae Confers Protective Immunity to Toxoplasma gondii in BALB/c Mice. Microb. Pathog. 2021, 155, 104925. [Google Scholar] [CrossRef]
- Redondo, A.; Wood, D.; Amaral, S.; Ferré, J.; Goti, D.; Bertran, J. Production of Toxoplasma gondii Recombinant Antigens in Genome-Edited Escherichia coli. Appl. Biochem. Microbiol. 2021, 57, 152–160. [Google Scholar] [CrossRef]
- Feldman, S.F.; Temkin, E.; Wulffhart, L.; Nutman, A.; Schechner, V.; Shitrit, P.; Shvartz, R.; Schwaber, M.J.; Carmeli, Y. Effect of Temperature on Escherichia coli Bloodstream Infection in a Nationwide Population-Based Study of Incidence and Resistance. Antimicrob. Resist. Infect. Control 2022, 11, 144. [Google Scholar] [CrossRef]
- Shin, M.-K.; Yoo, H.S. Animal Vaccines Based on Orally Presented Yeast Recombinants. Vaccine 2013, 31, 4287–4292. [Google Scholar] [CrossRef]
- Kim, K.; Bülow, R.; Kampmeier, J.; Boothroyd, J.C. Conformationally Appropriate Expression of the Toxoplasma Antigen SAG1 (P30) in CHO Cells. Infect. Immun. 1994, 62, 203–209. [Google Scholar] [CrossRef]
- Aghdasi, M.; Ghaffarifar, F.; Forooghi, F.; Dalimi Asl, A.H.; Sharifi, Z.; Maspi, N. Expression of Plasmid Encoded GRA4 Gene of Toxoplasma gondii RH Strain in CHO Eukaryotic Cells. Iran. J. Parasitol. 2018, 13, 392–398. [Google Scholar]
| Plasmid Vector | E. coli Host Strain | Protein | Expression Conditions | Yield | Application | Results | Reference, Year | |
|---|---|---|---|---|---|---|---|---|
| Diagnostic | Vaccine | |||||||
| pGEX-1N | JM105 | GST-H4 GST-H11 | − | − | + | − | Both antigens were highly specific for T. gondii antibodies. H4- and H11-ELISA can distinguish between acute and chronic phases of toxoplasmosis. | 1991, [32] |
| pGEX-2T | JM101 | P22a27–172 | LB, 37 °C, 3 h | − | + | − | Acute infection sera showed stronger IgG reaction with P22 protein (immunoblots and ELISA). IgA and IgM P22-ELISA showed no reactivity. | 1992, [33] |
| pGEX-3X | SURE | GST-GRA6 | − | − | + | − | GRA6-IgG EIA can distinguish between acute and chronic phases of toxoplasmosis. | 1998, [34] |
| pGEX-3X pGEX2T pGEX-3X | JM101 | GST-GRA1 GST-GRA6-Nt GST-GRA6-Ct | LB, 37 °C | − | + | − | GRA6-Ct IgG ELISA—10% sensitivity. GRA6-Nt IgG ELISA—96% sensitivity. GRA1 IgG ELISA—68% sensitivity | 2000, [35] |
| pGEX-6p-1 | BL21 Star (DE3) | SAG1/2, SAG1 SAG2 | 37 °C, 4 h | − | − | + | Vaccination with SAG1/2 protected 73% (11/15) of mice from a lethal challenge. SAG1 immunization—60% survival rate. SAG2 immunization—53% survival rate. | 2004, [37] |
| pGEX-SN | − | GST-EC2 (MIC2157–235-MIC3234–307-SAG1182–312) GST-EC3 (GRA336–134-GRA724–102-M2AP37–263) | − | GST-EC2 8 mg/L GST-EC3 5 mg/L induced bacterial culture | + | − | IgG and IgM ELISAs using EC2 and EC3 perform similarly to commercial assays. IgM-capture assays with chimeric antigens enhance postnatal congenital toxoplasmosis diagnosis. | 2006, [38] |
| pGEX-4T-1 | BL21 (DE3) | GST-GRA2-SAG1A | − | − | + | − | GRA2-SAG1A rapid diagnostic test showed 100% specificity 100% and 97.1% sensitivity. | 2013, [39] |
| pGEX-6P-1 | Rosetta (DE3) | GST-ROP17 | − | − | − | + | Intranasal ROP17 immunization in mice induces systemic and local immune responses. Provides protection against lethal T. gondii infections. Reduces tachyzoite burdens in host tissues. Increases animal survival rates. | 2014, [36] |
| pGEX-4T-1 | BL21 (DE3) pLysS | GST-GRA2 (aa 25–185, 25–135, 25–105, 75–185, 106–185, 25–105) GST-GRA3 (aa 39–138, 39–222) GST-ROP2 (aa 29–561, 29–323 324–561, 29–197, 324–483, 403–561, 324–430, 431–561) GST-MIC2 (aa 1–723, 1–651, 1–425, 219–651, 1–284, 142–425, 421–651, 1–215, 216–425, 142–284) GST-GRA231–71-MIC21–284 | 30 °C | − | + | − | GRA231–71-MIC21–284 shows a high diagnostic potential and may be used for developing a serological assay. | 2014, [40] |
| pGEX-6p-1 | BL21 | GST-GRA7 | LB, 37 °C, 6–8 h | − | + | − | GRA7-ELISA showed 92% sensitivity and 94% specificity. Results align closely with LAMP technique results. | 2016, [41] |
| pGEX-6p-1 | BL21 | GST-GRA5 | LB, 37 °C, 4 h | − | + | − | GRA5-ELISA with sera from hemodialysis patients showed 96% sensitivity and 93% specificity. | 2021, [42] |
| pGEX-4T1 | BL21 (DE3) | GRA15 | LB, 37 °C, 4 h | − | − | + | GRA15 immunization in mice induced IgG1 and IgG2c, boosted spleen cell proliferation and interferon γ (IFN-γ) production, improved survival rates, and reduced parasite burden against the Pru strain. | 2024, [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołowińska, K.; Holec-Gąsior, L. Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms 2024, 12, 1731. https://doi.org/10.3390/microorganisms12081731
Sołowińska K, Holec-Gąsior L. Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms. 2024; 12(8):1731. https://doi.org/10.3390/microorganisms12081731
Chicago/Turabian StyleSołowińska, Karolina, and Lucyna Holec-Gąsior. 2024. "Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis" Microorganisms 12, no. 8: 1731. https://doi.org/10.3390/microorganisms12081731
APA StyleSołowińska, K., & Holec-Gąsior, L. (2024). Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms, 12(8), 1731. https://doi.org/10.3390/microorganisms12081731






